This systematic review and meta-analysis investigates whether individuals with autism are at a higher associated risk of developing cardiometabolic diseases such as diabetes, hypertension, dyslipidemia, and macrovascular atherosclerotic disease.
Key Points
Question
Are individuals with autism at a higher associated risk of developing cardiometabolic diseases, including diabetes, hypertension, dyslipidemia, and macrovascular atherosclerotic disease?
Findings
In this systematic review and meta-analysis of 34 studies that included 276 173 participants with autism and 7 733 306 participants without autism, individuals with autism had a higher associated risk of developing diabetes, dyslipidemia, and heart disease, but not hypertension or stroke. The associated risks of developing diabetes and hypertension were even higher among children with autism.
Meaning
Study results suggest that having autism may be associated with a higher risk of developing diabetes, dyslipidemia, and heart disease.
Abstract
Importance
Although the increased risk of obesity among individuals with autism has been well established, evidence on the association between autism, cardiometabolic disorders, and obesity remains inconclusive.
Objective
To examine the association between autism spectrum disorders and cardiometabolic diseases in a systematic review and meta-analysis.
Data Sources
PubMed, Scopus, Web of Science, ProQuest, Embase, and Ovid databases were searched from inception through July 31, 2022, without restrictions on date of publication or language.
Study Selection
Observational or baseline data of interventional studies reporting the prevalence of cardiometabolic risk factors (ie, diabetes, hypertension, dyslipidemia, atherosclerotic macrovascular disease) among children and/or adults with autism and matched with participants without autism were included.
Data Extraction and Synthesis
Screening, data extraction, and quality assessment were performed independently by at least 2 researchers. DerSimonian-Laird random-effects meta-analyses were performed using the meta package in R.
Main Outcomes and Measures
Relative risks (RRs) of diabetes, hypertension, dyslipidemia, and atherosclerotic macrovascular disease among individuals with autism were the primary outcomes. Secondary outcomes included the RR of type 1 and type 2 diabetes, heart disease, stroke, and peripheral vascular disease.
Results
A total of 34 studies were evaluated and included 276 173 participants with autism and 7 733 306 participants without autism (mean [range] age, 31.2 [3.8-72.8] years; pooled proportion [range] of female individuals, 47% [0-66%]). Autism was associated with greater risks of developing diabetes overall (RR, 1.57; 95% CI, 1.23-2.01; 20 studies), type 1 diabetes (RR, 1.64; 95% CI, 1.06-2.54; 6 studies), and type 2 diabetes (RR, 2.47; 95% CI, 1.30-4.70; 3 studies). Autism was also associated with increased risks of dyslipidemia (RR, 1.69; 95% CI, 1.20-2.40; 7 studies) and heart disease (RR, 1.46; 95% CI, 1.42-1.50; 3 studies). Yet, there was no significantly associated increased risk of hypertension and stroke with autism (RR, 1.22; 95% CI, 0.98-1.52; 12 studies; and RR, 1.19; 95% CI, 0.63-2.24; 4 studies, respectively). Meta-regression analyses revealed that children with autism were at a greater associated risk of developing diabetes and hypertension compared with adults. High between-study heterogeneity was a concern for several meta-analyses.
Conclusions and Relevance
Results suggest that the associated increased risk of cardiometabolic diseases should prompt clinicians to vigilantly monitor individuals with autism for potential contributors, signs of cardiometabolic disease, and their complications.
Introduction
Autism spectrum disorders represent a group of neurodevelopmental differences commonly diagnosed in childhood, characterized by impaired communication and social interactions and restricted, repetitive behaviors.1 Autism has a prevalence of 17 per 1000 children in the US,2 and a global prevalence of 23 per 1000.3 Autism is associated with multiple medical, neurologic, and psychiatric comorbidities.4,5,6 These comorbidities often exacerbate disparities in the quality of life and life expectancy of individuals with autism.7
Obesity has emerged as an important comorbidity associated with autism. Several large-scale observational studies have repeatedly shown that the risk of obesity is increased among individuals with autism.8,9 This evidence has been corroborated in several recent meta-analyses.10,11 Accordingly, several studies have suggested that individuals with autism may be at a higher risk of developing obesity-associated comorbidities such as diabetes (DM),12 hypertension,8 hypercholesterolemia,5 and atherosclerotic macrovascular disease (eg, coronary artery disease and stroke).4,13
Evidence regarding the risk of these obesity-associated comorbidities remains ambiguous due to inconsistencies in findings of recent observational studies.8,9,12,14,15 Moreover, the overall impression is further complicated by the heterogeneity of study designs and patient samples used for studies examining associations between autism and obesity-associated comorbidities. Although a recently published systematic review and meta-analysis attempted to examine the association between autism and DM, the study pooled outcomes of case-control, cross-sectional, and longitudinal study designs to compute pooled odds ratios, thus limiting the inferences.16 Given that autism could be considered as an exposure present since birth, studies reporting the prevalence of obesity-associated complications in samples of individuals with autism and matched control groups without autism could be operationally considered as retrospective cohort designs. This allows computation of relative risks (RRs), which have superior interpretability compared with odds ratios, for each considered disease.
We aimed to examine the association between autism spectrum disorders and cardiometabolic disease (ie, DM, hypertension, dyslipidemia) and atherosclerotic macrovascular disease (ie, cardiovascular, cerebrovascular, and peripheral vascular disease) in a systematic review and meta-analysis. We further aimed to explore the sociodemographic factors that may moderate the association between autism and cardiometabolic disease. We hypothesized that there would be a significantly increased risk of cardiometabolic diseases associated with individuals with autism compared with those without autism.
Methods
This systematic review and meta-analysis was conducted from August 4, 2021, to August 31, 2022. The study protocol was preregistered in PROSPERO (CRD42021268892). All procedures were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (eTable 1 in Supplement 1) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines (eTable 2 in Supplement 1).
