Abstract
OBJECTIVE
To describe the relationships between the cumulative incidences of long-term complications in individuals with type 1 diabetes (T1D) and assess whether observed associations are independent of age, duration of diabetes, and glycemic levels.
METHODS
Proliferative diabetic retinopathy (PDR), clinically significant macular edema (CSME), reduced estimated glomerular filtration rate (eGFR), amputations, cardiovascular disease (CVD), and mortality were assessed in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study over ∼30 years.
RESEARCH DESIGN AND RESULTS
The cumulative incidence of complications ranged from 3% (amputations) to 37% (CSME). There were large differences in the cumulative incidence of PDR between participants with versus without prior CSME (66% vs. 15%), reduced eGFR (59% vs. 29%), and amputation (68% vs. 32%); reduced eGFR with or without prior PDR (25% vs. 9%), amputation (48% vs. 13%), and CVD (30% vs. 11%); CVD with or without prior reduced eGFR (37% vs. 14%) and amputation (50% vs. 16%); and mortality with or without prior reduced eGFR (22% vs. 9%), amputation (35% vs. 8%), and CVD (25% vs. 8%). Adjusted for age, duration of T1D, and mean updated HbA1c, the complications and associations with higher risk included PDR with CSME (hazard ratio [HR] 1.88; 95% CI 1.42, 2.50), reduced eGFR (HR 1.41; 95% CI 1.01, 1.97), and CVD (HR 1.43; 95% CI 1.06, 1.92); CSME with higher risk of PDR (HR 3.94; 95% CI 3.18 4.89), reduced eGFR (HR 1.49; 95% CI 1.10, 2.01), and CVD (HR 1.35; 95% CI 1.03, 1.78); reduced eGFR with higher risk of CVD (HR 2.09; 95% CI 1.44, 3.03), and death (HR 3.40; 95% CI 2.35, 4.92); amputation(s) with death (HR 2.97; 95% CI 1.70, 2.90); and CVD with reduced eGFR (HR 1.59; 95% CI 1.08, 2.34) and death (HR 1.95; 95% CI 1.32, 2.90).
CONCLUSIONS
Long-term micro- and macrovascular complications and mortality are highly correlated. Age, diabetes duration, and glycemic levels do not completely explain these associations.
Graphical Abstract
Introduction
Despite improvements in treatment and health management, individuals with type 1 diabetes (T1D) remain at higher risk of microvascular complications and cardiovascular disease (CVD) compared with those without diabetes (1–5). Mortality rates have also been higher among individuals with T1D compared with those without diabetes (2,6,7), with varying estimates of relative risk in more recent analyses (8–10).
With its thorough evaluation of microvascular and cardiovascular complications, and mortality over >30 years of follow-up, the Diabetes Control and Complications Trial (DCCT) and its observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC), provides a unique opportunity to investigate the incidence and co-occurrence of these long-term complications in individuals with T1D. Herein, we describe the relationships between the cumulative incidences of long-term complications in T1D and evaluate whether any observed associations are independent of age, duration of diabetes, and glycemia.
Research Design and Methods
Participants
The methods of the DCCT/EDIC study have been previously described (11,12). Briefly, the DCCT enrolled 1,441 participants with T1D who were randomly assigned to receive either intensive therapy (n = 711) aimed at achieving glycemic levels as close to the nondiabetic range as safely possible, or conventional therapy (n = 730) aimed at preventing symptoms of hypo- and hyperglycemia with no predefined glycemic targets. Participants were enrolled either into the primary prevention cohort (diabetes duration of 1–5 years, no retinopathy, based on stereoscopic fundus photography, and albuminuria <40 mg/24 h at baseline; n = 726) or the secondary intervention cohort (duration of T1D of 1–15 years, minimal to moderate nonproliferative retinopathy, and albuminuria <200 mg/24 h at baseline; n = 715). In 1993, after a mean follow-up of 6.5 years, the DCCT ended, and all participants were taught intensive therapy and referred to their health care providers for ongoing diabetes care. In 1994, the observational follow-up study, EDIC, enrolled 96% of the surviving DCCT cohort. Ninety-four percent of survivors still actively participate in the study after >25 years since the start of EDIC. In the present analyses, we used all available data in the full cohort (n = 1,441) over the combined DCCT/EDIC follow-up period up to 30 April 2018.
Risk Factors
HbA1c was measured quarterly during DCCT and annually during EDIC using high-performance liquid chromatography. To account for the different measurement frequencies during DCCT and EDIC, the models used the mean updated DCCT/EDIC HbA1c, which is a time-dependent exposure calculated by weighting each value by the time interval between measurements (13).
Microvascular Assessments and Outcomes
Serum creatinine was measured annually and used in combination with age, sex, and race to calculate estimated glomerular filtration rate (eGFR), which we calculated with the Chronic Kidney Disease Epidemiology Collaboration equation. End-stage renal disease (ESRD) was defined as the initiation of maintenance dialysis or kidney transplantation assessed yearly by questionnaire and adjudicated centrally. Reduced eGFR was defined as an eGFR <60 mL/min/1.73 m2 on at least one occasion or progression to ESRD (14).
