Abstract
Background.
More common in older women than younger women, rectoceles may be secondary to pelvic floor weakness and/or pelvic floor dysfunction with impaired rectal evacuation. Rectoceles may be small (< 2 cm), medium (2–4 cm), or large (> 4 cm). Arguably, large rectoceles are more likely to be associated with symptoms (eg, difficult defecation). It can be challenging to ascertain the extent to which a rectocele is secondary to pelvic floor dysfunction and/or whether a rectocele, rather than associated pelvic floor dysfunction, is responsible for symptoms. Surgical repair should be considered when initial treatment measures (eg, bowel modifying agents and pelvic floor biofeedback therapy) are unsuccessful.
Purpose.
We summarize the clinical features, diagnosis, and management of rectoceles, with an emphasis on outcomes after surgical repair. This review accompanies a retrospective analysis of outcomes after multidisciplinary, transvaginal rectocele repair procedures undertaken by 3 colorectal surgeons in 215 patients at a large teaching hospital in the UK. A majority of patients had a large rectocele. Some patients also underwent an anterior levatorplasty and/or an enterocele repair. All patients were jointly assessed, and some patients underwent surgery by colorectal and urogynecologic surgeons. In this cohort, the perioperative data, efficacy, and harms outcomes are comparable to historical data predominantly derived from retrospective series in which patients had a good outcome (67%−78%), symptoms of difficult defecation improved (30%−50%), and patients had a recurrent rectocele 2 years after surgery (17%). Building on these data, prospective studies that rigorously evaluate outcomes after surgical repair are necessary.
Introduction
Rarely observed in men,(1) rectoceles are protrusions of the anterior wall of the rectum into the vagina, which are more common in older than younger women. A summary of several large studies(2) suggests that our awareness of rectoceles has increased since the advent of defecography in the early 1980s.(3) Rectoceles indicate structural deficits (ie, weakening of the pelvic floor, especially the perineal body) and pelvic floor dysfunction with excessive straining and impaired rectal evacuation during defecation.(4) Infrequently observed in nulliparous younger women,(5) age, parity,(6) anal sphincter injury, and hysterectomy are the most frequently cited risk factors for a rectocele.(7) In older women, rectoceles smaller than 2 cm in size are common and generally asymptomatic. Larger rectoceles may be associated with symptoms of difficult defecation. When conservative management is unsuccessful, surgical repair may be necessary. In this issue, Ferrari et al(8) describe the long-term outcomes of transvaginal repair for symptomatic rectoceles in 215 patients. This review will focus on our current concepts of the clinical features, diagnosis, and management of rectoceles, with an emphasis on outcomes after surgical repair.
Symptoms
Rectoceles are common and frequently asymptomatic.(9) Among symptomatic patients, the association between symptoms of difficult defecation and a rectocele varies considerably among studies. In some studies, several symptoms (eg, anal digitation, incomplete emptying, and dyschezia) were associated with an ultrasound-documented rectocele.(10–12) In other studies, no symptoms(13) or only vaginal splinting(4, 14) was associated with a rectocele. Indeed, it can be challenging to determine if other symptoms (eg, excessive straining to defecate) are explained by a rectocele, another disturbance (eg, defecatory dyssynergia, rectal hypersensitivity, or hyposensitivity), or even to hard stools,(14–16) especially in patients with small rectoceles that empty completely during defecation.
Diagnosis and Classification
Rectoceles may be identified by physical examination, barium or magnetic resonance defecography, and translabial ultrasound.(2, 10, 17) Expert physical examination usually includes estimation of rectocele stage using the Pelvic Organ Prolapse Quantification System.(18) Stages 0 (none) to 4 (vaginal eversion) are defined as degrees of vaginal descent of the rectocele during maximum Valsalva maneuver or cough.(19) Among 200 symptomatic patients, the correlation between rectocele size on a standardized physical examination vs defecography was limited, but stronger in patients with a rectocele larger than 2 cm.(20) Arguably, these differences are at least partly explained by differences in techniques among these assessments (ie, Valsalva maneuver in dorsal lithotomy position for physical examination and ultrasound vs evacuation of contrast in seated position during defecography).
Rectoceles are radiologically characterized by the following features:
-
1
Dimensions. Based on the width, which is the most widely used parameter, during barium defecography rectoceles are regarded as small (<2 cm), medium (2–4 cm), or large (>4 cm)(21) (Figure). Among 28 healthy female volunteers, 26 (93%) had a rectocele with an average size of 2.5 cm (upper limit, 3.9 cm) during barium defecography. (22) By comparison, 70 of 113 asymptomatic women (62%) had a rectocele larger than 2.5 cm by magnetic resonance defecography; less than 10% were larger than 4 cm.(9) Based on these findings, rectoceles sized 4 cm or larger are abnormal, but may not cause symptoms. Indeed, in 2 small studies, the correlation between rectocele size and symptoms is relatively weak.(23, 24)
Other, less widely used measurements of a rectocele include the height of a rectocele, which is the length of a line running across the “mouth” of the rectocele, and the classification system suggested by Marti et al,(25) which is comprised of 3 types: type I (digitiform), type II (with a lax rectovaginal septum, an anterior mucosal prolapse, and a deep pouch of Douglas), and type III (associated with intussusception or even rectal prolapse).
