Abstract
The three genospecies Borrelia burgdorferi, Borrelia garinii, and Borrelia afzelii, all causative agents of Lyme disease, differ in their susceptibilities to human complement-mediated lysis. We recently reported that serum resistance of borrelias correlates largely with their ability to bind the human complement regulators FHL-1/reconectin and factor H. To date, two complement regulator-acquiring-proteins (CRASP-1 and CRASP-2) have been identified in serum-resistant B. afzelii isolates (P. Kraiczy, C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel, Eur. J. Immunol. 31:1674–1684, 2001). Here, we present a comprehensive study of the CRASPs detectable in both serum-resistant and intermediate serum-sensitive B. afzelii and B. burgdorferi isolates. These CRASPs were designated according to the genospecies either as BaCRASPs, when derived from B. afzelii, or as BbCRASPs, for proteins identified in B. burgdorferi isolates. Each borrelial isolate expresses distinct CRASPs that can be differentiated by their mobility and binding phenotypes. A detailed comparison reveals overlapping and even identical binding profiles for BaCRASP-1 (27.5 kDa), BbCRASP-1 (25.9 kDa), and BbCRASP-2 (23.2 kDa), which bind FHL-1/reconectin strongly and interact weakly with factor H. In contrast, two B. afzelii proteins (BaCRASP-4 [19.2 kDa] and BaCRASP-5 [22.5 kDa]) and three B. burgdorferi proteins (BbCRASP-3 [19.8 kDa], BbCRASP-4 [18.5 kDa], and BbCRASP-5 [17.7 kDa]) bind factor H but not FHL-1/reconectin. Most CRASPs bind both human immune regulators at their C-terminal ends. Temperature-dependent up-regulation of CRASPs (BaCRASP-1, BaCRASP-2, and BaCRASP-5) is detected in low-passage borrelias cultured at 33 or 37°C compared with those cultured at 20°C. The characterization of the individual CRASPs on the molecular level is expected to identify new virulence factors and potential vaccine candidates.
Borrelia burgdorferi, Borrelia garinii, and Borrelia afzelii are the causative agents of Lyme borreliosis or Lyme disease (38). Spirochetes transmitted to the human host by infected Ixodes ticks during a blood meal invade the host dermis. This initial infection often is followed by a local skin rash (erythema migrans), which usually disappears spontaneously. Untreated Lyme disease, however, can progress into a chronic, multisystemic disorder by hematogenous dissemination of the pathogen. This insidious disease primarily affects the joints, central nervous system, and skin, thereby presenting as Lyme arthritis, neuroborreliosis, or acrodermatitis chronica atrophicans (ACA) (38).
The first line of defense against many invading pathogens is provided by innate immunity, of which the complement system is a particularly important constituent (9, 29). Elimination of pathogens can be accomplished by complement in many different ways, and consequently, pathogens have developed a wide range of strategies in the course of evolution to avoid destructive complement attacks and to survive within the immunocompetent host (7, 14, 18, 46). One strategy of microorganisms that has recently attracted particular interest is the ability to acquire host fluid-phase complement regulatory proteins of the alternative pathway. Acquisition of these proteins allows control of the early steps in the complement activation cascade directly on the surface of the pathogen (28, 47, 48). This interference with the complement activation process permits survival of the invading microorganisms.
The two central human fluid-phase complement regulators of the alternative pathway are FHL-1/reconectin and factor H. The two proteins are structurally related, and their transcripts are derived by alternative processing of a nuclear RNA transcript, which is derived from a single human gene (11, 47). FHL-1/reconectin and factor H are composed exclusively of individually folding protein domains termed short consensus repeats (SCRs). The 42-kDa FHL-1/reconectin protein consists of seven SCRs, and the 150-kDa factor H protein includes 20 SCR domains. The SCRs of FHL-1/reconectin are identical to the N-terminal domain of factor H, and the protein has a unique C-terminal extension of 4 amino acids. Both proteins have the same complement regulatory functions: they control C3b formation and stability by acting as cofactors for factor I-mediated degradation of C3b and accelerate the decay of the C3 convertase. The complement regulatory domains of both proteins are located in the N-terminal SCRs 1 to 4 (12, 24, 26).
Protection against complement by binding of FHL-1/reconectin and/or complement factor H has been demonstrated elsewhere for several human pathogens: Echinococcus granulosus (10), Neisseria gonorrhoeae (33, 34), Neisseria meningitidis (35), Streptococcus pyogenes (17, 19, 31), Streptococcus pneumoniae (30), Yersinia enterocolitica (6), and the human immunodeficiency virus (41). For some pathogens, the microbial binding proteins responsible for the surface attachment of FHL-1/reconectin and for factor H have been identified, such as the sialylated lipooligosaccharide or porin, the major outer membrane protein of N. gonorrhoeae (33, 34), the M protein for S. pyogenes (35), the Hic protein for S. pneumoniae (16), and gp120 as well as gp41 from human immunodeficiency virus (41).
Recently published data provide direct evidence that serum-resistant B. burgdorferi isolates are also capable of binding FHL-1/reconectin as well as factor H to their surfaces (13, 22, 23). So far, two different borrelial proteins designated complement regulator-acquiring surface proteins (CRASPs), which serve as ligands for FHL-1/reconectin and factor H, have been identified in serum-resistant B. afzelii isolates. CRASP-1, a 27.5-kDa protein, preferentially binds FHL-1/reconectin, and CRASP-2, a 20- to 21-kDa protein, interacts preferably with factor H (23). Expression of CRASPs correlates directly with serum resistance inasmuch as all serum-resistant isolates analyzed express these proteins, whereas all serum-sensitive isolates analyzed to date do not possess proteins with such a binding activity (23). Recently published studies with recombinant OspE suggest that this surface protein also may function as a ligand for factor H (13). Thus, there is evidence that borrelias express more than one protein that serves as a ligand for the complement regulatory proteins FHL-1/reconectin and factor H. The binding domains of the complement regulators were located within SCRs 5 to 7 at the C-terminal end of FHL-1/reconectin (23) and within SCRs 15 to 20 at the C-terminal end of factor H (13).
In the present study, we extend our work on CRASPs to a larger number of borrelial isolates belonging to the group of serum-resistant and intermediate serum-sensitive isolates. Depending on the genospecies of the isolates tested, we were able to detect up to five additional CRASPs. These binding proteins differ greatly with respect to their reactivity with FHL-1/reconectin and/or factor H. Within one genospecies, however, the binding pattern is very consistent. Furthermore, we demonstrate that the regulation of some CRASPs is influenced by temperature and long-term cultivation. Finally, although most of the CRASPs bind the regulator proteins at the C-terminal domains, a few exceptions to this rule do exist.
MATERIALS AND METHODS
Borrelial isolates and culture conditions.
A panel totaling 14 B. burgdorferi and B. afzelii isolates was investigated. The designations, passages, and biological and geographical origins of the borrelial isolates appear in Table 1. Unless otherwise stated, all isolates and clone FEM1-D15 were cultured until mid-log phase (5 × 107 cells per ml) at 33°C in modified Barbour-Stoenner-Kelly medium (32). Samples of 1.8 ml then were dispensed into screw-cap tubes (Nunc, Wiesbaden, Germany), frozen at −70°C, and used as stock cultures. Prior to use, a frozen suspension of spirochetes was thawed and inoculated into fresh Barbour-Stoenner-Kelly medium. The density of spirochetes was determined using a Kova counting chamber (Hycor Biomedical, Garden Grove, Calif.) and dark-field microscopy.
