Abstract
Atomic/molecular layer deposition (ALD/MLD) allows for the direct gas-phase synthesis of crystalline metal–organic framework (MOF) thin films. Here, we show for the first time using krypton and methanol physisorption measurements that ALD/MLD-fabricated copper 1,4-benzenedicarboxylate (Cu-BDC) ultrathin films possess accessible porosity matching that of the corresponding bulk MOF.
Keywords: atomic layer deposition, molecular layer deposition, porous thin films, krypton physisorption, methanol physisorption, metal−organic framework
The landmark of the continuously expanding family of metal–organic frameworks (MOFs) is their highly porous structures.1 The open pores make these materials promising candidates for a variety of applications such as gas storage,2 separation,3 and catalysis.4 Solvothermal synthesis, together with pelletization and extrusion procedures, is typically utilized for these well-established uses.5 However, a more recent challenge is the integration of these porous MOF materials in microelectronics,6 which would require the development of industry-feasible nanoscale thin-film fabrication technologies.
In recent years, pioneering efforts have been made toward adopting cornerstone vapor-phase microfabrication techniques such as chemical vapor deposition (CVD) and atomic layer deposition (ALD) for the growth of MOF films.7 Hence, Stassen et al.8 developed the first MOF-CVD procedure to transform a metal oxide layer predeposited by ALD into a MOF structure via a solid–vapor reaction with organic linker vapor.
Alternatively, the combined atomic/molecular layer deposition (ALD/MLD) technique9,10 provides an elegant way to deposit metal–organic materials directly from gaseous precursors in a single process. Like ALD,11 the ALD/MLD technique is based on self-limiting gas-surface reactions of alternately supplied gaseous precursors, which enables thin-film uniformity and conformality as well as precise thickness control. So far, ALD/MLD processes have already been developed for more than a dozen in situ crystalline metal–organic materials, with both previously known and completely new crystal structures.12−16 Also, efforts have been made to crystallize the initially amorphous ALD/MLD-grown metal–organic films through various postdeposition treatments.17−19 This includes pioneering work by Ritala and co-workers for zinc 1,4-benzenedicarboxylate (Zn-BDC) films. Thus, a postdeposition treatment in a humidity-controlled chamber, followed by recrystallization with N,N-dimethylformamide in an autoclave, was required to obtain films of the cubic MOF-5 (also known as IRMOF-1) structure.17 Later, this work was followed by similar postdeposition crystallization efforts for IRMOF-8-structured zinc 2,6-naphthalenedicarboxylate films18 and UiO-66-structured zirconium 1,4-benzenedicarboxylate films.19
Some of the crystalline as-deposited ALD/MLD metal–organic films resemble the known MOF structures and thus are expected to be porous.20−22 However, their porous properties have not been experimentally verified. This can be mainly attributed to the experimental challenges of measuring the porosity on ultrathin (10–100 nm) films. The sensitivity of conventional volumetric N2 or Ar physisorption is too low to reliably characterize the minute amount of porous material on a typical substrate size (1–50 cm2). Recently, a number of alternative techniques have been employed to characterize the porous properties of thin films, such as positron annihilation lifetime spectroscopy, krypton physisorption (KrP),23 crystal microbalance (QCM) gravimetry,13 and ellipsometric porosimetry.24
In this paper, we for the first time show results from KrP porosity measurements for as-deposited crystalline ALD/MLD MOF films of copper 1,4-benzenedicarboxylate (Cu-BDC); the films were deposited following our previously reported ALD/MLD process based on Cu(thd)2 (thd = 2,2,6,6-tetramethyl-3,5-heptanedione) and 1,4-benzenedicarboxylic acid (H2BDC) precursors (Scheme 1).21 The porosity of our ALD/MLD Cu-BDC films was evaluated through KrP because of the accuracy of the technique for samples with extremely small surface areas (<1 m2), such as ultrathin films. In contrast to N2 and Ar, the sensitivity of KrP is significantly higher because of its much lower saturation pressure, thus reducing the number of molecules in the free space of the sample cell.23,25 In an earlier study, the porosity of Cu-BDC thin films was evaluated by QCM for samples prepared through a two-step CVD process consisting of a CuO/Cu precursor layer deposition and subsequent solid–vapor reaction of this layer with dicarboxylic acid linker vapor.26
Scheme 1. Schematic Illustration of a Crystalline ALD/MLD MOF Film of Cu-BDC Based on 1,4-Benzenedicarboxylic Acid and Cu(thd)2 Precursors Yielding Cu-BDC Films of the ZUBKEO27 Structure.
