This cohort study analyzes age- and sex-specific incidence rates of frontotemporal lobar degeneration–related disorders by phenotype across 9 European countries.
Key Points
Question
How common is frontotemporal lobar degeneration (FTLD) in Europe, by age and sex?
Findings
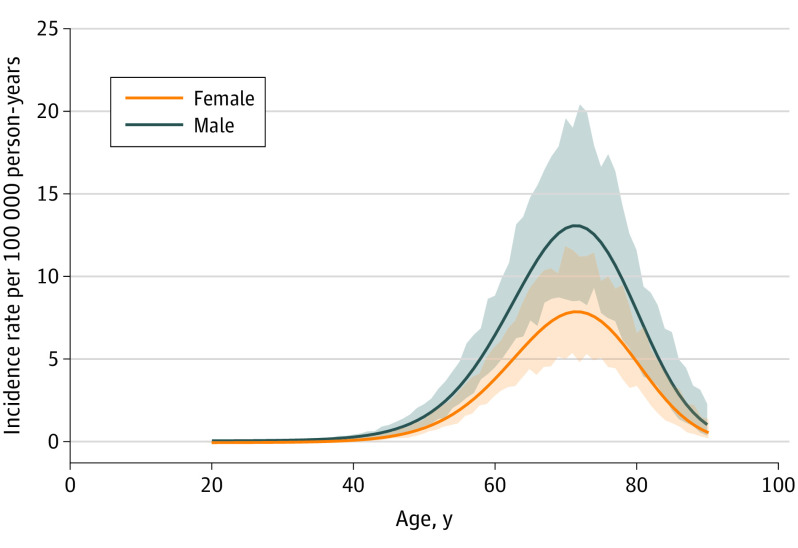
In this cohort study of 267 identified FTLD cases from 13 specialist FTLD research clinics in 9 European countries that drew a combined catchment population of 11 023 643 person-years, the incidence of FTLD reached its peak at the age of 71 years and was higher among males than females.
Meaning
The findings suggest that public health policies should consider FTLD-associated disorders as more common than previously described, and FTLD diagnosis should be considered at any age.
Abstract
Importance
Diagnostic incidence data for syndromes associated with frontotemporal lobar degeneration (FTLD) in multinational studies are urgent in light of upcoming therapeutic approaches.
Objective
To assess the incidence of FTLD across Europe.
Design, Setting, and Participants
The Frontotemporal Dementia Incidence European Research Study (FRONTIERS) was a retrospective cohort study conducted from June 1, 2018, to May 31, 2019, using a population-based registry from 13 tertiary FTLD research clinics from the UK, the Netherlands, Finland, Sweden, Spain, Bulgaria, Serbia, Germany, and Italy and including all new FTLD-associated cases during the study period, with a combined catchment population of 11 023 643 person-years. Included patients fulfilled criteria for the behavioral variant of frontotemporal dementia (BVFTD), the nonfluent variant or semantic variant of primary progressive aphasia (PPA), unspecified PPA, progressive supranuclear palsy, corticobasal syndrome, or frontotemporal dementia with amyotrophic lateral sclerosis (FTD-ALS). Data were analyzed from July 19 to December 7, 2021.
Main Outcomes and Measures
Random-intercept Poisson models were used to obtain estimates of the European FTLD incidence rate accounting for geographic heterogeneity.
Results
Based on 267 identified cases (mean [SD] patient age, 66.70 [9.02] years; 156 males [58.43%]), the estimated annual incidence rate for FTLD in Europe was 2.36 cases per 100 000 person-years (95% CI, 1.59-3.51 cases per 100 000 person-years). There was a progressive increase in FTLD incidence across age, reaching its peak at the age of 71 years, with 13.09 cases per 100 000 person-years (95% CI, 8.46-18.93 cases per 100 000 person-years) among men and 7.88 cases per 100 000 person-years (95% CI, 5.39-11.60 cases per 100 000 person-years) among women. Overall, the incidence was higher among men (2.84 cases per 100 000 person-years; 95% CI, 1.88-4.27 cases per 100 000 person-years) than among women (1.91 cases per 100 000 person-years; 95% CI, 1.26-2.91 cases per 100 000 person-years). BVFTD was the most common phenotype (107 cases [40.07%]), followed by PPA (76 [28.46%]) and extrapyramidal phenotypes (69 [25.84%]). FTD-ALS was the rarest phenotype (15 cases [5.62%]). A total of 95 patients with FTLD (35.58%) had a family history of dementia. The estimated number of new FTLD cases per year in Europe was 12 057.
Conclusions and Relevance
The findings suggest that FTLD-associated syndromes are more common than previously recognized, and diagnosis should be considered at any age. Improved knowledge of FTLD incidence may contribute to appropriate health and social care planning and in the design of future clinical trials.
