Abstract
Bacterial endotoxin or lipopolysaccharide (LPS) is an important causative agent of sepsis. This study determines the expression of defensins NP-2 and NP-5 and the function of polymorphonuclear leukocytes (PMN) in rabbits treated with LPS. PMN functional activity was assessed by measuring CD18 expression and H2O2 production and by examining the lungs. NP-2 and, to a minor extent, NP-5 of circulating PMN increase during endotoxemia. This early increase is concomitant with neutrophilia and elevated CD18 expression and H2O2 production, as well as with enhanced NP-2 immunoreactivity in pulmonary microvessels. A decline in defensins, shortly after the last LPS treatment, is associated with a decrease in the circulating activated PMN and enhanced immunoreactivity in the inflammatory cells, as well as with lung tissue damage. This study shows that LPS-induced changes in the defensins of circulating PMN correlate with the number and activated condition of these cells and suggests that PMN-derived products implement the inflammatory reaction that leads to lung injury and sepsis.
Endotoxin from gram-negative bacteria causes a number of pathophysiological effects that can lead to the adult respiratory distress syndrome (ARDS). Despite the progress made over the years, ARDS is still a major cause of death among patients who develop sepsis or pneumonia (17). A major factor that contributes to this high mortality is multiple organ failure associated with tissue injury. Lung injury typical of this syndrome has been associated with polymorphonuclear leukocytes (PMN) (12). These short-lived, bone marrow-derived cells are recruited to inflamed sites by responding to bacterial products and inflammatory mediators such as interleukins, tumor necrosis factor, and complement fragments. During this process PMN play a key role in the body's defense, but they can also damage the tissue that they are attempting to protect (33). Their granules carry a wide range of powerful antimicrobial enzymes and peptides (7, 19). Six of these neutrophil peptides (NP-1, -2, -3A, -3B, -4, and -5) or defensins have been isolated and characterized in rabbits. Among these, NP-2 of PMN is structurally and functionally identical to MCP-2 of adult rabbit alveolar macrophages (30). Defensins are known to mediate inflammation (40) and cause damage to endothelial (29), epithelial (23, 24), and tumor (20) cells. We have previously shown that immature marrow PMN contain elevated levels of NP-2 and NP-5 (16), and it has been shown that PMN transit time through the marrow decreases with bacterial infection (34, 37). The present study was designed to extend these observations by determining the level of defensins and the functional activity of circulating PMN during endotoxemia. PMN functional activity was assessed by measuring the expression of β2 integrin CD18 and production of H2O2 and by examining the lungs.
MATERIALS AND METHODS
Experimental protocol.
Adult female New Zealand White rabbits (2.2 ± 0.2 kg) were injected daily for 5 days via the marginal ear vein with either normal saline or lipopolysaccharide (LPS) from Esherichia coli 055:B5 (Sigma, St. Louis, Mo.). Increasing doses of LPS were given as 10 μg on the first two days, 20 μg on the following two days, and 30 μg on the last day of the experiment. Peripheral blood (2 ml) was collected at zero time (baseline), 24 h after each injection, and 1 h following the last treatment. Leukocyte cell counts were carried out in an S 880 Coulter Counter (Beckman Coulter, Inc., Miami, Fla.). Cells of PMN lineage were counted on Wright-stained blood smears. Animals were sacrificed with an excess of ketamine and xylazine at 49 or 97 h. The base of the heart was ligated, and the lungs were excised. The right lung was perfusion fixed with 1% paraformaldehyde (PFA) and inflated with an optimal cutting temperature embedding medium (Miles, Elkhart, Ind.) before storage at −70°C. The left lung was processed for transmission electron microscopy (TEM).
Flow cytometry. (i) Neutrophil peptides NP-2 and NP-5.