TABLE 1.
B. burgdorferi isolates analyzed for the expression of CRASPsa
| Genospecies and isolate | No. of passages | Origin
|
Complement resistanceb | |
|---|---|---|---|---|
| Biological | Geographical | |||
| B. afzelii | ||||
| EB1 | >50 | Skin (EM) | Germany | Resistant |
| FEM1-D15 | 31 | Skin (EM) | Germany | Resistant |
| FEM1 (WT) | <10 (LP) | Skin (EM) | Germany | Resistant |
| >85 (HP) | ||||
| PKo | 28 | Skin (EM) | Germany | Resistant |
| FAC1 | NA | Skin (ACA) | Germany | Intermediate |
| ACA1 | NA | Skin (ACA) | Sweden | Intermediate |
| MMS | 1 | Tick | Germany | Intermediate |
| VS461 | 3 | Tick | Switzerland | Intermediate |
| B. burgdorferi | ||||
| LW2 | 42 | Skin | Germany | Resistant |
| ZS7 | 1 | Tick | Germany | Intermediate |
| PKa-1 | 19 | CSF | Germany | Intermediate |
| B31 | 13 | Tick | United States | Intermediate |
| 297 | 3 | CSF | United States | Intermediate |
| N40 | 18 | Tick | United States | Intermediate |
| Sh-2-82 | 16 | Tick | United States | Intermediate |
Abbreviations: WT, wild type; LP, low passage; HP, high passage; NA, not available; EM, erythema migrans; CSF, cerebrospinal fluid.
Nonimmune human serum (NHS).
Sera from 20 healthy human blood donors without known histories of spirochetal infections were tested for the presence of immunoglobulin G (IgG) and IgM antibodies against B. burgdorferi by a commercial whole-cell enzyme-linked immunosorbent assay (Dade Behring, Marburg, Germany) and immunoblotting with recombinant proteins (Mikrogen, Martinsried, Germany). Only sera that proved negative in all assays were combined to form the NHS pool.
Expression of recombinant proteins.
Recombinant proteins were expressed in insect cells infected with recombinant baculovirus. The cloning of various deletion constructs, expression, and purification were performed as described previously (24, 25). Spodoptera frugiperda (Sf9) cells were grown at 28°C in monolayer cultures in protein-free expression medium (BioWhittaker, Verviers, Belgium) in the presence of streptomycin (100 μg/ml), penicillin (100 U/ml), and amphotericin B (250 ng/ml) (Life Technologies, Eggenstein, Germany). Adherent Sf9 cells were infected with recombinant virus using a multiplicity of infection of 5. The culture supernatant was harvested and used for ligand blotting.
SDS-PAGE and Western blot analysis for detection of FHL-1/reconectin and factor H borrelial binding proteins.
Whole-cell extracts of the above-mentioned borrelias were generated by harvesting cells from 12 ml of culture and washed twice with phosphate-buffered saline (PBS)–5 mM MgCl2. After centrifugation, the pellets were resuspended in 0.1 ml of PBS and whole-cell lysates were obtained by sonication of the cells using a Branson B-12 Sonifier (Heinemann, Schwäbisch Gmünd, Germany). The lysates (15 μg) were separated by Tricine-sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) via 4% stacking and 10% separating gels as described previously (21). Wide-range molecular mass markers were obtained from Sigma-Aldrich (Deisenhofen, Germany). For Western blot analysis, proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) by semidry blotting at 1 mA/cm2 for 180 min. Nonspecific binding was blocked by immersing the membranes in 5% (wt/vol) dried milk in TBS (50 mM Tris-HCl [pH 7.4], 200 mM NaCl, 0.1% Tween 20) for 6 h at room temperature. Subsequently, the membranes were rinsed four times in TBS and incubated at 4°C overnight either in 5 ml of pooled NHS or in 5 ml of culture supernatant containing recombinant FHL-1/reconectin or a variety of deletion mutants. By use of NHS as a source for factor H and FHL-1/reconectin, the concentrations of the fluid-phase proteins were approximately 500 and 40 μg/ml, respectively. The concentration of the recombinant FHL-1/reconectin protein in the culture supernatant was approximately 50 μg/ml. Equal concentrations of the other deletion mutants were used for each experiment in comparison to the recombinant FHL-1 protein. After four washing steps with 50 mM Tris-HCl (pH 7.5)–150 mM NaCl–0.2% Tween 20 (TBST), membranes were incubated for 3 h either with a polyclonal rabbit antibody recognizing SCRs 1 to 4 of FHL-1/reconectin and factor H or with the monoclonal mouse antibody VIG8 for specific detection of the C terminus (SCRs 19 and 20) of factor H. Following four washes with TBST, the strips were incubated with a secondary peroxidase-conjugated anti-rabbit IgG antibody or with a secondary peroxidase-conjugated anti-mouse IgG antibody (Dako, Glostrup, Denmark) for 60 min at room temperature. Detection of bound antibodies was performed by using 3,3′,5,5′-tetramethylbenzidine as substrate.
Immunofluorescence assay for detection of bound FHL-1/reconectin and factor H on intact borrelial cells.
For indirect immunofluorescence assays with unfixed cells, borrelias (108 cells/ml) were grown to mid-log phase, harvested by centrifugation (5,000 × g; 30 min; 4°C), washed, and resuspended in 200 μl of Veronal-buffered saline. Five hundred microliters of EDTA-supplemented NHS (EDTA-NHS) or culture supernatant of Sf9 insect cells containing recombinant FHL-1/reconectin was added to 5 × 107 counted cells. After incubation for 1 h at room temperature with gentle agitation, the cell suspension was washed three times with PBS containing 1% bovine serum albumin and incubated for 60 min with 1:10-diluted polyclonal rabbit anti-factor H SCR 1 to 4 antibody or with undiluted monoclonal mouse VIG8 antibody recognizing SCRs 19 and 20 of factor H. Following three washes with PBS, the borrelias were incubated for 60 min with a fluorescein isothiocyanate-conjugated swine anti-rabbit IgG antibody or with fluorescein isothiocyanate-conjugated goat anti-mouse IgG antibody (Dako) at a dilution of 1:50 in PBS containing 1% bovine serum albumin and then washed and mounted for microscopy. Microscopy was performed with an Olympus CX40 fluorescence microscope at a magnification of ×1,000.
Monoclonal antibodies used for the identification of borrelial antigens.
Monoclonal antibodies 93-196/01 against p41 (flagellin) and 93-193/0246 against OspC were generous gifts from Helmut Peters (Dade Behring). An additional monoclonal antibody, LA3, against HSP70 was kindly provided by Michael D. Kramer. The monoclonal antibody VIG8 was kindly provided by Wolfgang M. Prodinger.