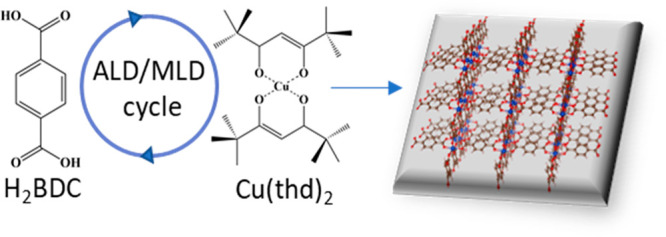
The literature for solution-synthesized Cu-BDC materials in bulk form is already extensive (Table S1). The porous ZUBKEO (Figure S1) structure consists of dinuclear CuII moieties bridged with BDC linkers, forming two-dimensional sheets and one-dimensional pores.27 As demonstrated below, our ALD/MLD process yields phase-pure Cu-BDC films of the ZUBKEO structure.
The deposition of Cu-BDC films from Cu(thd)2 and H2BDC precursor powders is detailed in the Supporting Information (SI). All of the depositions were carried out at a chamber temperature ranging from 180 to 220 °C, with varying precursor/N2 purge pulse lengths depending on the type of substrate used. The samples meant for general characterization were deposited on silicon (Si) substrates with the following parameters: 5 s Cu(thd)2/2 s N2/10 s BDC/20 s N2. Samples for the KrP experiments were deposited on high-aspect-ratio (HAR) pillars substrates (further described in the SI) with 700 ALD/MLD cycles and with significantly longer pulse lengths to ensure conformal growth: 40 s Cu(thd)2/30 s N2/60 s BDC/80 s N2. Samples for QCM measurements were deposited straight on a SiO2 sensor substrate with 900 ALD/MLD cycles using the following precursor/purge protocol: 5 s Cu(thd)2/3 s N2/15 s BDC/20 s N2.
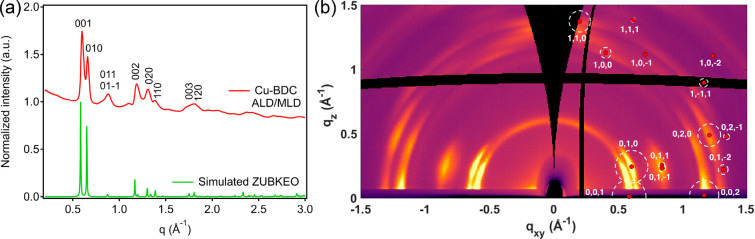
Next, we analyzed the crystallinity of our ALD/MLD Cu-BDC films. Because of their nanometric thicknesses, we measured synchrotron grazing-incidence X-ray diffraction (GIXRD). As shown in Figure 1a, the crystalline phase in our ALD/MLD Cu-BDC films could be readily assigned to the ZUBKEO Cu-BDC crystal structure, previously reported for powder Cu-BDC samples.27 A detailed inspection of the GIXRD reciprocal space maps revealed some degree of preferential orientation in the films, as denoted by the presence of small incomplete diffraction rings. As is visible in Figure 1b, their positions and intensities correspond to the ZUBKEO phase with a dominant (320) crystalline orientation. Thus, we assume that a significant percentage of the film’s crystallites are of that orientation, along with a minor fraction of other orientations and randomly oriented crystallites.
Figure 1.
(a) Synchrotron GIXRD diffractogram for our ALD/MLD Cu-BDC thin film compared to the simulated pattern of reported crystal structure ZUBKEO.27 (b) Reciprocal space map of an ALD/MLD Cu-BDC thin film obtained from synchrotron GIXRD. The simulated Bragg peaks for a (320) crystalline orientation are overlaid on the positive qxy side of the map. Red points at the center of circles give the expected positions of the diffraction peaks, and the areas inside the circles give the square of the structure factors, which are proportional to the expected intensities.
