Abstract
Objective
To provide a comprehensive overview of the efficacy, safety, and tolerability of antidepressants for pain according to condition.
Design
Overview of systematic reviews.
Data sources
PubMed, Embase, PsycINFO, and the Cochrane Central Register of Controlled Trials from inception to 20 June 2022.
Eligibility criteria for selecting studies
Systematic reviews comparing any antidepressant with placebo for any pain condition in adults.
Data extraction and synthesis
Two reviewers independently extracted data. The main outcome measure was pain; for headache disorders it was frequency of headaches. Continuous pain outcomes were converted into a scale of 0 (no pain) to 100 (worst pain) and were presented as mean differences (95% confidence intervals). Dichotomous outcomes were presented as risk ratios (95% confidence intervals). Data were extracted from the time point closest to the end of treatment. When end of treatment was too variable across trials in a review, data were extracted from the outcome or time point with the largest number of trials and participants. Secondary outcomes were safety and tolerability (withdrawals because of adverse events). Findings were classified from each comparison as efficacious, not efficacious, or inconclusive. Certainty of evidence was assessed with the grading of recommendations assessment, development, and evaluation framework.
Results
26 reviews (156 unique trials and >25 000 participants) were included. These reviews reported on the efficacy of eight antidepressant classes covering 22 pain conditions (42 distinct comparisons). No review provided high certainty evidence on the efficacy of antidepressants for pain for any condition. 11 comparisons (nine conditions) were found where antidepressants were efficacious, four with moderate certainty evidence: serotonin-norepinephrine reuptake inhibitors (SNRIs) for back pain (mean difference −5.3, 95% confidence interval −7.3 to −3.3), postoperative pain (−7.3, −12.9 to −1.7), neuropathic pain (−6.8, −8.7 to −4.8), and fibromyalgia (risk ratio 1.4, 95% confidence interval 1.3 to 1.6). For the other 31 comparisons, antidepressants were either not efficacious (five comparisons) or the evidence was inconclusive (26 comparisons).
Conclusions
Evidence of efficacy of antidepressants was found in 11 of the 42 comparisons included in this overview of systematic reviews—seven of the 11 comparisons investigated the efficacy of SNRIs. For the other 31 comparisons, antidepressants were either inefficacious or evidence on efficacy was inconclusive. The findings suggest that a more nuanced approach is needed when prescribing antidepressants for pain conditions.
Systematic review registration
PROSPERO CRD42022311073.
Introduction
Chronic pain is common and debilitating, affecting about one in five people globally.1 2 3 4 Musculoskeletal conditions such as back pain are typically the most common conditions leading to chronic pain, followed by headache, orofacial pain, and visceral pain (eg, abdominal, pelvic, or genital).
Chronic pain can be difficult to treat, and management is often suboptimal—for example, the efficacy of the most common non-opioid drug treatment, paracetamol (acetaminophen), is unknown for most pain conditions.5 Non-steroidal anti-inflammatory drugs may provide small benefits for pain reduction in some conditions, but they need to be used with caution and for short periods because of the risk of serious adverse events with longer term use.6 About a third of people with chronic non-cancer related pain are prescribed opioid analgesics.7 The benefits of such drugs for chronic pain are, however, limited, and the potential harms outweigh the small benefits.8 Antidepressants are frequently used for the treatment of chronic pain. In a Canadian study, about 9% of all antidepressant prescriptions were for pain conditions.9 In Portugal, 12% of people with back pain reported the use of an antidepressant to manage their condition.10 Among older people, recent data from Canada, the United States, the United Kingdom, and Taiwan suggest that chronic pain was the most common condition leading to an antidepressant prescription—even more so than for depression.11 In the Netherlands, prescription of the tricyclic antidepressants amitriptyline for people with osteoarthritis has increased by 17% over the past decade.12 In that study, long term use (≥3 months) was also observed in 40% of those prescribed an antidepressant.
The 2021 National Institute for Health and Care Excellence guideline for chronic primary pain explicitly recommends against the use of pain medicines, with the exception of antidepressants.13 14 Chronic primary pain is a recent diagnostic classification that encompasses a large number of conditions, such as fibromyalgia, complex regional pain syndrome, orofacial pain, visceral pain (eg, irritable bowel syndrome, bladder pain), and musculoskeletal pain (eg, back pain).15 Other types of pain, such as postsurgical, neuropathic, and cancer related are not classified under chronic primary pain, but the efficacy of antidepressants for these conditions has been investigated.16 17 18 19 To provide patients and clinicians with an updated and comprehensive resource on the efficacy, safety, and tolerability of antidepressants to treat pain, we conducted an overview of relevant systematic reviews. Given the heterogeneity in the types of pain conditions for which the efficacy of antidepressants has been documented by existing systematic reviews, we chose to conduct an overview of systematic reviews to appraise efficacy estimates for each condition individually.
Methods
Data sources and searches
This review was prospectively registered on PROSPERO and followed guidance from the preferred reporting items for overviews of reviews (PRIOR) statement,20 which defines an overview of systematic reviews as a review that uses explicit and systematic methods to search for and identify multiple systematic reviews on a similar topic for the purpose of extracting and analysing results across important outcomes. Thus, the unit of searching, inclusion, and data analysis is the systematic review itself.
We searched PubMed, Embase, PsycINFO, and the Cochrane Database of Systematic Reviews from inception to 16 February 2022, with searches updated on 20 June 2022. Supplementary file 1 provides details of the search strategy. We did not consider the grey literature. Two authors (GF and CAS or JZ) independently screened eligible studies by title and abstract and read the full text of potentially eligible studies. Disagreements were resolved by consensus. Supplementary file 2 provides a list of ineligible reviews after full text reading.
Eligibility criteria
We included systematic reviews with or without meta-analysis, published in peer reviewed journals, that investigated the efficacy of any antidepressant drug compared with placebo used for any pain condition in adults. We defined systematic reviews as peer reviewed studies with a clearly reported research question that used systematic methods to search the literature and synthesise data. Provided that an appropriate English translation could be obtained, we placed no restriction on antidepressant class, dose, or regimen, or on language of the review. Network meta-analyses were eligible if they provided direct effects for the antidepressant versus placebo comparison (to exclude indirect comparisons). Reviews that included populations of children or adolescents, or both, or that did not report pain or safety outcomes were excluded. When more than one review existed on the same topic, we selected the most comprehensive review—that is, the review with the most trials relevant to our comparison of interest (antidepressants versus placebo). When a Cochrane review and a non-Cochrane review included the same trials, we chose the Cochrane review because such reviews are typically of higher quality.21 22 The final decision on which reviews to include was based on consensus between two authors (GF and CAS or JZ). In line with the recommendations from Cochrane, we did not conduct trial level searches or a new systematic review within the overview.23
Data extraction
Two reviewers independently extracted data (GF, JZ). Data were extracted on characteristics of the review (eg, prospective registration, Cochrane or non-Cochrane review), information on antidepressant type and treatment regimen (eg, dose and duration of treatment), number of trials with industry ties, and outcomes for the current overview.
Outcomes
Our primary outcome was pain measured with any instrument and reported as between group differences along with corresponding 95% confidence intervals. We reported mean differences and 95% confidence intervals on a common 0-100 scale. Dichotomous outcomes were converted, if necessary, to risk ratios and corresponding 95% confidence intervals. When data were summarised as standardised mean differences, we examined the information available in the review to determine whether it was possible to convert the data to mean differences on a 0-100 scale. When multiple pain outcomes were available, we chose the one elected by the review as its primary outcome. When multiple time points existed for the same pain outcome within a review, we extracted data from the time point closest to the end of treatment in >50% of trials. For reviews of headache disorders, we extracted data from the headache frequency outcome in line with guidance on drug trials in chronic migraine,24 tension-type headache,25 and a core outcome set for migraine.26
Safety outcomes were considered as secondary outcomes. We extracted data for any adverse event and tolerability (ie, withdrawals due to adverse events) but not for individual trial adverse events as we anticipated too much variability in how these would be reported by each review. We also did not extract data on serious adverse events as we anticipated that most trials included in the eligible reviews would not be adequately powered to detect between group differences for these outcomes. For safety and tolerability outcomes, we defined appreciable benefits and harms when risk ratio estimates included 0.75 and 1.25 values, respectively.27
Methodological quality of systematic reviews
Two reviewers (GF, JZ) independently appraised the methodological quality of included systematic reviews with the AMSTAR-2 tool.28 This tool has 16 items, seven of which are considered critical. Using published guidance,28 we classified the quality of the included systematic reviews as high, moderate, low, or critically low. Supplementary file 3 shows the AMSTAR-2 ratings for the eligible reviews.