Search Strategy
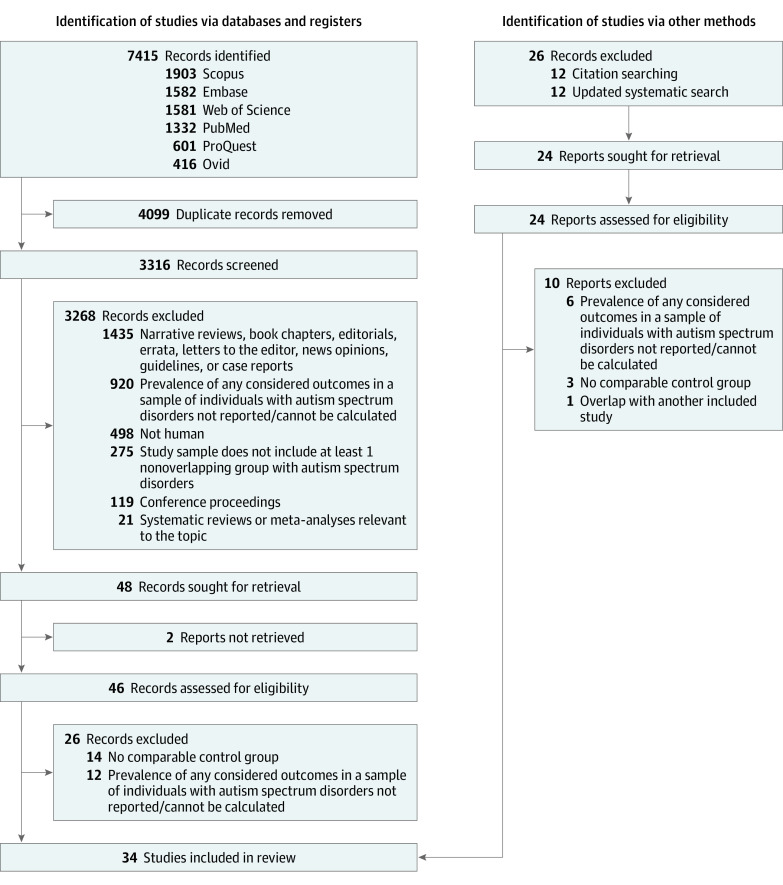
PubMed, Scopus, Web of Science, ProQuest, Embase, and Ovid databases were searched on August 4, 2021, for peer-reviewed observational or interventional studies using predefined keyword combinations (eTable 3 in Supplement 1). The search was not restricted by language or date of publication. Duplicates were removed using an in-house preprocessing pipeline.11,17 The title and the abstract of each record were screened by at least 2 independent screeners (D.A., L.C., and A.H.) using predefined criteria (Figure 1). Records published in other languages were translated into American English using Google Translate. Agreement between screening personnel was examined, and discrepancies in judgment were resolved by a tie breaker (C.S.D.). An additional systematic search was performed through July 31, 2022. Full-text articles of eligible records were screened by 2 screeners (C.S.D. and A.H.R.) independently, and any discrepancies were resolved by a third individual (C.N.K.).
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flowchart.
Quality Check
All eligible manuscripts were examined using the 14-item National Institutes of Health Study Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies by 2 independent study personnel.18 Eligible manuscripts were rated using the Oxford Centre for Evidence-based Medicine criteria for ratings of individual studies.19
Data Extraction
Data were extracted from eligible manuscripts into predefined data fields. The total number of participants with or without autism and participants in each group diagnosed with cardiometabolic diseases were recorded. The RRs of DM, hypertension, dyslipidemia, and atherosclerotic macrovascular disease among individuals with autism were the primary outcomes. Secondary outcomes included the RRs of type 1 and type 2 DM, heart disease, stroke, and peripheral vascular disease. Mean differences in fasting blood glucose (FBS), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol, and triglyceride levels between groups with autism compared with those without autism were considered as additional supportive outcomes. When means or SDs of outcomes were not reported but other summary statistics of central tendency and dispersion were available, means and SDs were estimated.20 When it was apparent that relevant data had been collected yet not reported, the corresponding authors were contacted via email. When reported in articles, the following were collected for consideration as sociodemographic variables in meta-regression analyses: mean age, mean body mass index, proportions of children and female individuals, White participants, patients with intellectual disability, patients with obesity in the autism groups, geographic location, and year of publication. Data regarding the proportion of White participants was consistently reported in most studies, however, data regarding other races and ethnicities were not adequately and consistently reported. As such, only the proportion of White participants were extracted for inclusion in meta-regressions.
Statistical Analysis
DerSimonian-Laird random-effects meta-analyses were performed for each outcome using the meta package, version 5.0-1 in R, version 4.1.2 (R Project for Statistical Computing). Additional random-effects meta-analyses were performed comparing the groups with and without autism on FBS, LDL, HDL, total cholesterol, and triglyceride levels. Consistency of the meta-analyses was examined in leave-1-out sensitivity analyses. Likelihood of publication bias was explored using funnel plots. Trim-and-fill method was used to impute effect sizes to correct funnel plot asymmetry.21 Heterogeneity of effect sizes was quantified.22 Exploratory univariate random-effects meta-regression analyses were performed, including the extracted sociodemographic variables to explain between-study heterogeneity.23 Statistical significance was set at a 2-sided P value = .05.
Results
Results of database search and screening for eligibility are summarized in a PRISMA flow diagram (Figure 1). A total of 34 studies4,5,6,7,8,9,12,13,14,15,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 on 276 173 individuals with autism and 7 733 306 individuals without autism (mean [range] age, 31.2 [3.8-72.8] years; pooled proportion [range] of female individuals, 47% [0%-66%]; pooled proportion [range] of male individuals, 53% [34%-100%]) were included. The proportions of females in the study samples ranged from 0% to 64%, with a pooled male:female ratio of 3:1. The mean age of individuals with autism was 22.8 years (range, 3.8-72.8 years). Of the 34 included studies, 22 reported statistics allowing computation of RRs of cardiometabolic disease in autism.4,5,6,7,8,9,12,13,14,15,24,25,26,27,28,29,30,31,32,33,34,35 Twelve other studies reported laboratory measurements (eg, FBS, total cholesterol, LDL, HDL, or triglyceride level) of individuals with autism compared with controls without autism.36,37,38,39,40,41,42,43,44,45,46,47 The characteristics of the included studies and pooled summary statistics of participants included in each meta-analysis are summarized in eTable 4 and eTable 5 in Supplement 1, respectively.