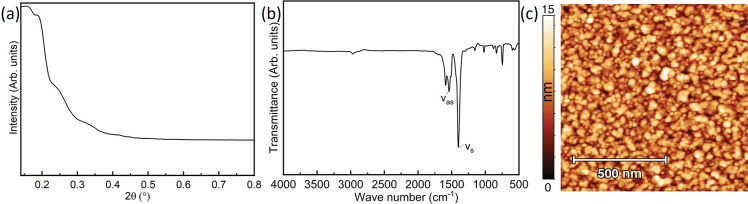
The film thickness determined through X-ray reflectivity (XRR) measurements was 44 nm for an ALD/MLD Cu-BDC film of 200 precursor pulse cycles (Figure 2a). The growth rate expressed as the so-called growth-per-cycle (GPC) value was thus 2.2 Å cycle–1 for samples deposited on Si wafer substrates. Densities were deduced from the critical angle θc in the XRR patterns using an equation detailed in the SI. The value obtained for our Cu-BDC film (1.5 g cm–3) is in reasonable agreement with the ideal density calculated for the ZUBKEO crystal structure (1.385 g cm–3).27 We ascribe the slightly higher density to a more strained structure in the ultrathin films versus the powder (Figure S2). The chemical bonding scheme expected for Cu-BDC was confirmed by Fourier transform infrared (FTIR; Figure 2b). The absence of the peak at 1720 cm–1 characteristic of the C=O stretch in a −COOH group indicates the completeness of the reaction between the precursors. Moreover, directly after the deposition, no features were seen around 3400 cm–1, which would indicate unintentionally adsorbed water. Finally, the bands around 1525 and 1390 cm–1 due to the asymmetric and symmetric vibrations of metal-coordinated carboxylate groups, respectively, confirm the expected bridging-type coordination of the carboxylate groups to the CuII atoms.27−29 Atomic force microscopy (AFM) images (Figures 2c and S3) show a smooth film surface over micrometric areas, composed of nanometric particles with a root-mean-square (RMS) roughness of 2.4 nm.
Figure 2.
(a) XRR pattern, (b) FTIR spectrum, and (c) 1 × 1 μm2 AFM topography image for an ALD/MLD Cu-BDC film deposited on a Si substrate.
The porosity of our ALD/MLD Cu-BDC films was evaluated through KrP by depositing the Cu-BDC film (ca. 154 nm, extrapolated using the GPC value) on a substrate with HAR pillars to enhance the surface area and thus yield improved diffusion kinetics in comparison to films deposited on a flat Si substrate.
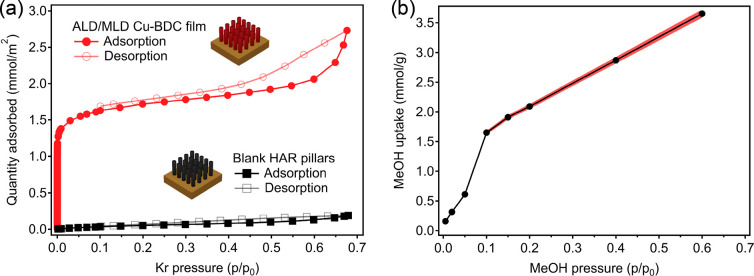
The Kr adsorption/desorption isotherms measured at 77 K for the sample after activation at 150 °C for 10 h compared with a blank HAR substrate are plotted in Figure 3a. According to the IUPAC classification,30 the ALD/MLD Cu-BDC film features a type I isotherm in the low-pressure region with a small H4-type hysteresis at P/P0 < 0.45, thus indicating a minor presence of mesoporosity likely originating from interparticle gaps. A specific surface area of 174 m2 cm–3 was obtained for the activated Cu-BDC film in the 0.001–0.07 P/P0 microporous region. Assuming that the film has a theoretical surface area of 1162 m2 cm–3 (calculated with Zeo++ for the ZUBKEO structure27,31), this specific surface area corresponds to a 150-nm-thick film, in perfect agreement with the film thickness estimated using the GPC value. Alternatively, considering a thickness of exactly 154 nm, we obtain an experimental surface area of 1166 m2 cm–3, again matching the theoretical value for ZUBKEO Cu-BDC. In addition, according to the dominant (320) orientation revealed by synchrotron GIXRD, the pore channels are mostly aligned roughly normal to the surface (Figure S4), which should facilitate guest accessibility and contribute to the high Brunauer–Emmett–Teller (BET) area recorded.