Standardized stereoscopic, seven-field fundus photographs were obtained every 6 months during DCCT and every fourth year (staggered from the start of the EDIC follow-up period) during EDIC. In addition, photographs were obtained in the full cohort at EDIC years 4 and 10. The photographs were graded centrally using the final Early Treatment of Diabetic Retinopathy Study severity grading scale. Graders were masked to treatment assignment and other risk factors. Proliferative diabetic retinopathy (PDR) was defined as neovascularization observed on fundus photograph grading or evidence of scatter photocoagulation. Clinically significant macular edema (CSME) was defined on the basis of fundus photography grading or the presence of focal photocoagulation scars (15,16). Lower extremity amputations at any level, including single toe, were identified as present or absent during the annual physical examination beginning in October 2005 (EDIC year 12).
Cardiovascular Outcomes
Annual medical histories and electrocardiograms were used to ascertain CVD events, and documentation from external medical records was sought for verification. These events were adjudicated by a within-study Mortality and Morbidity Review Committee masked to original DCCT treatment group assignment and glycemic levels as measured by HbA1c. The composite CVD outcome was defined as time to the first occurrence of CVD death, nonfatal myocardial infarction, nonfatal stroke, subclinical myocardial infarction on electrocardiogram (i.e., silent myocardial infarction), angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction documented on coronary angiography, revascularization (with angioplasty or coronary artery bypass), or congestive heart failure (paroxysmal nocturnal dyspnea, orthopnea, or marked limitation of physical activity caused by heart disease) (17).
Mortality
Deaths were reported to the Data Coordinating Center and external documentation provided (if available) for adjudication by the Mortality and Morbidity Review Committee (18). The primary mortality outcome was defined as all-cause mortality.
Statistical Analysis
The marginal cumulative incidence of each complication (e.g., the cumulative incidence of CVD) was estimated using the Kaplan-Meier estimator, whereas the joint cumulative incidence of multiple complications (e.g., the cumulative incidence of both PDR and CSME) was estimated using the Dabrowska estimator available in library(mhazard) in R (19–21).
Two metrics (conditional probabilities) describe the relationships between the incidences of complications (see Supplementary Material for details). Using PDR and CSME as examples, we estimated the probability of developing PDR prior to a specific time point (e.g., ≤15 years after randomization) separately for individuals with and without CSME up to that time point. In addition, we estimated the 5-year cumulative incidence of a complication separately on the basis of the presence or absence of another complication. For example, for a participant who is free of both PDR and CSME at 15 years from enrollment, we estimated the probability of developing PDR within 5 years (i.e., by year 20 = 15 + 5). Likewise, for a participant who is free of PDR but with CSME present at year 15, we estimated the probability of developing PDR within 5 years (i.e., by year 20 = 15 + 5).
The pairwise associations between complications were then assessed using separate Cox proportional hazards (PH) models for the risk of one of the complications as a function of the other complication included as a time-varying covariate capturing its status (present or absent) over time. For example, the association between reduced eGFR and the subsequent risk of CVD was assessed using reduced eGFR status as a time-varying covariate in a Cox PH model for the risk of CVD. The models were unadjusted and minimally adjusted for age, duration of T1D, and mean updated HbA1c.
Data and Resource Availability
Data collected for the DCCT/EDIC study through 30 June 2017 are available to the public through the National Institute of Diabetes and Digestive and Kidney Disease Central Repository (https://repository.niddk.nih.gov/studies/edic/). Data collected in the current cycle (July 2017–June 2022) will be available within 2 years after the end of the funding cycle.
Results
Briefly, at baseline, 53% of the participants were men, median age was 27 years, median duration of T1D was ∼4 years, and median HbA1c value was 8.7%. Supplementary Table 1 presents the baseline characteristics of the participants separately by outcome status over the entire DCCT/EDIC follow-up.