Risk of bias
We relied on the risk of bias assessment of reviews that assessed risk of bias using the original or revised version of the Cochrane risk of bias tool.29 30 For reviews that used a different risk of bias tool, such as the Jadad scale,17 or where risk of bias was not assessed,31 two independent reviewers (GF, JZ) assessed bias of each included trial that contained data for our outcomes of interest using the Cochrane risk of bias tool (see supplementary file 3). We used criteria described previously to assess selection (random sequence generation and allocation concealment), performance (blinding of participants and staff), detection (blinding of outcome assessors), attrition (incomplete outcome data), reporting (selective reporting), and other biases.32 For the other bias item, we assessed whether the review trials had ties with industry. We considered trials with industry ties to be at high risk of bias.33 A trial was considered to have industry ties if it was sponsored by industry or received funding from industry but did not include a disclosure that the funder had no role in any aspect of the study (ie, design and conduct; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication). For each trial in the included reviews, we classified ties with industry as either present, absent, or unclear. Supplementary file 4 shows all trials included in the eligible reviews that had industry ties.
Certainty of evidence
Two reviewers (GF, JZ) independently assessed the certainty of evidence for the primary outcome (pain) according to the grading of recommendations assessment, development, and evaluation (GRADE) criteria.34 If reviews used GRADE, we checked the ratings and reported our assessment when the ratings differed from the originals. If GRADE was not used in the review, we graded the certainty of evidence ourselves using prespecified criteria. Briefly, the certainty of evidence was initially set to high and downgraded by one level for each of the following domains: limitation of study design, inconsistency of results, imprecision, and publication bias. We did not upgrade the certainty of evidence for any reason, such as large effect size. Supplementary file 5 presents a detailed explanation of the criteria used, the reviews’ ratings, and our ratings.
Data synthesis
We reported data separately by pain condition and by antidepressant class. For each comparison, we calculated the median (minimum-maximum) dose and treatment duration (in weeks). When a trial provided a range of doses, we used the value of the final (highest) dose before tapering for the calculation. We reanalysed data from included reviews in the following instances: If a review reported pooled estimates combining data from different antidepressant classes,35 we reanalysed data and grouped trials by antidepressant class; if our team considered one or more trials included in a review as not appropriate for that comparison (eg, not placebo controlled), we excluded the trial from the analysis and re-ran the analysis35; if the primary outcome of the review was not pain but the included trials measured a pain outcome, we extracted data for the pain outcome from the trials included in the review36; and if a review reported data descriptively, we computed the pooled treatment effect when possible and provided a GRADE rating for that outcome.
A random effects model with a restricted maximum likelihood heterogeneity variance estimator was used to estimate the efficacy of antidepressants on pain (primary outcome) for all comparisons where meta-analysis was possible—that is, when at least two studies were pooled. The random effects model makes less stringent assumptions about the consistency of effects across studies, which is a more appropriate approach when studies within a meta-analysis might differ from each other in ways that could impact on the treatment effect.37 Restricted maximum likelihood is recommended to estimate the heterogeneity variance over other methods, including the widely used DerSimonian and Laird estimator.38 Reanalyses were performed in Stata version 17 using the metan package. We presented safety outcomes as reported by the reviews. Supplementary file 6 provides a detailed explanation of which reviews were reanalysed, details on our rationale to reanalyse data, and methods used in each reanalysis. To visually demonstrate the amount of overlap in trials included in more than one review in line, we created a citation matrix and calculated the amount of overlap using the corrected covered area as recommended by Cochrane (see supplementary file 7).39 Values of corrected covered area are interpreted as slight (0-5%), moderate (6-10%), high (11-15%), and very high (>15%).
From each comparison we classified findings as either efficacious, not efficacious, or inconclusive. An antidepressant was considered to be efficacious for a condition when the difference between intervention and placebo groups was statistically significantly in favour of the antidepressant and the certainty of evidence was at least low. We classified an antidepressant as not efficacious for a condition when the difference between intervention and placebo groups was not statistically significantly in favour of antidepressants and the certainty of evidence was at least low. When the certainty of evidence was very low or a comparison had only one small trial (defined arbitrarily as having a sample size <100 per arm40 41), or both, we considered the evidence of efficacy to be inconclusive regardless of statistical significance, magnitude, or direction of effect.42
Because this review presents data for a series of distinct pain conditions, we purposefully decided a priori not to make judgments about the clinical importance of observed effects for each condition. This was decided because commonly used thresholds (eg, the 10 point reduction on a 0-100 scale commonly used in musculoskeletal pain research43) are arbitrary and may not reflect patients’ views on whether an effect is meaningful to them.44 No threshold would universally apply to all conditions studied, potentially generating misleading interpretations about the importance of the observed effect.
Sensitivity analysis
To assess the robustness of our findings, we performed a sensitivity analysis. In this analysis we described the effect sizes and included trials of systematic reviews that were excluded owing to overlap with an eligible review and that were published either in the five years preceding the publication of the eligible review or after the eligible review. Five years was chosen as this is the recommended timeframe in which to update a Cochrane systematic review.23 Supplementary file 8 presents the data for the sensitivity analyses.
Protocol deviations
We stated in our protocol that we would extract data on disability and serious adverse events as secondary outcomes. During data extraction it became evident that most reviews did not have any data on disability and therefore we decided not to extract such data. We also did not extract data on serious adverse events as most trials in each review were not adequately powered to detect such events.
In the protocol we stated that in reviews reporting multiple data time points we would choose the time point at 3-12 months (or closest to six months when multiple time points existed within that time frame) as the primary endpoint. The team subsequently agreed that defining the intermediate term (3-12 months) as the primary time point was not appropriate for this review for several reasons, including: Most reviews only presented one measure of efficacy at the end of the treatment phase, and treatment phases typically had a duration of 6-12 weeks. For reviews that did present data for multiple time points (eg, at three months, six months, and one year), extracting data from the six month time point (as defined in our protocol) would have been detrimental as data from these reviews would be less comparable to data in others, and fewer trials would be included. As a solution we decided to only extract data from the time point closest to the end of treatment in most trials (>50%) included in each review.
Patient and public involvement
Owing to lack of funding, patients and members of the public were not involved in the design, conduct, or reporting of this study.
Results
Characteristics of included reviews
Overall, 1732 records were identified, of which 948 were duplicates and therefore excluded. The remaining 784 records were screened by title and abstract, and the full texts of 184 potentially eligible reviews were read. After the exclusion of a further 158 reviews (see supplementary file 2 for reasons), 26 studies were included (fig 1).
Fig 1.