Risk of DM Among Individuals With Autism
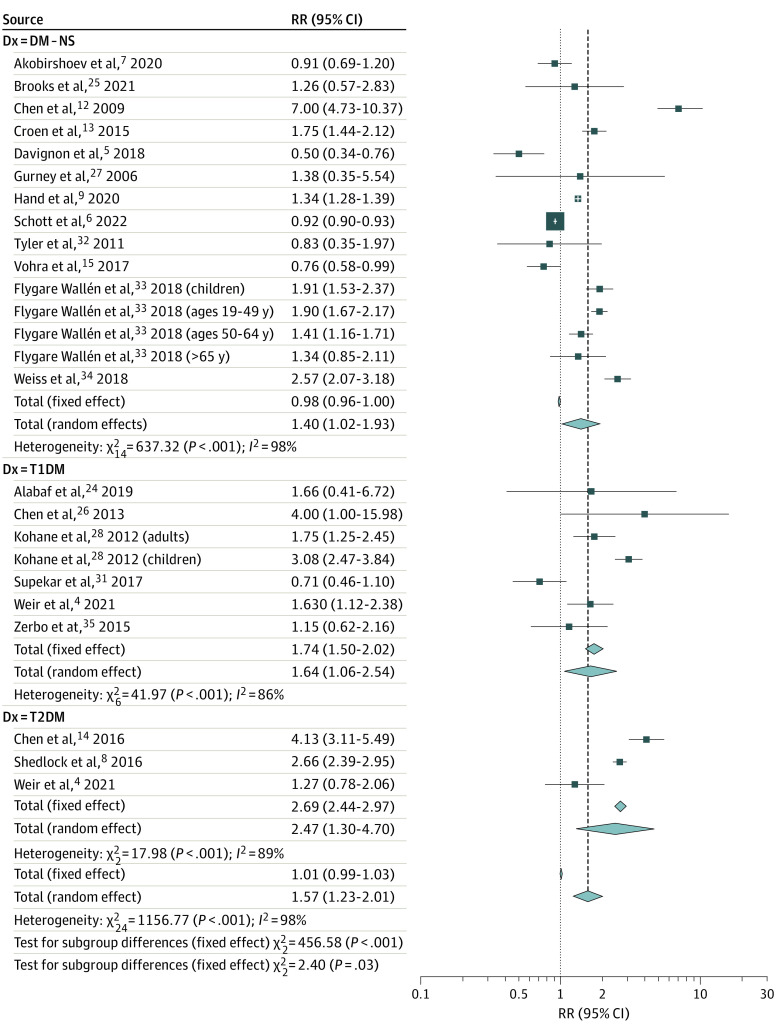
Individuals with autism had a 57.3% greater associated risk of developing DM compared with controls without autism (274 950 individuals with autism and 7 691 364 individuals without autism; RR, 1.57; 95% CI, 1.23-2.01; P < .001; 20 studies,4,5,6,7,8,9,12,13,14,15,24,25,26,27,28,31,32,33,34,35 25 study arms) (Figure 2). The risks of type 1 diabetes (RR, 1.64; 95% CI, 1.06-2.54; P = .03; 6 studies,4,24,26,28,31,35 7 study arms) and type 2 diabetes (RR, 2.47; 95% CI, 1.30-4.70; P = .006, 3 studies4,8,14) were even higher among individuals with autism in subgroup analyses. The observed pooled effect size was not significantly altered by omitting any of the 25 study arms in leave-1-out sensitivity analyses. The funnel plot was asymmetric, indicating possible publication bias (eFigure 1A in Supplement 1). Imputing 10 effect sizes to restore funnel plot symmetry using the trim-and-fill method and reanalyzing the data decreased the observed pooled RR estimate (RR, 1.01; 95% CI, 0.75-1.36; P = .93). Significant between-study heterogeneity was a concern (τ2 = 0.33; I2 = 97.9%; P < .001). Results of exploratory univariate meta-regression analyses aimed at explaining this heterogeneity suggest that the risk of developing DM in autism seems to be significantly greater among a pooled group of individuals of Arabic, Asian, Black, Hispanic, Jewish, multiracial, and nonspecified race/ethnicity compared with White individuals4,6,7,9,12,13,14,15,27,32 (β = 1.32; 95% CI, 0.18-2.46; P = .02; of note: studies were not consistent in reporting race and ethnicity); individuals with autism living outside of the US (β = 0.56; 95% CI, 1.01-0.11; P = .02); and children (β = 0.75; 95% CI, 0.20-1.31; P = .008) (eTable 6 in Supplement 1). Grouping the studies by age (ie, children vs adults) revealed that the RR of developing DM is significantly higher among children with autism compared with children without autism (RR, 2.84; 95% CI, 1.81-4.47; 6 study arms) (eFigure 2 in Supplement 1). Residual heterogeneity remained significant with all tested moderators. Due to a limited number of reports, we could not examine the association of several important potential moderators (eg, intellectual disability and psychotropic medication use). On further exploration, the FBS level of individuals with autism vs controls without autism was not significantly different (pooled mean difference [Δ] = 1.89 mg/dL; 95% CI, −2.05 to 5.83; P = .35) (eFigure 3 in Supplement 1), and this pooled mean difference did not exceed the minimal clinically important difference (MCID).48 Details of sensitivity analyses are summarized in eTable 7 in Supplement 1.
Figure 2. Forest Plot Depicting the Meta-analysis Examining the Association Between Autism and Diabetes (DM).
NS indicates not specified type of DM; T1DM, type 1 DM; T2DM, type 2 DM.
Risk of Hypertension Among Individuals With Autism
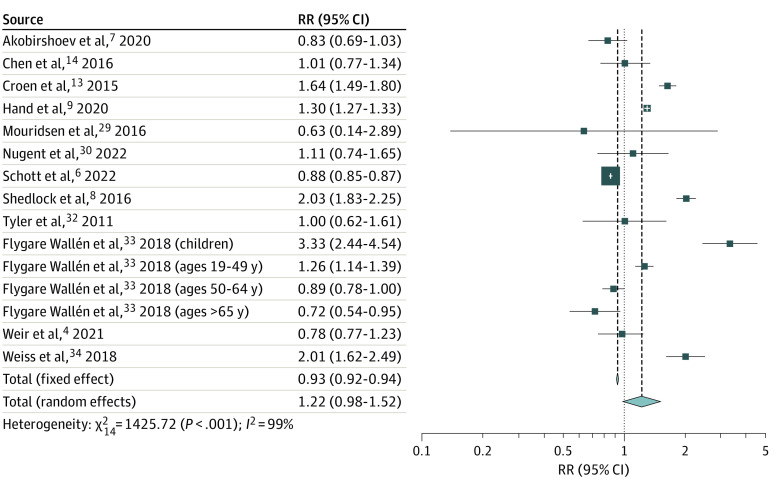
Autism was not associated with a significantly increased risk of hypertension (237 672 individuals with autism and 3 222 718 individuals without autism; RR, 1.22; 95% CI, 0.98-1.52; P = .08; 12 studies,9,13,14,29,30,32,33,34 15 study arms) (Figure 3). Leave-1-out sensitivity analyses did not significantly change this observation. The funnel plot revealed minimal asymmetry (eFigure 1B in Supplement 1); however, trim-and-fill analysis imputed 3 effect sizes. Conducting the meta-analysis including these effect sizes did not significantly alter the pooled RR (RR, 1.00; 95% CI, 0.76-1.32; P = .99). Between-study heterogeneity remained a concern (τ2 = 0.16; I2 = 99.0%; P < .001). Univariate meta-regression analyses revealed a negative moderator effect for age (β = −0.011; 95% CI, −0.02 to −0.02; P = .02) (eTable 8 in Supplement 1), which was apparent in an exploratory subgroup meta-analysis performed based on the age groups. Although the risk of hypertension was not increased in adults with autism (RR, 1.03; 95% CI, 0.85-1.26; P = .75; 8 studies,6,7,9,13,29,32,33,34 10 study arms), the risk was significantly greater among children with autism compared with age-matched controls (RR, 2.54; 95% CI, 1.56-4.12; P < .001; 2 studies8,33) (eFigure 4 in Supplement 1). All univariate meta-regression analyses failed to decrease the residual heterogeneity significantly.