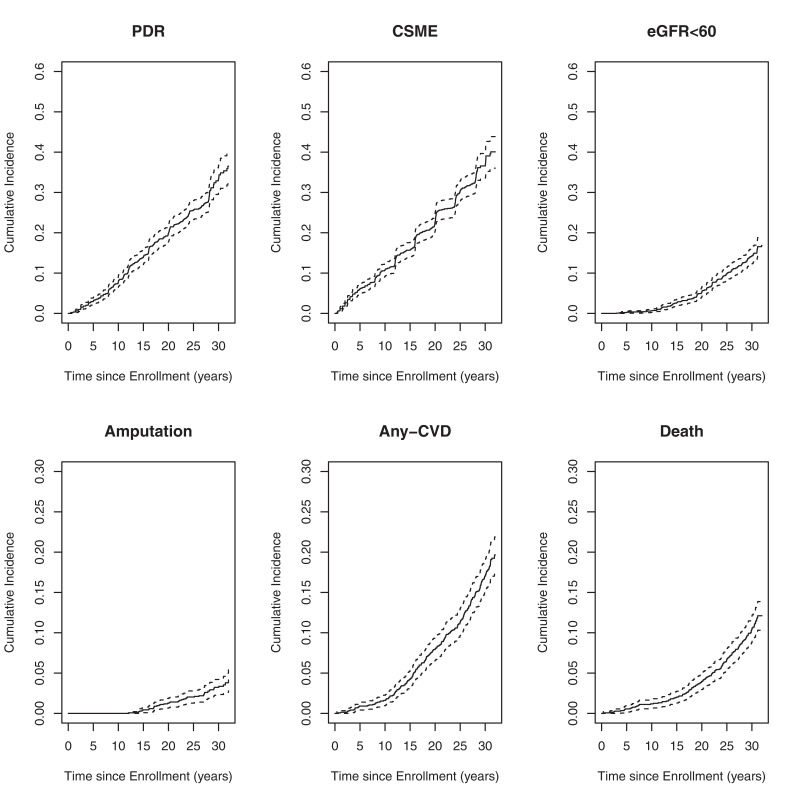
The 30-year cumulative incidence of PDR and CSME was 33.4% and 36.6%, respectively; reduced eGFR was 14.1%; amputation was 3.2%; CVD was 17.1%; mortality was 10.4% (Fig. 1). The highest numbers of documented events were for CSME (n = 448; rate is 14.2 events per 1,000 individuals at risk for 1 year) and PDR (n = 394; rate is 12 events per 1,000 individuals at risk for 1 year), and the lowest number of events was for amputations (n = 47; rate is 1.1 events per 1,000 individuals at risk for 1 year) (Table 1). There were 639 participants (44%) who did not experience any of the 6 long-term complications during follow-up; 367 participants developed 1 complication, 247 participants developed 2 complications, 110 participants developed 3 complications, 59 participants developed 4 complications, 14 participants developed 5 complications, and 5 participants developed all 6 complications.
Figure 1.
Cumulative incidence of PDR, CSME, eGFR <60 mL/min/1.73 m2, amputations, any CVD, and mortality. Note the different scaling of the top row (0.6) versus the bottom row (0.3) figures reflect the difference in cumulative incidence of outcomes. eGFR<60, estimated GFR <60 mL/min/1.73 m2 on at least one occasion or progression to ESRD.
Table 1.
Number of events, rate of each complication, and the probability of developing a complication by specific time points from DCCT randomization for individuals without and with a prior complication†
| Outcome (n; rate**) | Time (years) | Prior PDR (%) | Prior CSME (%) | Prior reduced eGFR (%) | Prior amputation (%) | Prior CVD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||
| PDR (395; 12) | 10 | 3.9 | 40.4 | 7.4 | 66.9 | 7.7 | NA* | 7.8 | 9.7 | ||
| 15 | 6.5 | 54.6 | 12.8 | 62.1 | 13.9 | 16.6 | 13.6 | 22.9 | |||
| 20 | 8.9 | 57.5 | 17.7 | 60.4 | 19.4 | 48.2 | 19.0 | 30.5 | |||
| 25 | 11.5 | 57.9 | 22.2 | 56.7 | 24.7 | 61.8 | 23.8 | 39.5 | |||
| 30 | 14.9 | 65.5 | 29.2 | 58.8 | 32.1 | 68.2 | 31.3 | 43.6 | |||
| CSME (448; 14.2) | 10 | 6.9 | 55.6 | 10.5 | 44.6 | 10.5 | NA | 10.7 | 14.2 | ||
| 15 | 8.3 | 61.0 | 14.4 | 61.7 | 15.4 | 50.1 | 15.0 | 29.7 | |||
| 20 | 12.0 | 65.5 | 20.4 | 64.7 | 22.1 | 53.6 | 21.6 | 35.3 | |||
| 25 | 17.1 | 68.6 | 26.9 | 61.9 | 29.5 | 64.9 | 28.2 | 47.2 | |||
| 30 | 18.9 | 71.7 | 32.2 | 63.0 | 35.3 | 71.5 | 33.5 | 51.6 | |||
| Reduced eGFR (204; 5.1) | 10 | 0.2 | 5.5 | 0.4 | 2.7 | 0.6 | NA | 0.5 | 9.0 | ||
| 15 | 1.1 | 11.5 | 1.2 | 10.2 | 2.5 | 0.1 | 2.2 | 12.2 | |||
| 20 | 2.6 | 15.8 | 2.4 | 14.9 | 4.9 | 18.4 | 4.2 | 17.0 | |||
| 25 | 5.7 | 21.8 | 5.4 | 20.1 | 8.8 | 54.0 | 7.8 | 26.1 | |||
| 30 | 8.8 | 25.0 | 8.3 | 24.5 | 13.0 | 47.9 | 10.9 | 30.2 | |||
| Amputation (47; 1.1) | 10 | NA | NA | NA | NA | NA | NA | NA | NA | ||
| 15 | 0.4 | 0.5 | 0.3 | 1.4 | 0.4 | 0.0 | 0.3 | 3.6 | |||
| 20 | 0.8 | 3.1 | 0.8 | 3.0 | 1.1 | 4.6 | 1.0 | 4.8 | |||
| 25 | 1.0 | 4.9 | 1.0 | 4.4 | 1.0 | 11.3 | 1.3 | 8.2 | |||
| 30 | 1.5 | 6.6 | 1.4 | 6.3 | 2.0 | 11.0 | 1.9 | 9.5 | |||
| CVD (261; 6.3) | 10 | 1.6 | 2.0 | 1.5 | 2.1 | 1.5 | 22.7 | 1.4 | NA | ||
| 15 | 3.8 | 6.9 | 3.5 | 8.0 | 3.8 | 19.8 | 3.9 | 33.4 | |||
| 20 | 6.9 | 12.2 | 6.7 | 12.4 | 7.0 | 26.1 | 7.5 | 29.6 | |||
| 25 | 9.2 | 17.5 | 8.6 | 17.6 | 9.3 | 30.0 | 10.4 | 45.1 | |||
| 30 | 14.4 | 22.3 | 13.0 | 24.0 | 13.9 | 36.3 | 15.9 | 49.9 | |||
| Mortality (173; 4) | 10 | 1.1 | 1.6 | 1.0 | 1.8 | 1.0 | 11.7 | 0.1 | NA | 0.9 | 17.8 |
| 15 | 1.8 | 3.1 | 1.6 | 4.0 | 1.7 | 9.5 | 0.9 | 0.7 | 1.6 | 10.8 | |