RESULTS
Previously, we showed that B. afzelii isolates are capable of acquiring FHL-1/reconectin and factor H from NHS (23). In these earlier studies, we identified two outer surface proteins of 27.5 kDa (CRASP-1) and 20 kDa (CRASP-2), which are responsible for this binding activity. Here, we extend these findings to an additional number of serum-resistant and intermediate serum-sensitive borrelial isolates of the genospecies B. afzelii and B. burgdorferi. For the sake of clarity, therefore, we add a prefix to the CRASPs according to the expressing genospecies, e.g., BaCRASP for CRASP of B. afzelii and BbCRASPs for the binding proteins of B. burgdorferi.
Identification and characterization of CRASPs expressed by B. afzelii isolates (BaCRASPs).
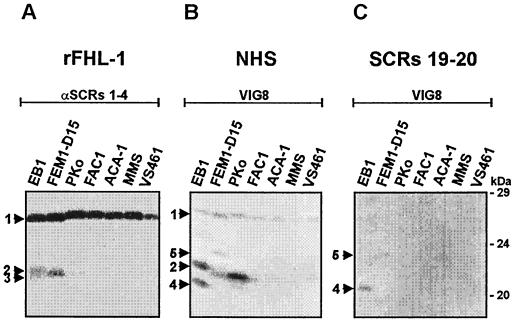
In this series of experiments, we first analyzed binding of FHL-1/reconectin to various B. afzelii isolates. Cell extracts generated from B. afzelii isolates EB1, FEM1-D15, PKo, FAC1, ACA1, MMS, and VS461 were separated by a 10% Tris-Tricine gel, transferred to nitrocellulose membranes, and incubated with recombinant FHL-1/reconectin (rFHL-1). The membranes were developed using a rabbit antiserum that detects SCRs 1 to 4 of FHL-1/reconectin (Fig. 1A). Upon incubation of the membranes with rFHL-1, a 27.5-kDa protein termed BaCRASP-1 and a second borrelial protein of 20.7 kDa (BaCRASP-2) were identified. BaCRASP-1 was present in all seven B. afzelii isolates analyzed (Fig. 1A), while expression of BaCRASP-2 was restricted. BaCRASP-1 displayed strong binding and was the most prominent ligand for FHL-1/reconectin, whereas the binding of BaCRASP-2 was weaker. Isolates EB1, FEM1-D15, and VS461 also possessed an additional protein of 20.4 kDa, termed BaCRASP-3, which displayed very weak binding (Fig. 1A).
FIG. 1.
Identification of BaCRASPs expressed within B. afzelii isolates. Protein extracts (15 μg) obtained from seven B. afzelii isolates (EB1, FEM1-D15, PKo, FAC1, ACA1, MMS, and VS461) were separated by 10% Tris-Tricine SDS-PAGE and transferred to nitrocellulose. The membranes were incubated with either rFHL-1 (A), NHS (B), or factor H deletion mutant SCRs 19-20 (C). Binding of the proteins was detected with the indicated antisera, i.e., polyclonal serum specific for SCRs 1 to 4 of FHL-1/reconectin (A) and monoclonal antibody VIG8 specific for SCR 20 of factor H (B and C). The arrowheads and the numbers point to the corresponding borrelial proteins: BaCRASP-1 (27.5 kDa) (1), BaCRASP-2 (20.7 kDa) (2), BaCRASP-3 (20.4 kDa) (3), BaCRASP-4 (19.2 kDa) (4), and BaCRASP-5 (22.6 kDa) (5). The mobilities of the marker proteins (in kilodaltons) are indicated on the right.
In the second series of experiments, the same preparations of B. afzelii isolates were tested for binding of factor H. As a source of factor H, either NHS or the deletion mutant SCRs 19-20 of this complement regulator were employed. Binding of factor H was detected by monoclonal antibody VIG8, which is specific for SCR 20. Figure 1B summarizes the results obtained with NHS. Factor H binding to BaCRASP-1 was detectable at low intensities with all isolates studied. Binding of factor H to BaCRASP-2 was strong in isolates EB1 and PKo but weak in isolate FEM1-D15 and not detectable in isolates FAC1, ACA1, MMS, and VS461. This approach identified two additional factor H binding proteins of low molecular mass termed BaCRASP-4 (19.2 kDa) and BaCRASP-5 (22.5 kDa), which were detectable only in isolates EB1 and FEM1-D15. Binding studies with the C-terminal fragment of factor H SCRs 19 and 20 (Fig. 1C) showed that both BaCRASP-4 and BaCRASP-5 bind the C-terminal region of factor H. Although intact factor H bound BaCRASP-1 and BaCRASP-2, the C-terminal deletion mutant SCRs 19-20 did not do so. This reveals that BaCRASP-1 and BaCRASP-2 bind different domains of factor H. The dissimilar binding characteristics of the individual CRASPs and the location of their binding domains within both human host regulators are summarized in Table 2.
TABLE 2.
Binding characteristics of CRASPs of various B. afzelii isolatesa
| Isolate | BaCRASP-1 (27.5 kDa)
|
BaCRASP-2 (20.7 kDa)
|
BaCRASP-3 (20.4 kDa)
|
BaCRASP-4 (19.2 kDa)
|
BaCRASP-5 (22.6 kDa)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FHL-1 | Factor H
|
FHL-1 | Factor H
|
FHL-1 | Factor H
|
FHL-1 | Factor H
|
FHL-1 | Factor H
|
||||||
| FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | ||||||
| EB1 | ++ | ± | − | + | ++ | − | + | − | − | − | ++ | + | − | − | − |
| FEM1-D15 | ++ | ± | − | + | + | − | − | − | − | − | − | − | − | + | ± |
| PKo | ++ | ± | − | ± | ++ | − | − | − | − | − | − | − | − | − | − |
| FAC1 | ++ | ± | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ACA1 | ++ | ± | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MMS | ++ | ± | − | − | − | − | − | − | − | − | − | − | − | − | − |
| VS461 | ++ | ± | − | − | − | − | − | − | − | − | − | − | − | − | − |
EB1 was used as a reference isolate for the estimation of the molecular masses of B. afzelii CRASPs. For calculation, the Wincam gel styler software version 2.2 was employed. FH, serum factor H. ++, strong binding intensity; +, moderate binding intensity; +/−, weak binding intensity; −, no binding.
Localization of the domains of FHL-1/reconectin and factor H interacting with BaCRASPs.