Peripheral blood cell suspension (4.0 × 106 cells/ml) from saline (n = 3)- or LPS (n = 5)-treated rabbits was fixed with 0.8% PFA. Red blood cells (RBCs) were lysed with an immunolysing agent (commercial kit from Beckman Coulter, Inc.). After being washed with phosphate-buffered saline, pH 7.3, leukocytes were simultaneously fixed and permeabilized (1 h) with 0.7% PFA and 50 μg of l-α-lysophosphatidylcholine/ml. Cells were incubated (1 h) with 9 μg of mouse monoclonal antibodies B9 (anti-NP-2 and anti-MCP-2) or R5-3 (anti-NP-5) (25) or the nonspecific mouse immunoglobulin G1 (IgG1) (Sigma) per ml. Cells were then labeled (1 h) with a 1/50 dilution from stock of fluorescein isothiocyanate-conjugated anti-mouse secondary antibody (Sigma). After fixing with 0.8% PFA, and to exclude cell aggregates, cells were stained (15 min) with 4 μg of propidium iodide per ml (16). The mean fluorescence intensity of 5,000 to 30,000 cells was measured using analysis gates for PMN in a flow cytometer (Epics XL; Beckman Coulter, Inc.).
(ii) CD18.
Blood samples from saline (n = 4)- or LPS (n = 6)-treated rabbits were collected into tubes containing acid-citrate-dextrose. Aliquots (1 ml) of these samples were added to 1.5 ml of Hank's balanced salt solution, pH 7.3. After being fixed (10 s) with 1.6% PFA, cells were incubated (15 min) with l μg of mouse anti-human LFA-1, β-chain CD18 (Dako, Glostrup, Denmark), or mouse IgG (Sigma) per ml. Samples were incubated (15 min) with 7.5 μg of goat anti-mouse fluorescein isothiocyanate per ml. After lysing of the RBCs, cells were fixed with 1% PFA and the mean fluorescence intensity of 3,000 cells was measured by flow cytometry.
(iii) H2O2.
Blood (2 ml) from saline (n = 3)- or LPS (n = 3)-treated rabbits was drawn into EDTA-containing tubes. Aliquots (50 μl) of blood cell suspension (3.5 × 10 6 cells/ml) or cellZyme (positive control) were incubated (5 min) at 37 oC, with 25 μl of dichlofluorescein hydrogen diacetate (Cellprobe; Coulter Electronics) or phosphate-buffered saline (control) and then placed on ice. After the cells were stained with 5 μg of propidium iodide/ml, RBCs were lysed and the shift of the peak position from the control to the test was measured by flow cytometry.
(iv) NP-2 (MCP-2) immunoreactivity.
The modified version of the alkaline phosphatase anti-alkaline phosphatase method (16) was used to detect NP-2 (MCP-2) in the lungs of rabbits treated with LPS or saline. Right lung frozen sections (4 μm) were obtained on a cryostat (Frigocut 2800 N; Leica) and mounted on 3-aminopropyltriethoxysilane-coated slides. After blocking of the nonspecific binding with 5% rabbit serum, specimens were labeled (1 h) with 0.5 μg of B9 (anti-NP-2 and anti-MCP-2) antibody per ml. Dilutions were made in 0.05 M Tris-buffered saline, pH 7.6, containing 1% bovine serum albumin, and the nonspecific mouse IgG1 was used as a negative control. After rinsing, specimens were incubated (30 min) with a 1:20 dilution of rabbit anti-mouse IgG (Dako). Specific labeling was detected by incubating (30 min) with a 1:50 dilution of a mouse alkaline phosphatase anti-alkaline phosphatase complex (Dako) followed by a new fuchsin-based red substrate solution. Specimens were counterstained with Mayer's hematoxylin, dehydrated with ethanol, and mounted in Entalan (BDH, Mississauga, Ontario, Canada). Photographs were obtained using 100 ASA Kodak film in a Zeiss light microscope.
(v) TEM.
The left lung was inflated and immersion fixed (1 h) with 2.5% glutaraldehyde using 0.1 M Na cacodylate, pH 7.3. Lung tissue samples (∼2 mm3) were further fixed (1 h) in 2.5% glutaraldehyde and postfixed (1 h) in 1% osmium tetroxide. Samples were then dehydrated with ethanol and embedded in LRWhite. Ultrathin sections were stained with uranyl acetate and lead citrate and examined on a Philips 400 electron microscope.
(vi) Statistical analysis.