Figure 3.
(a) KrP isotherms for the ALD/MLD Cu-BDC thin film and a blank HAR reference. (b) Methanol adsorption on a Cu-BDC-coated QCM sensor calculated for the 5th overtone. The red shaded area represents the 95% confidence interval based on the uncertainty in the MOF layer mass determination.
Methanol adsorption was measured by placing a Cu-BDC-coated sensor into a dedicated QCM cell, followed by exposure to methanol vapor of different concentrations. During the experiment, the sensor resonant frequencies (fn) and bandwidths (Γn) for different overtones (n = 1, 3, 5, 7, 9, 11, and 13) were monitored. Because the change in the bandwidth (ΔΓn) was much smaller than the resonant frequency shift (Δfn) for all of the measured n (ΔΓn/Δfn < 0.01), the Sauerbrey equation was applied to calculate the change in the layer’s mass corresponding to methanol adsorption.32 To determine the mass of the deposited Cu-BDC layer, it was first dissolved in piranha [1:3 (v/v) H2O2/H2SO4], and then fn and Γn before and after were compared. Similarly, because ΔΓn/Δfn < 0.01, the Sauerbrey equation was used to calculate the mass of the MOF layer. The specific methanol uptake calculated for n = 7 is shown in Figure 3b as an isotherm of shape similar to those of others of previously reported Cu-BDC thin films.26 The maximum uptake is in agreement with a rough estimation of 3.7 mmol g–1 based on the calculated ZUBKEO structure volume fraction (0.149 cm3 g–1) and density of liquid methanol (0.792 g cm–3).
In conclusion, we were able to directly produce crystalline and phase-pure thin films of a well-known Cu-BDC MOF via our ALD/MLD process. Moreover, synchrotron GIXRD measurements showed a preferential orientation of the film crystallites. The accessible porosity of these MOF thin films was demonstrated via KrP and methanol physisorption, with the total BET surface area and methanol uptake matching the theoretical values calculated for the ZUBKEO structure. Thus, we foresee that these positive results could serve as a strong motivation for further ALD/MLD process development for other promising porous MOF materials.
Acknowledgments
We acknowledge the use of the RawMatters Finland Infrastructure at Aalto University as well as Elettra Sincrotrone Trieste for providing access to its synchrotron radiation facilities (Proposal 20210538) and thank Luisa Barba and Nicola Demitri for assistance using beamline XRD1. We thank Prof. Roland Resel for assistance in using GIDVis software.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsanm.2c04934.
Additional experimental details and descriptions of the materials, substrates, characterization, and methods (PDF)
Author Contributions
† These authors contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie Grant Agreement 765378 and from Academy of Finland (PREIN). V.R.-G. thanks the Research Foundation Flanders for a Junior Postdoctoral Fellowship (1263622N). The research leading to this result has been supported by the project CALIPSOplus under Grant Agreement 730872 from the EU Framework Programme for Research and Innovation Horizon 2020.
The authors declare no competing financial interest.