-
2
Amount of contrast emptied from the rectocele during defecation. Contrast trapping refers to contrast retained in a rectocele on images obtained immediately after defecation. This assessment is typically based on visual interpretation rather than a semiquantitative assessment.(22) Trapping of barium contrast was common even in asymptomatic women.(22) When images were obtained after patients had defecated on a toilet, the trapped contrast had been evacuated in 43% of patients.(26) Such postdefecation images may also reveal features of pelvic organ prolapse (eg, enterocele or peritoneocele) that may not be evident on images during defecation (Figure). Barium trapping was more frequently observed with larger rectoceles in some(23, 26) but not all studies.(20) Moreover, barium trapping does not predict the outcome after rectocele repair.(27, 28) Hence, the utility of this feature to identify patients for surgery gis unclear.
-
3
Perineal descent during defecation. Among constipated patients, rectoceles are associated with increased perineal descent.(29, 30) A score that is derived from 3 variables (anorectal descent during evacuation + rectal pressure during evacuation − patulous anal canal) predicted rectoceles larger than 3 cm.(30)
-
4
Other structural abnormalities. Patients with rectoceles often have other abnormalities (eg, rectal intussusception, increased perineal descent, enterocele, cystocoele, or uterine prolapse)(2, 30, 31) (Figure).
-
5
Impaired rectal evacuation. Some patients with rectoceles have impaired rectal evacuation documented with defecography or an abnormal rectal balloon expulsion test.(4) However, in one study, 91% of patients with a rectocele larger than 3 cm and 94% of patients with an enterocele, a peritoneocele, and/or a sigmoidocele had a normal balloon expulsion time.(30) Only defecography, not the balloon expulsion test, can visualize structural abnormalities. Hence, arguably a balloon expulsion test is less useful than defecography in this setting. Among patients with defecatory disorders, excessive straining during defecation is associated with a higher rectal pressure,(32) which may, in turn, predispose the patient to a greater rectal-vaginal pressure gradient,(33) resulting in a rectocele.
Management
The management of a rectocele is guided by a detailed assessment of bowel symptoms, the characteristics of the rectocele, and anorectal functions with manometry.(34) Although there are no published society guidelines, experts recommend conservative management of bowel symptoms before surgery.(35) Hard stools predispose to excessive straining and a defecatory disorder.(14, 36) Hence, conservative management includes, as appropriate, measures to ensure soft stools that are generally easier to defecate and anorectal biofeedback therapy in patients with a defecatory disorder.(15, 37) Among 90 patients with a rectocele, 64 (71%) responded to such therapy.(35) Surgical repair should only be considered in patients who have symptoms that are probably due to a rectocele and have not responded to a robust trial of conservative approaches (eg, they do not have residual pelvic floor dysfunction).
Surgical Repair
In this month’s issue, Ferrari et al,(8) describe their experience of transvaginal rectocele repair at a large teaching hospital in the UK. The study is a retrospective series of transvaginal rectocele repair performed by 3 colorectal surgeons. This large series of 215 patients, of whom 71.6%, 27.5%, and 0.9% of patients respectively had a large (>4cm), medium (2–4cm), or a small rectocele. provides valuable information on current surgical practice, including joint surgery with gynecology, complications, and basic expectations in terms of outcome. However, outcomes were assessed with global rating scores rather than validated symptom scoring instruments.
Methods of Surgical Repair and Rationale.
Ferrari et al (8) describe patients undergoing a transvaginal rectocele repair using a full posterior suture repair of the vagina as opposed to more limited options such as site-specific repair. In addition, 211 of the 215 patients (98.1%) had an anterior levatorplasty and 42 (19.5%) had an enterocele repair. A significant proportion (58 [27%]) of patients had a joint procedure with an attending urogynecologist who performed a variety of adjunctive procedures (mainly combination of transvaginal obturator tape; anterior repair, hysterectomy) to address anterior and middle compartment prolapse at the same procedure.
