Controlling hyperglycemia is foundational to diabetes management and is necessary to reduce the risks of long-term diabetes complications and death (1). However, people with diabetes also need to consider more immediate harms posed by dysglycemia. Contemporary data on emergency department (ED) visits and hospitalizations for hypoglycemia and hyperglycemia in the general U.S. population of adults with type 1 and type 2 diabetes, particularly in the context of the coronavirus disease 2019 (COVID-19) pandemic, are scarce.
We used claims data of privately insured and Medicare Advantage beneficiaries across the U.S. included in the OptumLabs Data Warehouse between 1 January 2011 and 31 December 2020 to characterize annual trends in hypoglycemia- and hyperglycemia-related ED visits/hospitalizations (ascertained as previously described [2,3] and reported as the number of events per 1,000 person-years [1,000PY]) adjusted for patient age, sex, race, ethnicity, and U.S. region, with specific attention paid to 2020 as the first year of the COVID-19 pandemic. All study data are deidentified, and the study was exempt from Mayo Clinic Institutional Review Board review.
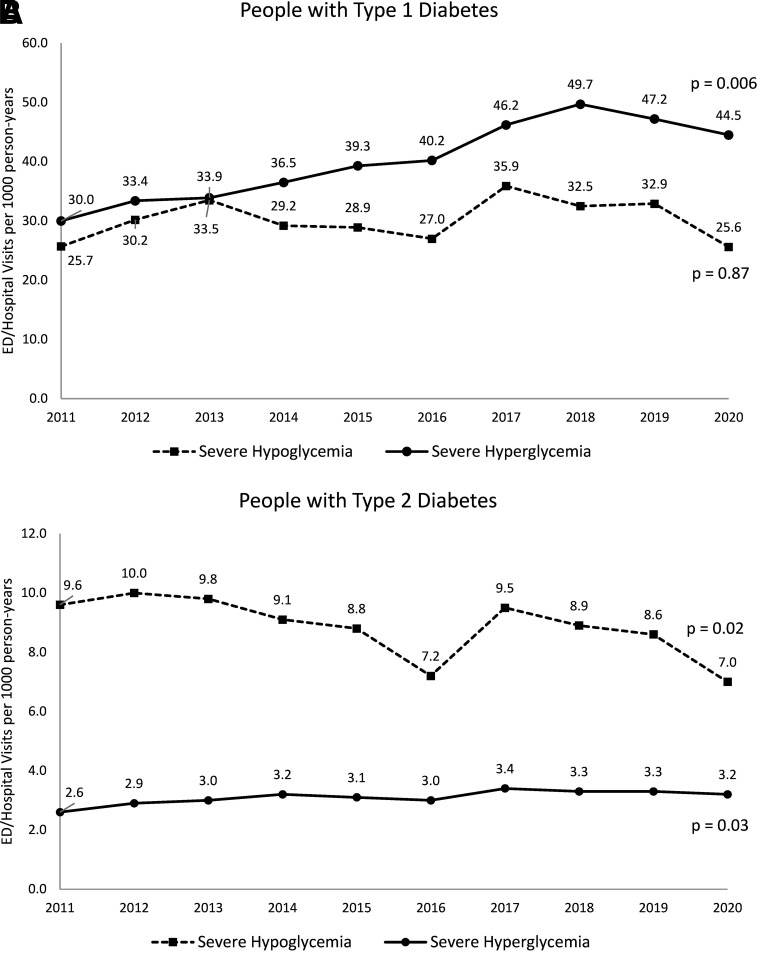
The study population included 67,901 adults with type 1 diabetes (mean age 43.2 [SD 16.1] years, 52.8% male, 71.8% non-Hispanic White) and 2,483,951 adults with type 2 diabetes (mean age 62.6 [SD 12.5] years, 50.5% male, 56.3% non-Hispanic White) (Table 1). Between 2011 and 2019, adjusted rates of severe hypoglycemia among people with type 1 diabetes increased from 25.7 to 32.9/1,000PY and then decreased to 25.6/1,000PY in 2020 (P = 0.87 for overall trend) (Fig. 1A). Concurrently, their adjusted rates of severe hyperglycemia increased from 30.0 to 47.2/1,000PY and then decreased to 44.5/1,000PY (P = 0.006 for overall trend).
Table 1.