| 20 | 3.5 | 5.4 | 3.0 | 6.9 | 3.0 | 19.8 | 2.7 | 7.4 | 2.7 | 18.9 | |
| 25 | 5.7 | 9.4 | 5.2 | 9.8 | 5.2 | 19.9 | 5.3 | 13.4 | 5.2 | 18.7 | |
| 30 | 9.1 | 13.0 | 8.6 | 13.5 | 8.5 | 21.5 | 8.3 | 35.1 | 7.5 | 24.6 | |
NA, not applicable.
For example, by 10 years from randomization, there was a 3.9% chance an individual without CSME developed PDR, and a 40.4% chance an individual with prior CSME developed PDR. Likewise, by 30 years from randomization, there was a 7.5% chance of mortality for an individual without a prior CVD event compared with a 24.6% chance of mortality for an individual with prior CVD.
There were no amputations prior to year 10; therefore, these probabilities are not estimable.
Rate = events per 1,000 patient-years.
The marginal and joint cumulative incidences of complications are presented in Supplementary Table 2. By 30 years from DCCT baseline, 33.4% of the participants experienced PDR and 36.6% experienced CSME, with 23.9% of the participants experiencing both PDR and CSME. The cumulative incidence for reduced eGFR was 14.1% by year 30, with 8.9% of the participants experiencing both reduced eGFR and CSME, and 8.4% experiencing both reduced eGFR and PDR. Only 3.2% of the participants had an amputation by year 30, with 2.2% and 2.3% of the participants having also experienced PDR and CSME, respectively. By year 30, 17.1% of the participants had a CVD event, with 8.8% and 5.0% also experiencing CSME and reduced eGFR, respectively. Cumulative incidence of mortality at year 30 was 10.4%, with 5.0% and 4.2% also experiencing CSME and CVD, respectively.
By year 30, there were large differences in the cumulative incidence of PDR between participants with and without prior CSME (65.5% vs. 14.9%), prior reduced eGFR (58.8% vs. 29.2%), and prior amputation (68.2% vs. 32.1%) (Table 1). Similar patterns were observed for CSME. For reduced eGFR, the cumulative incidence at year 30 was 25% vs. 8.8% among participants with versus without prior PDR, 47.9% vs. 13% among participants with versus without prior amputation, and 30.2% vs. 10.9% among participants with versus without prior CVD. For CVD, the cumulative incidence at year 30 was 36.6% vs. 13.9% among participants with versus without prior reduced eGFR, and 49.9% vs. 15.9% among participants with versus without prior amputation. For mortality, the cumulative incidence at year 30 was 21.5% vs. 8.5% among participants with versus without prior reduced eGFR, 35.1% vs. 8.3% among participants with versus without prior amputation, and 24.6 vs. 7.5% among participants with versus without prior CVD.
Table 2 describes the 5-year risk (%) of a complication from a specific time point (e.g., 15 years from randomization) for an individual with versus without another complication present at that time point (i.e., year 15). Presence of CSME was an important predictor of subsequent 5-year risk of PDR; for example, at year 25, the probability of developing PDR in the next 5 years (i.e., between years 25 and 30 of follow-up) was 21.4% for a participant with CSME present at year 25, and only 8.2% for a participant without CSME at year 25. Presence of PDR and a prior amputation were predictive of future 5-year risk of CSME. Note that prior reduced eGFR and amputations were more predictive of 5-year risk of CVD than either of the eye outcomes. Prior amputations and prior CVD were predictive of 5-year risk of mortality.
Table 2.