One-way analysis of variance and a paired t test were used for multiple- and two-group comparisons. Data are presented as mean ± standard error of the mean, and statistical significance is defined as P < 0.05.
RESULTS
Peripheral blood leukocytes.
Rabbits received daily injections of LPS or saline for 5 days. Total leukocyte, PMN, and band cell counts were carried out at the baseline (0 h), 24 h following each dose, and 1 h after the last injection (97 h). We observed that leukocyte counts increase during LPS treatment. As shown in Table 1, PMN increase at 24 and 48 h and band forms rise at 24 h (P < 0.05). As expected, PMN counts decrease at 1 h after the last dose of LPS (97 h) (P < 0.05) with no change in the band cells. By comparison, cell counts do not change with saline treatment (P > 0.05).
TABLE 1.
PMN and band forms (as % of total white blood cells)a
| Cell type | Treatment | % Of total white blood cells at:
|
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 h | 48 h | 72 h | 96 h | 97 h | ||
| PMN | Saline | 31.8 ± 2.9 | 29.3 ± 4.2 | 23.8 ± 0.9 | 25.5 ± 1.6 | 35.7 ± 3.6 | 34.7 ± 5.0 |
| LPS | 38.0 ± 3.1 | 64.6 ± 5.2* | 57.6 ± 3.4* | 49.1 ± 4.6 | 45.4 ± 5.2 | 17.9 ± 5.6* | |
| Bands | Saline | 1.2 ± 0.7 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.5 ± 0.2 | 1.7 ± 0.5 | 0.7 ± 0.2 |
| LPS | 2.1 ± 0.7 | 6.3 ± 1.3* | 2.1 ± 0.6 | 1.0 ± 0.2 | 1.0 ± 0.6 | 2.6 ± 1.0 | |
Note that circulating PMN increase at 24 and 48 h and decrease at 97 h of LPS treatment. By comparison, band forms increase only at 24 h. Values represent mean ± standard errors of the means. Saline, n = 6, LPS, n = 8. P < 0.05 from the baseline (*).
Flow cytometry (i) NP-2 and NP-5.
Figure 1A shows that NP-2 in circulating PMN increases 1.8-fold at 24 and 48 h of LPS treatment. A sharp drop (6.9-fold) in this peptide is observed following the last dose of LPS (97 h) (P < 0.05). Figure 1B shows that the amount of NP-5 tends to rise at 48 h (P = 0.19) and declines (3.2-fold) at 97 h (P < 0.05). The amount of neither of these peptides changes with saline treatment (P > 0.05).
FIG. 1.
(A and B) Kinetics of NP-2 and NP-5 in circulating PMN. NP-2 increases by 24 to 48 h (P < 0.05) (A), and NP-5 rises at 48 h (P = 0.19) (B). Both of these peptides fall at 97 h. Values show means ± standard errors of the means. P < 0.05 from the baseline (∗).
(ii) CD18 and H2O2.
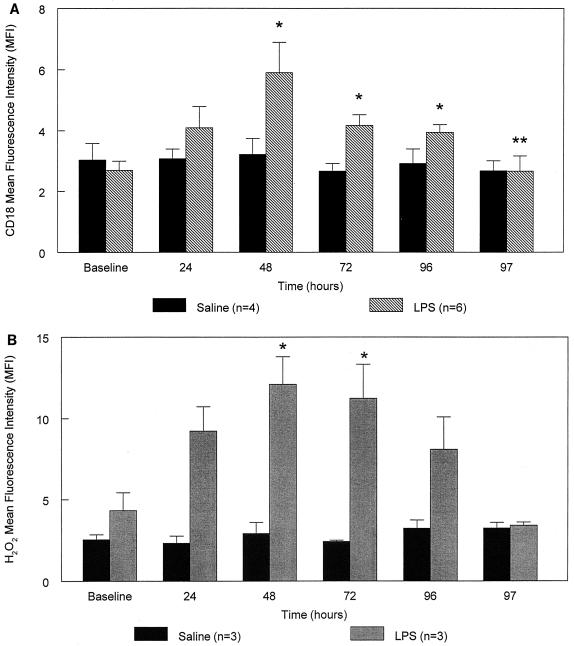
Figure 2A shows that the expression of CD18 on the surface of circulating PMN increases during LPS treatment and reaches a peak (2.2-fold) at 48 h. A sharp decline in this integrin is observed after the last LPS exposure (97 h compared to 96 h) (P < 0.05). Figure 2B shows that H2O2 production also increases with repeated LPS injections, peaking (2.8-fold) at 48 h (P < 0.05) and falling at 97 h (P = 0.08). Neither of these markers of PMN activation changes during saline treatment (P > 0.05).