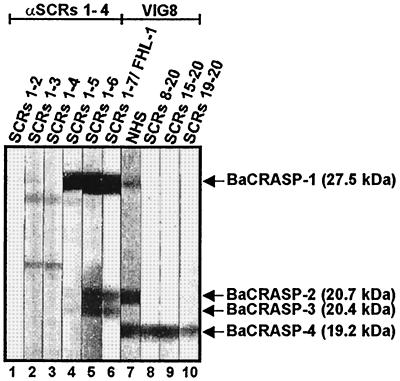
Having demonstrated the existence of additional BaCRASPs as well as the distinct binding properties of each of these proteins for FHL-1/reconectin, factor H, and a truncated fragment of factor H (Fig. 1), we wanted to differentiate the individual borrelial proteins more precisely and localize the binding domains of FHL-1/reconectin and factor H that interact with the corresponding BaCRASPs. To this end, we used deletion mutants of FHL-1/reconectin and factor H for ligand blotting with B. afzelii isolate EB1. These deletion mutants represent truncated proteins of both complement regulators, which are exclusively composed of repetitive folding protein domains termed SCRs (24–26). BaCRASP-1 bound rFHL-1 (i.e., SCRs 1-7) and deletion mutants SCRs 1-6 and SCRs 1-5 with high intensity, whereas binding of deletion mutants SCRs 1-4, SCRs 1-3, and SCRs 1-2 was much weaker (Fig. 2). BaCRASP-2 and -3 bound intact rFHL-1 protein and the mutant SCRs 1-6 but did not bind SCRs 1-5 or further deletion mutants. Accordingly, the region of FHL-1/reconectin that interacts with BaCRASP-1 to BaCRASP-3 is localized within SCRs 5 to 7. Factor H binding was observed to BaCRASP-1, BaCRASP-2, and BaCRASP-4. For BaCRASP-1 and BaCRASP-2, the binding regions of FHL-1/reconectin as well as factor H were localized within the C-terminal domain SCRs 5 to 7 of FHL-1/reconectin. In contrast, BaCRASP-4 clearly bound the C-terminal region of factor H insofar as deletion mutants SCRs 8-20, SCRs 15-20, and SCRs 19-20 bound to this protein. Taken together, these experiments reveal the existence of a group of borrelial proteins in B. afzelii that (i) display different binding characteristics and affinities to the human complement regulators FHL-1/reconectin and factor H and (ii) interact with different domains of these proteins.
FIG. 2.
Localization of the binding domains within FHL-1/reconectin and factor H for CRASPs of B. afzelii isolate EB1. Protein extract (15 μg) obtained from B. afzelii isolate EB1 was separated by 10% Tris-Tricine SDS-PAGE and transferred to nitrocellulose. The membranes were incubated with the indicated proteins, i.e., FHL-1/reconectin deletion mutants SCRs 1-2, SCRs 1-3, SCRs 1-4, SCRs 1-5, SCRs 1-6, and SCRs 1-7/rFHL-1; NHS; and factor H deletion mutants SCRs 8-20, SCRs 15-20, and SCRs 19-20. Bound proteins were visualized by staining with antisera specific for FHL-1/reconectin (anti-SCRs 1 to 4) or factor H (VIG8). The sizes of the indicated binding molecules are derived from the mobilities of marker proteins.
Identification and characterization of CRASPs expressed by B. burgdorferi isolates (BbCRASPs).
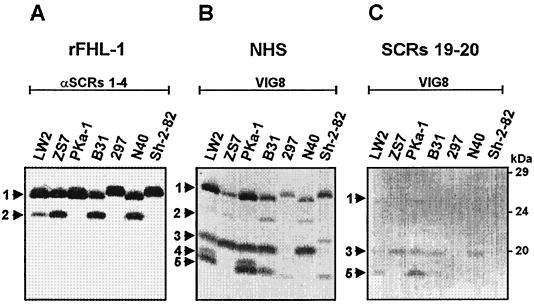
After establishing the existence of several distinct CRASPs on B. afzelii isolates, we wanted to characterize similar binding proteins in B. burgdorferi isolates. We investigated binding of FHL-1/reconectin to various serum-resistant and intermediate serum-sensitive B. burgdorferi isolates. Following incubation with rFHL-1, a 25.9-kDa (BbCRASP-1) protein was present in all seven B. burgdorferi isolates analyzed, and a 23.2-kDa (BbCRASP-2) protein was identified in isolates PKa-1, 297, B31, and Sh-2-82 (Fig. 3A). Differences in the mobilities of BbCRASP-1 were detected between isolates 297 and Sh-2-82 and isolates LW2, ZS7, PKa-1, B31, and N40. A similarly strong binding to rFHL-1 could be observed for both BbCRASP-1 and BbCRASP-2.
FIG. 3.
Identification of BbCRASPs expressed by B. burgdorferi isolates. Protein extracts (15 μg) obtained from seven B. burgdorferi isolates (LW2, ZS7, PKa-1, B31, 297, N40, and Sh-2-82) were separated by 10% Tris-Tricine SDS-PAGE and transferred to nitrocellulose. The membranes were incubated with either rFHL-1 (A), NHS (B), or factor H deletion mutant SCRs 19-20 (C). Binding of the proteins was detected with the indicated antisera, i.e., polyclonal serum specific for SCRs 1 to 4 of FHL-1/reconectin (A) and monoclonal antibody VIG8 specific for SCR 20 of factor H (B and C). The arrowheads and the numbers point to the corresponding borrelial proteins: BbCRASP-1 (25.9 kDa) (1), BbCRASP-2 (23.2 kDa) (2), BbCRASP-3 (19.8 kDa) (3), BbCRASP-4 (18.5 kDa) (4), and BbCRASP-5 (17.7 kDa) (5). The mobilities of the marker proteins (in kilodaltons) are indicated on the right.
Binding of factor H to B. burgdorferi proteins was detected upon incubation of the membranes in NHS and development with the factor H-specific monoclonal antibody VIG8. As shown in Fig. 3B, the FHL-1/reconectin binding proteins BbCRASP-1 and -2 that are present in all seven isolates also bound factor H. Three additional proteins of 19.8 kDa (BbCRASP-3), 18.5 kDa (BbCRASP-4), and 17.7 kDa (BbCRASP-5) were detected, all of which exclusively bound factor H. Different expression patterns of these five proteins were present in the isolates analyzed. Isolate 297 expressed only two proteins (BbCRASP-1 and -5), and isolates LW2 and PKa-1 expressed all five proteins.
We next analyzed the binding properties of the C-terminal factor H fragment SCRs 19 and 20 to the seven B. burgdorferi isolates. The results in Fig. 3C show that only BbCRASP-3 and -5 served as ligands for deletion mutant SCRs 19-20. No differences in the binding intensities were detectable between these two proteins. The respective binding characteristics of the individual B. burgdorferi CRASPs and the location of their binding domains within FHL-1/reconectin and factor H are summarized in Table 3.
TABLE 3.
Binding characteristics of CRASPs of various B. burgdorferi isolatesa
| Isolate | BbCRASP-1 (25.9 kDa)
|
BbCRASP-2 (23.2 kDa)
|
BbCRASP-3 (19.8 kDa)
|
BbCRASP-4 (18.5 kDa)
|
BbCRASP-5 (17.7 kDa)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FHL-1 | Factor H
|
FHL-1 | Factor H
|
FHL-1 | Factor H
|
FHL-1 | Factor H
|
FHL-1 | Factor H
|
||||||
| FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | FH | SCRs 19 and 20 | ||||||
| LW2 | ++ | ++ | − | + | + | − | − | + | ± | − | + | − | − | ++ | ± |
| ZS7 | ++ | + | − | ++ | + | − | − | ++ | + | − | − | − | − | − | − |
| PKa-1 | ++ | ++ | − | − | − | − | − | ++ | + | − | + | − | − | ++ | + |
| B31 | ++ | + | − | ++ | + | − | − | ++ | + | − | − | − | − | + | ± |
| 297 | ++ | + | − | − | − | − | − | − | − | − | − | − | − | ± | − |
| N40 | ++ | + | − | ++ | + | − | − | ++ | + | − | − | − | − | − | − |
| Sh-2-82 | ++ | + | − | − | − | − | − | − | − | − | − | − | − | ± | − |
LW2 was used as a reference isolate for the estimation of the molecular masses of B. burgdorferi CRASPs. For calculation, the Wincam gel styler software version 2.2 was employed. FH, serum factor H. ++, strong binding intensity; +, moderate binding intensity; +/−, weak binding intensity; −, no binding.