Introduction
Frontotemporal lobar degeneration (FTLD) is a complex family of neuropathological conditions characterized by a spectrum of focal neurodegeneration with atrophy of the frontal and temporal lobes and a wide range of clinical, genetic, and neuropathological features.1,2,3 Different clinical phenotypes have been classically defined on the basis of presenting clinical symptoms: the behavioral variant of frontotemporal dementia (BVFTD), with early behavioral and personality changes4; the nonfluent variant of primary progressive aphasia (NFVPPA), with progressive deficits in speech, grammar, and word output; and the semantic variant of PPA (SVPPA), a progressive disorder of semantic knowledge and naming.5 Progressive supranuclear palsy (PSP),6 corticobasal syndrome (CBS),7 and frontotemporal dementia with amyotrophic lateral sclerosis (FTD-ALS) are also usually caused by subtypes of FTLD.8
In recent years, the adoption of operationalized clinical diagnostic criteria,4,5 the growing use of robust biomarkers aimed to exclude Alzheimer disease (AD),9 and the ever-improving description of imaging features related to different clinical phenotypes10 have prompted a wider awareness and recognition of FTLD-associated syndromes. This in turn may have increased the estimated incidence of this group of rare diseases. However, few studies assessing FTLD incidence are available.11,12 Some studies have been restricted to age-delimited populations or specific phenotypes in isolation or carried out considering outdated diagnostic criteria (before current consensus guidelines for each FTLD-related disorder).13,14,15 While some studies focused only on the FTLD incidence below the age of 70 years14 or FTLD prevalence below the age of 65 years,13 a population-based study considering the whole age spectrum and updated diagnostic criteria estimated FTLD-related incidence to be 1.61 per 100 000 person-years.12
In light of recent developments in pharmacological and nonpharmacological treatment approaches16,17,18,19 and in order to promote appropriate public health service policies, it is essential to quantify the incidence and prevalence of FTLD-associated disorders. This observation prompted the formation of the Frontotemporal Dementia Incidence European Research Study (FRONTIERS), a collaborative European research initiative aimed at assessing FTLD incidence across Europe, aligning multinational population-based disease registries.20
In the present retrospective analysis, FRONTIERS aimed to address 6 critical issues in the field: (1) to assess the incidence of FTLD-related disorders across Europe; (2) to assess the geographic heterogeneity of the disease presentations; (3) to define age- and sex-specific incidence rates of FTLD; (4) to describe the distribution of different FTLD phenotypes, related clinical features, and phenotype heterogeneity; (5) to assess the frequency of a positive family history across phenotypes; and (6) to estimate the numbers of new cases in Europe on the basis of the data obtained.
Methods
FRONTIERS Centers and Study Design
This retrospective cohort study was based on data collected in 13 population-based FTLD registries across 9 European countries: Bulgaria, Finland, Germany, Italy, the Netherlands, Serbia, Spain, Sweden, and the UK (eFigure 1 in Supplement 1). In 4 countries (Italy, Finland, Germany, and the Netherlands), the registries are split into 2 distinct administrative areas. In the present study, we did not include data from Dublin, Ireland, a center within the FRONTIERS network, because of administrative constraints. Each tertiary referral center was selected on the basis of both long-lasting experience in the FTLD field and the ability to cover a well-defined geographical area with comprehensive referral pathways.20 FRONTIERS investigators carried out patient assessments. To ensure that each case had been evaluated by the referral center, additional sources of information, such as other hospitals or dementia centers in the referral geographic area, local lay associations, and charities, were contacted. The presence of national health systems with free public medicine in most of the participating countries meant that nearly the entire population of those with FTLD-associated disorders was likely to be visited at some stage during illness and thus to be ascertained by their respective registry. Using the described system for inception of cases, we identified all residents in the registries’ catchment populations over a 1-year period from June 1, 2018, to May 31, 2019. Written informed consent was obtained from all participants or caregivers according to the Declaration of Helsinki.21 The central ethics committee in Lecce and the local ethics committee at each site approved the study protocol. The study was compliant with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and Standards of Reporting of Neurologic Disorders22 guideline requirements.