The surgical procedures described for rectocele in this paper (8) are with understanding that surgical treatment of a rectocele is not the only operation. Rather, there are multiple methods described to address the barrier between the rectum and vagina (rectovaginal septum) to obliterate the symptomatic bulge into the vagina. The surgeon also has at their disposal procedures that hitch up the redundant prolapsing rectum (forms of rectopexy with or without mesh) and procedures that excise the redundant rectal wall, either using standard dissection methods or staplers (eg, stapled transanal rectal resection [STARR]). A detailed discussion of rectopexy and STARR is not relevant here, but, in brief, rectopexy is mostly reserved for instances where a high-grade rectal intussusception or uterovaginal/vaginal vault prolapse directs hitching as the primary procedure(38, 39). The vagina may also be addressed ie, by sacrocolpopexy +/− hysterectomy or hysteropexy. STARR has considerably reduced in popularity due to concerns around harmful outcomes such as chronic anorectal urgency and pain.(40)
There are 3 main approaches for rectovaginal reinforcement: the posterior vagina (posterior repair) (Figure 2),(27) the perineum (transperineal repair),(41) or via the anus (transanal repair).(42, 43) Vaginal repairs all involve an incision in the posterior wall either longitudinally to open the entire length of the rectocele or transversely to produce a broad based flap and expose the entirety of the rectocele, or a combination of the 2 incisions in the shape of an inverted ‘T’ (as employed in the Ferrari et al study) (Figure 2).(8, 44) In most repairs the redundant rectal wall is plicated outside the bowel wall. The vaginal wall is then reconstructed with resection of any excess vaginal mucosa. Many repairs include some degree of approximation of the levator ani and pelvic side wall muscles to formally reinforce the reconstruction of the rectovaginal septum (the described anterior levatoplasty). More recently attempts have been made to localize specific defects in the rectovaginal septum. This has led to site-specific repairs,(45, 46) where the individual defects are repaired before the vaginal wall is closed. All of these types of repairs may be augmented by mesh reinforcement, usually using one of a variety of collagen meshes, although placement of mesh via the vagina is now banned in many countries due to well-documented risks (47).
Figure 2.
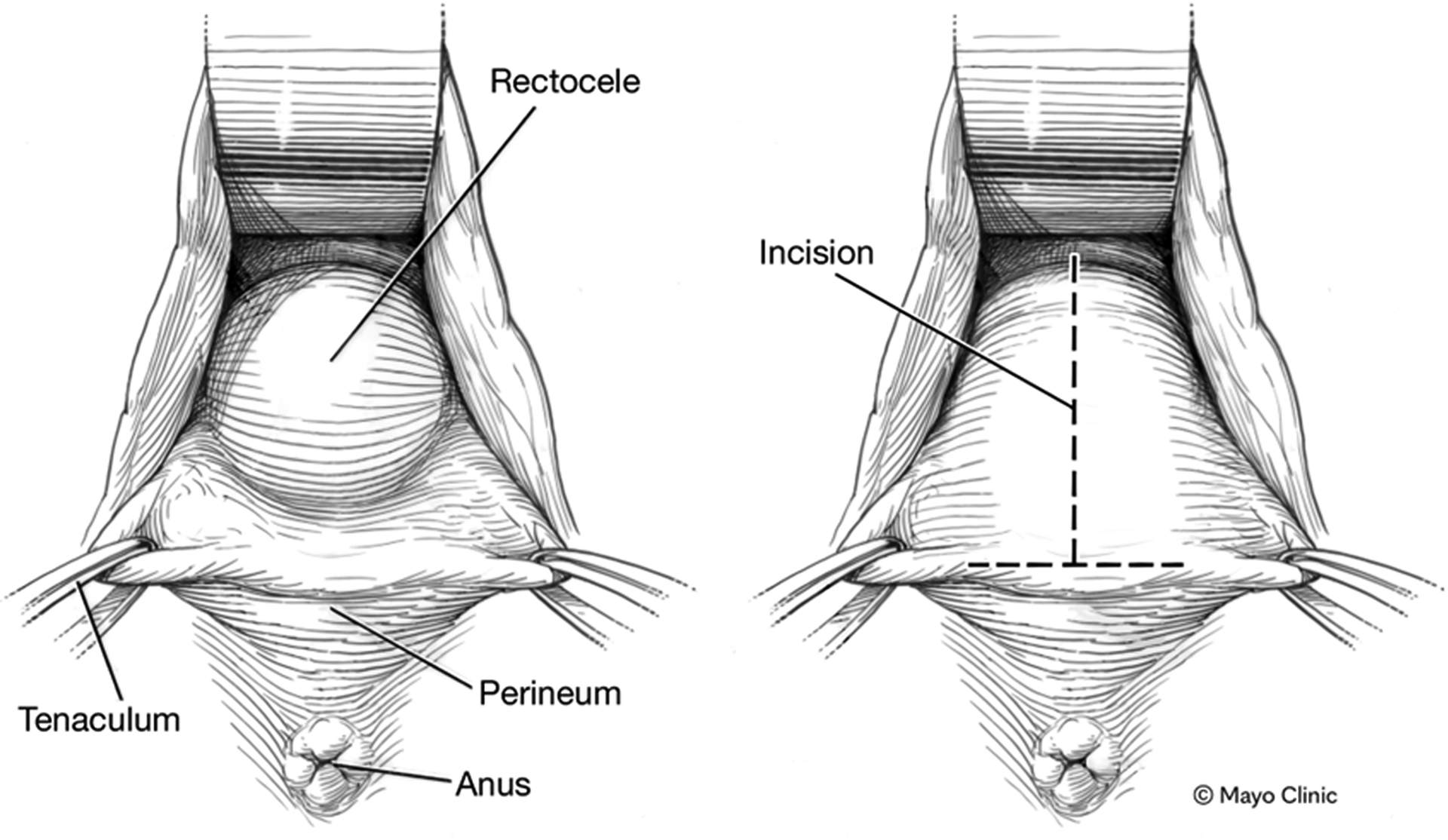
Transvaginal repair of rectocele. Left panel. Intra-operative of rectocele in posterior wall of vagina. Right panel. A T-shaped incision has been made in the posterior wall of the vagina to affect repair the rectocele.