Localization of the domains of FHL-1/reconectin and factor H interacting with BbCRASPs.
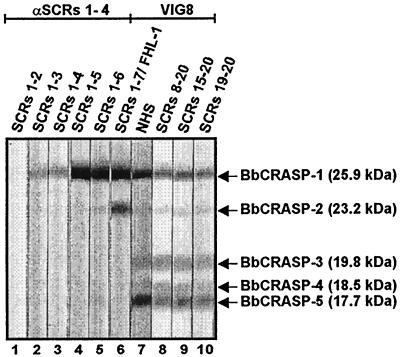
To localize further the domains of FHL-1/reconectin that interact with the CRASPs of B. burgdorferi, isolate LW2 and a set of deletion mutants of this immune regulator were used for ligand blotting. As shown in Fig. 4, BbCRASP-1 bound rFHL-1 (SCRs 1-7) as well as the deletion mutants containing SCRs 1 to 6 and SCRs 1 to 5 but did not bind mutants comprising SCRs 1 to 4, SCRs 1 to 3, or SCRs 1 and 2. BbCRASP-2 bound rFHL-1 and deletion mutant SCRs 1-6. This finding demonstrates that both proteins bind the C-terminal region of FHL-1/reconectin within SCRs 5 to 7. As anticipated, BbCRASP-3 to BbCRASP-5 did not bind intact rFHL-1 or any of the deletion mutants of this immune regulator.
FIG. 4.
Localization of the binding domains within FHL-1/reconectin and factor H for CRASPs of B. burgdorferi isolate LW2. Protein extract (15 μg) obtained from B. burgdorferi isolate LW2 was separated by 10% Tris-Tricine SDS-PAGE and transferred to nitrocellulose. The membranes were incubated with the indicated proteins, i.e., FHL-1/reconectin deletion mutants SCRs 1-2, SCRs 1-3, SCRs 1-4, SCRs 1-5, SCRs 1-6, and SCRs 1-7/rFHL-1; NHS; and factor H deletion mutants SCRs 8-20, SCRs 15-20, and SCRs 19-20. Bound proteins were visualized by staining with antisera specific for FHL-1/reconectin (anti-SCRs 1-4) or factor H (VIG8). The sizes of the indicated binding molecules are derived from the mobilities of marker proteins.
Next, we aimed to localize the binding domains within factor H to all five BbCRASPs. All identified CRASPs of B. burgdorferi isolate LW2, however, bound native factor H with different affinities. BbCRASP-3, -4, and -5 interact exclusively via the C-terminal region of factor H (SCRs 19 and 20). In contrast, BbCRASP-1 and -2 bound both the N-terminal region of factor H and the deletion mutant SCRs 19-20, thus proving that these two borrelial proteins attach to factor H at two different sites.
Influence of temperature and long-term cultivation on the expression of CRASPs.
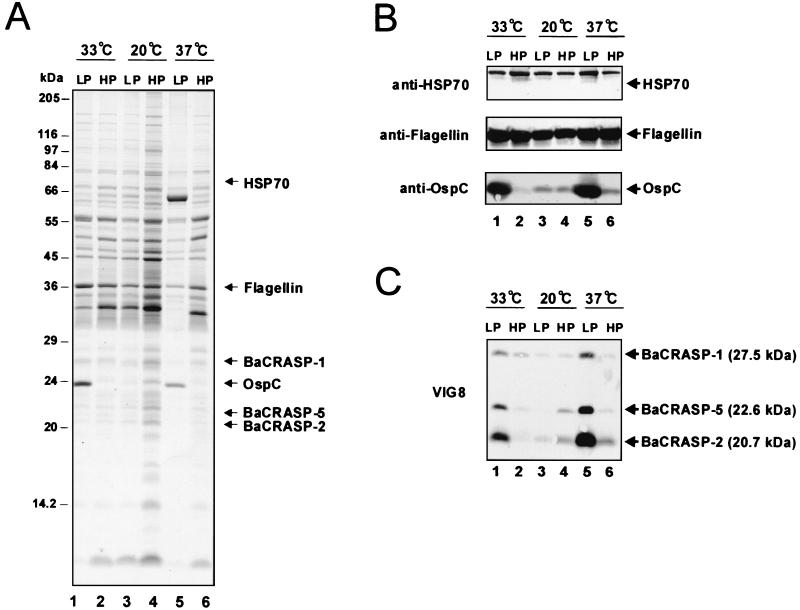
Expression of several borrelial proteins, such as OspA, -C, -E, and -F and DbpA, depends on culture time and temperature (1, 5, 36, 39). We therefore asked whether these parameters also affect expression of CRASPs of the wild-type B. afzelii isolate FEM1. For this purpose, cell extracts were generated from both low- and high-passage spirochetes grown at 20, 33, and 37°C for SDS-PAGE and immunoblot analysis (Fig. 5). HSP70 and flagellin served as controls for temperature-independent proteins, and OspC served as a control for a temperature-dependent protein (21, 36, 39). SDS-PAGE followed by Coomassie blue staining confirmed expression of HSP70 and flagellin independently of temperature and passage time. In contrast, borrelial cells expressed OspC at significantly higher levels at 33 and 37°C than at 20°C (Fig. 5A). This temperature effect, however, was observed only in low-passage cultures. In addition to Coomassie blue staining, the same set of protein samples was examined by Western blotting with specific antibodies. The expression pattern of these three control proteins observed on Western blots was consistent with the Coomassie blue-stained gel (Fig. 5B).
FIG. 5.
Effect of temperature and culture time on BaCRASP expression of B. afzelii isolate FEM1. Whole-cell extracts of low-passage (LP; less than 10 passages) and high-passage (HP; more than 85 passages) cultures from B. afzelii wild-type isolate FEM1 grown at 20, 33, and 37°C were separated by SDS-PAGE and subsequently transferred to nitrocellulose. Lanes 1, LP FEM1 grown at 33°C; lanes 2, HP FEM1 grown at 33°C; lanes 3, LP FEM1 grown at 20°C; lanes 4, HP FEM1 grown at 20°C; lanes 5, LP FEM1 grown at 37°C; and lanes 6, HP FEM1 grown at 37°C. (A) Coomassie blue staining of a 10% Tris-Tricine SDS-polyacrylamide gel. (B) Western blot analysis for HSP70, flagellin, and OspC detected with specific monoclonal antibodies. (C) Detection of factor H-binding CRASPs. After incubation of the membranes in NHS, BaCRASPs were identified by using the monoclonal antibody VIG8 specific for SCR 20 of factor H. The identified BaCRASPs and their corresponding molecular masses are indicated on the right.