FIG. 2.
(A and B) CD18 expression and H2O2 production in circulating PMN. CD18 expression (A) and H2O2 production (B) increase with repeated doses of LPS and exhibit a peak at 48 h. These parameters fall at 97 h and do not change with saline treatment. Values represent means ± standard errors of the means. P < 0.05 from the baseline (∗) or from 96 h (∗∗).
(iii) NP-2 (MCP-2) immunoreactivity.
Light microscopy results show that, at 49 h, the NP-2 immunoreactivity in pulmonary microvessels of random sections from control rabbits is moderate (Fig. 3A, arrows) compared to that of LPS-treated animals (Fig. 3B, arrows). By comparison, at 97 h of LPS treatment, NP-2 (MCP-2)-positive cells appear in the interstitium (Fig. 3C, arrows) and alveoli (Fig. 3D) and are less evident in the capillaries (Fig. 3E). IgG-incubated lung sections show no evidence of staining (e.g., specimens from LPS-treated animals shown in Fig. 3F).
FIG. 3.
(A to F) NP-2 (MCP-2) immunoreactivity. PMN-containing defensins are scarce in the lungs of control rabbits (arrows) (A) and accumulate in the capillaries of animals treated with LPS for 49 h (arrows) (B). Defensin-rich phagocytes are prominent in the interstitial (arrows) (C) and alveolar (D) spaces and are less evident in the capillaries of animals treated with LPS for 97 h (E). (F) IgG1-negative control. Magnifications are as follows: ×200 (A, B, E, and F), ×700 (C), and ×850 (D).
(iv) TEM.
Ultrastructural examination of random lung sections at 49 h shows that leukocytes, mostly PMN and monocytes, form clusters inside microvessels and that some of these PMN are closely attached to the endothelium (Fig. 4A). At 97 h of LPS treatment, endothelial cells display vacuolization and membrane blebbing (Fig. 4B, arrows). Inflammatory cells appear in the interstitium and alveoli (Fig. 4C, arrows), and the walls of the alveolar septa exhibit electron lucent spaces that suggest edema. Epithelial type II cells are flattened, extend protrusions over type I cells, and display reduced microvilli. The alveolar space shows fibrin deposits typical of hyaline membrane formation (Fig. 4D, arrows).
FIG. 4.
(A to D) Changes in the lungs of rabbits treated with LPS for 49 h (A) and 97 h (B to D). Note PMN adhered to the endothelium (A), endothelial cell vacuolization (B), interstitial cell infiltrates (C), and alveolar epithelial cell damage with fibrillar deposits (D). Magnifications are as follows: ×7,700 (A), ×2,150 (B), ×2,750 (C), and ×10,000 (D).
DISCUSSION
This study shows that E. coli endotoxin (LPS) increases the expression of defensins NP-2 and NP-5 in circulating PMN. This increase is associated with elevated numbers of segmented and nonsegmented PMN. We have previously shown that immature marrow PMN contain high levels of defensins (16). In the present study, a rise in defensins is likely due to maturational and/or conformational changes in the peptide precursors of less mature PMN. Results indicate that these changes are not synchronized with the transformation of the nucleus from a band to a multilobed form, which supports the notion that nuclear morphology is not always concordant with cellular function (18).
Earlier investigations demonstrated that human defensins are synthesized as large 94-amino-acid (aa) precursors which must undergo cleavage to yield 75-aa and 56-aa prodefensins (9, 22, 36). These intermediate forms of defensins are present in circulating PMN (22) and are pH sensitive in their processing to smaller peptides (7, 9). Other studies showed that bacterial LPS recognizes PMN surface receptors and is internalized into phagosomes (15, 26, 27). It is possible that the following incorporation of primary granule contents into phagosomes (3) sets the ideal environment for the proteolytic processing that promotes defensin expression and microbial destruction. In this respect, it is interesting that Chediak-Higashi syndrome patients show reduced transfer of lysosomal enzymes to phagocytic vacuoles and are highly susceptible to microbial infections (33).