Using the transperineal route, a transverse or curved incision is made in the perineal body toward the vagina and the dissection extended anterior to the sphincter complex to gain access to the rectovaginal septum. Having gained exposure, a repair is made in the same way as in a transvaginal approach. The potential advantage to this route is that it may be combined with a sphincteroplasty in those women with a deficient sphincter and a concurrent rectocele.
Finally, rather than a weakness in the rectovaginal septum, a rectocele can be regarded as a redundant pocket of rectum, leading to herniation of the rectum. Transanal repairs with either an anterior Delorme procedure, as described by Sarle et al,(43) or with a sutured pexy of the anterior rectal wall, described by Block,(42) address such rectal redundancy. In the Delorme procedure, the mucosa is dissected free from the rectal muscle and excised. Thereafter, the rectal muscle coat is plicated longitudinally to obliterate the rectocele pocket and the mucosa is re-approximated to close the defect. The repair used by Block entails placement of full thickness longitudinal sutures anteriorly to draw together the redundant anterior rectal pocket and thereby close the rectocele.(42) Both procedures necessitate a degree of rectal wall excision or suspension.(44)
Armed with such a spectrum of approaches available, what does the surgeon choose and what evidence to we have to support decision making? In 2017, a systematic review of rectovaginal reinforcement surgery (44) included 43 articles (3 randomized clinical trials [RCTs] and 40 observational studies) published between 1990 and 2016 with outcome data in 3,346 patients (study mean, 78; range, 13–307). There was one good quality RCT (level IB) with a low level of susceptibility to bias, and two with less well described methodology (level IIB). The 40 observational studies included 8 good quality cohort studies with low susceptibility to bias (level IIB). Other studies were a mix of prospective and retrospective case series. The mean study follow-up was 2.1 years (range, 0.7–6.2 years). The studies covered all types of repairs and 17 studies evaluated a standard transvaginal repair, of which the largest included 231 patients. Hence, the current Ferrari et al(8) study is the second largest to date.
The 2017 systematic review by Grossi et al(44) carefully documented all available perioperative data, efficacy, and harms outcomes for 8 different surgical approaches. However, the authors were unable to draw any strong statistically-based inferences due to inconsistency of reporting and poor study quality (generally level IV). Surgery times averaged approximately 1 hour (vs 102 minutes in this study) and the average hospital stay was approximately 4 days (vs 3 days in this study).(8)
Data on efficacy in the systematic review(44) were inconsistently measured and findings heterogeneous, making estimates tentative and imprecise (all level IV). Although inconsistent, global assessments suggest a good outcome in about 67% to 78% of patients. The review by Grossi et al(44) noted that “Findings for global improvement, derived from global satisfaction rating scales, provide insufficient evidence to prefer one type of procedure over another, and validated symptom scores were inconsistently reported. Approximately 30% to 50% of patients experienced reduced symptoms of straining, incomplete emptying or reduced vaginal digitation (level IV). Anatomical recurrence (as judged by a variety of measures) occurred in approximately 17% patients at mean follow up of 23.4 months (range, 12 – 74).” (44) By comparison, improvement in global symptoms (88%) and several specific prolapse and bowel symptoms were greater in the Ferrari et al study.(8) For instance, vaginal splinting, vaginal bulging, and post-defecatory soiling improved in 98 of 99, 173 of 176, and 136 of 146 patients, respectively. Other symptoms such as constipation and incomplete evacuation improved in approximately half of patients.
Also emanating from observational studies and comparisons (level IV evidence), the assessment of harms outcomes in the systematic review observed overall procedural complication rates ranging from 0% to 61%, with these occurring in about 7% to 17% of procedures.(44) The incidence of bleeding, hematoma, and fistulation ranged from 0% to 4%. New onset dyspareunia was reported too inconsistently (with wide variations eg, 0%−33%) to make meaningful comparisons between procedures. Overall, there was insufficient evidence to prefer one type of procedure over another. In the Ferrari et al study,(8) the commonest adverse event was urinary retention (8.4%) followed by dyspareunia (7.9%). In keeping with the review there were low rates of hematoma and fistulation (<1%).