Figure 3. Forest Plot Depicting the Meta-analysis Examining the Association Between Autism and Hypertension.
Risk of Dyslipidemia Among Individuals With Autism
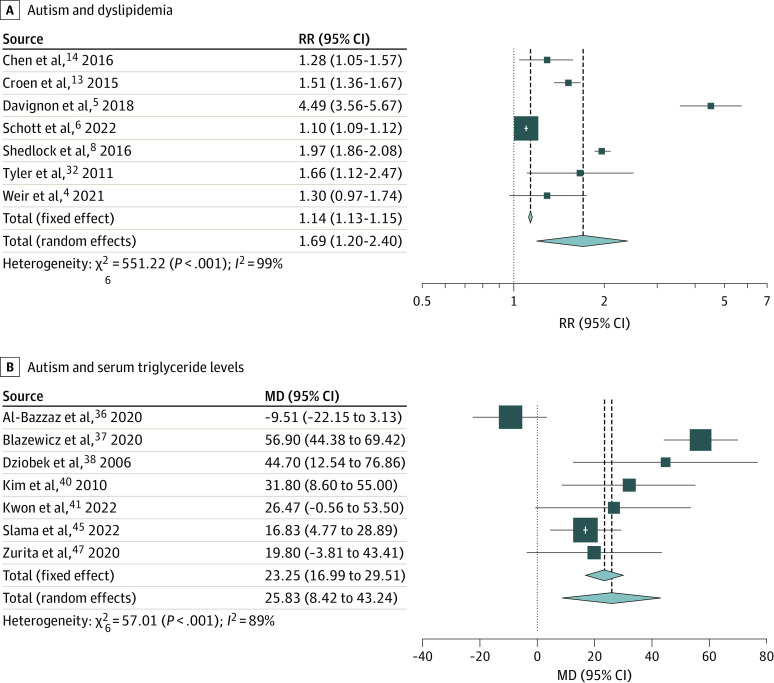
The RR of dyslipidemia was 69.4% higher among individuals with autism than among controls without autism (217 395 individuals with autism and 772 252 individuals without autism; RR, 1.69; 95% CI, 1.20-2.40; P = .003; 7 studies4,5,6,8,13,14,32) (Figure 4A). Leave-1-out sensitivity analyses did not significantly change the observed estimates. The funnel plot indicated possible publication bias (eFigure 1C in Supplement 1). Imputing 4 additional effect sizes to correct for publication bias resulted in loss of statistical significance (RR, 1.14; 95% CI, 0.74-1.74; P = .56). Significant between-study heterogeneity was noted (τ2 = 0.21; I2 = 98.9%; P < .001); however, subgroup analyses or meta-regressions were not attempted due to the limited number of studies.22
Figure 4. Forest Plots Depicting the Meta-analyses Examining the Association Between Autism and Lipid Metabolism.
A, The association between autism and dyslipidemia. B, The mean difference of serum triglyceride levels between individuals with autism and those without autism.
Meta-analyses comparing individuals with autism vs controls without autism revealed that triglyceride level was significantly increased (Δ = 25.83 mg/dL; 95% CI, 8.42-43.24 mg/dL; P = .004; to convert to millimoles per liter, multiply by 0.0113), whereas HDL level was significantly decreased (Δ = −9.35 mg/dL; 95% CI, −13.42 to −5.28 mg/dL; P < .001; to convert to millimoles per liter, multiply by 0.0259) among individuals with autism vs controls without autism (Figure 4B and eFigure 5 in Supplement 1). These between-group differences also exceeded the MCIDs for triglyceride and HDL levels (7.99 mg/dL and 3.67 mg/dL, respectively).48,49 However, between-group differences for LDL and total cholesterol levels were not significant (Δ = −5.22 mg/dL; 95% CI, −15.61 to 5.18 mg/dL; P = .33; and Δ = 9.07 mg/dL; 95% CI, −9.56 to 27.70 mg/dL; P = .34, respectively; to convert to millimoles per liter, multiply by 0.0259) (eFigure 6 and eFigure 7 in Supplement 1) and did not exceed MCIDs.48
Risk of Macrovascular Disease Among Individuals With Autism
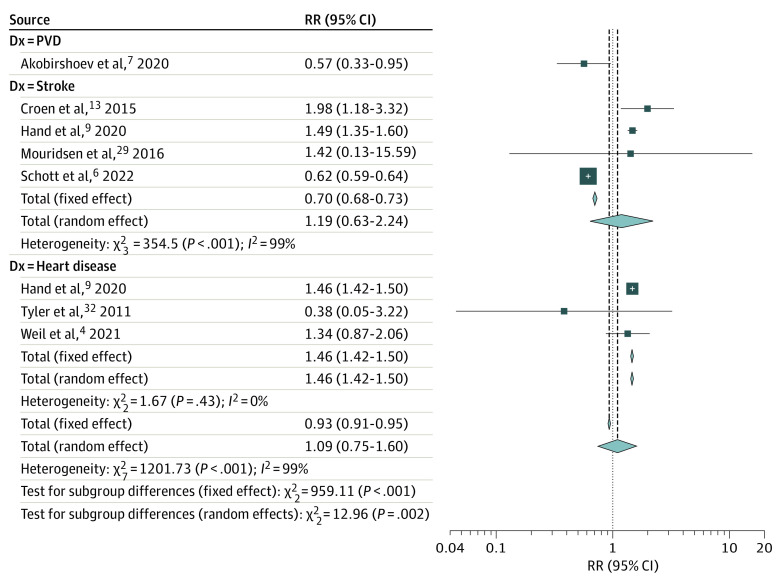
Autism was not associated with a significantly increased risk of macrovascular disease (163 653 individuals with autism and 531 492 individuals without autism; RR, 1.09; 95% CI, 0.75-1.60; P = .65; 7 studies,4,6,7,9,13,29,32 8 study arms) (Figure 5). Subgroup analyses indicated that autism may significantly increase the risk of heart disease (RR, 1.46; 95% CI, 1.42-1.50; P < .001; 3 studies4,9,32) but not stroke (RR, 1.19; 95% CI, 0.63-2.24; P = .592; 4 studies6,9,13,29). In leave-1-out sensitivity analyses, omitting 1 study6 resulted in a significantly increased pooled risk of macrovascular disease (RR, 1.46; 95% CI, 1.42-1.50; P < .001) and stroke (RR, 1.51; 95% CI, 1.28-1.78; P < .001) among individuals with autism. Furthermore, with omission of this study, residual heterogeneity substantially decreased (τ2 = 0.21; I2 = 99.4%; P < .001 vs τ2 = 0; I2 = 61.6%, P = .01) and the heterogeneity was completely eliminated from the subgroup analysis on stroke (τ2 = 0; I2 = 0%, P = .54). Trim-and-fill analyses including all studies and after omitting6 did not significantly affect the pooled outcomes (RR, 1.13; 95% CI, 0.78-1.64; P = .54; and RR, 1.50; 95% CI, 0.99-2.29; P = .06, respectively) (eFigure 1D in Supplement 1). Due to the limited number of studies, meta-regressions were not attempted.22
Figure 5. Forest Plot Depicting the Meta-analyses Examining the Association Between Autism and Macrovascular Disease.