Participants and Inclusion and Exclusion Criteria
Patients with a new FTLD-related diagnosis in the defined ascertainment time window and in the defined geographical boundaries were considered. Diagnosis was made by FRONTIERS investigators according to standard procedures at each tertiary referral center, including clinical examination, standardized neuropsychological assessment at each site, structural or functional imaging, and in selected cases, assessment of cerebrospinal fluid biomarkers to exclude AD and/or genetic screening. Patients fulfilling current clinical criteria and with features indicating BVFTD, NFVPPA or SVPPA, PSP, CBS, or FTD-ALS were considered.4,5,6,7,8 FRONTIERS included mixed or indeterminate PPA with negative AD markers (unspecified PPA) but did not include the logopenic variant of PPA, as the majority of logopenic PPA cases have AD as the underlying pathology. Moreover, patients in a prodromal stage or asymptomatic patients with FTLD-associated pathogenetic sequence variations were excluded.23
FRONTIERS achieved consensus on a standardized set of data recorded by each center, which were checked and confirmed by the coordinating center (G.L.). Family history was assessed according to the modified Goldman score (GS), ranging from 1-4 with 1 indicating an autosomal dominant inheritance pattern and 4 indicating no known family history of neurodegenerative disorders (eMethods in Supplement 1).24 Disease severity at the time of diagnosis was measured by the Clinical Dementia Rating (CDR) Dementia Staging Instrument plus behavior and language domains from the National Alzheimer Coordinating Center and Frontotemporal Lobar Degeneration modules (CDR plus NACC FTLD) sum of boxes (range, 0-24, with higher scores indicating more severe disease).25 Age at estimated time of symptom onset and age at diagnosis were recorded. Disease duration at diagnosis was the period between symptom onset (based on patient and/or informant report) and the diagnosis of an FTLD-associated syndrome.
Inclusion criteria were the following: (1) participant was aged 18 years or older; (2) participant fulfilled current clinical criteria of the FTLD spectrum4,5,6,7,8; (3) diagnosis of the FTLD-related disorder was made in the referral period of the current retrospective study; (4) participant was living in a referral geographical area selected in each country for the purpose of the present study; (5) participant had an identified informant if necessary; and (6) participant had no significant medical or psychiatric illness, such as major depression, schizophrenia, or bipolar disorder.
Statistical Analysis
Patients’ characteristics were reported as the mean and standard deviation or median and range for quantitative variables and as the frequency and percentage for categorical variables. The Kruskal-Wallis test, followed by the post hoc Dunn test with Bonferroni-adjusted P values, was used to compare the GS distribution between FTLD subtypes. Incidence rates were computed as the ratio of the number of new FTLD cases recorded in 1 year to the number of residents in the area (approximation for the number of person-years spent at risk by the population of interest in 1 year). Incidence rates were computed for each combination of age group (5-year age categories, with the last category ≥90 years), sex (male, female), and residence area (13 catchment areas). Incidence rates were reported as the number of cases per 100 000 person-years along with their 95% CIs based on Γ distribution.
In order to model the FTLD incidence rate based on age and sex, we fitted a random-intercept Poisson model with the number of cases as the dependent variable and the logarithm of the number of residents as the offset. A random intercept was included for each residence area to account for heterogeneity (details of the model are reported in the eMethods in Supplement 1). We considered the incidence rate per 100 000 person-years derived from the fixed effects only as a summary measure representing the incidence rate of the average European region. Confidence intervals for the average region’s incidence rate by age (0-120 years) and sex were obtained by parametric bootstrapping using the bootMer function (lmer R package; R Project for Statistical Computing) with 100 bootstrapped data sets.
We also fitted an intercept-only random-intercept Poisson model with the same characteristics. Consistently, the exponentiated intercept was interpreted as an overall summary measure of the European FTLD incidence rates. A random-intercept Poisson model with only sex (or age group) as the covariate was instead used to investigate sex (or age) differences and estimate the sex-specific (or age-specific) average European FTLD incidences. The average European incidence rate was estimated and the sex differences were investigated for the 4 FTLD diagnostic groups separately (BVFTD, CBS or PSP, FTD-ALS, and PPA) using the same methods.
The 2013 European Standard Population26 with 19 age bands, as available in the esp2013 function of the PHEindicatormethods R package, was used as standard. Directly standardized rates and 95% Γ CIs27 were computed using the function dsr from the dsr R package.28 All analyses were conducted using R, version 4.0.3, and RStudio, version 1.1.456 (RStudio, PBC). Two-sided P < .05 was considered significant. Data were analyzed from July 19, to December 7, 2021.
Results
Incidence of FTLD in Europe
Overall, 267 incident cases (mean [SD] patient age, 66.70 [9.02] years; 111 females [41.57%] and 156 males [58.43%]) were identified in the 13 European residence areas (demographic features are shown in Table 1). The incidence of FTLD in each of the 13 catchment areas is shown in Table 2. The overall incidence rate was comparable in 9 out of 13 centers (69.23%), with incidence-rate estimates ranging from 1.77 cases per 100 000 person-years (95% CI, 1.20-2.51 cases per 100 000 person-years) to 4.40 cases per 100 000 person-years (95% CI, 3.07-6.12 cases per 100 000 person-years). The lowest estimated incidence rates were found in the Netherlands (Zuid-Holland Zuid: 0.44 cases per 100 000 person-years; 95% CI, 0.05-1.58 cases per 100 000 person-years; Rotterdam Rijnmond: 0.84 cases per 100 000 person-years; 95% CI, 0.42-1.51 cases per 100 000 person-years) and in Stockholm, Sweden (0.83 cases per 100 000 person-years; 95% CI, 0.36-1.63 cases per 100 000 person-years), whereas the highest incidence rate was found in 1 of the 2 catchment areas of Finland, that is, Northern Savo (8.14 cases per 100 000 person-years; 95% CI, 4.97-12.58 cases per 100 000 person-years) (eFigure 2 and eTable in Supplement 1). The combined catchment population of the 13 FTLD registries was 11 023 643 person-years. The average European incidence rate of FTLD was estimated to be 2.36 cases per 100 000 person-years (95% CI, 1.59-3.51 cases per 100 000 person-years).