Summary
In summary, the Ferrari et al(8) study provides yet more level IV observational evidence to a field that really needs some high-quality clinical trials. One area where the study does contribute progressive thinking is in the multidisciplinary approach to surgery. The concept of joint patient assessment by colorectal and urogynecologic colleagues, followed where relevant by joint operating, is now well engrained in the UK but perhaps less so elsewhere, depending on service constraints and other drivers. Conceivably, the greater improvement in symptoms in the Ferrari et al study(8) than the systematic review(44) is partly because 59 of the 215 patients in the Ferrari et al study underwent a joint procedure. Lack of focus in addressing vaginal or bladder symptoms by a colorectal surgeon, or the reverse for bowel symptoms by a urogynecologist, must be a flaw in a surgical environment, where a common sheet of muscle (with 3 holes in a female) is subject to common risk factors for unsuccessful procedures. It is also known that some procedures may distort collateral anatomy leading to onset of new problems in other compartments if these compartments are not operated upon at the same time.(48) The controversy around this area is evidenced by the inclusion of a key debate at the forthcoming European Society of Coloproctology Annual meeting (September 21–23, 2022, Dublin, Ireland). The multidisciplinary team can also include a gastroenterologist to optimize management of defecatory disorders and coexistent IBS (49). Such input may help avoid suboptimal outcomes associated with surgery in constipated patients among whom chronic abdominal and/or pelvic pain are major symptoms (50).
Of particular interest, colorectal surgeons were performing vaginal rather than transanal surgery in the Ferrari et al study.(8) This is not uncommon in the UK but might be viewed as an anathema in other parts of the world. It does, however, pose another problem for future research seeking to find the optimal approach to rectocele —namely that expertise is also a variable. Arguably, the data in Ferrari et al(8) suggest that appropriately trained colorectal surgeons can perform vaginal surgery. Indeed, there are many advantages to this approach from the perspective of access, especially in the lithotomy position where the back wall of the vagina is much easier to access than the anterior wall of the anorectum. Perhaps it is time to compare vaginal and transanal approaches using an expertise-based design. However, this raises the issue of comparing like with like. The inclusion criteria for the 2017 systematic review(44) necessitated the reporting of at least some bowel symptoms (the basis for the review series was constipation). It is acknowledged that a separate body of gynecologic literature, including some higher quality studies,(51) exists for transvaginal rectocele repair, where the surgical intent was entirely focused on addressing vaginal prolapse symptoms. While it may just be an issue of asking the right questions, it is also possible that there are 2 groups of patients: one with predominantly bowel symptoms who come to a colorectal surgeon and one with prolapse symptoms that present to urogynecologists. This poses a further problem for research into optimal approaches because the baseline may be a covariate in differential responses from different surgical procedures. One advantage of the Ferrari et al(8) paper is that an equitable approach has been taken in recording some vaginal symptoms as well as those of obstructed defecation; however, the patients were still drawn from a population presenting with obstructed defecation. A UK cohort study is planned to evaluate this question. In keeping with earlier remarks about patient selection, the 215 patients were drawn from a much a larger number (1,888) with ODS.
In conclusion, the study by Ferrari and colleagues(8) suggests that a majority of patients have an overall good outcome after a rectocele repair.(8) Prospective studies with standardized assessments of symptoms and anorectal functions will provide more rigorous data on the relationship between rectocele characteristics, symptoms, and outcomes after surgery.