Probability of a complication occurring within the next 5 years from a specific time point from DCCT randomization for individuals without and with a prior complication by time t†
| Outcome in the next 5 years | Time (years) | Prior PDR (%) | Prior CSME (%) | Prior reduced eGFR (%) | Prior amputation (%) | Prior CVD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||
| PDR | 10 | 4.8 | 33.4 | 6.8 | 0.0 | 6.8 | NA* | 6.8 | 6.3 | ||
| 15 | 5.2 | 24.8 | 6.6 | 25.4 | 6.6 | 62.0 | 6.6 | 13.0 | |||
| 20 | 5.7 | 17.5 | 6.9 | 13.5 | 7.0 | 13.4 | 6.7 | 11.6 | |||
| 25 | 8.2 | 21.4 | 10.2 | 16.2 | 10.2 | 37.6 | 11.0 | 5.9 | |||
| CSME | 10 | 5.1 | 15.5 | 5.6 | 0.0 | 5.5 | NA* | 5.4 | 11.7 | ||
| 15 | 7.8 | 15.6 | 8.1 | 24.5 | 8.2 | 34.6 | 8.2 | 12.0 | |||
| 20 | 9.1 | 17.9 | 9.4 | 29.1 | 9.8 | 15.4 | 9.5 | 14.5 | |||
| 25 | 7.0 | 24.6 | 9.0 | 8.3 | 8.7 | 41.1 | 8.8 | 10.8 | |||
| Reduced eGFR | 10 | 1.7 | 6.0 | 1.4 | 6.9 | 1.9 | NA* | 1.8 | 10.3 | ||
| 15 | 1.9 | 7.9 | 1.9 | 7.5 | 2.6 | 17.1 | 2.3 | 12.5 | |||
| 20 | 4.1 | 8.4 | 3.9 | 8.6 | 4.5 | 38.2 | 4.6 | 8.9 | |||
| 25 | 3.5 | 9.7 | 3.9 | 7.4 | 4.8 | 9.9 | 4.6 | 7.0 | |||
| Amputation | 10 | NA* | NA* | NA* | NA* | NA* | NA* | NA* | NA* | ||
| 15 | 0.7 | 1.6 | 0.5 | 2.5 | 0.8 | 3.3 | 0.7 | 3.9 | |||
| 20 | 0.5 | 2.0 | 0.3 | 2.5 | 0.5 | 6.5 | 0.6 | 3.2 | |||
| 25 | 0.8 | 2.5 | 0.7 | 2.5 | 1.0 | 3.0 | 1.0 | 2.9 | |||
| CVD | 10 | 2.5 | 4.6 | 2.2 | 6.1 | 2.7 | 0.3 | 2.7 | NA* | ||
| 15 | 3.5 | 6.7 | 3.8 | 4.6 | 3.9 | 7.6 | 3.8 | 25.0 | |||
| 20 | 3.5 | 4.3 | 3.0 | 6.0 | 3.4 | 8.3 | 3.5 | 17.0 | |||
| 25 | 5.2 | 10.4 | 5.6 | 8.6 | 6.0 | 12.2 | 6.3 | 21.2 | |||
| Mortality | 10 | 0.8 | 1.0 | 0.7 | 2.1 | 0.9 | 0.1 | 0.7 | NA* | 0.9 | 0.0 |
| 15 | 1.9 | 2.8 | 1.8 | 3.0 | 1.4 | 27.5 | 1.9 | 0.1 | 1.5 | 14.8 | |
| 20 | 2.4 | 4.7 | 2.3 | 4.6 | 2.3 | 13.8 | 2.7 | 12.7 | 2.7 | 4.3 | |
| 25 | 3.7 | 5.1 | 3.7 | 4.7 | 3.7 | 7.7 | 3.4 | 29.4 | 3.2 | 11.7 | |
NA, not applicable.
For example, an individual free of PDR at 10 years from randomization has a 4.8% chance of developing PDR in the next 5 years if they did not have CSME by year 10, compared with a 33.4% chance if they had CSME by year 10. Likewise, an individual free of CVD at 25 years from randomization has a 3.2% chance of death in the next 5 years if they did not have CVD by year 25, compared with an 11.7% chance if they had CVD by year 10.
There were no amputations prior to year 10; therefore, these probabilities are not estimable.