PVD indicates peripheral vascular disease.
Discussion
To our knowledge, this was the first comprehensive systematic review and meta-analysis exploring the autism-associated risks of DM, hypertension, dyslipidemia, and macrovascular disease. Based on our results, individuals with autism seem to be at a greater associated risk of developing DM by 57.3%, type 1 DM by 64.1%, type 2 DM by 146.7%, dyslipidemia by 69.4%, and atherosclerotic heart disease by 45.9%. The risks of developing DM and hypertension were even higher among children with autism compared with children without autism (ie, 184.2% and 153.7%, respectively), even though the overall risk of hypertension was not significantly increased in autism. Individuals with autism had low HDL and high triglyceride levels of statistical and clinical significance, compared with individuals without autism.
Observed autism-associated high risk of type 1 DM is likely due to increased risk of autoimmune disease in autism.26,28,50 Similarly, early development of type 2 DM in individuals with autism was evident in several studies. After a 10-year follow-up, Chen et al14 revealed hazard ratios of 2.71 (95% CI, 1.64-4.48) and 5.31 (95% CI, 2.85-9.90) for DM 2 among children and young adults with autism, respectively. Cortese et al16 substantiated this association in a recent meta-analysis of case-control, cohort, and survival studies (odds ratio, 2.57; 95% CI, 1.65-4.03). However, statistical significance was diminished after adjusting for study-level confounders. We selectively included prospective and retrospective cohort studies, decreasing the risk of biases associated with case-control studies. Yet, our subgroup analyses on type 2 DM in autism were limited by the paucity of studies. Thus, we emphasize the need to conduct large-scale, prospective, multicenter cohort studies to confirm the association between autism and particularly type 2 DM and the potential factors that may contribute to this association (eg, obesity, psychotropic medications, gestational diabetes).
Our observation of markedly increased risk of DM and hypertension, particularly among children with autism, is worth further exploration. One possible explanation could be that as age progresses, the prevalence of these diseases increases in the entire population,51 decreasing the group differences between those with and without autism. Similarly, individuals with autism tend to have a shorter life span52,53; thus, the studies comprising older patients may underrepresent individuals with autism.
Etiology of the observed increased risks of type 2 DM and dyslipidemia in individuals with autism and hypertension, particularly among children with autism, could be multifactorial and may be partially associated with the factors contributing to increased risk of obesity in autism.8,11 Several etiological factors such as genetic variants (eg, 16p11.2 deletion, microdeletion 11p14.1)54,55; prenatal infections,56 medications,56,57 toxins,58,59 maternal obesity,60 and maternal diabetes61; and prematurity and intrauterine growth retardation62,63 may contribute to obesity and related cardiometabolic disease in autism. Furthermore, behaviors and characteristics common among people with autism (eg, food selectivity,64 physical limitations,65 sedentary behavior,66 and sleep disturbances67) and medications intended to improve challenging behaviors (eg, atypical antipsychotics68) could be mediating or moderating the association between autism and cardiometabolic disease. In addition, a few risk factors contributing to dyslipidemia in autism have been documented, including variations in genes NPC1 and DHCR24 associated with cholesterol metabolism,69 altered proteins involved in lipid transport (eg, apolipoprotein B100)70 and metabolism pathways,71 and increased lipoprotein lipase activity.72 Unfortunately, the associations between these factors and cardiometabolic disease in autism could not be determined in meta-regression analyses as these variables were not reported in most included studies.
Strengths and Limitations
This study has several strengths. The novelty of the study and findings, robust methods, meticulous exclusion of case-control designs (eTable 9 in Supplement 1) identifying potential moderators (eg, age) of the explored associations, and examining differences between groups with autism vs control groups without autism in relevant metabolic parameters are notable strengths of our study. Furthermore, summary statistics of demographic parameters of individuals included in our meta-analyses were consistent with overall demographic data of autistic individuals (ie, predominance in male individuals, White individuals, and in developed countries), strengthening the validity.73
Our study also has several limitations. First, several included studies did not clearly distinguish between type 1 and type 2 DM.5,7,12,13,25 Only a few studies specified relevant numerators and denominators required to compute the RR of type 2 DM.4,8,14 Second, due to inconsistencies in definitions (eg, cardiovascular disease), we had to exclude several population-based studies from final analyses on macrovascular disease.6,13,15 Third, some included studies relied on self- or parent-reported data on comorbidities, potentially introducing systematic biases associated with self-report.24,27 Moreover, biases associated with retrospective analysis of medical records (eg, data quality, missing data, and sampling biases) were of concern. Fourth, due to limitations in reported data, we were unable to examine the association with several critical potential moderators (eg, intellectual disability, body mass index, psychotropic medication use). Specifically, albeit several studies explored how intellectual disability affects cardiometabolic outcomes in autism,33,74,75 due to study-level limitations (eg, lack of control groups and not reporting prevalence of intellectual disability in samples), we were unable to explore the association of intellectual disability. Fifth, due to the limited number of studies, we could not explore and adjust for between-study heterogeneity, small study effect, and publication bias in several meta-analyses. The explored moderators also failed to explain between-study heterogeneity in most analyses sufficiently. Sixth, paucity of studies on middle-aged and older adults hindered our ability to examine the association of autism in this age range. Finally, we considered separate age groups of 2 studies as independent data points28,33 despite the likelihood of the presence of shared variance between groups.