Figure 1.
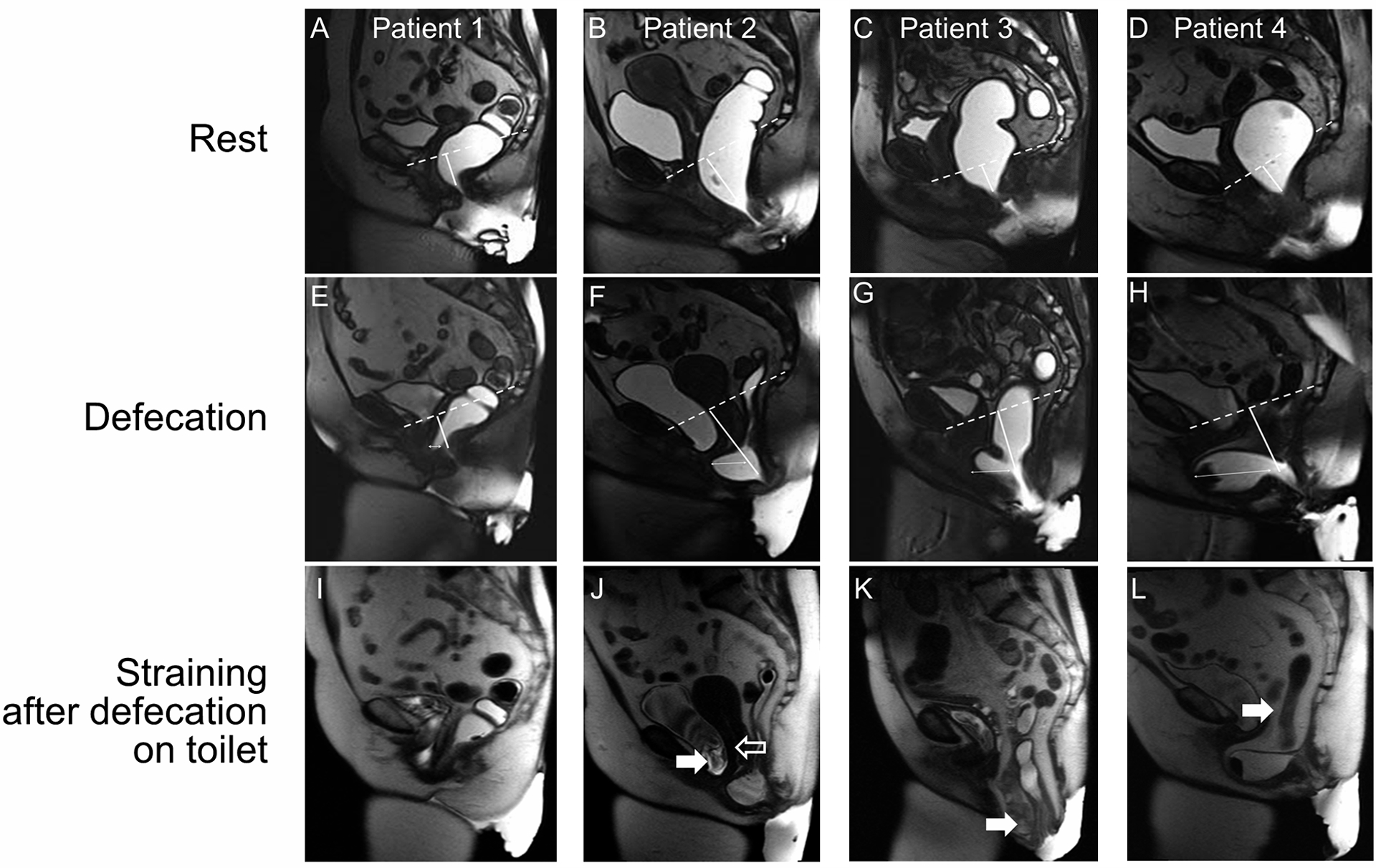
Sagittal magnetic resonance images at rest (A-D), during defecation (E-H), and while straining after defecation on the toilet (I-L) in 4 patients. Rectoceles (thin arrows) measuring 1.0 cm (E), 2.4 cm (F), 3.9 cm (G), and 6.7 cm (H) did not completely empty during defecation in the magnetic resonance image scanner (J, K, and L) or thereafter on the toilet (J and L). Approximately 90% (Patient 1), 70% (Patient 2), 95% (Patient 3), and 80% (Patient 4) of the ultrasound gel was emptied from the rectum during defecation. During defecation, the anorectal junction was 3.2 cm (E), 7.4 cm (F), 7.6 cm (G), and 7.5 cm (H) below the pubococcygeal line, i.e., perineal descent was increased in panels F, G, and H. Associated findings include a cystocele (J, filled arrow), excessive uterine descent (J, open arrow), enterocele (K, filled arrow), and peritoneocele (L, filled arrow).
Key Messages.
Rectoceles are protrusions of the anterior rectal wall into the vagina, predominantly observed in women and more frequently in older people.
Rectoceles smaller than 2 cm are generally asymptomatic, rectoceles greater than 4 cm may be associated with symptoms of difficult defecation, as well as of prolapse.
Initially, rectoceles should be managed with bowel modifying agents and pelvic floor muscle training (with biofeedback therapy for bowel symptoms). When conservative management is unsuccessful, surgical repair may be necessary.
Acknowledgment
The Scientific Publications staff at Mayo Clinic provided copyediting support. Dr. Bharucha is supported by US Public Health Service National Institutes of Health grant R01DK078924.
Abbreviations
- RCT
randomized clinical trial
- STARR
stapled transanal rectal resection
Footnotes
Conflicts: The authors have no conflicts of interest.
REFERENCES
- 1.Chen HH, Iroatulam A, Alabaz O, Weiss EG, Nogueras JJ, Wexner SD. Associations of defecography and physiologic findings in male patients with rectocele. Techniques in Coloproctology 2001; 5: 157–161. [DOI] [PubMed] [Google Scholar]
- 2.Grossi U, Heinrich H, Di Tanna GL, et al. Systematic Characterization of Defecographic Abnormalities in a Consecutive Series of 827 Patients With Chronic Constipation. Diseases of the Colon & Rectum 2021; 64: 1385–1397. [DOI] [PubMed] [Google Scholar]
- 3.Mahieu P, Pringot J, Bodart P. Defecography: II. Contribution to the diagnosis of defecation disorders. Gastrointestinal Radiology 1984; 9: 253–261. [DOI] [PubMed] [Google Scholar]
- 4.Hicks CW, Weinstein M, Wakamatsu M, Pulliam S, Savitt L, Bordeianou L. Are rectoceles the cause or the result of obstructed defaecation syndrome? A prospective anorectal physiology study. Colorectal Disease 2013; 15: 993–999. [DOI] [PubMed] [Google Scholar]
- 5.Dietz HP, Clarke B. Prevalence of rectocele in young nulliparous women. Australian & New Zealand Journal of Obstetrics & Gynaecology 2005; 45: 391–394. [DOI] [PubMed] [Google Scholar]
- 6.Dietz HP, Gomez M, Atan IK, Ferreira CSW. Association between vaginal parity and rectocele. International Urogynecology Journal 2018; 29: 1479–1483. [DOI] [PubMed] [Google Scholar]
- 7.Zbar AP, Lienemann A, Fritsch H, Beer-Gabel M, Pescatori M. Rectocele: pathogenesis and surgical management. International Journal of Colorectal Disease 2003; 18: 369–384. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari L, Cuinas K, Hainsworth A, et al. Transvaginal rectocoele repair for the surgical treatment of a “symptomatic” rectocoele when conservative measures fail: a 12 year experience of 215 patients. Neurogastroenterology & Motility 2022; Under Peer Review. [DOI] [PubMed] [Google Scholar]
- 9.Tirumanisetty P, Prichard D, Fletcher JG, Chakraborty S, Zinsmeister AR, Bharucha AE. Normal values for assessment of anal sphincter morphology, anorectal motion, and pelvic organ prolapse with MRI in healthy women. Neurogastroenterology & Motility 2018; 30: e13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz HP, Korda A. Which bowel symptoms are most strongly associated with a s rectocele? Australian and New Zealand Journal of Obstetrics and Gynaecology 2005; 45: 505–508. [DOI] [PubMed] [Google Scholar]