In Table 3, we report the associations between each complication and the subsequent risk of the other complications, using separate Cox PH models, first unadjusted and then minimally adjusted for age, duration of T1D, and mean updated HbA1c. In the unadjusted analyses, presence of a complication was associated with higher subsequent risk of other complications, with the exception of CVD for the subsequent risk of PDR, and reduced eGFR, amputation, and CVD for the subsequent risk of CSME. Adjusted for age, duration of T1D, and mean updated HbA1c, the presence versus absence of PDR remained associated with higher risk of CSME (hazard ratio [HR] 1.88; P < 0.0001), reduced eGFR (HR 1.41; P = 0.0434), and CVD (HR 1.43; P = 0.0182); presence versus absence of CSME was associated with higher risk of PDR (HR 3.94; P < 0.0001), reduced eGFR (HR 1.49; P = 0.0092), and CVD (HR 1.35; P = 0.0309); presence versus absence of reduced eGFR was associated with higher risk of CVD (HR 2.09; P = 0.0001) and death (HR 3.40; P < 0.0001); presence versus absence of amputation(s) was associated with higher risk of death (HR 2.97; P = 0.0001); presence versus absence of CVD was associated with higher risk of reduced eGFR (HR 1.59; P = 0.0188) and death (HR 1.95; P = 0.0009). A sensitivity analysis using models further adjusted for DCCT baseline cohort assignment (primary prevention versus secondary intervention) revealed similar results (data not shown). An additional sensitivity analysis that excluded the CVD deaths without a prior (nonfatal) CVD event (n = 20) from the analyses investigating the association between CVD and mortality yielded virtually identical results (data not shown).
Table 3.
The association between each complication and the subsequent risk of the other complications, using unadjusted and then minimally adjusted Cox PH models
| Unadjusted models | Adjusted models* | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | z | P | HR (95% CI) | z | P | |
| Risk of PDR | ||||||
| PDR | NA (NA, NA) | NA | NA | NA (NA, NA) | NA | NA |
| CSME | 6.86 (5.56, 8.46) | 18.02 | <0.0001 | 3.94 (3.18, 4.89) | 12.45 | <0.0001 |
| eGFR<60 | 1.98 (1.07, 3.68) | 2.18 | 0.0296 | 1.48 (0.79, 2.78) | 1.22 | 0.2220 |
| AMP | 3.44 (1.27, 9.31) | 2.44 | 0.0148 | 1.89 (0.68, 5.24) | 1.23 | 0.2186 |
| CVD | 1.39 (0.85, 2.28) | 1.31 | 0.1895 | 1.00 (0.61, 1.65) | 0.02 | 0.9858 |
| Risk of CSME | ||||||
| PDR | 3.66 (2.80, 4.79) | 9.44 | <0.0001 | 1.88 (1.42, 2.50) | 4.37 | <0.0001 |
| CSME | NA (NA, NA) | NA | NA | NA (NA, NA) | NA | NA |
| eGFR<60 | 1.48 (0.76, 2.88) | 1.14 | 0.2544 | 1.02 (0.52, 1.99) | 0.05 | 0.9641 |
| AMP | 2.39 (0.76, 7.49) | 1.49 | 0.1354 | 2.06 (0.66, 6.49) | 1.24 | 0.2143 |
| CVD | 1.25 (0.75, 2.08) | 0.85 | 0.3979 | 0.93 (0.56, 1.56) | −0.26 | 0.7944 |
| Risk of eGFR<60 | ||||||
| PDR | 2.87 (2.12, 3.88) | 6.84 | <0.0001 | 1.41 (1.01, 1.97) | 2.02 | 0.0434 |
| CSME | 2.86 (2.16, 3.80) | 7.30 | <0.0001 | 1.49 (1.10, 2.01) | 2.61 | 0.0092 |
| eGFR<60 | NA (NA, NA) | NA | NA | NA (NA, NA) | NA | NA |
| AMP | 4.82 (2.45, 9.49) | 4.55 | <0.0001 | 1.26 (0.62, 2.57) | 0.64 | 0.5191 |
| CVD | 2.74 (1.88, 4.00) | 5.25 | <0.0001 | 1.59 (1.08, 2.34) | 2.35 | 0.0188 |
| Risk of amputations | ||||||
| PDR | 3.39 (1.89, 6.05) | 4.12 | <0.0001 | 1.10 (0.58, 2.11) | 0.30 | 0.7668 |
| CSME | 3.74 (2.09, 6.68) | 4.46 | <0.0001 | 1.52 (0.83, 2.80) | 1.34 | 0.1786 |
| eGFR<60 | 4.34 (2.11, 8.94) | 3.99 | <0.0001 | 1.74 (0.84, 3.64) | 1.48 | 0.1386 |
| AMP | NA (NA, NA) | NA | NA | NA (NA, NA) | NA | NA |
| CVD | 3.10 (1.52, 6.34) | 3.11 | 0.0019 | 1.49 (0.71, 3.13) | 1.05 | 0.2958 |
| Risk of CVD | ||||||
| PDR | 2.00 (1.52, 2.62) | 5.00 | <0.0001 | 1.43 (1.06, 1.92) | 2.36 | 0.0182 |
| CSME | 1.94 (1.49, 2.53) | 4.93 | <0.0001 | 1.35 (1.03, 1.78) | 2.16 | 0.0309 |
| eGFR<60 | 3.06 (2.12, 4.41) | 5.95 | <0.0001 | 2.09 (1.44, 3.03) | 3.87 | 0.0001 |
| AMP | 3.10 (1.52, 6.32) | 3.11 | 0.0018 | 1.83 (0.89, 3.75) | 1.65 | 0.1000 |
| CVD | NA (NA, NA) | NA | NA | NA (NA, NA) | NA | NA |
| Risk of death | ||||||
| PDR | 1.72 (1.23, 2.40) | 3.20 | 0.0014 | 1.03 (0.72, 1.48) | 0.16 | 0.8759 |
| CSME | 1.95 (1.42, 2.68) | 4.13 | <0.0001 | 1.25 (0.89, 1.75) | 1.31 | 0.1902 |
| eGFR<60 | 5.40 (3.78, 7.71) | 9.29 | <0.0001 | 3.40 (2.35, 4.92) | 6.48 | <0.0001 |
| AMP | 6.14 (3.58, 10.53) | 6.58 | <0.0001 | 2.97 (1.70, 5.22) | 3.80 | 0.0001 |
| CVD | 2.95 (2.02, 4.31) | 5.61 | <0.0001 | 1.95 (1.32, 2.90) | 3.32 | 0.0009 |
P values in bold indicate statistical significance at P < 0.05. AMP, amputation; NA, not applicable.