Table 1. Demographic Features and Goldman Scores in Frontotemporal Lobar Degeneration Subtypes.
| Feature | Incident cases | ||||
|---|---|---|---|---|---|
| BVFTD (n = 107) | PPA (n = 76) | CBS or PSP (n = 69) | FTD-ALS (n = 15) | Total (N = 267) | |
| Age, mean (SD), y | 64.19 (10.10) | 67.12 (7.63) | 70.38 (7.32) | 65.53 (9.10) | 66.70 (9.02) |
| Sex, No. (%) | |||||
| Female | 45 (42.06) | 31 (40.79) | 30 (43.48) | 5 (33.33) | 111 (41.57) |
| Male | 62 (57.94) | 45 (59.21) | 39 (56.52) | 10 (66.67) | 156 (58.43) |
| Educational level, y | |||||
| Missing data, No. (%) | 9 (8.41) | 4 (5.26) | 10 (14.49) | 1 (6.67) | 24 (8.99) |
| Mean (SD) | 11.50 (3.74) | 11.93 (3.31) | 10.64 (4.18) | 12.00 (3.88) | 11.45 (3.75) |
| Disease duration, mean (SD), mo | 36.89 (21.53) | 33.41 (19.38) | 33.23 (19.77) | 20.93 (15.04) | 34.06 (20.39) |
| AD ruled out, No. (%)a | 49 (45.79) | 28 (36.84) | 15 (21.74)b | 7 (46.67) | 99 (37.08) |
| Goldman score, No. (%) | |||||
| 1 | 7 (6.54) | 1 (1.32) | 1 (1.45) | 1 (6.67) | 10 (3.75) |
| 2 | 9 (8.41) | 3 (3.95) | 1 (1.45) | 1 (6.67) | 14 (5.24) |
| 3 | 17 (15.89) | 5 (6.58) | 6 (8.70) | 0 (0.00) | 28 (10.49) |
| 3.5 | 14 (13.08) | 18 (23.68) | 10 (14.49) | 1 (6.67) | 43 (16.10) |
| 4 | 60 (56.07) | 49 (64.47) | 51 (73.91) | 12 (80.00) | 172 (64.42) |
| CDR plus NACC FTLD sum of boxes | |||||
| Missing, No. (%) | 15 (14.02) | 7 (9.21) | 7 (10.14) | 3 (20.00) | 32 (11.99) |
| Median (range) | 6.0 (1.0-20.0) | 3.0 (0.5-21.0) | 3.5 (0.5-23.0) | 4.5 (1.0-20.0) | 4.0 (0.5-23.0) |
Abbreviations: AD, Alzheimer disease; BVFTD, behavioral variant of frontotemporal dementia; CBS, corticobasal syndrome; CDR plus NACC FTLD, Clinical Dementia Rating Dementia Staging Instrument plus behavior and language domains from the National Alzheimer Coordinating Centre and Frontotemporal Lobar Degeneration modules; FTD-ALS, frontotemporal dementia with amyotrophic lateral sclerosis; PPA, primary progressive aphasia; PSP, progressive supranuclear palsy.
Ruled out by either cerebrospinal fluid markers (amyloid-β, tau, and P-tau), positron emission tomography image negative for amyloid, demonstration of FTLD-related pathogenetic sequence variations, or following autopsy.
AD ruled out for 11 of the 27 CBS cases (40.74%) and for 4 of the 42 PSP cases (9.52%).