Study population
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| Number of patients | 67,901 | 2,483,951 |
| Age, years, mean (SD) | 43.2 (16.1) | 62.6 (12.5) |
| Age category, years | ||
| 18–44 | 37,221 (54.8) | 225,315 (9.1) |
| 45–64 | 22,558 (33.2) | 1,011,475 (40.7) |
| 65–74 | 6,021 (8.9) | 800,946 (32.2) |
| ≥75 | 2,101 (3.1) | 446,215 (18.0) |
| Sex | ||
| Female | 32,017 (47.2) | 1,229,944 (49.5) |
| Male | 35,884 (52.8) | 1,254,007 (50.5) |
| Race and ethnicity | ||
| White | 48,770 (71.8) | 1,398,818 (56.3) |
| Black | 6,724 (9.9) | 425,951 (17.1) |
| Hispanic | 4,501 (6.6) | 317,685 (12.8) |
| Asian | 1,374 (2.0) | 98,619 (4.0) |
| Other/unknown | 6,532 (9.6) | 242,878 (9.8) |
| Region | ||
| Midwest | 19,792 (29.1) | 592,215 (23.8) |
| Northeast | 7,683 (11.3) | 342,322 (13.8) |
| South | 28,872 (42.5) | 1,283,051 (51.7) |
| West | 11,497 (16.9) | 264,142 (10.6) |
| Unknown | 57 (0.1) | 2,221 (0.1) |
| Comorbidities | ||
| Retinopathy | 18,109 (26.7) | 331,917 (13.4) |
| Neuropathy | 14,821 (21.8) | 574,782 (23.1) |
| Peripheral vascular disease | 5,511 (8.1) | 368,854 (14.8) |
| Dementia | 693 (1.0) | 88,575 (3.6) |
| Cardiovascular disease | 8,322 (12.3) | 765,674 (30.8) |
| Heart failure | 2,210 (3.3) | 267,238 (10.8) |
| Cerebrovascular disease | 2,908 (4.3) | 288,150 (11.6) |
| COPD | 3,533 (5.2) | 355,740 (14.3) |
| Cancer | 2,400 (3.5) | 212,300 (8.5) |
| Cirrhosis | 288 (0.4) | 26,692 (1.1) |
| Hypertension | 28,261 (41.6) | 2,056,367 (82.8) |
| Depression | 8,492 (12.5) | 330,208 (13.3) |
| Chronic kidney disease | 3,316 (4.9) | 247,233 (10.0) |
| Severe hyperglycemia | 3,812 (5.6) | 13,056 (0.5) |
| Severe hypoglycemia | 2,176 (3.2) | 22,020 (0.9) |
| Glucose-lowering medications filled within 120 days of index date | ||
| Any insulin | 62,264 (91.7) | 442,410 (17.8) |
| Basal insulin | 30,864 (45.5) | 380,343 (15.3) |
| Bolus insulin | 58,330 (85.9) | 206,093 (8.3) |
| Sulfonylurea | 0 (0.0) | 558,202 (22.5) |
| Metformin | 3,440 (5.1) | 1,201,566 (48.4) |
| DPP-4 inhibitor | 354 (0.5) | 241,402 (9.7) |
| SGLT2 inhibitor | 541 (0.8) | 83,794 (3.4) |
| GLP-1 receptor agonist | 847 (1.2) | 120,449 (4.8) |
| Glitazone | 379 (0.6) | 141,740 (5.7) |
| Other medications | 523 (0.8) | 19,568 (0.8) |
| No medication fills | 5,253 (7.7) | 771,517 (31.1) |
Data are presented as N (%), except when noted otherwise. All comorbidities were ascertained from medical claims during 1 year prior to cohort entry, while medications were ascertained from pharmacy claims during 120 days prior to cohort entry. COPD, chronic obstructive pulmonary disease; DPP-4, dipeptidyl-peptidase 4; GLP-1, glucagon-like peptide 1; SGLT2, sodium–glucose cotransporter 2.
Figure 1.
Trends in the rates of severe hypoglycemic and hyperglycemic events among people with type 1 diabetes (A) and type 2 diabetes (B) from 2011 to 2020. Rates of severe hypoglycemia and hyperglycemia were calculated as marginal probabilities using logistic regression models (individual models for type 1 and type 2 diabetes for each outcome of severe hypoglycemia and hyperglycemia), adjusted for patient age, sex, race, ethnicity, and U.S. region. P values assess trends in event rates over time, with the null hypothesis of no change over time. Coding methodology for severe hypoglycemia and hyperglycemia changed between 2015 and 2016 due to transition from ICD-9 to ICD-10 codes, affecting hypoglycemia ascertainment more than severe hyperglycemia due to greater availability of hypoglycemia ICD-10 codes than ICD-9 codes.
Adjusted rates of severe hypoglycemia among people with type 2 diabetes decreased from 9.6 to 8.6/1,000PY and then declined further to 7.0/1,000PY (P = 0.02 for overall trend) (Fig. 1B). In contrast, the adjusted rate of severe hyperglycemia increased from 2.6 to 3.3/1,000PY and then stayed stable in 2020 at 3.2/1,000PY (P = 0.03 for overall trend).