- 11.Guzman Rojas R, Kamisan Atan I, Shek KL, Dietz HP. The prevalence of abnormal posterior compartment anatomy and its association with obstructed defecation symptoms in urogynecological patients. International Urogynecology Journal 2016; 27: 939–944. [DOI] [PubMed] [Google Scholar]
- 12.Tan C, Geng J, Tang J, Yang X. The relationship between obstructed defecation and true rectocele in patients with pelvic organ prolapse. Scientific Reports 2020; 10: 5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenton K, Shott S, Brubaker L. The anatomic and functional variability of rectoceles in women. International Urogynecology Journal 1999; 10: 96–99. [DOI] [PubMed] [Google Scholar]
- 14.Dietz HP. Rectocele or stool quality: what matters more for symptoms of obstructed defecation? Techniques in Coloproctology 2009; 13: 265–268. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha AE, Lacy BE. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020; 158: 1232–1249.e1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharucha AE, Coss-Adame E. Diagnostic Strategy and Tools for Identifying Defecatory Disorders. Gastroenterology Clinics of North America 2022; 51: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietz HP. Ultrasound in the investigation of pelvic floor disorders. Current Opinion in Obstetrics & Gynecology 2020; 32: 431–440. [DOI] [PubMed] [Google Scholar]
- 18.Muir TW, Stepp KJ, Barber MD. Adoption of the pelvic organ prolapse quantification system in peer-reviewed literature. American Journal of Obstetrics & Gynecology 2003; 189: 1632–1635; discussion 1635–1636. [DOI] [PubMed] [Google Scholar]
- 19.Madhu C, Swift S, Moloney-Geany S, Drake MJ. How to use the Pelvic Organ Prolapse Quantification (POP-Q) system? Neurourology & Urodynamics 2018; 37: S39–S43. [DOI] [PubMed] [Google Scholar]
- 20.Wallace SL, Torosis M, Rogo-Gupta L. Does Rectocele on Defecography Equate to Rectocele on Physical Examination in Patients With Defecatory Symptoms? Female Pelvic Medicine & Reconstructive Surgery 2021; 27: 18–22. [DOI] [PubMed] [Google Scholar]
- 21.Mellgren A, Bremmer S, Johansson C, et al. Defecography. Results of investigations in 2,816 patients. Diseases of the Colon & Rectum 1994; 37: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 22.Palit S, Bhan C, Lunniss PJ, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Disease 2014; 16: 538–546. [DOI] [PubMed] [Google Scholar]
- 23.Carter D, Gabel MB. Rectocele--does the size matter? International Journal of Colorectal Disease 2012; 27: 975–980. [DOI] [PubMed] [Google Scholar]
- 24.Dietz HP, Zhang X, Shek KL, Guzman RR. How large does a rectocele have to be to cause symptoms? A 3D/4D ultrasound study. International Urogynecology Journal 2015; 26: 1355–1359. [DOI] [PubMed] [Google Scholar]
- 25.Marti Roche, Deleaval. Rectoceles: value of videodefaecography in selection of treatment policy. Colorectal Disease 1999; 1: 324–329. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg T, Kelvin FM, Maglinte DD. Barium trapping in rectoceles: are we trapped by the wrong definition? Abdominal Imaging 2001; 26: 587–590. [DOI] [PubMed] [Google Scholar]
- 27.Mellgren A, Anzen B, Nilsson BY, et al. Results of rectocele repair. A prospective study. Diseases of the Colon & Rectum 1995; 38: 7–13. [DOI] [PubMed] [Google Scholar]
- 28.van Dam JH, Ginai AZ, Gosselink MJ, et al. Role of defecography in predicting clinical outcome of rectocele repair. Diseases of the Colon & Rectum 1997; 40: 201–207. [DOI] [PubMed] [Google Scholar]
- 29.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology 2005; 128: 1199–1210. [DOI] [PubMed] [Google Scholar]
- 30.Prichard DO, Lee T, Parthasarathy G, Fletcher JG, Zinsmeister AR, Bharucha AE. High-resolution Anorectal Manometry for Identifying Defecatory Disorders and Rectal Structural Abnormalities in Women. Clinical Gastroenterology & Hepatology 2017; 15: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durnea CM, Khashan AS, Kenny LC, Durnea UA, Smyth MM, O’Reilly BA. Prevalence, etiology and risk factors of pelvic organ prolapse in premenopausal primiparous women. International Urogynecology Journal 2014; 25: 1463–1470. [DOI] [PubMed] [Google Scholar]