Conclusions
Results of this systematic review and meta-analysis suggest that autism seems to be associated with an increased risk of DM, dyslipidemia, and atherosclerotic heart disease. Children with autism seem to possess a higher risk of developing DM and hypertension compared with children without autism. Because developing cardiometabolic disease at an early age raises morbidity and health concerns, the need for health care, and mortality, clinicians should vigilantly monitor individuals with autism for early signs of cardiometabolic disease and their complications. Future studies should aim to identify factors contributing to the established associations and to develop preventive strategies.
eTable 1. PRISMA 2020 Checklist
eTable 2. MOOSE Checklist
eTable 3. Keywords and Keyword Combinations Used to Screen the PubMed, Scopus, Web of Science, ProQuest, Embase, and Ovid Electronic Databases
eTable 4. Characteristics of the Studies Meeting Eligibility for the Systematic Review and Primary Meta-analyses
eTable 5. Demographic Summaries of Individual Meta-analyzed Samples
eTable 6. Results of the Random-Effects Meta-regression Analyses That Examined the Heterogeneity and Moderator Effects of the Association Between Autism and Relative Risk of Diabetes
eTable 7. Summary of Assessments for Publication Bias and Adjusted Outcomes
eTable 8. Results of the Random-Effects Meta-regression Analyses That Examined the Heterogeneity and Moderator Effects of the Association Between Autism and Relative Risk of Hypertension
eTable 9. List of Excluded Studies
eFigure 1. Funnel Plot Depicting Publication Bias and Imputed Effect Sizes to Correct for Publication Bias for Cardiometabolic Risk Factors
eFigure 2. Results of Random-Effects Meta-analysis Examining the Relative Risk of Diabetes With Subgroup Analysis for Age Groups
eFigure 3. Results of Random-Effects Meta-analysis Examining the Mean Difference of Fasting Blood Glucose Levels Between Autistic and Nonautistic Individuals
eFigure 4. Results of Random-Effects Meta-analysis Examining the Relative Risk Hypertension With Subgroup Analysis for Age Groups
eFigure 5. Results of Random-Effects Meta-analysis Examining the Mean Difference of High-Density Cholesterol Levels Between Autistic and Nonautistic Individuals
eFigure 6. Results of Random-Effects Meta-analysis Examining the Mean Difference of Low-Density Cholesterol Levels Between Autistic and Nonautistic Individuals
eFigure 7. Results of Random-Effects Meta-analysis Examining the Mean Difference of Total Cholesterol Levels Between Autistic and Nonautistic Individuals
eReferences
Data Sharing Statement
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. American Psychiatric Association; 2013. [Google Scholar]
- 2.Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 y—Autism and Developmental Disabilities Monitoring Network, 11 sites, US, 2014. MMWR Surveill Summ. 2018;67(6):1-23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maenner MJ, Shaw KA, Bakian AV, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70(11):1-16. doi: 10.15585/mmwr.ss7011a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir E, Allison C, Warrier V, Baron-Cohen S. Increased prevalence of noncommunicable physical health conditions among autistic adults. Autism. 2021;25(3):681-694. doi: 10.1177/1362361320953652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davignon MN, Qian Y, Massolo M, Croen LA. Psychiatric and medical conditions in transition-aged individuals with ASD. Pediatrics. 2018;141(suppl 4):S335-S345. doi: 10.1542/peds.2016-4300K [DOI] [PubMed] [Google Scholar]
- 6.Schott W, Tao S, Shea L. Co-occurring conditions and racial-ethnic disparities: Medicaid enrolled adults on the autism spectrum. Autism Res. 2022;15(1):70-85. doi: 10.1002/aur.2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akobirshoev I, Mitra M, Dembo R, Lauer E. In-hospital mortality among adults with autism spectrum disorder in the US: a retrospective analysis of US hospital discharge data. Autism. 2020;24(1):177-189. doi: 10.1177/1362361319855795 [DOI] [PubMed] [Google Scholar]
- 8.Shedlock K, Susi A, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Nylund CM. Autism spectrum disorders and metabolic complications of obesity. J Pediatr. 2016;178:183-187.e1. [DOI] [PubMed] [Google Scholar]
- 9.Hand BN, Angell AM, Harris L, Carpenter LA. Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism. 2020;24(3):755-764. doi: 10.1177/1362361319890793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YJ, Xie XN, Lei X, Li YM, Lei X. Global prevalence of obesity, overweight and underweight in children, adolescents and adults with autism spectrum disorder, attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Obes Rev. 2020;21(12):e13123. doi: 10.1111/obr.13123 [DOI] [PubMed] [Google Scholar]
- 11.Kahathuduwa CN, West BD, Blume J, Dharavath N, Moustaid-Moussa N, Mastergeorge A. The risk of overweight and obesity in children with autism spectrum disorders: a systematic review and meta-analysis. Obes Rev. 2019;20(12):1667-1679. doi: 10.1111/obr.12933 [DOI] [PubMed] [Google Scholar]
- 12.Chen CY, Chen KH, Liu CY, Huang SL, Lin KM. Increased risks of congenital, neurologic, and endocrine disorders associated with autism in preschool children: cognitive ability differences. J Pediatr. 2009;154(3):345-350.e1. [DOI] [PubMed] [Google Scholar]
- 13.Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism. 2015;19(7):814-823. doi: 10.1177/1362361315577517 [DOI] [PubMed] [Google Scholar]
- 14.Chen MH, Lan WH, Hsu JW, et al. Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care. 2016;39(5):788-793. doi: 10.2337/dc15-1807 [DOI] [PubMed] [Google Scholar]
- 15.Vohra R, Madhavan S, Sambamoorthi U. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism. 2017;21(8):995-1009. doi: 10.1177/1362361316665222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortese S, Gabellone A, Marzulli L, et al. Association between autism spectrum disorder and diabetes: systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;136:104592. doi: 10.1016/j.neubiorev.2022.104592 [DOI] [PubMed] [Google Scholar]
- 17.Dhanasekara CS, Nelson A, Spradley M, et al. Effects of consumption of coconut oil or coconut on glycemic control and insulin sensitivity: a systematic review and meta-analysis of interventional trials. Nutr Metab Cardiovasc Dis. 2022;32(1):53-68. doi: 10.1016/j.numecd.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 18.National Heart Lung, and Blood Institute . Study quality assessment tool. Accessed August 25, 2021. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 19.The Oxford Center for Evidence-Based Medicine . Oxford Centre for evidence-based medicine: levels of evidence. Accessed August 25, 2021. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- 20.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89-98. [Google Scholar]
- 22.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 23.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693-2708. doi: [DOI] [PubMed] [Google Scholar]