We subsequently performed ligand blotting with NHS to determine whether expression of BaCRASPs was affected by temperature and by passage numbers as well (Fig. 5C). This approach revealed that BaCRASP-1, BaCRASP-2, and BaCRASP-5 were expressed in large amounts at both 33 and 37°C, as indicated by a strong binding intensity for factor H. In contrast, these three CRASPs were expressed only weakly in spirochetes grown at 20°C. Moreover, this temperature-regulated high expression of CRASPs occurred solely in low-passage cultures (Fig. 5C). In summary, expression levels of BaCRASP-1, BaCRASP-2, and BaCRASP-5 were subject to the influence of both temperature and passage number.
Distribution of rFHL-1 and factor H on the surface of intact borrelias.
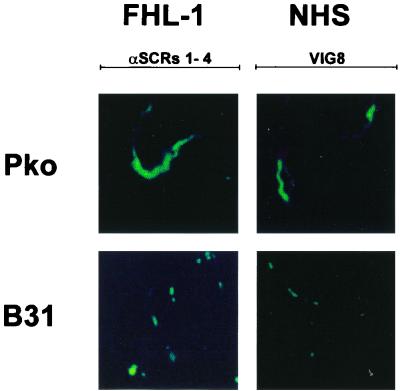
Since both serum-resistant and intermediate serum-sensitive isolates expressed several CRASPs, we asked whether serum sensitivity correlates with CRASP surface expression and distribution. Accordingly, we used an immunofluorescence assay to analyze the distributions of FHL-1/reconectin and factor H on intact, unfixed cells of serum-resistant B. afzelii isolate PKo and the intermediate serum-sensitive B. burgdorferi isolate B31. Following incubation with rFHL-1/reconectin or EDTA-NHS and staining with specific antisera, the distribution of both complement regulators was assayed. With respect to FHL-1/reconectin cells from the serum-resistant isolate, PKo displayed strong fluorescent staining, which was distributed evenly over the entire spirochete (Fig. 6). In contradistinction to this, cells from the intermediate serum-sensitive isolate B31 did not show such a uniform distribution but exhibited a punctate fluorescent pattern, which was concentrated at both ends of the microorganism. These differences suggest higher expression levels of FHL-1/reconectin binding proteins on the surface of the serum-resistant isolate PKo than on the intermediate serum-sensitive isolate B31. This staining pattern was considered specific insofar as it was not detected after incubation with buffer.
FIG. 6.
Detection of FHL-1/reconectin and factor H on the surface of intact borrelias. Serum-resistant B. afzelii isolate PKo and intermediate serum-sensitive B. burgdorferi isolate B31 were incubated with rFHL-1 (left panels) or pooled NHS (right panels). Bound proteins were detected by immunofluorescence microscopy after incubation with an antiserum specific for FHL-1/reconectin (anti-SCRs 1-4) or factor H (VIG8). The spirochetes were observed at an original magnification of ×100. The data were recorded via a charge-coupled device camera mounted on an Olympus CX40 fluorescence microscope. Panels shown are representative for at least 20 microscope fields examined in each of three separate experiments.
By analysis of factor H binding, cells from the serum-resistant isolate PKo as well as cells from the intermediate serum-sensitive isolate B31 showed weak staining patterns. Again, fluorescence was uniformly distributed on isolate PKo, whereas isolate B31 displayed a punctate fluorescent staining, thereby suggesting that factor H-binding CRASPs were localized at certain points along the bacterial surface. No staining of bacteria was detected after incubation with Veronal-buffered saline instead of rFHL-1 or EDTA-NHS.
DISCUSSION
In order to understand the pathogenesis of Lyme disease, it is important to elucidate the immune evasion mechanisms of B. burgdorferi. Here, we show for the first time that borrelias of the human pathogenic genospecies B. afzelii and B. burgdorferi express up to five different proteins termed CRASPs, which interact with the human fluid-phase complement regulators FHL-1/reconectin and factor H. Each of the CRASPs was designated according to species origin using the prefix Ba for proteins derived from B. afzelii and Bb for proteins derived from B. burgdorferi. It is evident from our data that proteins with similar or even identical binding profiles are present in both genospecies: BaCRASP-1, BbCRASP-1, and BbCRASP-2 bind FHL-1/reconectin strongly but interact weakly with factor H. In contrast to these, both of the B. afzelii proteins, BaCRASP-4 and BaCRASP-5, and the three B. burgdorferi proteins, BbCRASP-3, BbCRASP-4, and BbCRASP-5, bind factor H but not FHL-1/reconectin. Four out of five proteins bind the human immune regulators at their C-terminal ends (Fig. 2 and 4; Tables 2 and 3). This attachment orients the N-terminal complement regulatory domains of FHL-1/reconectin and factor H in such a way that they maintain their complement regulatory function (23) and, consequently, prevent formation of toxic activation products on the bacterial surface. The existence of several surface proteins, which bind the two related host complement regulators, indicates that the acquisition of FHL-1/reconectin and factor H is of paramount importance for borrelial complement resistance. In support of this conclusion, we performed additional experiments to corroborate our earlier observation (23) that serum-sensitive B. garinii isolates do not express CRASPs (data not shown).
Individual CRASPs show considerable variability in their binding properties at both the inter- and the intraspecies level. With respect to their binding characteristics, CRASPs can be divided into three groups: group I consists of four proteins (BaCRASP-1, BaCRASP-2, BbCRASP-1, and BbCRASP-2) that bind FHL-1/reconectin and factor H; group II is represented by BaCRASP-3, which binds FHL-1/reconectin exclusively; and group III includes BaCRASP-4, BaCRASP-5, BbCRASP-3, BbCRASP-4, and BbCRASP-5, all of which interact specifically with factor H (Fig. 1 and 3; Tables 2 and 3). Ligand blot experiments reveal rather similar binding intensities on the part of group I proteins for FHL-1/reconectin, whereas group III proteins bind factor H with different affinities (Fig. 1 and 3). These differences may be explained by different expression levels of group III proteins on the bacterial surface. Finally, the strong binding, e.g., of FHL-1/reconectin to BaCRASP-1 and BbCRASP-2, compared with the weak binding of factor H to the same proteins clearly points to different affinities for single host complement regulators.
We previously reported that BaCRASP-1 binds to the C-terminal SCRs 5 to 7 of FHL-1/reconectin (23). The availability of additional deletion mutants of both FHL-1/reconectin and factor H now allows more precise analysis of the binding regions that attach to the individual CRASPs. We performed these studies with B. afzelii EB1 and B. burgdorferi LW2 as representative borrelial isolates. With this approach, we localized the binding domains of each CRASP. BaCRASP-1 and BbCRASP-1 bind FHL-1/reconectin to SCRs 5 to 7, whereas BaCRASP-2, BaCRASP-3, and BbCRASP-2 interact with the C-terminal SCRs 6 and 7. Obviously, the majority of CRASPs bind this immune regulator at the C-terminal end. With respect to factor H, borrelial proteins BaCRASP-4, BbCRASP-3, BbCRASP-4, and BbCRASP-5 interact also with the C-terminal end, i.e., SCRs 19 and 20 (summarized in Table 4).
TABLE 4.