Since circulating PMN retain considerable ability to synthesize proteins (3, 22, 33), we cannot rule out the possibility that increased amount of defensins could, at least in part, result from de novo biosynthesis. Under normal circumstances, mature PMN have a short life span and contain vast quantities of granule proteins; thus, biosynthesis may not be necessary. However, during inflammation, de novo protein synthesis may be required to sustain functional activity (21) and compensate for rapid granule turnover (33). It has been previously shown that LPS triggers the expression of mRNA for β-defensins, which may serve as a protective mechanism to defend the host against invading microorganisms (5, 28).
In the present study, the early (24 to 48 h) rise in PMN defensins is concomitant with cell activation, increased PMN adherence to the vascular endothelium, and enhanced NP-2 immunoreactivity inside microvessels, which creates opportunity for cell toxicity via oxidative and nonoxidative mechanisms. Defensins are known to increase their cytotoxicity by acting synergistically with hydrogen peroxide (24). The combined effect of these PMN-derived products is illustrated in specific-granule-deficiency patients, who show normal respiratory burst but are virtually devoid of defensins, or in patients with chronic granulomatous disease, who express defensins but fail to generate reactive oxygen intermediates and are predisposed to life-threatening infections (6).
The observed decline in the defensins of circulating PMN at 97 h is associated with a drop in the number of activated circulating PMN and correlates with PMN exudation from the vascular space as well as endothelial and epithelial cell damage. Strong immunoreactivity for defensins in the phagocytes of the interstitium and alveoli is associated with amplified inflammatory reaction and is attributed to upregulation of the gene encoding MCP-2 (8) or to phagocytosis of apoptotic PMN.
Furthermore, the weak NP-2 immunoreactivity observed in PMN of pulmonary microvessels suggests extracellular degranulation. Extracellular granule release has been previously demonstrated during phagocytosis (39) and following cell activation (2, 6). High concentrations of defensins have been detected in the plasma and other body fluids of patients with bacterial infections, chronic bronchitis, cystic fibrosis, idiopathic pulmonary fibrosis, ARDS, chronic obstructive pulmonary disease, and α1-antitrypsin deficiency (1, 2, 10, 13, 25, 31, 32). Although the in vivo role of high concentration of defensins is presently unclear, recent studies showed that these peptides mediate lung inflammation and dysfunction (40). Furthermore, data from in vitro studies suggest that defensins may contribute to cytotoxicity by promoting cytokine production and leukocyte recruitment, inducing mast cell degranulation, decreasing antioxidant levels, or altering the permeability and potential of the cell membrane (10, 14, 23, 24, 35, 38). Other studies indicate that an exacerbation of the latter events may take place near cholesterol-enriched membranes (4) in the presence of lipoproteins (11) or in areas of difficult access to naturally occurring inhibitors, such as α2 macroglobulin and α1 proteinases (6). Since excessive amounts of defensins promote inflammation and bacterial adhesion (10, 40), their role in the pathogenesis of clinical disorders associated with bacterial infection must be further investigated.
In summary this study shows that bacterial endotoxin causes changes in the defensins and functional activity of PMN and indicates that the excessive turnover of PMN-derived products elicits an amplified inflammatory reaction that is detrimental to the lung.
ACKNOWLEDGMENTS
This work was funded by Canadian Institutes of Health Research CIHR-4219 and the British Columbia Lung Association.
We thank T. Ganz from the University of California at Los Angeles School of Medicine for insightful reading of the manuscript and the kind supply of B9 and R5-3 antibodies.