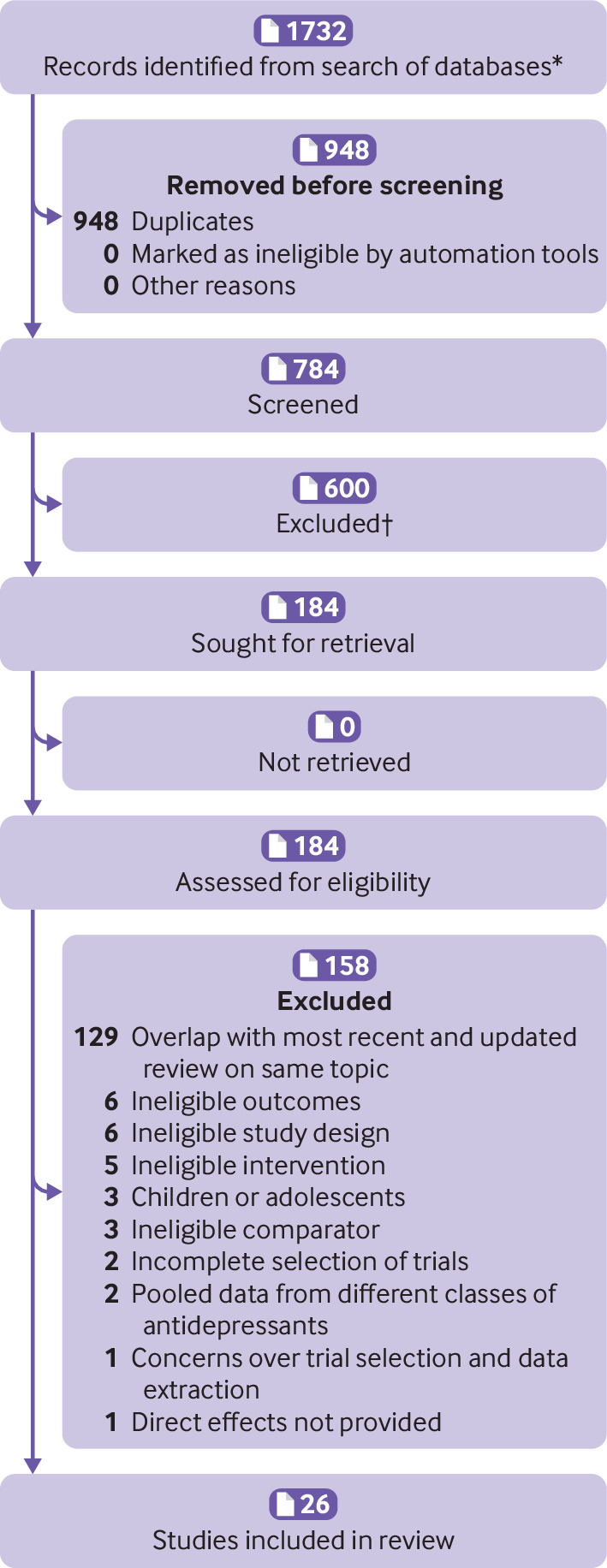
Flow of studies through review
The 26 reviews, published between 2012 and 2022, included 156 unique trials and >25 000 participants and covered 22 distinct pain conditions representing 42 distinct antidepressant versus placebo comparisons. Each condition was covered by one review, except for fibromyalgia (five reviews),45 46 47 48 49 neuropathic pain (two reviews),17 35 and chronic tension-type headache (two reviews).50 51 For these three conditions, each review provided data for a different antidepressant class. Table 1 describes the included reviews. In only three of the 156 trials did data overlap in more than one review (see supplementary file 7), yielding a corrected covered area of 0.07%. We explored the effect of removing the overlapping trials from the treatment effect estimates in the reviews in which they were included (see supplementary file 9).
Table 1.
Characteristics of included reviews (n=26)
| Reference | Condition | Antidepressant class | Prospective registration | Type of review | No of trials (No of participants) with data for pain | No (%) of trials with industry ties* |
|---|---|---|---|---|---|---|
| Wang 202216 | Postoperative pain | SNRI, SSRI | Yes | Non-Cochrane | 17 (1242) | 0 |
| Roberts 202252 | Aromatase inhibitor therapy induced pain in breast cancer | SNRI | Yes | Cochrane | 1 (255) | 0 |
| Ferreira 202132 | Back pain, sciatica, knee osteoarthritis | SNRI, SSRI, TCA, NDRI, SARI, tetracyclic/atypical | Yes | Non-Cochrane | 30 (4445) | 14 (47) |
| Farag 202153 | Burning mouth syndrome | SARI | No | Non-Cochrane | 1 (37) | 1 (100) |
| Ford 202136 | Functional dyspepsia | SSRI, TCA, tetracyclic/atypical | No | Non-Cochrane | 5 (618) | 0 |
| Do 202154 | Atypical chronic orofacial pain | SNRI, TCA | Yes | Non-Cochrane | 2 (60) | 1 (100) |
| Imamura 202055 | Bladder pain syndrome | TCA | Yes | Cochrane | 2 (279) | 0 |
| Ford 201956 | Irritable bowel syndrome | SSRI, TCA | Yes | Non-Cochrane | 7 (351) | 1 (14) |
| Caruso 201935 | Neuropathic pain | SNRI | Yes | Non-Cochrane | 12 (3010) | 9 (75) |
| Perez-Lopez 201957 | Vulvodynia | TCA | No | Non-Cochrane | 1 (58) | 0 |
| Christophorou 201958 | Acute oral mucositis | TCA | No | Non-Cochrane | 1 (140) | 0 |
| Franco 201959 | Chronic prostatitis | SSRI | Yes | Cochrane | 1 (42) | 1 (100) |
| Welsch 201845 | Fibromyalgia | SNRI | Yes | Cochrane | 15 (6918) | 14 (93) |
| Welsch 201846 | Fibromyalgia | Tetracyclic/atypical | Yes | Cochrane | 3 (591) | 2 (66) |
| Jackson 201751 | Chronic tension-type headache | TCA, tetracyclic/atypical | No | Non-Cochrane | 4 (197) | 0 |
| Gebhardt 201631 | Depression and comorbid chronic pain | SNRI, SSRI | No | Non-Cochrane | 14 (4380) | 15 (79) |
| Alviar 201660 | Phantom limb pain | TCA | Yes | Cochrane | 1 (39) | 0 |
| Walitt 201547 | Fibromyalgia | SSRI | Yes | Cochrane | 6 (343) | 5 (83) |
| Moore 201549 | Fibromyalgia | TCA | Yes | Cochrane | 4 (275) | 1 (25) |
| Banzi 201561 | Chronic migraine | SSRI | Yes | Cochrane | 2 (82) | 1 (20) |
| Banzi 201550 | Chronic tension-type headache | SNRI, SSRI | Yes | Cochrane | 2 (127) | 1 (50) |
| Finnerup 201517 | Neuropathic pain | TCA | No | Non-Cochrane | 14 (948) | 0 |
| Atluri 201562 | Non-cardiac chest pain | SSRI | No | Non-Cochrane | 4 (184) | 2 (50) |
| Cheong 201463 | Chronic pelvic pain | SSRI | Yes | Cochrane | 1 (23) | 0 |
| Richards 201164 | Rheumatoid arthritis | TCA | Yes | Cochrane | 7 (482) | 1 (17) |
| Tort 201248 | Fibromyalgia | MAOI | Yes | Cochrane | 2 (121) | 1 (50) |
SNRI=serotonin-norepinephrine reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants; NDRI=noradrenaline-dopamine reuptake inhibitors; SARI=serotonin antagonist and reuptake inhibitors; MAOI=monoamine oxidase inhibitors.
Pain data (primary outcome).
Treatment regimens
The 26 reviews provided efficacy estimates for eight classes of antidepressants. Efficacy estimates for tricyclic antidepressants (TCAs) were provided in 12 reviews,17 32 36 49 51 54 55 56 57 58 60 64 followed by selective serotonin reuptake inhibitors (SSRIs) in 11 reviews,16 31 32 36 47 50 56 59 61 62 63 serotonin-norepinephrine reuptake inhibitors (SNRIs) in eight reviews,16 31 32 35 45 50 52 54 tetracyclic/atypical antidepressants in four reviews,32 36 46 51 serotonin antagonist and reuptake inhibitors (SARIs) in two reviews,32 53 and noradrenaline-dopamine reuptake inhibitors (NDRIs)32 and monoamine oxidase inhibitors (MAOIs)48 in one trial each.
Ties with industry
Table 1 describes the number of trials, number of participants, and percentage of included trials in each review with ties to industry. Of the 156 unique trials that were included in at least one included review, we were able to locate 154 (98%) and investigate their ties with industry. Industry ties were present in 69 (45%) reviews, absent in 45 (29%), and unclear in 40 (26%). Of the trials with industry ties, 47 (68%) investigated SNRIs, 13 (18%) investigated SSRIs, 4 (6%) investigated TCAs, 2 (3%) investigated atypical antidepressants and SARIs each, and one (1%) investigated NDRIs and MAOIs.
Conditions for which antidepressants are efficacious
Nine reviews provided evidence that some antidepressants were efficacious compared with placebo for nine conditions in 11 distinct comparisons.16 17 31 32 35 45 51 52 56 Most reviews providing evidence that antidepressants were efficacious for pain were of SNRIs—six reviews covering seven conditions.16 31 32 35 45 52
No review reported high certainty evidence about the effects of antidepressants for pain. Moderate certainty of evidence suggested that SNRIs were efficacious for chronic back pain,32 postoperative pain (most trials were in orthopaedic surgery),16 fibromyalgia,45 and neuropathic pain.35
Low certainty evidence supported the efficacy of SNRIs, SSRIs, and TCAs for some other conditions. SNRIs were efficacious for aromatase inhibitor therapy induced pain in breast cancer,52 depression and comorbid chronic pain,31 and knee osteoarthritis.32 SSRIs were efficacious for depression and comorbid chronic pain.31 TCAs were efficacious for irritable bowel syndrome,56 neuropathic pain,17 and chronic tension-type headache.51 Table 2 shows typical treatment regimens, effect estimates, number of trials, and number of participants.