Adjusted for age, duration of T1D, and mean updated HbA1c value.
Conclusions
Our analyses of this well-characterized cohort of individuals with T1D followed for >30 years demonstrate strong correlations among long-term microvascular and cardiovascular complications, including mortality. Noteworthy is that many of these associations (e.g., PDR with CSME; PDR, CSME, and CVD with reduced eGFR; PDR, CSME, and reduced eGFR with CVD; and reduced eGFR, amputations, and CVD with mortality) were independent of age, duration of diabetes, and glycemic exposure. These results suggest there is within-individual susceptibility to develop micro- and macrovascular complications and mortality independent of glycemic exposure.
Many of these relationships have been individually demonstrated in previous studies. The increased risk of kidney disease in participants with diabetic retinopathy was shown previously in the DCCT/EDIC study (22), in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) (23,24), and several other studies (25–27) of both type 1 and type 2 diabetes. Likewise, retinopathy has also been associated with increased CVD and mortality risk in WESDR (28) and other studies (29–31). Finally, increased CVD and mortality risk among those with kidney disease has been well established (32,33) and subsequently has been verified in the DCCT/EDIC (34) and several other studies (35–38).
In contrast to these previous studies, herein we describe the relationships among complications not only on the relative risk scale (i.e., using Cox PH models) but also on the absolute risk scale. More specifically, we report cumulative incidence and 5-year risk of complications separately on the basis of the status (presence or absence) of a different complication.
Strengths of our study include the detailed phenotyping of our participants over a prolonged period of time. At baseline, the participants were in the early stages of disease progression, with none of the participants in the primary cohort (n = 726) having any complications, and the participants in the secondary cohort (n = 715) only having very early microvascular disease present (e.g., 28% with very mild to moderate nonproliferative retinopathy, and 22% and 2% with albumin excretion rate >30 mg/24 h and >100 mg/24 h, respectively). Furthermore, the outcomes chosen for this study were advanced long-term complications. Although we used eGFR <60 mL/min/1.73 m2 and/or progression to ESRD, prior studies often used the development of microalbuminuria or macroalbuminuria as kidney outcomes. However, we previously showed that such development often reverses over time rather than progresses (34).
Our study has limitations as well. The low number of participants with amputations in our cohort (n = 47) may have limited our ability to detect its associations with the other complications. Likewise, we were underpowered to assess associations with individual CVD events (e.g., stroke or myocardial infarction) or with cause-specific mortality (e.g., cardiovascular mortality).
In summary, we have demonstrated strong correlations among advanced long-term microvascular and cardiovascular complications, including mortality. The unifying modifiable factor underlying the development of both microvascular and macrovascular complications is glycemic control (16,18,22,39). Previously, we also demonstrated that the earlier better glycemic control is achieved after diagnosis, the lower the risk of long-term microvascular and cardiovascular outcomes (40). However, age, duration of diabetes, and glycemia do not completely explain the joint incidence of these complications. This suggests there is within-individual susceptibility to both microvascular and macrovascular complications. More studies are needed to define the causes of this individual susceptibility to complications. Possibilities include genetic, clinical, and behavior factors, as well as social or environmental exposures. The results do suggest that surveillance for additional complications should be intensified in patients with existing microvascular and cardiovascular complications.
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2022) and contracts (1982–2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK; current grants U01 DK094176 and U01 DK094157), and through support from the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006–present), Bethesda, MD. The following industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Duality of Interest. B.A.P. has received speaker honoraria from Abbott, Medtronic, Insulet, and Novo Nordisk, served as an advisor to Boehringer Ingelheim, Insulet, Sanofi, and Abbott, and has received research support to his research institute from Novo Nordisk, and the Bank of Montreal. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. I.B. and M.E.M. designed the study with input from all coauthors. I.B. conducted the statistical analyses. I.B. and M.E.M. wrote the initial draft of the manuscript. All authors contributed revisions to the article and approve the final content. I.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00360893 and NCT00360815, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.21583773.
A complete list of participants in the DCCT/EDIC Research Group can be found in the supplementary material online.