Our study has important limitations, including lack of data on causes of death, focus on patients with established diabetes (excluding those newly diagnosed and potentially presenting with severe hyperglycemia), and inclusion of individuals with private insurance (not public health plans; rates of severe hypoglycemia and hyperglycemia may be higher among individuals on public health plans). This epidemiologic study also cannot identify precisely why rates of severe dysglycemia changed over time.
Nevertheless, these are the most recent estimates of the trends in severe hypoglycemia and hyperglycemia in the U.S., building on publicly available data through 2016 (4). We found that as rates of severe hypoglycemia among people with type 2 diabetes decreased, rates of severe hyperglycemia increased, signaling potentially inappropriate undertreatment of this population and disproportionate focus on preventing hypoglycemia rather than more holistically pursuing optimal time in range. Concerningly, rates of severe hypoglycemia and hyperglycemia among people with type 1 diabetes were both high and increasing, although rates of severe hyperglycemia among them appear to have peaked in 2018. Omission of insulin is among the most common and preventable reasons for severe hyperglycemia, and increased event rates may reflect financial toxicity and rationing of therapy stemming from high insulin prices in the U.S., although further research will be needed to elucidate the multitude of factors likely driving severe dysglycemia in type 1 diabetes.
Despite concerns about deferred care during the COVID-19 pandemic (5), we did not find increased rates of ED visits/hospitalizations for severe hypoglycemia or hyperglycemia in 2020. However, our study population was limited to patients who had and did not lose health insurance coverage during this period. These individuals are less vulnerable to severe dysglycemic events than individuals without consistent health insurance access. Additionally, events managed outside the hospital, which may have increased due to patients’ avoidance of hospitals during the pandemic, are missed in the data, resulting in an underestimate of event rates.
Thus, our findings underscore the importance of preventing severe hypoglycemia and hyperglycemia, closely monitoring patients who experience them, and intervening to prevent recurrence. Further research is needed to probe for the reasons for the observed deterioration of glycemic management among people with type 1 diabetes, with higher rates of both severe hypoglycemia and hyperglycemia, and why people with type 2 diabetes who saw improvements in the rates of severe hypoglycemia did not see similar declines in severe hyperglycemia.
Article Information
Funding. This effort was funded by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant number K23DK114497 (R.G.M.).
Duality of Interest. In the past 36 months, R.G.M. received support from the NIDDK, the Patient-Centered Outcomes Research Institute, and AARP for research unrelated to this work. She also serves as a consultant to Emmi (Wolters Kluwer) on the development of patient education materials related to prediabetes and diabetes. R.J.G. received research support to Emory University for investigator-initiated studies outside of this work from Novo Nordisk, Dexcom, and Eli Lilly and consulting fees from Sanofi, Eli Lilly, Pfizer, Boehringer, and Weight Watchers. He is also funded by the NIDDK. G.E.U. is partly supported by research grants from the Clinical and Translational Science Award program and the NIDDK and has received research support (to Emory University) unrelated to this work from AstraZeneca, Bayer, and Dexcom. P.J.O. has received research support from the NIDDK, National Heart, Lung, and Blood Institute, National Cancer Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute on Drug Abuse, and Patient-Centered Outcomes Research Institute and has served as an unpaid consultant to the World Health Organization. S.H.G. has received research support from the NIDDK and serves on the Health Equity Advisory Committee for Medtronic, Inc., and for Abbott.
Study contents are the sole responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions. R.G.M. designed the study, interpreted the data, and wrote the manuscript. J.H. analyzed the data and reviewed and edited the manuscript. K.S.S. managed the data, assisted with analyses, and reviewed and edited the manuscript. R.J.G., G.E.U., S.H.G., and P.J.O. contributed to interpretation of results and reviewed and edited the manuscript. R.G.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented as a poster at the American Diabetes Association 82nd Scientific Sessions in New Orleans, LA, 4–7 June 2022.
References
- 1. American Diabetes Association . 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45:S83–S96 [DOI] [PubMed] [Google Scholar]
- 2. McCoy RG, Galindo RJ, Swarna KS, et al. Sociodemographic, clinical, and treatment-related factors associated with hyperglycemic crises among adults with type 1 or type 2 diabetes in the US from 2014 to 2020. JAMA Netw Open 2021;4:e2123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Association of cumulative multimorbidity, glycemic control, and medication use with hypoglycemia-related emergency department visits and hospitalizations among adults with diabetes. JAMA Netw Open 2020;3:e1919099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . United States Diabetes Surveillance System. Accessed 21 July 2020. Available from https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html#
- 5. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med 2021;181:269–271 [DOI] [PMC free article] [PubMed] [Google Scholar]