Table 2.
Conditions for which antidepressants show evidence of efficacy
| Condition by drug class | Treatment regimen: median (minimum-maximum) dose and duration | Outcome | Effect estimate (95% CI)* | No of trials (No of participants) | Certainty of evidence |
|---|---|---|---|---|---|
| SNRI | |||||
| Back pain32 | Duloxetine 60 (20-120) mg for 13 (13-13) weeks (4 trials) | Pain intensity | MD −5.3 (−7.3 to −3.3) | 4 (1415) | Moderate |
| Postoperative pain16 | Duloxetine 60 (30-60) mg pre-surgery and/or post-surgery for 3 days (1 day to 26 weeks) (15 trials); venlafaxine 37.5 mg for 10 days (1 trial) | Pain intensity (24 hours post-surgery) | MD −7.3 (−12.9 to −1.7) | 16 (1128) | Moderate |
| Fibromyalgia45 | Duloxetine 120 (30-120) mg for 12 (12-27) weeks (7 trials); milnacipran 150 (100-200) mg for 12 (6-27) weeks (8 trials) | Pain reduction ≥50% | RR 1.4 (1.3 to 1.6) | 15 (6918) | Moderate† |
| Neuropathic pain35 | Duloxetine 120 (60-120) mg for 12 (6-12) weeks (8 trials); milnacipran 200 mg for 12 weeks (1 trial); venlafaxine 172.5 (150-225) for 7 (6-8) weeks (2 trials); desvenlafaxine (400 mg for 13 weeks) (1 trial) | Pain intensity | MD −6.8 (−8.7 to −4.8) | 12 (3010) | Moderate† |
| Aromatase inhibitor therapy induced pain in breast cancer52 | Duloxetine 60 mg for 12 weeks (1 trial) | Pain intensity | MD −6.3 (−9.7 to −2.9) | 1 (255) | Low† |
| Depression and comorbid chronic pain31 | Duloxetine 60 (60-60) mg for 9 (8-10 weeks) (9 trials); venlafaxine 177 mg for 12 weeks (1 trial); desvenlafaxine 50 mg for 10 weeks (1 trial) | Pain intensity | MD −6.4 (−7.7 to −5.1) | 11 (3520) | Low† |
| Knee osteoarthritis32 | Duloxetine 60 (60-120) mg for 13 (8-14) weeks (7 trials); milnacipran 200 mg for 8 weeks (1 trial) | Pain intensity | MD −9.6 (−12.3 to −6.9) | 8 (1941) | Low† |
| SSRI | |||||
| Depression and comorbid chronic pain31 | Paroxetine 20 (20-20) mg for 8 weeks (2 trials); fluoxetine 20 mg for 7 weeks (1 trial); escitalopram 10 mg for 12 weeks (1 trial) | Pain intensity | MD −5.9 (−10.1 to −1.7) | 4 (947) | Low† |
| TCA | |||||
| Irritable bowel syndrome56 | Amitriptyline 10 mg for 8 weeks (1 trial); nortriptyline 10 mg for 8 weeks (1 trial); doxepin 75 mg for 6 weeks (1 trial); desipramine 150 mg for 8 weeks (1 trial) | Abdominal pain not improving | RR 0.6 (0.4 to 0.8) | 4 (184) | Low† |
| Neuropathic pain17 | Amitriptyline 75 (65-150) for 5 (4-8) weeks (9 trials); desipramine 201 (160-250) for 6 (6-8) weeks; (3 trials); imipramine 150 mg for 4 weeks (1 trial); maprotiline 75 mg for 4 weeks (1 trial); nortriptyline 100 mg for 9 weeks (1 trial) | Pain reduction ≥30% | RR 3.4 (2.1 to 5.5) | 14 (948) | Low |
| Chronic tension-type headache51 | Amitriptyline 100 (50-150) mg for 12 (8-26) weeks | Headache frequency (days) | MD −4.8 (−6.6 to −3) | 4 (197) | Low† |
CI=confidence interval: MD=mean difference; RR=risk ratio; SNRI=serotonin-norepinephrine reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants.
Pain intensity outcomes are on a 0-100 scale unless stated otherwise.
Grading of recommendations assessment, development, and evaluation determined by current authors (see supplementary file 5 for details of the assessment).
Conditions for which antidepressants are not efficacious
Four reviews provided evidence that some antidepressants were not efficacious compared with placebo for four conditions in five distinct comparisons.32 36 47 62 No review reported high certainty evidence. Most reviews providing evidence that antidepressants were not efficacious for pain were of SSRIs, with four reviews covering three conditions.32 36 47 62
One review provided moderate certainty evidence that TCAs were not efficacious for functional dyspepsia.36 The certainty of evidence for all other reviews was low. SSRIs were not efficacious for back pain,32 fibromyalgia,47 functional dyspepsia,36 and non-cardiac chest pain.62 Table 3 shows typical treatment regimens, effect estimates, number of trials, and number of participants.
Table 3.
Conditions for which antidepressants are not efficacious, by class and certainty of evidence
| Condition by drug class | Treatment regimen: median (minimum-maximum) dose and duration | Outcome | Effect estimate (95% CI)* | No of trials (No of participants) | Certainty of evidence |
|---|---|---|---|---|---|
| SSRI | |||||
| Back pain32 | Paroxetine 25 (20-30) mg for 8 weeks (2 trials); fluoxetine 16-514 ng/mL for 12 weeks (1 trial) | Pain intensity | MD 1.5 (−5.4 to 8.5) | 3 (170) | Low |
| Fibromyalgia47 | Fluoxetine 62.5 (20-80) mg for 12 (6-12) weeks (3 trials); citalopram 40 mg for 12 (8-16) weeks (2 trials); paroxetine 20 mg for 8 weeks (1 trial) | Pain reduction ≥30% | RR 1.4 (1 to 2) | 6 (343) | Low |
| Functional dyspepsia36 | Venlafaxine 150 mg for 8 weeks (1 trial); sertraline 50 mg for 8 weeks (1 trial) | Pain intensity | MD 1.3 (−5.9 to 8.5) | 2 (291) | Low† |
| Non-cardiac chest pain62 | Paroxetine 45 (40-50) mg for 10 (8-12) (2 trials); sertraline 200 for 9 (8-10) weeks mg (2 trials) | Pain intensity | MD −3.8 (−9.8 to 2.3) | 4 (184) | Low† |
| TCA | |||||
| Functional dyspepsia36 | Amitriptyline 37.5 (25-50) mg for 10 (8-12) weeks (2 trials); imipramine 50 mg for 12 weeks (1 trial) | Pain intensity | MD −2.3 (−7.3 to 2.8) | 3 (293) | Moderate† |
CI=confidence interval; MD=mean difference; RR=risk ratio; SSRI=selective serotonin reuptake inhibitors, TCA=tricyclic antidepressants.
Pain intensity outcomes are on a 0-100 scale unless stated otherwise.
Grading of recommendations assessment, development, and evaluation determined by current authors (see supplementary file 5 for details of the assessment).
Conditions for which evidence is inconclusive
Eighteen reviews provided inconclusive evidence about the efficacy of antidepressants for 17 conditions in 26 distinct comparisons.16 32 36 46 48 49 50 53 54 55 56 57 58 59 60 61 63 64 Most reviews presenting inconclusive evidence were of TCAs, with eight reviews covering nine conditions (nine comparisons).32 49 54 55 57 58 60 64 For 17 comparisons, only one trial formed the body of evidence for the comparisons. For comparisons with more than one trial available, evidence for SNRIs was inconclusive for sciatica.32 Evidence for SSRIs was inconclusive for irritable bowel syndrome.56 Evidence for TCAs was inconclusive for back pain,32 bladder pain syndrome,55 fibromyalgia,49 rheumatoid arthritis,64 and sciatica.32 Evidence for atypical and MAOI antidepressants was inconclusive for fibromyalgia.46 48 Table 4 shows typical treatment regimens, effect estimates, number of trials, and number of participants.
Table 4.