Table 2. Raw and Age-Standardized Incidence of FTLD per 100 000 Person-Years by Catchment Area.
| Registry | Catchment area | Cases, No. (N = 267) | Denominator, person-years | Incidence (95% CI), cases per 100 000 person-years | Standardized incidence (95% CI), cases per 100 000 person-yearsa |
|---|---|---|---|---|---|
| Italy | Lecce | 35 | 795 134 | 4.40 (3.07-6.12) | 3.79 (2.64-5.27) |
| Brescia | 30 | 1 265 954 | 2.37 (1.60-3.38) | 2.26 (1.53-3.23) | |
| Finland | Northern Ostrobothnia | 17 | 412 161 | 4.12 (2.40-6.60) | 4.16 (2.42-6.68) |
| Northern Savo | 20 | 245 602 | 8.14 (4.97-12.58) | 7.01 (4.24-10.91) | |
| Spain | Donostialdea | 15 | 388 091 | 3.87 (2.16-6.37) | 3.50 (1.95-5.79) |
| Bulgaria | Sofia City and Sofia Region | 52 | 1 557 161 | 3.34 (2.49-4.38) | 3.52 (2.62-4.62) |
| Serbia | Belgrade and Vojvodina | 40 | 1 690 193 | 2.37 (1.69-3.22) | 2.25 (1.60-3.07) |
| UK | Cambridgeshire and Norfolk | 31 | 1 753 964 | 1.77 (1.20-2.51) | 1.70 (1.15-2.41) |
| The Netherlands | Zuid-Holland Zuid | 2 | 456 891 | 0.44 (0.05-1.58) | 0.42 (0.05-1.52) |
| Rotterdam Rijnmond | 11 | 1 305 717 | 0.84 (0.42-1.51) | 0.88 (0.44-1.58) | |
| Sweden | Stockholm | 8 | 967 160 | 0.83 (0.36-1.63) | 1.02 (0.44-2.00) |
| Germany | Ulm | 4 | 126 476 | 3.16 (0.86-8.10) | 3.25 (0.86-8.45) |
| Neu-Ulm | 2 | 59 139 | 3.38 (0.41-12.22) | 3.23 (0.39-11.69) |
Abbreviation: FTLD, frontotemporal lobar degeneration.
European Standard Population 2013.
Age- and Sex-Specific FTLD Incidence Rates
European FTLD incidence was different between sexes. The estimated European incidence was 1.91 cases per 100 000 person-years (95% CI, 1.26-2.91 cases per 100 000 person-years) among women and 2.84 cases per 100 000 person-years (95% CI, 1.88-4.27 cases per 100 000 person-years) among men (P = .001).
FTLD incidence increased with age, reaching its peak at the age of 71 years with an incidence rate of 13.09 cases per 100 000 person-years (95% CI, 8.46-18.93 cases per 100 000 person-years) among men and 7.88 cases per 100 000 person-years (95% CI, 5.39-11.60 cases per 100 000 person-years) among women (Figure). The estimated variance of the random effect in the random-intercept model with age and sex as covariates was 0.35 (95% CI, 0.13-1.02), indicating the presence of variability across the registries. Per the age- and sex-specific European incidence estimates, we expect 12 057 new FTLD cases each year in Europe based on the European Union 28 countries population size and age-sex structure (January 1, 2019).29
Figure. Sex- and Age-Specific Incidence Rates of Frontotemporal Lobar Degeneration in Europe per 100 000 Person-Years.
Estimates were derived from the fixed effects of a random-intercept Poisson model including age (cubic polynomial) and sex as covariates and a random intercept for residence area. Shading indicates 95% bootstrapped CIs.
Phenotypes Distribution and Clinical Features of FTLD in Europe
Overall, the most common phenotype was BVFTD, diagnosed in 107 cases (40.07%). Language phenotypes represented 76 incident cases (28.46%), with NFVPPA in 33 patients (12.36%), SVPPA in 23 (8.61%), and an unspecified PPA subtype in 20 (7.49%). Extrapyramidal phenotypes were diagnosed in 69 cases (25.84%), with PSP in 42 (15.73%) and CBS in 27 (10.11%). FTD-ALS was the rarest phenotype, found in 15 patients (5.62%).
The youngest age at diagnosis was 21 years (BVFTD), while the oldest was 87 years (PSP). The median disease duration from onset to diagnosis was 29 months (range, 2-122 months), with the highest median disease duration found for SVPPA (median, 36 months; range, 14-96 months) and BVFTD (median, 35 months; range, 6-101 months). Disease severity at diagnosis, measured with the median CDR plus NACC FTLD sum of boxes, was 4 (range, 0.5-23), the highest being in the BVFTD group (median, 6; range, 1-20).
We further explored the time of a switch from the predominant phenotype at onset to the development of a second cluster of symptoms. The median time to develop a second cluster of symptoms was 12 months (range, 0-72 months) and was particularly short in NFVPPA (median, 8.5 months; range, 0-24 months) and CBS (median, 9 months; range, 0-48 months).
FTLD inheritance was measured with the GS, with 95 patients (35.58%) presenting a family history for dementia (GS < 4) and 24 (8.99%) presenting either autosomal dominant disorder (GS = 1; 10 patients [3.75%]) or familial aggregation of 3 or more affected relatives (GS = 2; 14 patients [5.24%]). We found a statistically significant difference in the degree of heritability among FTLD subtypes (χ2, 9.97; P = .02), with lower GS (ie, higher heritability) in BVFTD as compared with CBS or PSP (Z, −2.87; adjusted P = .02) (Table 1).