- 24.Alabaf S, Gillberg C, Lundström S, et al. Physical health in children with neurodevelopmental disorders. J Autism Dev Disord. 2019;49(1):83-95. doi: 10.1007/s10803-018-3697-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks JD, Bronskill SE, Fu L, et al. Identifying children and youth with autism spectrum disorder in electronic medical records: examining health system utilization and comorbidities. Autism Res. 2021;14(2):400-410. doi: 10.1002/aur.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MH, Su TP, Chen YS, et al. Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: a nationwide population-based study. Res Autism Spectr Disord. 2013;7(2):205-212. doi: 10.1016/j.rasd.2012.08.008 [DOI] [Google Scholar]
- 27.Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: National Survey of Children’s Health. Arch Pediatr Adolesc Med. 2006;160(8):825-830. doi: 10.1001/archpedi.160.8.825 [DOI] [PubMed] [Google Scholar]
- 28.Kohane IS, McMurry A, Weber G, et al. The comorbidity burden of children and young adults with autism spectrum disorders. PLoS One. 2012;7(4):e33224. doi: 10.1371/journal.pone.0033224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouridsen SE, Rich B, Isager T. Diseases of the circulatory system among adult people diagnosed with infantile autism as children: a longitudinal case control study. Res Dev Disabil. 2016;57:193-200. doi: 10.1016/j.ridd.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Nugent JT, Bakhoum C, Ghazi L, Greenberg JH. Screening for hypertension in children with and without autism spectrum disorder. JAMA Netw Open. 2022;5(4):e226246-e226246. doi: 10.1001/jamanetworkopen.2022.6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Supekar K, Iyer T, Menon V. The influence of sex and age on prevalence rates of comorbid conditions in autism. Autism Res. 2017;10(5):778-789. doi: 10.1002/aur.1741 [DOI] [PubMed] [Google Scholar]
- 32.Tyler CV, Schramm SC, Karafa M, Tang AS, Jain AK. Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed. Am J Intellect Dev Disabil. 2011;116(5):371-380. doi: 10.1352/1944-7558-116.5.371 [DOI] [PubMed] [Google Scholar]
- 33.Flygare Wallén E, Ljunggren G, Carlsson AC, Pettersson D, Wändell P. High prevalence of diabetes mellitus, hypertension and obesity among persons with a recorded diagnosis of intellectual disability or autism spectrum disorder. J Intellect Disabil Res. 2018;62(4):269-280. doi: 10.1111/jir.12462 [DOI] [PubMed] [Google Scholar]
- 34.Weiss JA, Isaacs B, Diepstra H, et al. Health concerns and health service utilization in a population cohort of young adults with autism spectrum disorder. J Autism Dev Disord. 2018;48(1):36-44. doi: 10.1007/s10803-017-3292-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, Croen LA. Immune mediated conditions in autism spectrum disorders. Brain Behav Immun. 2015;46:232-236. doi: 10.1016/j.bbi.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Bazzaz A, Kahtan A, Almashhadani A, Al-Ani I. Estimation of fasting serum levels of glucose, zinc, copper, zinc/copper ratio and their relation to the measured lipid profile in autistic patients and nonautistic controls in Jordan. Biomed Pharmacol J. 2020;13(1):481-488. doi: 10.13005/bpj/1909 [DOI] [Google Scholar]
- 37.Błażewicz A, Szymańska I, Astel A, Stenzel-Bembenek A, Dolliver WR, Makarewicz A. Assessment of changes over time of lipid profile, C-reactive protein level, and body mass index in teenagers and young adults on different diets belonging to autism spectrum disorder. Nutrients. 2020;12(9):2594. doi: 10.3390/nu12092594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziobek I, Gold SM, Wolf OT, Convit A. Hypercholesterolemia in Asperger syndrome: independence from lifestyle, obsessive-compulsive behavior, and social anxiety. Psychiatry Res. 2007;149(1-3):321-324. doi: 10.1016/j.psychres.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 39.Hassan MH, Desoky T, Sakhr HM, Gabra RH, Bakri AH. Possible metabolic alterations among autistic male children: clinical and biochemical approaches. J Mol Neurosci. 2019;67(2):204-216. doi: 10.1007/s12031-018-1225-9 [DOI] [PubMed] [Google Scholar]
- 40.Kim E-K, Neggers YH, Shin C-S, Kim E, Kim EM. Alterations in lipid profile of autistic boys: a case control study. Nutr Res. 2010;30(4):255-260. doi: 10.1016/j.nutres.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 41.Kwon SJ, Hong K-W, Choi S, et al. Association of 3-hydroxy-3-methylglutaryl-coenzyme A reductase gene polymorphism with obesity and lipid metabolism in children and adolescents with autism spectrum disorder. Metab Brain Dis. 2022;37(2):319-328. doi: 10.1007/s11011-021-00877-3 [DOI] [PubMed] [Google Scholar]
- 42.Manco M, Guerrera S, Ravà L, et al. Cross-sectional investigation of insulin resistance in youths with autism spectrum disorder—any role for reduced brain glucose metabolism? Transl Psychiatry. 2021;11(1):229. doi: 10.1038/s41398-021-01345-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moses L, Katz N, Weizman A. Metabolic profiles in adults with autism spectrum disorder and intellectual disabilities. Eur Psychiatry. 2014;29(7):397-401. doi: 10.1016/j.eurpsy.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 44.Pearson J. Disordered cholesterol metabolism in autism spectrum disorders: sterol and genetic analyses. Accessed June 6, 2022. https://scholararchive.ohsu.edu/downloads/c247ds117?locale=en
- 45.Slama S, Bahia W, Soltani I, Gaddour N, Ferchichi S. Risk factors in autism spectrum disorder: a Tunisian case-control study. Saudi J Biol Sci. 2022;29(4):2749-2755. doi: 10.1016/j.sjbs.2021.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tierney E, Remaley AT, Thurm A, et al. Sterol and lipid analyses identifies hypolipidemia and apolipoprotein disorders in autism associated with adaptive functioning deficits. Transl Psychiatry. 2021;11(1):471. doi: 10.1038/s41398-021-01580-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zurita MF, Cárdenas PA, Sandoval ME, et al. Analysis of gut microbiome, nutrition, and immune status in autism spectrum disorder: a case-control study in Ecuador. Gut Microbes. 2020;11(3):453-464. doi: 10.1080/19490976.2019.1662260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeraattalab-Motlagh S, Jayedi A, Shab-Bidar S. The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: an umbrella review of meta-analyses of randomized controlled trials. Am J Clin Nutr. 2021;114(5):1675-1685. doi: 10.1093/ajcn/nqab250 [DOI] [PubMed] [Google Scholar]
- 49.Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman SJ, Roberts W, Daneman D. Type 1 diabetes and autism: is there a link? Diabetes Care. 2005;28(4):925-926. doi: 10.2337/diacare.28.4.925 [DOI] [PubMed] [Google Scholar]
- 51.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: US, 2017–2018. NCHS Data Brief, no 364. National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 52.Catalá-López F, Hutton B, Page MJ, et al. Mortality in persons with autism spectrum disorder or attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. JAMA Pediatr. 2022:176(4):e216401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, Bölte S. Premature mortality in autism spectrum disorder. Br J Psychiatry. 2016;208(3):232-238. doi: 10.1192/bjp.bp.114.160192 [DOI] [PubMed] [Google Scholar]