Conditions for which antidepressants show inconclusive evidence for pain
| Condition by drug class | Treatment regimen: median (minimum-maximum) dose and duration | Outcome | Effect estimate (95% CI)* | No of trials (No of participants) | Certainty of evidence |
|---|---|---|---|---|---|
| SNRI | |||||
| Atypical chronic orofacial pain54 | Venlafaxine 37.5-75 mg for 4 weeks (1 trial) | Pain intensity | MD −13 (−26.1 to 0.1) | 1 (36) | Low† |
| Chronic migraine61 | Venlafaxine 75-150 mg for 10 weeks | Headache frequency (days) | MD −1.4 (−2.8 to −0.1) | 1 (49) | Low† |
| Sciatica32 | Duloxetine 120 mg for 4 weeks (1 trial); milnacipran 100 mg for 8 (6-10) weeks (2 trials) | Pain intensity | MD −17.8 (−45.5 to 9.9) | 3 (96) | Very low |
| Chronic tension-type headache50 | Venlafaxine 150 mg for 12 weeks (1 trial) | Headache frequency (days) | MD −2.3 (−7.3 to 2.7) | 1 (59) | Low |
| SSRI | |||||
| Chronic pelvic pain63 | Sertraline 100 mg for 6 weeks (1 trial) | Pain intensity | MD 0 (−6 to 6) | 1 (23) | Low† |
| Chronic prostatitis59 | Fluvoxamine 50-300 mg for 8 weeks (1 trial) | Pain intensity | MD −45 (−77.6, −12.4) | 1 (42) | Low† |
| Irritable bowel syndrome56 | Fluoxetine 20 mg for 9 (6-12) weeks (2 trials); paroxetine 40 mg for 12 weeks (1 trial) | Abdominal pain not improving | RR 0.6 (0.3 to 1.3) | 3 (167) | Very low† |
| Chronic migraine61 | S-fluoxetine 40 mg for 12 weeks (1 trial) | Headache frequency (days) | MD −1.4 (−2.7, −0.1) | 1 (33) | Low† |
| Postoperative pain16 | Escitalopram 10 mg for 7 days (including day of surgery) (1 trial) | Pain 24 hours post-surgery | MD 0 (−7.1 to 7.1) | 1 (114) | Low |
| Chronic tension-type headache50 | Citalopram 20 mg for 24 weeks (1 trial) | Headache frequency (days) | MD −0.2 (−3.9 to 3.5) | 1 (68) | Low |
| TCA | |||||
| Acute oral mucositis58 | Doxepin mouth rinse, single dose of 10 mg/mL×2.5 mL, diluted to 5 mL with 2.5 mL of sterile or distilled water (1 trial) | Pain intensity | MD −44 (−67 to −21) | 1 (140) | Low† |
| Atypical chronic orofacial pain54 | Amitriptyline 10 mg for 8 weeks (1 trial) | Pain intensity | MD −15.7 (−32.4 to 1.1) | 1 (42) | Low† |
| Back pain32 | Amitriptyline 15 (5-25) mg for 17 (8-26) weeks (2 trials); desipramine 60 mg for 12 weeks (2 trials); nortriptyline 100 mg for 8 weeks (1 trial); imipramine 75 mg for 4 weeks (1 trial); doxepin 300 mg for 6 weeks | Pain intensity | MD −10.3 (−18.8 to −1.9) | 7 (591) | Very low |
| Bladder pain syndrome55 | Amitriptyline 62.5 (25-100) mg for 14 (12-16) weeks | Pain intensity | MD −12.7 (−33.1 to 7.6) | 2 (279) | Very low† |
| Fibromyalgia49 | Amitriptyline 37.5 (25-50) mg for 8.5 (8-24) weeks | 50% pain reduction | RR 2.1 (1.2 to 3.6) | 4 (275) | Very low |
| Phantom limb pain60‡ | Amitriptyline 10-125 mg for 6 weeks (1 trial) | Pain intensity | MD 0 (−17.6 to 17.6) | 1 (39) | Low† |
| Rheumatoid arthritis64 | Amitriptyline 50 (25-75) mg for 6 (1 day to 12 weeks) weeks (3 trials); dothiepin 75-150 mg for 7-10 weeks (2 trial); imipramine 75 mg for 6 weeks (1 trial); trimipramine 75 mg for 12 weeks (1 trial) | Pain intensity | NE. No benefit of TCA in the descriptive analysis | 7 (482) | Very low |
| Sciatica32§ | Amitriptyline 50 mg for 26 weeks (1 trial); nortriptyline 100 mg for 9 weeks (1 trial) | Pain intensity | MD −16 (−31.5 to −0.4) | 2 (114) | Very low |
| Vulvodynia57 | Desipramine 25-150 mg for 12 weeks (1 trial) | Pain intensity | MD 8.2 (−11.8, 28.2) | 1 (112) | Low† |
| NDRI | |||||
| Back pain32 | Bupropion 300 mg for 7 weeks (1 trial) | Pain intensity | MD −1 (−12.2 to 10.2) | 1 (44) | Low |
| SARI | |||||
| Back pain32 | Trazodone 600 mg for 6 weeks (1 trial) | Pain intensity | MD −5.4 (−22.9 to 12.1) | 1 (40) | Low |
| Burning mouth syndrome53 | Trazodone 200 mg for 8 weeks (1 trial) | Pain intensity | MD −1.6 (−6.8 to 3.6) | 1 (37) | Low† |
| Tetracyclic/atypical | |||||
| Back pain32 | Maprotiline 150 mg for 8 weeks (1 trial) | Pain intensity | MD −4.5 (−20.4 to 11.4) | 1 (34) | Low |
| Fibromyalgia46 | Mirtazapine 30 (30-45) mg for 13 (7-13) weeks (3 trials) | Pain reduction ≥50% | RR 1.3 (0.9 to 1.9) | 3 (591) | Very low |
| Functional dyspepsia36 | Mirtazapine 15 mg for 8 weeks (1 trial) | Pain intensity | MD 1.7 (−21.2 to 24.6) | 1 (34) | Low† |
| MAOI | |||||
| Fibromyalgia48 | Pirlindole 150 mg for 4 weeks (1 trial); moclobemide 600 mg for 12 weeks (12 weeks) | Pain intensity | MD −14.5 (−27.1 to −2) | 2 (121) | Very low† |
CI=confidence interval; MAOI=monoamine oxidase inhibitors; MD=mean difference; NDRI=noradrenaline-dopamine reuptake inhibitors; NE=not estimable; RR=risk ratio; SARI=serotonin antagonist and reuptake inhibitors; SNRI=serotonin-norepinephrine reuptake inhibitors; SSRI=selective serotonin reuptake inhibitors; TCA=tricyclic antidepressants.
Pain intensity outcomes are on a 0-100 scale unless stated otherwise.
Grading of recommendations assessment, development, and evaluation determined by current authors (see supplementary file 5 for details of the assessment).
Phantom pain is a form of neuropathic pain, although the trial included in this review was not included in the meta-analysis estimates of either of the neuropathic pain reviews (Finnerup et al 201517; Caruso et al 201935).
Sciatica may be classified as a form of neuropathic pain; however, the sciatica review included substantially more trials (n=6) than previous reviews (eg, Finnerup et al 201517 only included one trial).
Sensitivity analysis
The sensitivity analysis identified 57 reviews covering 13 conditions that were excluded owing to overlap with another eligible review. Many excluded reviews did not conduct meta-analysis. Of the ones that did, effect sizes reported by the excluded reviews were broadly similar to the ones reported in the included reviews (see supplementary file 8).
Safety and tolerability
Supplementary files 10 and 11 present data on safety and tolerability. Most safety and tolerability data were imprecise. SNRIs appeared to increase the risk of any adverse event in patients with chemotherapy induced pain and with back pain, sciatica, and osteoarthritis (data combined from all three conditions), but not with postoperative pain or tension-type headache. Use of TCAs increased the risk of any adverse events for functional dyspepsia,36 irritable bowel syndrome,56 acute oral mucositis,58 and some adverse events in vulvodynia57 and tension-type headache.51 NDRIs increased the risk of any adverse event in people with back pain in one trial only.32 Estimates for SSRIs,16 32 50 56 61 tetracyclic/atypical antidepressants,32 36 46 51 and MAOIs48 were all imprecise and not informative.
People tolerated SNRIs less than placebo in reviews of back pain, sciatica, and osteoarthritis (data combined),32 functional dyspepsia,36 neuropathic pain,35 and fibromyalgia.45 For SSRIs, TCAs, and tetracyclic/atypical antidepressants, only the review on functional dyspepsia,36 neuropathic pain,17 and tension-type headache51 showed lower tolerability compared with placebo, respectively.