The estimated European average incidence of each clinical phenotype per 100 000 person-years is reported in Table 3. The FTLD subtype characterized by the largest incidence variability across European registries was the CBS or PSP phenotype (random-intercept variance, 1.94), while the one with the smallest variability was PPA (random-intercept variance, 0.15).
Table 3. European Incidence Rate of FTLD Phenotype Cases per 100 000 Person-Yearsa.
| Phenotype | Total | Rate (95% CI), cases per 100 000 person-years | Random-intercept variance, sex-adjusted model | ||
|---|---|---|---|---|---|
| Rate (95% CI), cases per 100 000 person-years | Random-intercept variance | Male | Female | ||
| BVFTD | 0.83 (0.52-1.32) | 0.48 | 0.99 (0.60-1.62) | 0.67 (0.40-1.13) | 0.48 |
| PPA | 0.61 (0.42-0.88) | 0.15 | 0.74 (0.49-1.12) | 0.48 (0.31-0.76) | 0.15 |
| CBS or PSP | 0.51 (0.22-1.20) | 1.94 | 0.59 (0.25-1.41) | 0.44 (0.18-1.06) | 1.93 |
| FTD-ALS | 0.11 (0.05-0.26) | 0.81 | 0.16 (0.06-0.39) | 0.08 (0.03-0.23) | 0.80 |
Abbreviations: BVFTD, behavioral variant frontotemporal dementia; CBS, corticobasal syndrome; FTD-ALS, frontotemporal dementia with amyotrophic lateral sclerosis; FTLD, frontotemporal lobar degeneration; PPA, primary progressive aphasia; PSP, progressive supranuclear palsy.
Estimates of incidence and sex-specific incidence are derived from random-intercept Poisson models. The estimated variance of the random intercept (measure of incidence heterogeneity across registries) is also reported.
Discussion
In this retrospective study, we present, to our knowledge, the first multinational effort to estimate the incidence of FTLD-associated disorders in Europe. The data collected in 13 well-defined geographical areas across 9 countries over a period of 1 year drew on a pooled catchment population of more than 11 000 000 people.
The diagnostic incidence of FTLD-associated disorders was more than 2 cases per 100 000 person-years, with 9 out of 13 registries in the range between 1.77 and 4.40 cases per 100 000 person-years. Indeed, from a geographical point of view, estimated FTLD incidence captured a relative homogeneity among different countries, with the exception of 1 district surveyed in Finland (Northern Savo) with the highest incidence rate and comparatively low incidence rates found in the Netherlands and Sweden. As recently demonstrated, the high rate found in Northern Savo may be linked to the prevalence of C9orf72 sequence variations specifically found in this area, as compared with other Finnish areas (ie, Northern Ostrobothnia).30 Conversely, the low incidence detected in the Netherlands and in Stockholm might be due to incomplete ascertainment and nonuniform assessments in place across registries, which could also influence bias toward specific phenotypes. This interpretation needs to be confirmed in future prospective studies.
The second aim of the present study was to assess age- and sex-specific FTLD incidence. A relevant finding of this study was that more than 60% of patients were 65 years or older, with an incidence peak at the age of 71 years. This age distribution was comparable with previous population-based studies,11,12 further suggesting that FTLD cannot be merely considered presenile neurodegenerative dementia but that its diagnosis should be considered at any age. In older age groups, greater awareness of late-onset FTLD and the use of biological markers may be especially important in order to avoid misidentification with AD. Indeed, the age distribution in FRONTIERS differed from autopsy series and clinical-based series,1,3 which typically do not take into account reference data of the source populations.
In terms of sex differences, FTLD diagnostic incidence was found to be higher in male than in female patients. This was to be expected, although sex has not been shown to be a biological determinant in FTLD based on previous population-based studies in Europe and the US. In the Rochester study, for example, the gap between males and females in terms of FTLD incidence increased after the year 2000.31 Similarly, the incidence of ALS, a disease that shares genetic risk factors with FTLD, including C9orf72 pathogenetic sequence variations, is generally higher in males.32 The structural heterogeneity of FTLD needs to be further explored, with sex as a possible determinant,33 even though referral biases cannot be excluded.
Another task of the present work was to establish FTLD phenotype distribution, a goal that may only be examined in population-based studies considering new cases over a period of time and that cannot be evaluated in prevalent clinical-based studies. We found that BVFTD was the most common phenotype, followed by comparable rates in language and extrapyramidal variants; FTD-ALS was found to be a rare phenotype. Phenotypic diagnosis within the FTLD spectrum remains challenging, considering the relatively early occurrence of symptoms belonging to other phenotypes, as demonstrated even by the present series. Interestingly, among FTLD subtypes, PPA and CBS or PSP were characterized by the smallest and the highest variance in incidence of diagnosis, respectively, across the European registries. This result could be explained by a higher degree of robustness of diagnostic criteria for PPA compared with CBS or PSP across centers, by possible differences in coverage of movement-disorders phenotypes across referral centers, or by real heterogeneity in terms of new case occurrence of CBS or PSP phenotypes (or by a combination of these factors).