Binding characteristics and localization of the binding domains of CRASPs from representative B. afzelii and B. burgdorferi isolatesa
| Isolate and CRASP | FHL-1/reconectin
|
Factor H
|
||
|---|---|---|---|---|
| Binding intensity | Binding domain | Binding intensity | Binding domain | |
| B. afzelii EB1 | ||||
| BaCRASP-1 | ++ | SCRs 5 to 7 | + | SCRs 1 to 7 |
| BaCRASP-2 | + | SCRs 6 and 7 | + | SCRs 1 to 7 |
| BaCRASP-3 | + | SCRs 6 and 7 | − | |
| BaCRASP-4 | − | ++ | SCRs 19 and 20 | |
| B. burgdorferi LW2 | ||||
| BbCRASP-1 | ++ | SCRs 5 to 7 | ++ | SCRs 19 and 20 |
| BbCRASP-2 | ++ | SCRs 6 and 7 | + | SCRs 19 and 20 |
| BbCRASP-3 | − | + | SCRs 19 and 20 | |
| BbCRASP-4 | − | + | SCRs 19 and 20 | |
| BbCRASP-5 | − | ++ | SCRs 19 and 20 | |
++, strong binding intensity; +, weak binding intensity; −, no binding.
The binding observed at the C-terminal end is typical for the attachment of the human immune regulators FHL-1/reconectin and factor H to microorganisms. Borrelial CRASPs bind SCRs 5 to 7 (present study), and streptococcal M protein binds SCR 7 of FHL-1/reconectin. Similarly, binding of factor H to CRASPs (our data), to OspE of B. burgdorferi (13), and to the M protein of S. pyogenes (19, 31) requires the C-terminal region SCRs 19 and 20; binding of factor H to the lipooligosaccharide of N. gonorrhoeae (33) involved the C-terminal region SCRs 16 to 20. The complement regulatory domains of both FHL-1/reconectin and factor H reside in the N-terminal region, i.e., SCRs 1 to 4 (12, 24, 26). Thus, upon binding via the C-terminal regions, both human regulators maintain the ability to control alternative pathway activation. It needs to be demonstrated that acquisition of these two host regulators favors long-term survival in immunocompetent hosts.
In order to survive within the human host, B. burgdorferi has to adapt to a different environment. The different expression of proteins owing to temperature changes has been described already as an adaptive mechanism and is reported for a number of outer surface lipoproteins, e.g., OspA, -C, -E, and -F and members of the OspE- and -F-related proteins designated Erps (36, 37, 39). Some of these proteins are selectively expressed during mammalian infection but not in ticks (39, 42). To examine the differential expression of borrelial proteins, the incubation of spirochetes at various temperatures is a convenient method for mimicking environmental stimuli in ticks (lower temperature) and in the mammalian host (higher temperature) in vitro. Using B. afzelii FEM1, we observed an up-regulation of the factor H binding proteins BaCRASP-1, BaCRASP-2, and BaCRASP-5 in low-passage spirochetes grown at 33 and 37°C (Fig. 5C). The increased synthesis of these three CRASPs was not sustained in high-passage borrelial cultures at 33 and 37°C. Studies of OspC expression that were included in our studies produced the same results and confirmed earlier observations (15, 37, 39). Our findings suggest that up-regulation of CRASPs may be particularly relevant in maintaining bacterial integrity during infection and adaptation to the human host. Preliminary studies, including those of several isolates of the genospecies B. afzelii and B. burgdorferi, suggest that up-regulation of CRASPs is a property of B. afzelii isolates (data not shown). Serum-sensitive isolates of the genospecies B. garinii totally lack CRASP expression under the conditions of these experiments (data not shown). Apparently, the latter genospecies does not require this strategy for survival in the infected human host.
The fact that recombinant OspE binds factor H (13) raises the question whether additional OspE-related proteins, such as the Erps (OspE- and -F-related proteins) (39, 40, 43) and Elps (OspE- and -F-like leader peptides) (2) or other OspE homologs like p21 (8), also interact with complement regulators. It is tempting to speculate that these OspE homologs and CRASPs are identical molecules. To date, the biological function of Erps, Elps, and other OspE homologs is unknown, and their role in the pathogenesis of Lyme disease is poorly understood. It is noteworthy that these proteins are expressed at the initial stages of mammalian infection, as evidenced by the appearance of antibodies within the first 2 to 4 weeks of infection (39, 40, 43, 45) and by reverse transcriptase PCR analyses (8). Since these proteins are expressed on the borrelial surface directly after transmission in the human host, the binding of fluid-phase complement regulatory proteins, such as factor H and FHL-1/reconectin, seems advantageous for evading complement-mediated killing and opsonophagocytosis.
According to the sizes and the mobilities of the proteins identified in this work, it can be hypothesized that BaCRASP-4 and OspE are identical proteins, inasmuch as both proteins bind exclusively factor H but not FHL-1/reconectin (Fig. 4) (13) and have similar molecular masses of 19.2 kDa. Furthermore, according to our ligand blot analysis (Fig. 1 to 4), the molecular masses of the identified CRASPs, i.e., BaCRASP-1 (27.5 kDa), BaCRASP-2 (20.7 kDa), BaCRASP-3 (20.4 kDa), BaCRASP-4 (19.2 kDa), BaCRASP-5 (22.6 kDa), BbCRASP-1 (25.9 kDa), BbCRASP-2 (23.2 kDa), BbCRASP-3 (19.8 kDa), BbCRASP-4 (18.5 kDa), and BbCRASP-5 (17.7 kDa), are similar to those of Erps, such as ErpA (19.4 kDa), ErpI (19.8 kDa), ErpC (20.2 kDa), ErpL (26.1 kDa), and ErpK (28.9 kDa) (40). Thus, these proteins may be identical or may belong to the same protein families. A clear identification of the individual CRASPs and a correlation with characterized borrelial proteins, such as OspE, await the sequence data and cloning of each individual CRASP.
As a further point of interest, we analyzed the distribution of CRASPs on the surface of borrelias differing in their complement resistance. From published work, it is known that B. afzelii isolates are mainly serum resistant, whereas the majority of B. burgdorferi isolates are intermediate serum sensitive, and isolates of the genospecies B. garinii are mostly serum sensitive (3, 4, 20, 27, 44). The strong and uniform staining pattern for FHL-1/reconectin and factor H on serum-resistant isolate EB1 clearly differs from the spotted staining pattern on the intermediate serum-sensitive isolate B31 (Fig. 6). This in turn suggests that the distribution of CRASPs on the entire surface of isolate EB1 enhances the concentration of bound host complement regulators and thereby efficiently inhibits the formation of the complement convertase. In contrast, no staining could be observed on the surface of serum-sensitive B. garinii isolates (data not shown). As such, the distribution and concentration of complement regulators on the surface of borrelias seem to correlate with the complement susceptibility pattern of the corresponding isolate.
In summary, we identified and characterized five distinct borrelial CRASPs, which are expressed by B. afzelii and B. burgdorferi isolates and which interact with the two central human complement regulators of the alternative pathway FHL-1/reconectin as well as factor H of complement activation. Based on their function, CRASPs may represent novel virulence factors involved in immune evasion strategies by B. burgdorferi and may serve as vaccine candidates for prevention of Lyme disease. Current investigations are aimed at identifying CRASPs and their encoding genes to generate recombinant expressed proteins.
ACKNOWLEDGMENTS
We thank Christa Hanssen-Hübner and Angelika Sames for skillful and expert technical assistance and Michael Stappenbeck for the photography.