Discussion
This overview of systematic reviews summarised evidence on the efficacy, safety, and tolerability of antidepressants for 26 pain conditions. No review presented high certainty evidence on the efficacy of antidepressants. Moderate certainty evidence supported the efficacy of SNRIs, mostly duloxetine at median doses of 60-120 mg for back pain, postoperative pain, fibromyalgia, and neuropathic pain. We found low certainty evidence that SNRIs were efficacious for aromatase inhibitor therapy induced pain in breast cancer, depression and comorbid chronic pain, and knee osteoarthritis; SSRIs, mostly paroxetine at a median dose of 20 mg, for people with depression and comorbid chronic pain; and that TCAs were efficacious for irritable bowel syndrome, neuropathic pain, and chronic tension-type headache. We found moderate certainty evidence that TCAs were not efficacious for functional dyspepsia, and low certainty evidence that SSRIs were not efficacious for back pain, fibromyalgia, functional dyspepsia, and non-cardiac chest pain. For the other 26 comparisons, evidence for the efficacy of antidepressants was inconclusive.
Strengths and limitations of this review
We performed a comprehensive literature search, conducted every step of the review in duplicate, investigated industry ties for all trials included in the 26 reviews that contributed with an effect estimate for pain, registered our overview prospectively, and reported the few protocol deviations in line with guidelines for research transparency. For 26 comparisons, evidence on the efficacy of antidepressants was inconclusive. Of these, 17 comparisons from 12 reviews were formed by a single small trial (<100 participants in each trial arm).16 32 36 50 53 54 57 58 59 60 61 63 This finding highlights the limited body of evidence supporting the use of several types of antidepressants for various pain conditions. The assessment of publication bias is challenging given that most comparisons had a limited number of trials. For example, only five out of the 42 comparisons in our review had 10 or more trials, which is the minimum number of studies recommended when assessing publication bias with a funnel plot. The GRADE working group recognises the challenge of assessing publication bias.65
Only nine of the 26 included reviews were considered to have high methodological quality as assessed by the AMSTAR-2 tool. We took several steps to minimise the impact of the low methodological quality of most reviews on treatment effect estimates. For example, when a Cochrane and a non-Cochrane review included the same trials on the same topic, we included the Cochrane review, which typically has higher methodological standards. We reviewed GRADE ratings for all comparisons or graded the certainty of evidence ourselves when a review did not assess the certainty of evidence. We also reanalysed the data of several reviews and conducted a sensitivity analysis contrasting trials and effect sizes from the 26 included reviews with 57 other excluded reviews.
We identified a systematic review and network meta-analysis investigating the efficacy of various drugs for migraine, including several antidepressant classes.66 For various reasons we excluded that review after closer examination of its included trials. Many of those trials, for example, did not report data on headache frequency, which is the recommended outcome for clinical trials of headache.25 26 Other trials had important issues with the unit of analysis67 or were not randomised.68
For some conditions included in this overview, pain might not be the main reason why patients seek care—such is the case for functional dyspepsia, where around four in 10 patients experience some degree of epigastric pain.36 For others, symptoms might include early satiety or postprandial fullness, or both. Findings of this review may therefore not be applicable to clinicians who are considering prescribing an antidepressant with the goal of reducing symptoms of postprandial fullness or another symptom in people with another pain condition. For some patients with pain conditions included in this review, pain might not be the only important outcome to be measured. For example, the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group recommends that outcomes other than pain, such as tenderness, fatigue, patient functional ability, and sleep disturbance be measured in all clinical trials.69 Our findings therefore may not apply to clinicians who are considering prescribing antidepressants to patients with fibromyalgia whose primary symptom is fatigue or sleep disturbance. In the review on pain and comorbid depression that we included,31 nine of the 14 included trials (64%) did not require participants to have pain in addition to depression as part of the inclusion criteria. Findings from this review therefore may not be applicable to patients who seek care primarily because of pain but also have depression.
Meaning of the study
The use of antidepressants has doubled in OECD countries from 2000 to 2015,70 and the off-label (unapproved) use of these drugs for pain is thought to be a contributing factor to that increase. In a Canadian study, 29% of antidepressant prescriptions were off-label, and about 26% of these were for treatment indications covered by our review (eg, pain, fibromyalgia, headache, digestive and urinary system disorders).9 Antidepressants are only approved for a few pain conditions. For example, in the UK71 and Australia,72 diabetic neuropathic pain is the only pain condition for which the SNRI duloxetine is approved. In the US, the Food and Drug Administration has approved duloxetine for fibromyalgia and chronic musculoskeletal pain.73 Amitriptyline is approved in the UK for neuropathic pain and prophylactic treatment of headache (tension-type and migraine),74 whereas in Australia it is not approved for any pain condition.75 For many conditions covered in our review, the use of antidepressants is therefore off-label.
TCAs are the most commonly prescribed off-label antidepressant for pain, with a study showing that 74% of antidepressant prescriptions for a pain condition concerned a TCA.9 In our review, we provided efficacy estimates of TCAs for 14 conditions. Of these, we found evidence of efficacy for only three conditions—all with low certainty evidence. For the other 11 conditions, TCAs were either ineffective (one condition, moderate certainty evidence) or the evidence was inconclusive (10 conditions, four with only one small trial). The off-label use of drugs, including antidepressants, has been associated with a higher incidence of adverse drug events.76 Given that most safety and tolerability estimates reported in our review were not informative owing to small sample sizes and imprecision (eg, around 63-66% of safety and tolerability estimates had total sample sizes <300), the safety profile of antidepressants for several conditions, many of which are used off-label, is unknown.
Implications for clinicians and future research
Caution is needed in interpreting our findings because 45% of the trials forming the body of evidence for this review had ties to industry. This is particularly relevant for the evidence on the efficacy of SNRIs, where 68% of trials were identified as having industry ties. The influence of industry on outcomes of clinical trials has been recognised as a source of bias in trials.77 78 Only one review, however, investigated the influence of industry sponsorship on patient outcomes.32 It is important that future reviews carry out sensitivity analysis based on industry funding when possible. For some pain conditions, where most or all available trials are funded by industry (eg, SNRIs for neuropathic pain and fibromyalgia), future trials free of industry ties are needed. For the three conditions with evidence of efficacy from TCA use (neuropathic pain, chronic tension-type headache, and irritable bowel syndrome), 15 out of 22 trials (68%) were published >20 years ago compared with four out of 67 (6%) trials on SNRIs. This is important as a recent study has identified that older trials of drugs for neuropathic pain (including antidepressants) are likely to have overestimated treatment effects.79 We would encourage clinicians, researchers, and policy makers to be aware of these caveats when reading our review and considering the use of SNRIs and TCAs to treat pain.
For conditions where we found that antidepressants are efficacious, it is unclear whether the effects are clinically relevant. For example, in conditions where SNRIs were deemed efficacious with moderate certainty evidence, the reduction of pain compared with placebo was smaller than 10 points on a 0-100 scale (back pain, postoperative pain, and neuropathic pain).16 32 35 For fibromyalgia, where outcomes were measured as the proportion of participants with at least a 50% reduction in pain, 31% of people receiving an SNRI improved (compared with around 21% in the placebo group), which translates to an absolute risk reduction of 9% (95% confidence interval 7% to 9%).45 We purposefully chose not to make judgments about the clinical importance of observed effects for each condition because commonly used thresholds, such as the 10 point reduction on a 0-100 scale commonly used in musculoskeletal pain research,43 are arbitrary, context specific (specific condition, treatment, comparison, and outcome), and potentially misleading if interpreted inappropriately.80 Given the challenges of making judgment calls about the clinical relevance of treatment effects, we encourage clinicians first to conduct a holistic assessment of the evidence, which includes an appraisal of the effect size, certainty of available evidence, and trade-offs between benefits and harms of each antidepressant, and then to involve patients in these discussions.
Our review identified a series of conditions for which the evidence base is scarce and high quality trials are needed. For example, no drug treatment has been shown to provide clear benefits for sciatica.81 Efficacy estimates for SNRIs and TCAs presented in our reviews may, however, contain clinically important effects, which would warrant further investigation by future trials.
Conclusions
We found evidence of efficacy of antidepressants in 11 (26%) of the 42 comparisons included in this overview of systematic reviews—seven (64%) investigated the efficacy of SNRIs. In four (37%) of the comparisons where antidepressants were efficacious, evidence was of moderate certainty: SNRIs for back pain, postoperative pain, fibromyalgia, and neuropathic pain. For the other 31 (74%) comparisons, antidepressants were either inefficacious or evidence on their efficacy was inconclusive. Our findings suggest that a more nuanced approach is needed when prescribing antidepressants for pain.