REFERENCES
- 1.Ashitani J, Mukae H, Ihiboshi H, Taniguchi H, Mashimoto H, Nakazato M, Matsukura S. Defensin in plasma and in bronchoalveolar lavage fluid from patients with acute respiratory distress syndrome. Nihon Kyobu Shikkan Gakkai Zasshi. 1996;34:1349–1353. [PubMed] [Google Scholar]
- 2.Ashitani J, Mukae H, Nakazato M, Ihi T, Mashimoto H, Kadota J, Kohno S, Matsukura S. Elevated concentrations of defensins in bronchoalveolar lavage fluid in diffuse panbronchiolitis. Eur Respir J. 1998;11:104–111. doi: 10.1183/09031936.98.11010104. [DOI] [PubMed] [Google Scholar]
- 3.Bradley S G. Cellular and molecular mechanisms of action of bacterial endotoxins. Annu Rev Microbiol. 1979;33:67–94. doi: 10.1146/annurev.mi.33.100179.000435. [DOI] [PubMed] [Google Scholar]
- 4.Chasovnikova L V, Formaziuk V E, Sergienko V I, Kokriakov V N. Interaction of myeloperoxidase and defensins with lipid monolayers. Biokhimiia. 1992;57:97–102. [PubMed] [Google Scholar]
- 5.Diamond G, Bevins C L. Beta-defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987;55:568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz T, Liu L, Valore E V, Oren A. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood. 1993;82:641–650. [PubMed] [Google Scholar]
- 8.Ganz T, Rayner J R, Valore E V, Tumolo A, Talmadge K, Fuller F. The structure of the rabbit macrophage defensin genes and their organ-specific expression. J Immunol. 1989;143:1358–1365. [PubMed] [Google Scholar]
- 9.Harwig S S, Park A S, Lehrer R I. Characterization of defensin precursors in mature human neutrophils. Blood. 1992;79:1532–1537. [PubMed] [Google Scholar]
- 10.Hiemstra P S, van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J. 1998;12:1200–1208. doi: 10.1183/09031936.98.12051200. [DOI] [PubMed] [Google Scholar]
- 11.Higazi A A, Lavi E, Bdeir K, Ulrich A M, Jamieson D G, Rader D J, Usher D C, Kane W, Ganz T, Cines D B. Defensin stimulates the binding of lipoprotein (a) to human vascular endothelial and smooth muscle cells. Blood. 1997;89:4290–4298. [PubMed] [Google Scholar]
- 12.Hogg J. The interaction between polymorphonuclear cells and pulmonary endothelium. New York, N.Y: Marcel Dekker, Inc; 1989. [Google Scholar]
- 13.Ihi T, Nakazato M, Mukae H, Matsukura S. Elevated concentrations of human neutrophil peptides in plasma, blood, and body fluids from patients with infections. Clin Infect Dis. 1997;25:1134–1140. doi: 10.1086/516075. [DOI] [PubMed] [Google Scholar]
- 14.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klut M E, van Eeden S F, Hogg J C. Neutrophil structural changes associated with chronic endotoxemia and lung injury. J Inflamm. 1998;48:1–12. [PubMed] [Google Scholar]
- 16.Klut M E, Whalen B A, Hogg J C. Flow cytometric analysis of defensins in blood and marrow neutrophils. Eur J Haematol. 2000;64:114–120. doi: 10.1034/j.1600-0609.2000.90069.x. [DOI] [PubMed] [Google Scholar]
- 17.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald R D, Koc D, Schneider B, Hammerle A F, Steltzer H. The acute respiratory distress syndrome: definitions, severity and clinical outcome. An analysis of 101 clinical investigations. Intern Care Med. 1996;22:519–529. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 18.Krause P J, Todd M B, Hancock W W, Pastuszak W T, Maderazo E G, Hild D H, Kosciol C M. The role of cellular maturation in neutrophil heterogeneity. Blood. 1990;76:1639–1646. [PubMed] [Google Scholar]
- 19.Lehrer R I, Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990;76:2169–2181. [PubMed] [Google Scholar]
- 20.Lichtenstein A, Ganz T, Selsted M E, Lehrer R I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68:1407–1410. [PubMed] [Google Scholar]
- 21.Mori S, Goto K, Goto F, Murakami K, Ohkawara S, Yoshinaga M. Dynamic changes in mRNA expression of neutrophils during the course of acute inflammation in rabbits. Int Immunol. 1994;6:149–156. doi: 10.1093/intimm/6.1.149. [DOI] [PubMed] [Google Scholar]