This work was funded by the Thüringer Ministerium für Wissenschaft, Forschung und Kultur and the Deutsche Forschungsgemeinschaft DFG, Project Zi 342/5 and Br 446/11-1.
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Caimano M J, Yang X, Cerna F, Norgard M V, Radolf J D. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brade V, Kleber I, Acker G. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology. 1992;185:453–465. doi: 10.1016/S0171-2985(11)80087-2. [DOI] [PubMed] [Google Scholar]
- 4.Breitner-Ruddock S, Würzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
- 5.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.China B, Sory M P, N′guyen B T, De Bruyere M, Cornelis G R. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect Immun. 1993;61:3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper N R. Complement evasion strategies of microorganisms. Immunol Today. 1991;12:327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Barthold S W, Stocker Giles S, Montgomery R R, Telford S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey P W, Allison M E, Akkaraju S, Goodnow C C, Fearon D T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 10.Diaz A, Ferreira A, Sim R B. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J Immunol. 1997;158:3779–3786. [PubMed] [Google Scholar]
- 11.Friese M A, Hellwage J, Jokiranta T S, Meri S, Peter H H, Eibel H, Zipfel P F. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol. 1999;36:809–818. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 12.Gordon D L, Kaufman R M, Blackmore T K, Kwong J, Lublin D M. Identification of complement regulatory domains in human factor H. J Immunol. 1995;155:348–356. [PubMed] [Google Scholar]
- 13.Hellwage J, Meri T, Heikkila A, Panelius J, Lahdenne P, Seppälä I, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- 14.Horstmann R D. Target recognition failure by the nonspecific defense system: surface constituents of pathogens interfere with the alternative pathway of complement activation. Infect Immun. 1992;60:721–727. doi: 10.1128/iai.60.3.721-727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu C M, Simon M, Kramer M D, Gern L. Tick factors and in vitro cultivation influence the protein profile, antigenicity and pathogenicity of a cloned Borrelia garinii isolate from Ixodes ricinus hemolymph. Infection. 1996;24:251–254. doi: 10.1007/BF01781105. [DOI] [PubMed] [Google Scholar]
- 16.Janulcyk R, Iannelli F, Sjöholm A G, Pozzi G, Björck L. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J Biol Chem. 2000;47:37257–37263. doi: 10.1074/jbc.M004572200. [DOI] [PubMed] [Google Scholar]
- 17.Johnsson E, Berggard K, Kotarsky H, Hellwage J, Zipfel P F, Sjöbring U, Lindahl G. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J Immunol. 1998;161:4894–4901. [PubMed] [Google Scholar]
- 18.Joiner K A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 19.Kotarsky H, Hellwage J, Johnsson E, Skerka C, Svensson H G, Lindahl G, Sjobring U, Zipfel P F. Identification of a domain in human factor H and factor H-like protein-1 required for the interaction with streptococcal M proteins. J Immunol. 1998;160:3349–3354. [PubMed] [Google Scholar]
- 20.Kraiczy P, Hunfeld K-P, Breitner-Ruddock S, Würzner R, Acker G, Brade V. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology. 2000;201:406–419. doi: 10.1016/S0171-2985(00)80094-7. [DOI] [PubMed] [Google Scholar]
- 21.Kraiczy P, Hunfeld K-P, Peters S, Würzner R, Acker G, Wilske B, Brade V. Borreliacidal activity of early Lyme disease sera against complement-resistant Borrelia afzelii FEM1 wild-type and an OspC-laking FEM1 variant. J Med Microbiol. 2000;49:917–928. doi: 10.1099/0022-1317-49-10-917. [DOI] [PubMed] [Google Scholar]
- 22.Kraiczy P, Skerka C, Kirschfink M, Zipfel P F, Brade V. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int Immunopharmacol. 2001;1:393–401. doi: 10.1016/s1567-5769(00)00041-2. [DOI] [PubMed] [Google Scholar]
- 23.Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel P F. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur J Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Kühn S, Skerka C, Zipfel P F. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H. J Immunol. 1995;155:5663–5670. [PubMed] [Google Scholar]
- 25.Kühn S, Zipfel P F. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene. 1995;162:225–229. doi: 10.1016/0378-1119(95)00360-i. [DOI] [PubMed] [Google Scholar]
- 26.Kühn S, Zipfel P F. Mapping of the domains required for decay acceleration activity of the human factor H-like protein 1 and factor H. Eur J Immunol. 1996;26:2383–2387. doi: 10.1002/eji.1830261017. [DOI] [PubMed] [Google Scholar]
- 27.Kurtenbach K, Sewell H-S, Ogden N H, Randolph S E, Nuttall P A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66:1248–1251. doi: 10.1128/iai.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindahl G, Sjöbring U, Johnsson E. Human complement regulators: a major target for pathogenic microorganisms. Curr Opin Immunol. 2000;12:44–51. doi: 10.1016/s0952-7915(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 30.Neeleman C, Geelen S P, Aerts P C, Daha M R, Mollnes T E, Roord J J, Posthuma G, van Dijk H, Fleer A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999;67:4517–4524. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-Caballero D, Alberti S, Vivanco F, Sánchez-Corral P, Rodríguez de Córdoba S. Assessment of the interaction of human complement regulatory proteins with group A Streptococcus: identification of a high affinity group A Streptococcus binding site in FH-1. Eur J Immunol. 2000;30:1243–1253. doi: 10.1002/(SICI)1521-4141(200004)30:4<1243::AID-IMMU1243>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks: culture conditions and antibiotic susceptibility. Zentbl Bakteriol Mikrobiol Hyg A. 1986;263:112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 33.Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ram S, McQuillen D P, Gulati S, Elkins C, Pangburn M K, Rice P A. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ram S, Mackinnon F G, Gulati S, McQuillen D P, Vogel U, Frosch M, Elkins C, Guttormsen H-K, Wetzler L M, Oppermann M, Pangburn M K, Rice P A. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol Immunol. 1999;36:915–928. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 36.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan T G, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–388. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson B, Bono J L, Schwan T G, Rosa P A. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoiber H, Ebenbichler C, Schneider R, Janatova J, Dierich M P. Interaction of several complement proteins with gp120 and gp41, the two envelope glycoproteins of HIV-1. AIDS. 1995;9:19–26. doi: 10.1097/00002030-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung S Y, McDowell J V, Carlyon J A, Marconi R T. Mutation and recombination in the upstream homology box-flanked ospE-related genes of the Lyme disease spirochetes result in the development of new antigenic variants during infection. Infect Immun. 2000;68:1319–1327. doi: 10.1128/iai.68.3.1319-1327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Dam A P, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Würzner R. Evasion of pathogens by avoiding recognition or eradication by complement, in part via molecular mimicry. Mol Immunol. 1999;36:249–260. doi: 10.1016/s0161-5890(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 47.Zipfel P F, Skerka C. FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol Today. 1999;20:135–140. doi: 10.1016/s0167-5699(98)01432-7. [DOI] [PubMed] [Google Scholar]
- 48.Zipfel P F, Jokiranta S T, Hellwage J, Koistinen V, Meri S. The factor H protein family. Immunopharmacology. 1999;42:53–60. doi: 10.1016/s0162-3109(99)00015-6. [DOI] [PubMed] [Google Scholar]