What is already known on this topic
Antidepressants are commonly used to treat a variety of pain conditions
The National Institute for Health and Care Excellence guideline for chronic primary pain has explicitly recommended against the use of pain medicines, with the exception of antidepressants
What this study adds
Some antidepressants were efficacious for some pain conditions; however, efficacy appears to depend on the condition and class of antidepressant
Although three quarters of antidepressants prescribed to treat a pain condition are tricyclic antidepressants, evidence suggests that their efficacy is inconclusive for most pain conditions
The findings suggest that a more nuanced approach is needed when prescribing antidepressants for pain
Web extra.
Extra material supplied by authors
Supplementary information: Supplementary files 1-11
Contributors: GF and CAS designed the review protocol. GF, CAS, and JZ developed the search strategy and selected studies. GF and JZ extracted data. GF and JZ analysed the data. CAS, MU, NF, RD, AM, SE, JZ, and CM made substantial contributions to the interpretation of the data. GF drafted the manuscript. CAS, MU, NF, RD, AM, SE, JZ, and CM revised the manuscript critically for important intellectual content. All authors approved the final version of the article. All authors had access to all the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. GF is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors. GEF, JZ, and CM are supported by National Health and Medical Research Council (NHMRC) fellowships (APP2009808, APP1194105, APP11094283, respectively). CM is supported by Centre for Research Excellence grants (APP1134856, APP1171459, APP2006545). NBF is supported by the Lundbeck Foundation (R359-2020-2620).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; support from the following organisations that may have an interest in the submitted work in the previous three years: The Sydney Pharmacy School receives funding from GlaxoSmithKline for a postgraduate scholarship supervised by AM. CM has received research grants from various government and not for profit agencies. Flexeze provided heat wraps at no cost for the SHaPED trial for which he is an investigator. Outside the submitted work, NBF has done consultancy work for Merck, Almirall, NeuroPN, Vertex, Novartis Pharma, and Nanobiotix; has undertaken consultancy work for Aarhus University with remuneration from Biogen, Merz, and Confo Therapeutics; and has received grants from IMI2PainCare, an EU IMI 2 (Innovative medicines initiative) public-private consortium and the companies involved are: Grunenthal, Bayer, Eli Lilly, Esteve, and Teva. MU is chief investigator or co-investigator on multiple previous and current research grants from the UK National Institute for Health Research (NIHR), and Arthritis Research UK and is a co-investigator on grants funded by the Australian National Health and Medical Research Council and Norwegian Medical Research Council. He was a senior investigator for NIHR until March 2021. He has received travel expenses for speaking at conferences from the professional organisations hosting the conferences. He is a director and shareholder of Clinvivo that provides electronic data collection for health services research. He is part of an academic partnership with Serco, funded by the European Social Fund, related to return to work initiatives. He receives some salary support from University Hospitals Coventry and Warwickshire. He is a co-investigator on two current studies and one completed study funded by NIHR that are, or have had, additional support from Stryker. Until March 2020 he was an editor of the NIHR journal series, and a member of the NIHR Journal Editors Group, for which he received a fee. No other relationships or activities that could appear to have influenced the submitted work.
The lead author (GF) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted.
Dissemination to participants and related patient and public communities: We will disseminate our findings to public, patient, and clinician organisations. Diverse media will be used, including mainstream media (eg, through press releases), social media, plain language summaries, and conference presentations.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1. Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain 2022;163:e328-32. 10.1097/j.pain.0000000000002291 [DOI] [PubMed] [Google Scholar]
- 2. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333. 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3. Kamerman PR, Bradshaw D, Laubscher R, et al. Almost 1 in 5 South African adults have chronic pain: a prevalence study conducted in a large nationally representative sample. Pain 2020;161:1629-35. 10.1097/j.pain.0000000000001844. [DOI] [PubMed] [Google Scholar]
- 4.Australian Institute of Health and Welfare. Chronic pain in Australia. 2020. https://www.aihw.gov.au/reports/chronic-disease/chronic-pain-in-australia
- 5. Abdel Shaheed C, Ferreira GE, Dmitritchenko A, et al. The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews. Med J Aust 2021;214:324-31. 10.5694/mja2.50992. [DOI] [PubMed] [Google Scholar]
- 6. Finnerup NB. Nonnarcotic Methods of Pain Management. N Engl J Med 2019;380:2440-8. 10.1056/NEJMra1807061. [DOI] [PubMed] [Google Scholar]
- 7. Mathieson S, Wertheimer G, Maher CG, et al. What proportion of patients with chronic noncancer pain are prescribed an opioid medicine? Systematic review and meta-regression of observational studies. J Intern Med 2020;287:458-74. 10.1111/joim.13026. [DOI] [PubMed] [Google Scholar]
- 8. Busse JW, Wang L, Kamaleldin M, et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA 2018;320:2448-60. 10.1001/jama.2018.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong J, Motulsky A, Eguale T, Buckeridge DL, Abrahamowicz M, Tamblyn R. Treatment Indications for Antidepressants Prescribed in Primary Care in Quebec, Canada, 2006-2015. JAMA 2016;315:2230-2. 10.1001/jama.2016.3445. [DOI] [PubMed] [Google Scholar]
- 10. Gouveia N, Rodrigues A, Ramiro S, et al. The Use of Analgesic and Other Pain-Relief Drugs to Manage Chronic Low Back Pain: Results from a National Survey. Pain Pract 2017;17:353-65. 10.1111/papr.12455. [DOI] [PubMed] [Google Scholar]
- 11. Tamblyn R, Bates DW, Buckeridge DL, et al. Multinational comparison of new antidepressant use in older adults: a cohort study. BMJ Open 2019;9:e027663. 10.1136/bmjopen-2018-027663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Driest JJ, Schiphof D, de Wilde M, Bindels PJE, van der Lei J, Bierma-Zeinstra SMA. Antidepressant and anticonvulsant prescription rates in patients with osteoarthritis: a population-based cohort study. Rheumatology (Oxford) 2021;60:2206-16. 10.1093/rheumatology/keaa544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carville S, Constanti M, Kosky N, Stannard C, Wilkinson C, Guideline Committee . Chronic pain (primary and secondary) in over 16s: summary of NICE guidance. BMJ 2021;373:n895. 10.1136/bmj.n895. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. NICE, 2021. https://www.nice.org.uk/guidance/ng193 [PubMed]
- 15. Nicholas M, Vlaeyen JWS, Rief W, et al. IASP Taskforce for the Classification of Chronic Pain . The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain 2019;160:28-37. 10.1097/j.pain.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Tobe J, Au E, et al. Selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors as adjuncts for postoperative pain management: systematic review and meta-analysis of randomised controlled trials. Br J Anaesth 2022;128:118-34. 10.1016/j.bja.2021.08.032. [DOI] [PubMed] [Google Scholar]
- 17. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162-73. 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leenstra JL, Miller RC, Qin R, et al. Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: a phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance]). J Clin Oncol 2014;32:1571-7. 10.1200/JCO.2013.53.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henry NL, Unger JM, Schott AF, et al. Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. J Clin Oncol 2018;36:326-32. 10.1200/JCO.2017.74.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ 2022;378:e070849. 10.1136/bmj-2022-070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Useem J, Brennan A, LaValley M, et al. Systematic Differences between Cochrane and Non-Cochrane Meta-Analyses on the Same Topic: A Matched Pair Analysis. PLoS One 2015;10:e0144980. 10.1371/journal.pone.0144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moseley AM, Elkins MR, Herbert RD, Maher CG, Sherrington C. Cochrane reviews used more rigorous methods than non-Cochrane reviews: survey of systematic reviews in physiotherapy. J Clin Epidemiol 2009;62:1021-30. 10.1016/j.jclinepi.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (updated February 2022). 2022. [Google Scholar]