Supplementary Material
References
- Rowsell J. L. C.; Yaghi O. M. Metal–Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73 (1–2), 3–14. 10.1016/j.micromeso.2004.03.034. [DOI] [Google Scholar]
- He Y.; Zhou W.; Qian G.; Chen B. Methane Storage in Metal–Organic Frameworks. Chem. Soc. Rev. 2014, 43 (16), 5657–5678. 10.1039/C4CS00032C. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Wang Y.; Li D. S.; Bu X.; Feng P.. Metal–Organic Frameworks for Separation. Advanced Materials; John Wiley & Sons, Ltd., September 27, 2018; p 1705189. 10.1002/adma.201705189. [DOI] [PubMed] [Google Scholar]
- Corma A.; García H.; Llabrés i Xamena F. X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110 (8), 4606–4655. 10.1021/cr9003924. [DOI] [PubMed] [Google Scholar]
- Yeskendir B.; Dacquin J.-P.; Lorgouilloux Y.; Courtois C.; Royer S.; Dhainaut J. From Metal–Organic Framework Powders to Shaped Solids: Recent Developments and Challenges. Mater. Adv. 2021, 2 (22), 7139–7186. 10.1039/D1MA00630D. [DOI] [Google Scholar]
- Shekhah O.; Liu J.; Fischer R. A.; Wöll C. MOF Thin Films: Existing and Future Applications. Chem. Soc. Rev. 2011, 40 (2), 1081. 10.1039/c0cs00147c. [DOI] [PubMed] [Google Scholar]
- Su P.; Tu M.; Ameloot R.; Li W. Vapor-Phase Processing of Metal–Organic Frameworks. Acc. Chem. Res. 2022, 55 (2), 186–196. 10.1021/acs.accounts.1c00600. [DOI] [PubMed] [Google Scholar]
- Stassen I.; Styles M.; Grenci G.; Gorp H.; Vanderlinden W.; Feyter S.; Falcaro P.; Vos D.; Vereecken P.; Ameloot R. Chemical Vapour Deposition of Zeolitic Imidazolate Framework Thin Films. Nat. Mater. 2016, 15 (3), 304–310. 10.1038/nmat4509. [DOI] [PubMed] [Google Scholar]
- Multia J.; Karppinen M. Atomic/Molecular Layer Deposition for Designer’s Functional Metal–Organic Materials. Adv. Mater. Interfaces 2022, 9 (15), 2200210. 10.1002/admi.202200210. [DOI] [Google Scholar]
- George S. M.; Lee B. H.; Yoon B.; Abdulagatov A. I.; Hall R. A. Metalcones: Hybrid Organic-Inorganic Films Fabricated Using Atomic and Molecular Layer Deposition Techniques. J. Nanosci. Nanotechnol. 2011, 11 (9), 7948–7955. 10.1166/jnn.2011.5034. [DOI] [PubMed] [Google Scholar]
- George S. M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110 (1), 111–131. 10.1021/cr900056b. [DOI] [PubMed] [Google Scholar]
- Nisula M.; Karppinen M. Atomic/Molecular Layer Deposition of Lithium Terephthalate Thin Films as High Rate Capability Li-Ion Battery Anodes. Nano Lett. 2016, 16 (2), 1276–1281. 10.1021/acs.nanolett.5b04604. [DOI] [PubMed] [Google Scholar]
- Lausund K. B.; Olsen M. S.; Hansen P.-A.; Valen H.; Nilsen O. MOF Thin Films with Bi-Aromatic Linkers Grown by Molecular Layer Deposition. J. Mater. Chem. A 2020, 8 (5), 2539–2548. 10.1039/C9TA09303F. [DOI] [Google Scholar]
- Multia J.; Heiska J.; Khayyami A.; Karppinen M. Electrochemically Active In Situ Crystalline Lithium-Organic Thin Films by ALD/MLD. ACS Appl. Mater. Interfaces 2020, 12 (37), 41557–41566. 10.1021/acsami.0c11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahvenniemi E.; Karppinen M. In Situ Atomic/Molecular Layer-by-Layer Deposition of Inorganic–Organic Coordination Network Thin Films from Gaseous Precursors. Chem. Mater. 2016, 28 (17), 6260–6265. 10.1021/acs.chemmater.6b02496. [DOI] [Google Scholar]
- Gikonyo B.; Liu F.; De S.; Journet C.; Marichy C.; Fateeva A. Investigating the Vapour Phase Synthesis of Copper Terephthalate Metal Organic Framework Thin Films by Atomic/Molecular Layer Deposition. Dalt. Trans. 2022, 52 (1), 211–217. 10.1039/D2DT03216C. [DOI] [PubMed] [Google Scholar]
- Salmi L. D.; Heikkilä M. J.; Puukilainen E.; Sajavaara T.; Grosso D.; Ritala M. Studies on Atomic Layer Deposition of MOF-5 Thin Films. Microporous Mesoporous Mater. 2013, 182, 147–154. 10.1016/j.micromeso.2013.08.024. [DOI] [Google Scholar]