- 32.Mazor Y, Hansen R, Prott G, Kellow J, Malcolm A. The importance of a high rectal pressure on strain in constipated patients: implications for biofeedback therapy. Neurogastroenterology and Motility 2017; 29. [DOI] [PubMed] [Google Scholar]
- 33.Tan C, Tan M, Geng J, Tang J, Yang X. Rectal-vaginal pressure gradient in patients with pelvic organ prolapse and symptomatic rectocele. BMC Women’s Health 2021; 21: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharucha AE, Basilisco G, Malcolm A, et al. Review of the indications, methods, and clinical utility of anorectal manometry and the rectal balloon expulsion test. Neurogastroenterol Motil 2022: e14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hicks CW, Weinstein M, Wakamatsu M, Savitt L, Pulliam S, Bordeianou L. In patients with rectoceles and obstructed defecation syndrome, surgery should be the option of last resort. Surgery 2014; 155: 659–667. [DOI] [PubMed] [Google Scholar]
- 36.Bharucha AE, Seide BM, Zinsmeister AR, Melton LJ 3rd. Insights into normal and disordered bowel habits from bowel diaries. American Journal of Gastroenterology 2008; 103: 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wald A, Bharucha AE, Limketkai B, et al. ACG Clinical Guidelines: Management of Benign Anorectal Disorders. Am J Gastroenterol 2021; 116: 1987–2008. [DOI] [PubMed] [Google Scholar]
- 38.Grossi U, Knowles CH, Mason J, et al. Surgery for constipation: systematic review and practice recommendations: Results II: Hitching procedures for the rectum (rectal suspension). Colorectal Dis 2017; 19 Suppl 3: 37–48. [DOI] [PubMed] [Google Scholar]
- 39.Corsetti M, Brown S, Chiarioni G, et al. Chronic constipation in adults: Contemporary perspectives and clinical challenges. 2: Conservative, behavioural, medical and surgical treatment. Neurogastroenterol Motil 2021: e14070. [DOI] [PubMed] [Google Scholar]
- 40.Mercer-Jones M, Grossi U, Pares D, et al. Surgery for constipation: systematic review and practice recommendations: Results III: Rectal wall excisional procedures (Rectal Excision). Colorectal Dis 2017; 19 Suppl 3: 49–72. [DOI] [PubMed] [Google Scholar]
- 41.Farid M, Madbouly KM, Hussein A, Mahdy T, Moneim HA, Omar W. Randomized controlled trial between perineal and anal repairs of rectocele in obstructed defecation. World journal of surgery 2010; 34: 822–829. [DOI] [PubMed] [Google Scholar]
- 42.Block IR. Transrectal repair of rectocele using obliterative suture. Dis Colon Rectum 1986; 29: 707–711. [DOI] [PubMed] [Google Scholar]
- 43.Sarles JC, Arnaud A, Selezneff I, Olivier S. Endo-rectal repair of rectocele. Int J Colorectal Dis 1989; 4: 167–171. [DOI] [PubMed] [Google Scholar]
- 44.Grossi U, Horrocks EJ, Mason J, et al. Surgery for constipation: systematic review and practice recommendations: Results IV: Recto-vaginal reinforcement procedures. Colorectal Dis 2017; 19 Suppl 3: 73–91. [DOI] [PubMed] [Google Scholar]
- 45.Miklos JR, Kohli N, Lucente V, Saye WB. Site-specific fascial defects in the diagnosis and surgical management of enterocele. American journal of obstetrics and gynecology 1998; 179: 1418–1422; discussion 1822–1413. [DOI] [PubMed] [Google Scholar]
- 46.Abramov Y, Gandhi S, Goldberg RP, Botros SM, Kwon C, Sand PK. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstetrics and gynecology 2005; 105: 314–318. [DOI] [PubMed] [Google Scholar]
- 47.Cumberlege J First Do No Harm. The Report of the Independent Medicines and Medical Devices Safety Review. London, England: Crown Copyright, 2020. [Google Scholar]
- 48.Pham T, Kenton K, Mueller E, Brubaker L. New pelvic symptoms are common after reconstructive pelvic surgery. American Journal of Obstetrics & Gynecology 2009; 200: 88.e81–85. [DOI] [PubMed] [Google Scholar]
- 49.Bharucha AE, Wald A. Chronic Constipation. Mayo Clinic Proceedings 2019; 94: 2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaichanavichkij P, Vollebregt PF, Tee SZY, Scott SM, Knowles CH. Slow-transit constipation and criteria for colectomy: a cross-sectional study of 1568 patients. Bjs Open 2021; 5: 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2013: CD004014. [DOI] [PubMed] [Google Scholar]