I.B. is chair and M.E.M. is co-chair of the DCCT/EDIC Writing Committee.
References
- 1. Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 2. Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish Registry Linkage Study. PLoS Med 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association . Addendum. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl. 1):S151–S167 [DOI] [PubMed] [Google Scholar]
- 4. Margolis DJ, Jeffcoate W. Epidemiology of foot ulceration and amputation: can global variation be explained? Med Clin North Am 2013;97:791–805 [DOI] [PubMed] [Google Scholar]
- 5. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern®. Ophthalmology 2020;127:P66. [DOI] [PubMed] [Google Scholar]
- 6. Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health 1991;81:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County Type 1 Diabetes Registry. Diabetes Care 2010;33:2573–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rawshani A, Rawshani A, Gudbjörnsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;377:300–301 [DOI] [PubMed] [Google Scholar]
- 9. Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study. Diabetes Care 2016;39:2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group . Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care 2016;39:1378–138327411699 [Google Scholar]
- 11. The DCCT Research Group . The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 12. The DCCT/EDIC Research Group . Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Writing Group for the DCCT/EDIC Research Group . Coprogression of cardiovascular risk factors in type 1 diabetes during 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2016;39:1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perkins BA, Bebu I, de Boer IH, et al.; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Risk factors for kidney disease in type 1 diabetes. Diabetes Care 2019;42:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DCCT/EDIC Research Group. Nathan DM, Bebu I, Hainsworth D, et al. Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl J Med 2017;376:1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group; Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diabetes Control and Complications Trial (DCCT)-Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Nathan DM, Bebu I, Braffett BH, et al. Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 2016;65:1370–137926895792 [Google Scholar]
- 18. Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dabrowska DM. Kaplan-Meier estimate on the plane. Ann Stat 1988;16:1475–1489 [Google Scholar]
- 20. Prentice RL, Zhao S. The Statistical Analysis of Multivariate Failure Time Data: A Marginal Modeling Approach. Boca Raton, FL, CRC Press, 2019 [Google Scholar]
- 21. Prentice RL, Zhao S. Regression models and multivariate life tables. J Am Stat Assoc 2021;116:1330–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Boer IH, Rue TC, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lecaire TJ, Klein BE, Howard KP, Lee KE, Klein R. Risk for end-stage renal disease over 25 years in the population-based WESDR cohort. Diabetes Care 2014;37:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein R, Zinman B, Gardiner R, et al.; Renin-Angiotensin System Study . The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes 2005;54:527–533 [DOI] [PubMed] [Google Scholar]
- 25. Romero P, Salvat M, Fernández J, Baget M, Martinez I. Renal and retinal microangiopathy after 15 years of follow-up study in a sample of type 1 diabetes mellitus patients. J Diabetes Complications 2007;21:93–100 [DOI] [PubMed] [Google Scholar]
- 26. Hong J, Surapaneni A, Daya N, et al. Retinopathy and risk of kidney disease in persons with diabetes. Kidney Med 2021;3:808–815.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karlberg C, Falk C, Green A, Sjølie AK, Grauslund J. Proliferative retinopathy predicts nephropathy: a 25-year follow-up study of type 1 diabetic patients. Acta Diabetol 2012;49:263–268 [DOI] [PubMed] [Google Scholar]
- 28. Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med 2004;164:1917–1924 [DOI] [PubMed] [Google Scholar]
- 29. Targher G, Bertolini L, Tessari R, Zenari L, Arcaro G. Retinopathy predicts future cardiovascular events among type 2 diabetic patients: the Valpolicella Heart Diabetes Study. Diabetes Care 2006;29:1178. [DOI] [PubMed] [Google Scholar]
- 30. Pongrac Barlovic D, Harjutsalo V, Gordin D, et al.; FinnDiane Study Group . The association of severe diabetic retinopathy with cardiovascular outcomes in long-standing type 1 diabetes: a longitudinal follow-up. Diabetes Care 2018;41:2487–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu XH, Sun B, Zhong S, Wei DD, Hong Z, Dong AQ. Diabetic retinopathy predicts cardiovascular mortality in diabetes: a meta-analysis. BMC Cardiovasc Disord 2020;20:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borch-Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1985;28:590–596 [DOI] [PubMed] [Google Scholar]
- 33. Jensen T, Borch-Johnsen K, Kofoed-Enevoldsen A, Deckert T. Coronary heart disease in young type 1 (insulin-dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia 1987;30:144–148 [DOI] [PubMed] [Google Scholar]
- 34. de Boer IH, Gao X, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clin J Am Soc Nephrol 2016;11:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 1997;157:1413–1418 [PubMed] [Google Scholar]
- 36. Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 2000;160:1093–1100 [DOI] [PubMed] [Google Scholar]
- 37. Fox CS, Matsushita K, Woodward M, et al.; Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Groop PH, Thomas MC, Moran JL, et al.; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 40. Lachin JM, Bebu I; DCCT/EDIC Research Group . The beneficial effects of earlier versus later implementation of intensive therapy in type 1 diabetes. Diabetes Care 2021;44:2225–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]