- 54.Maillard AM, Ruef A, Pizzagalli F, et al. ; 16p11.2 European Consortium . The 16p11.2 locus modulates brain structures common to autism, schizophrenia, and obesity. Mol Psychiatry. 2015;20(1):140-147. doi: 10.1038/mp.2014.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shinawi M, Sahoo T, Maranda B, et al. 11p14.1 microdeletions associated with ADHD, autism, developmental delay, and obesity. Am J Med Genet A. 2011;155A(6):1272-1280. doi: 10.1002/ajmg.a.33878 [DOI] [PubMed] [Google Scholar]
- 56.Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447-e1454. doi: 10.1542/peds.2012-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section, and risk of childhood obesity. Int J Obes (Lond). 2015;39(4):665-670. doi: 10.1038/ijo.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raz R, Levine H, Pinto O, Broday DM, Yuval, Weisskopf MG. Traffic-related air pollution and autism spectrum disorder: a population-based nested case-control study in Israel. Am J Epidemiol. 2018;187(4):717-725. doi: 10.1093/aje/kwx294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung Y, Lee AM, McKee SA, Picciotto MR. Maternal smoking and autism spectrum disorder: meta-analysis with population smoking metrics as moderators. Sci Rep. 2017;7(1):4315. doi: 10.1038/s41598-017-04413-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchez CE, Barry C, Sabhlok A, et al. Maternal prepregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev. 2018;19(4):464-484. doi: 10.1111/obr.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wan H, Zhang C, Li H, Luan S, Liu C. Association of maternal diabetes with autism spectrum disorders in offspring: a systemic review and meta-analysis. Medicine (Baltimore). 2018;97(2):e9438. doi: 10.1097/MD.0000000000009438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr. 2009;30(2):122-130. doi: 10.1097/DBP.0b013e31819e6a16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindberg J, Norman M, Westrup B, Öhrman T, Domellöf M, Berglund SK. Overweight, obesity, and body composition in 3.5- and 7-year-old Swedish children born with marginally low birth weight. J Pediatr. 2015;167(6):1246-52.e3. doi: 10.1016/j.jpeds.2015.08.045 [DOI] [PubMed] [Google Scholar]
- 64.Sharp WG, Berry RC, McCracken C, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. 2013;43(9):2159-2173. doi: 10.1007/s10803-013-1771-5 [DOI] [PubMed] [Google Scholar]
- 65.Shetreat-Klein M, Shinnar S, Rapin I. Abnormalities of joint mobility and gait in children with autism spectrum disorders. Brain Dev. 2014;36(2):91-96. doi: 10.1016/j.braindev.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 66.Murakami K, Livingstone MBE. Prevalence and characteristics of misreporting of energy intake in US adults: NHANES 2003-2012. Br J Nutr. 2015;114(8):1294-1303. doi: 10.1017/S0007114515002706 [DOI] [PubMed] [Google Scholar]
- 67.Kodak T, Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2008;17(4):887-905, x-xi. doi: 10.1016/j.chc.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 68.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773. doi: 10.1001/jama.2009.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall TA, Steiner RD, Wright H, et al. Lipid and sterol gene sequence variation in autism and correlates with neurodevelopmental status: a pilot study. New Horiz Transl Med. 2015;2(6-7):137-146. doi: 10.1016/j.nhtm.2015.09.001 [DOI] [Google Scholar]
- 70.Corbett BA, Kantor AB, Schulman H, et al. A proteomic study of serum from children with autism showing differential expression of apolipoproteins and complement proteins. Mol Psychiatry. 2007;12(3):292-306. doi: 10.1038/sj.mp.4001943 [DOI] [PubMed] [Google Scholar]
- 71.Schwarz E, Guest PC, Rahmoune H, et al. Sex-specific serum biomarker patterns in adults with Asperger syndrome. Mol Psychiatry. 2011;16(12):1213-1220. doi: 10.1038/mp.2010.102 [DOI] [PubMed] [Google Scholar]
- 72.Hirai T, Usui N, Iwata K, et al. Increased plasma lipoprotein lipase activity in males with autism spectrum disorder. Res Autism Spectr Disord. 2020;77:101630. doi: 10.1016/j.rasd.2020.101630 [DOI] [Google Scholar]
- 73.Zeidan J, Fombonne E, Scorah J, et al. Global prevalence of autism: A systematic review update. Autism Res. 2022;15(5):778-790. doi: 10.1002/aur.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilmore D, Harris L, Longo A, Hand BN. Health status of Medicare-enrolled autistic older adults with and without co-occurring intellectual disability: an analysis of inpatient and institutional outpatient medical claims. Autism. 2021;25(1):266-274. doi: 10.1177/1362361320955109 [DOI] [PubMed] [Google Scholar]
- 75.Ptomey LT, Walpitage DL, Mohseni M, et al. Weight status and associated comorbidities in children and adults with Down syndrome, autism spectrum disorder, and intellectual and developmental disabilities. J Intellect Disabil Res. 2020;64(9):725-737. doi: 10.1111/jir.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PRISMA 2020 Checklist
eTable 2. MOOSE Checklist
eTable 3. Keywords and Keyword Combinations Used to Screen the PubMed, Scopus, Web of Science, ProQuest, Embase, and Ovid Electronic Databases
eTable 4. Characteristics of the Studies Meeting Eligibility for the Systematic Review and Primary Meta-analyses
eTable 5. Demographic Summaries of Individual Meta-analyzed Samples
eTable 6. Results of the Random-Effects Meta-regression Analyses That Examined the Heterogeneity and Moderator Effects of the Association Between Autism and Relative Risk of Diabetes
eTable 7. Summary of Assessments for Publication Bias and Adjusted Outcomes
eTable 8. Results of the Random-Effects Meta-regression Analyses That Examined the Heterogeneity and Moderator Effects of the Association Between Autism and Relative Risk of Hypertension
eTable 9. List of Excluded Studies
eFigure 1. Funnel Plot Depicting Publication Bias and Imputed Effect Sizes to Correct for Publication Bias for Cardiometabolic Risk Factors
eFigure 2. Results of Random-Effects Meta-analysis Examining the Relative Risk of Diabetes With Subgroup Analysis for Age Groups
eFigure 3. Results of Random-Effects Meta-analysis Examining the Mean Difference of Fasting Blood Glucose Levels Between Autistic and Nonautistic Individuals
eFigure 4. Results of Random-Effects Meta-analysis Examining the Relative Risk Hypertension With Subgroup Analysis for Age Groups
eFigure 5. Results of Random-Effects Meta-analysis Examining the Mean Difference of High-Density Cholesterol Levels Between Autistic and Nonautistic Individuals
eFigure 6. Results of Random-Effects Meta-analysis Examining the Mean Difference of Low-Density Cholesterol Levels Between Autistic and Nonautistic Individuals
eFigure 7. Results of Random-Effects Meta-analysis Examining the Mean Difference of Total Cholesterol Levels Between Autistic and Nonautistic Individuals
eReferences
Data Sharing Statement