- Salmi L. D.; Heikkilä M. J.; Vehkamäki M.; Puukilainen E.; Ritala M.; Sajavaara T. Studies on Atomic Layer Deposition of IRMOF-8 Thin Films. J. Vac. Sci. Technol. A 2015, 33 (1), 01A121. 10.1116/1.4901455. [DOI] [Google Scholar]
- Lausund K. B.; Nilsen O. All-Gas-Phase Synthesis of UiO-66 through Modulated Atomic Layer Deposition. Nat. Commun. 2016, 7 (1), 13578. 10.1038/ncomms13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen A.; Karppinen M. Iron-Terephthalate Coordination Network Thin Films Through In-Situ Atomic/Molecular Layer Deposition. Sci. Rep. 2018, 8 (1), 8976. 10.1038/s41598-018-27124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahvenniemi E.; Karppinen M. Atomic/Molecular Layer Deposition: A Direct Gas-Phase Route to Crystalline Metal-Organic Framework Thin Films. Chem. Commun. 2016, 52 (6), 1139–1142. 10.1039/C5CC08538A. [DOI] [PubMed] [Google Scholar]
- Silva R. M.; Carlos L. D.; Rocha J.; Silva R. F. Luminescent Thin Films of Eu-Bearing UiO-66 Metal Organic Framework Prepared by ALD/MLD. Appl. Surf. Sci. 2020, 527, 146603. 10.1016/j.apsusc.2020.146603. [DOI] [Google Scholar]
- Stassin T.; Verbeke R.; Cruz A. J.; Rodríguez-Hermida S.; Stassen I.; Marreiros J.; Krishtab M.; Dickmann M.; Egger W.; Vankelecom I. F. J.; Furukawa S.; De Vos D.; Grosso D.; Thommes M.; Ameloot R. Porosimetry for Thin Films of Metal–Organic Frameworks: A Comparison of Positron Annihilation Lifetime Spectroscopy and Adsorption-Based Methods. Adv. Mater. 2021, 33 (17), 2006993. 10.1002/adma.202006993. [DOI] [PubMed] [Google Scholar]
- Dendooven J.; Devloo-Casier K.; Levrau E.; Van Hove R.; Pulinthanathu Sree S.; Baklanov M. R.; Martens J. A.; Detavernier C. In Situ Monitoring of Atomic Layer Deposition in Nanoporous Thin Films Using Ellipsometric Porosimetry. Langmuir 2012, 28 (8), 3852–3859. 10.1021/la300045z. [DOI] [PubMed] [Google Scholar]
- Thommes M.; Cychosz K. A. Physical Adsorption Characterization of Nanoporous Materials: Progress and Challenges. Adsorption 2014, 20 (2–3), 233–250. 10.1007/s10450-014-9606-z. [DOI] [Google Scholar]
- Stassin T.; Rodríguez-Hermida S.; Schrode B.; Cruz A. J.; Carraro F.; Kravchenko D.; Creemers V.; Stassen I.; Hauffman T.; De Vos D.; Falcaro P.; Resel R.; Ameloot R. Vapour-Phase Deposition of Oriented Copper Dicarboxylate Metal–Organic Framework Thin Films. Chem. Commun. 2019, 55 (68), 10056–10059. 10.1039/C9CC05161A. [DOI] [PubMed] [Google Scholar]
- Carson C. G.; Brunnello G.; Lee S. G.; Jang S. S.; Gerhardt R. A.; Tannenbaum R. Structure Solution from Powder Diffraction of Copper 1,4-Benzenedicarboxylate. Eur. J. Inorg. Chem. 2014, 2014 (12), 2140–2145. 10.1002/ejic.201301543. [DOI] [Google Scholar]
- Klepper K. B.; Nilsen O.; Francis S.; Fjellvåg H. Guidance of Growth Mode and Structural Character in Organic–Inorganic Hybrid Materials – a Comparative Study. Dalt. Trans. 2014, 43 (9), 3492–3500. 10.1039/C3DT52391H. [DOI] [PubMed] [Google Scholar]
- Deacon G. B. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33 (3), 227–250. 10.1016/S0010-8545(00)80455-5. [DOI] [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87 (9–10), 1051–1069. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Willems T. F.; Rycroft C. H.; Kazi M.; Meza J. C.; Haranczyk M. Algorithms and Tools for High-Throughput Geometry-Based Analysis of Crystalline Porous Materials. Microporous Mesoporous Mater. 2012, 149 (1), 134–141. 10.1016/j.micromeso.2011.08.020. [DOI] [Google Scholar]
- Reviakine I.; Johannsmann D.; Richter R. P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83 (23), 8838–8848. 10.1021/ac201778h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.