- 24. Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Task Force of the International Headache Society Clinical Trials Subcommittee . Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008;28:484-95. 10.1111/j.1468-2982.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 25. Bendtsen L, Bigal ME, Cerbo R, et al. International Headache Society Clinical Trials Subcommittee . Guidelines for controlled trials of drugs in tension-type headache: second edition. Cephalalgia 2010;30:1-16. 10.1111/j.1468-2982.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- 26. Haywood K, Potter R, Froud R, et al. CHESS COSMIG group . Core outcome set for preventive intervention trials in chronic and episodic migraine (COSMIG): an international, consensus-derived and multistakeholder initiative. BMJ Open 2021;11:e043242. 10.1136/bmjopen-2020-043242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 2011;64:1283-93. 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 28. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 31. Gebhardt S, Heinzel-Gutenbrunner M, König U. Pain Relief in Depressive Disorders: A Meta-Analysis of the Effects of Antidepressants. J Clin Psychopharmacol 2016;36:658-68. 10.1097/JCP.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira GE, McLachlan AJ, Lin CC, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ 2021;372:m4825. 10.1136/bmj.m4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MR000033. 10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schünemann H BJ, Guyatt G, Oxman A. GRADE Handbook. 2013. [Google Scholar]
- 35. Caruso R, Ostuzzi G, Turrini G, et al. Beyond pain: can antidepressants improve depressive symptoms and quality of life in patients with neuropathic pain? A systematic review and meta-analysis. Pain 2019;160:2186-98. 10.1097/j.pain.0000000000001622 [DOI] [PubMed] [Google Scholar]
- 36. Ford AC, Moayyedi P, Black CJ, et al. Systematic review and network meta-analysis: efficacy of drugs for functional dyspepsia. Aliment Pharmacol Ther 2021;53:8-21. 10.1111/apt.16072. [DOI] [PubMed] [Google Scholar]
- 37. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97-111. 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 38. Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods 2019;10:83-98. 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- 39. Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol 2014;67:368-75. 10.1016/j.jclinepi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care 2013;17:R2. 10.1186/cc11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nüesch E, Trelle S, Reichenbach S, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. 10.1136/bmj.c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santesso N, Glenton C, Dahm P, et al. GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020;119:126-35. 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 43. Reginster JY, Reiter-Niesert S, Bruyère O, et al. Recommendations for an update of the 2010 European regulatory guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis and reflections about related clinically relevant outcomes: expert consensus statement. Osteoarthritis Cartilage 2015;23:2086-93. 10.1016/j.joca.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 44. Ferreira ML, Herbert RD, Ferreira PH, et al. A critical review of methods used to determine the smallest worthwhile effect of interventions for low back pain. J Clin Epidemiol 2012;65:253-61. 10.1016/j.jclinepi.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 45. Welsch P, Üçeyler N, Klose P, Walitt B, Häuser W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst Rev 2018;2:CD010292. 10.1002/14651858.CD010292.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Welsch P, Bernardy K, Derry S, Moore RA, Häuser W. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst Rev 2018;8:CD012708. 10.1002/14651858.CD012708.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev 2015;2015:CD011735. 10.1002/14651858.CD011735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tort S, Urrútia G, Nishishinya MB, Walitt B. Monoamine oxidase inhibitors (MAOIs) for fibromyalgia syndrome. Cochrane Database Syst Rev 2012;(4):CD009807. 10.1002/14651858.CD009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for fibromyalgia in adults. Cochrane Database Syst Rev 2015;(7). 10.1002/14651858.CD011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev 2015;2015:CD011681. 10.1002/14651858.CD011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jackson JL, Mancuso JM, Nickoloff S, Bernstein R, Kay C. Tricyclic and Tetracyclic Antidepressants for the Prevention of Frequent Episodic or Chronic Tension-Type Headache in Adults: A Systematic Review and Meta-Analysis. J Gen Intern Med 2017;32:1351-8. 10.1007/s11606-017-4121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberts KE, Adsett IT, Rickett K, Conroy SM, Chatfield MD, Woodward NE. Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev 2022;1:CD013167. 10.1002/14651858.CD013167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farag AM, Kuten-Shorrer M, Natto Z, et al. WWOM VII: Effectiveness of systemic pharmacotherapeutic interventions in the management of BMS: A systematic review and meta-analysis. Oral Dis 2021. 10.1111/odi.13817. [DOI] [PubMed] [Google Scholar]
- 54. Do TM, Unis GD, Kattar N, Ananth A, McCoul ED. Neuromodulators for Atypical Facial Pain and Neuralgias: A Systematic Review and Meta-Analysis. Laryngoscope 2021;131:1235-53. 10.1002/lary.29162. [DOI] [PubMed] [Google Scholar]
- 55. Imamura M, Scott NW, Wallace SA, et al. Interventions for treating people with symptoms of bladder pain syndrome: a network meta-analysis. Cochrane Database Syst Rev 2020;7:CD013325. 10.1002/14651858.CD013325.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-Analysis. Am J Gastroenterol 2019;114:21-39. 10.1038/s41395-018-0222-5. [DOI] [PubMed] [Google Scholar]
- 57. Pérez-López FR, Bueno-Notivol J, Hernandez AV, Vieira-Baptista P, Preti M, Bornstein J. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care 2019;24:337-46. 10.1080/13625187.2019.1643835. [DOI] [PubMed] [Google Scholar]
- 58. Christoforou J, Karasneh J, Manfredi M, et al. World Workshop on Oral Medicine VII: Non-opioid pain management of head and neck chemo/radiation-induced mucositis: A systematic review. Oral Dis 2019;25(Suppl 1):182-92. 10.1111/odi.13074. [DOI] [PubMed] [Google Scholar]
- 59. Franco JV, Turk T, Jung JH, et al. Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev 2019;10:CD012552. 10.1002/14651858.CD012552.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev 2016;10:CD006380. 10.1002/14651858.CD006380.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev 2015;4:CD002919. 10.1002/14651858.CD002919.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Atluri DK, Chandar AK, Fass R, Falck-Ytter Y. Systematic review with meta-analysis: selective serotonin reuptake inhibitors for noncardiac chest pain. Aliment Pharmacol Ther 2015;41:167-76. 10.1111/apt.13015. [DOI] [PubMed] [Google Scholar]
- 63. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev 2014;(3):CD008797. 10.1002/14651858.CD008797.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev 2011;(11):CD008920. 10.1002/14651858.CD008920.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 2011;64:1277-82. 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 66. Jackson JL, Cogbill E, Santana-Davila R, et al. A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache. PLoS One 2015;10:e0130733. 10.1371/journal.pone.0130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gomersall JD, Stuart A. Amitriptyline in migraine prophylaxis. Changes in pattern of attacks during a controlled clinical trial. J Neurol Neurosurg Psychiatry 1973;36:684-90. 10.1136/jnnp.36.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Couch JR, Ziegler DK, Hassanein R. Amitriptyline in the prophylaxis of migraine. Effectiveness and relationship of antimigraine and antidepressant effects. Neurology 1976;26:121-7. 10.1212/WNL.26.2.121. [DOI] [PubMed] [Google Scholar]
- 69. Mease P, Arnold LM, Choy EH, et al. OMERACT Fibromyalgia Working Group . Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol 2009;36:2318-29. 10.3899/jrheum.090367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Organisation for Economic Co-operation and Development. Antidepressant drugs consumption, 2000 and 2015 (or nearest year). OECD, 2017. 10.1787/health_glance-2017-en [DOI]
- 71.Electronic Medicines Compendium. Cymbalta 60mg hard gastro-resistant capsules. Accessed 29/04/2022. emc, 2022. https://www.medicines.org.uk/emc/product/7450/pil
- 72. Therapeutic Goods Administration . Duloxetine 60 mg. 2007. https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=12AE7483B02D13B9CA2586D50042281F&agid=(PrintDetailsPublic)&actionid=1
- 73. United States Food and Drug Administration . Medication guide. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021427s056lbl.pdf#page=38
- 74.Electronic Medicines Compendium. Amitriptyline 25 mg. 2022. https://www.medicines.org.uk/emc/product/10850/smpc
- 75.Therapeutic Goods Administration. Amitriptyline hydrochloride 10mg. 2021. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2010-PI-04558-3&d=20230117172310101
- 76. Eguale T, Buckeridge DL, Verma A, et al. Association of Off-label Drug Use and Adverse Drug Events in an Adult Population. JAMA Intern Med 2016;176:55-63. 10.1001/jamainternmed.2015.6058. [DOI] [PubMed] [Google Scholar]
- 77. Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167-70. 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MR000033. 10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Finnerup NB, Haroutounian S, Baron R, et al. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain 2018;159:2339-46. 10.1097/j.pain.0000000000001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Olsen MF, Bjerre E, Hansen MD, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med 2017;15:35. 10.1186/s12916-016-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jensen RK, Kongsted A, Kjaer P, Koes B. Diagnosis and treatment of sciatica. BMJ 2019;367:l6273. 10.1136/bmj.l6273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Supplementary files 1-11
Data Availability Statement
No additional data available.