In the same vein, the proportion of familial aggregation may be properly addressed only in incident population-based studies. We found a high proportion of patients with positive family history as measured with the GS, with 35.58% of patients presenting family history for dementia and almost 9% of patients showing high familial aggregation. This is in line with previous reports from a cohort study,34 but only future prospective population-based surveys considering extensive genetic screening may define the rate of monogenic disease and the rate of the different pathogenetic sequence variations within the FTLD spectrum. Moreover, integrating population-based data with findings from European cohort studies, such as Genetic Frontotemporal Dementia Initiative (GENFI),35 may give further insights into monogenic disease.
Strengths and Limitations
The main strength of the present study is the large and multinational population-based approach using registries with a design seeking to minimize diagnostic differences through shared diagnostic published criteria. All FTLD diagnoses were finalized in clinical centers with expertise in FTLD-associated disorders. The presence of a public health system and multiple sources of case detection in the designated geographic areas are key elements to support complete case ascertainment, a linchpin in population-based registry studies. We also used complex and advanced statistical methods to summarize information from different registries, properly accounting for the intrinsic heterogeneity.
We acknowledge that this work also has limitations. First, FTLD-associated disorders are rare and heterogeneous conditions. Some cases may have been missed or misdiagnosed, and this is especially true for cases with behavioral presentation that may overlap with psychiatric disorders or movement disorders.36 Second, there will be people whose phenotypic expression of FTLD is not one of the classic disorders described here (eg, ataxia, Parkinson disease mimics, primary amnestic syndromes, or psychotic depression). Third, tracking small numbers of incident cases and defining an appropriate source population are extremely challenging tasks. For this reason, FRONTIERS relied on the reconstructed cohort design, using case information from a well-defined geographic region collected through a complex surveillance system and administrative data about the population structure, to identify a theoretical cohort of interest.37
Conclusions
The findings of this multinational European study suggest that FTLD-associated disorders are more common than previously described, and their diagnosis needs to be considered also in the elderly, beyond the age of 70 years. We estimated that the projected number of newly diagnosed FTLD cases in Europe is approximately 12 000 per year. This is a substantial burden on the European health and welfare system that should be seriously considered to provide appropriate health and social care planning and to design future clinical trials. Further confirmatory prospective studies and further studies with similar design in different geographic areas are needed.
eMethods. Details About the Modified Goldman Score and the Random Intercept Poisson Model
eFigure 1. FRONTIERS Centres, Principal Investigators, and Source Populations
eFigure 2. FTLD Incidence Rates in the 13 Referral Geographical Areas
eTable. FTLD Incidence Rates in the 13 Referral Geographical Areas by Age Groups
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996-2005. doi: 10.1093/brain/awh598 [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1-4. doi: 10.1007/s00401-009-0612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohrer JD, Lashley T, Schott JM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134(Pt 9):2565-2581. doi: 10.1093/brain/awr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höglinger GU, Respondek G, Stamelou M, et al. ; Movement Disorder Society-endorsed PSP Study Group . Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society criteria. Mov Disord. 2017;32(6):853-864. doi: 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503. doi: 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis–frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3-4):153-174. doi: 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simrén J, Ashton NJ, Blennow K, Zetterberg H. An update on fluid biomarkers for neurodegenerative diseases: recent success and challenges ahead. Curr Opin Neurobiol. 2020;61:29-39. doi: 10.1016/j.conb.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 10.Filippi M, Agosta F. MRI of non-Alzheimer’s dementia: current and emerging knowledge. Curr Opin Neurol. 2018;31(4):405-414. doi: 10.1097/WCO.0000000000000571 [DOI] [PubMed] [Google Scholar]
- 11.Logroscino G, Piccininni M, Binetti G, et al. Incidence of frontotemporal lobar degeneration in Italy: the Salento-Brescia Registry study. Neurology. 2019;92(20):e2355-e2363. doi: 10.1212/WNL.0000000000007498 [DOI] [PubMed] [Google Scholar]
- 12.Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi: 10.1212/WNL.0000000000002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621. doi: 10.1212/WNL.58.11.1615 [DOI] [PubMed] [Google Scholar]