- 22.Nakazato M, Shiomi K, Date Y, Matsukura S, Kangawa K, Minamino N, Matsuo H. Isolation and sequence determination of 6- and 8-kDa precursors of human neutrophil peptides from bone marrow, plasma and peripheral blood neutrophils. Biochem Biophys Res Commun. 1995;211:1053–1062. doi: 10.1006/bbrc.1995.1918. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard S D, Ganz T, Peterson M W. Defensins reduce the barrier integrity of a cultured epithelial monolayer without cytotoxicity. Am J Respir Cell Mol Biol. 1993;8:193–200. doi: 10.1165/ajrcmb/8.2.193. [DOI] [PubMed] [Google Scholar]
- 24.Okrent D G, Lichtenstein A K, Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990;141:179–185. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- 25.Panyutich A V, Panyutich E A, Krapivin V A, Baturevich E A, Ganz T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J Lab Clin Med. 1993;122:202–207. [PubMed] [Google Scholar]
- 26.Pedron T, Girard R, Turco S J, Chaby R. Phosphatidylinositol-anchored molecules and inducible lipopolysaccharide binding sites of human and mouse bone marrow cells. J Biol Chem. 1994;269:2426–2432. [PubMed] [Google Scholar]
- 27.Risco C, Pinto da Silva P. Cellular functions during activation and damage by pathogens: immunogold studies of the interaction of bacterial endotoxins with target cells. Microsc Res Tech. 1995;31:141–158. doi: 10.1002/jemt.1070310206. [DOI] [PubMed] [Google Scholar]
- 28.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schluesener H, Meyermann R. Neutrophilic defensins penetrate the blood-brain barrier. J Neurosci Res. 1995;42:718–723. doi: 10.1002/jnr.490420515. [DOI] [PubMed] [Google Scholar]
- 30.Selsted M E, Brown D M, DeLange R J, Lehrer R I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983;258:14485–14489. [PubMed] [Google Scholar]
- 31.Shiomi K, Nakazato M, Ihi T, Kangawa K, Matsuo H, Matsukura S. Establishment of radioimmunoassay for human neutrophil peptides and their increases in plasma and neutrophil in infection. Biochem Biophys Res Commun. 1993;195:1336–1344. doi: 10.1006/bbrc.1993.2190. [DOI] [PubMed] [Google Scholar]
- 32.Soong L B, Ganz T, Ellison A, Caughey G H. Purification and characterization of defensins from cystic fibrosis sputum. Inflamm Res. 1997;46:98–102. doi: 10.1007/s000110050114. [DOI] [PubMed] [Google Scholar]
- 33.Steven E W. Biochemistry and physiology of the neutrophil. Cambridge, England: Cambridge University Press; 1994. [Google Scholar]
- 34.Terashima T, English D, Hogg J C, van Eeden S F. Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8. Blood. 1998;92:1062–1069. [PubMed] [Google Scholar]
- 35.Territo M C, Ganz T, Selsted M E, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Investig. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valore E V, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–1544. [PubMed] [Google Scholar]
- 37.van Eeden S F, Kitagawa Y, Klut M E, Lawrence E, Hogg J C. Polymorphonuclear leukocytes released from the bone marrow preferentially sequester in lung microvessels. Microcirculation. 1997;4:369–380. doi: 10.3109/10739689709146801. [DOI] [PubMed] [Google Scholar]
- 38.van Wetering S, Sterk P J, Rabe K F, Hiemstra P S. Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 1999;104:1131–1138. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 39.Yomogida S, Nagaoka I, Saito K, Yamashita T. Evaluation of the effects of defensins on neutrophil functions. Inflamm Res. 1996;45:62–67. doi: 10.1007/BF02265117. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Porro G, Orzech N, Mullen B, Liu M, Slutsky A S. Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose-related fashion. Am J Physiol Lung Cell Mol Physiol. 2001;280:L947–L954. doi: 10.1152/ajplung.2001.280.5.L947. [DOI] [PubMed] [Google Scholar]