- 14.Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology. 2004;62(3):506-508. doi: 10.1212/01.WNL.0000106827.39764.7E [DOI] [PubMed] [Google Scholar]
- 15.Garre-Olmo J, Genís Batlle D, del Mar Fernández M, et al. ; Registry of Dementia of Girona Study Group (ReDeGi Study Group) . Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology. 2010;75(14):1249-1255. doi: 10.1212/WNL.0b013e3181f5d4c4 [DOI] [PubMed] [Google Scholar]
- 16.Panza F, Lozupone M, Seripa D, et al. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat Rev Neurol. 2020;16(4):213-228. doi: 10.1038/s41582-020-0330-x [DOI] [PubMed] [Google Scholar]
- 17.Benussi A, Dell’Era V, Cosseddu M, et al. Transcranial stimulation in frontotemporal dementia: a randomized, double-blind, sham-controlled trial. Alzheimers Dement (N Y). 2020;6(1):e12033. doi: 10.1002/trc2.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsapkini K, Webster KT, Ficek BN, et al. Electrical brain stimulation in different variants of primary progressive aphasia: a randomized clinical trial. Alzheimers Dement (N Y). 2018;4:461-472. doi: 10.1016/j.trci.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotelli M, Manenti R, Petesi M, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis. 2014;39(4):799-808. doi: 10.3233/JAD-131427 [DOI] [PubMed] [Google Scholar]
- 20.Borroni B, Graff C, Hardiman O, et al. Frontotemporal Dementia Incidence European Research Study—FRONTIERS: rationale and design. Alzheimers Dement. 2022;18(3):498-506. doi: 10.1002/alz.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Bennett DA, Brayne C, Feigin VL, et al. Explanation and elaboration of the Standards of Reporting of Neurological Disorders Checklist: a guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Neuroepidemiology. 2015;45(2):113-137. doi: 10.1159/000439132 [DOI] [PubMed] [Google Scholar]
- 23.Benussi A, Alberici A, Samra K, et al. ; GENFI Consortium . Conceptual framework for the definition of preclinical and prodromal frontotemporal dementia. Alzheimers Dement. 2022;18(7):1408-1423. doi: 10.1002/alz.12485 [DOI] [PubMed] [Google Scholar]
- 24.Goldman JS, Farmer JM, Wood EM, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65(11):1817-1819. doi: 10.1212/01.wnl.0000187068.92184.63 [DOI] [PubMed] [Google Scholar]
- 25.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131(Pt 11):2957-2968. doi: 10.1093/brain/awn234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Commission. Revision of the European Standard Population: report of Eurostat’s task force. 2013. Accessed January 14, 2022. https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF/e713fa79-1add-44e8-b23d-5e8fa09b3f8f
- 27.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791-801. doi: [DOI] [PubMed] [Google Scholar]
- 28.R Project for Statistical Computing. Index of /src/contrib/Archive/dsr. Accessed January 14, 2022. https://cran.r-project.org/src/contrib/Archive/dsr/
- 29.Eurostat. European Union. Accessed December 14, 2022. https://ec.europa.eu/eurostat/web/main/eurostat/web/main/help/faq/data-services
- 30.Rostalski H, Korhonen V, Kuulasmaa T, et al. A novel genetic marker for the C9orf72 repeat expansion in the Finnish population. J Alzheimers Dis. 2021;83(3):1325-1332. doi: 10.3233/JAD-210599 [DOI] [PubMed] [Google Scholar]
- 31.Turcano P, Stang CD, Mielke MM, et al. Incidence of frontotemporal disorders in Olmsted County: a population-based study (1995–2010). Alzheimers Dement. 2020;16(3):482-490. doi: 10.1016/j.jalz.2019.08.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logroscino G, Traynor BJ, Hardiman O, et al. ; EURALS . Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81(4):385-390. doi: 10.1136/jnnp.2009.183525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Illán-Gala I, Casaletto KB, Borrego-Écija S, et al. Sex differences in the behavioral variant of frontotemporal dementia: a new window to executive and behavioral reserve. Alzheimers Dement. 2021;17(8):1329-1341. doi: 10.1002/alz.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73(18):1451-1456. doi: 10.1212/WNL.0b013e3181bf997a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal Dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14(3):253-262. doi: 10.1016/S1474-4422(14)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ducharme S, Dols A, Laforce R, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. 2020;143(6):1632-1650. doi: 10.1093/brain/awaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logroscino G, Kurth T, Piccininni M. The reconstructed cohort design: a method to study rare neurodegenerative diseases in population-based settings. Neuroepidemiology. 2020;54(2):114-122. doi: 10.1159/000502863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Details About the Modified Goldman Score and the Random Intercept Poisson Model
eFigure 1. FRONTIERS Centres, Principal Investigators, and Source Populations
eFigure 2. FTLD Incidence Rates in the 13 Referral Geographical Areas
eTable. FTLD Incidence Rates in the 13 Referral Geographical Areas by Age Groups
Nonauthor Collaborators
Data Sharing Statement