Abstract
Individuals with an elevated fasting glucose level, elevated glucose level after glucose challenge, or elevated hemoglobin A1c level below the diagnostic threshold for diabetes (collectively termed prediabetes) are at increased risk for type 2 diabetes. More than one-third of U.S. adults have prediabetes but fewer than one in five are aware of the diagnosis. Rigorous scientific research has demonstrated the efficacy of both intensive lifestyle interventions and metformin in delaying or preventing progression from prediabetes to type 2 diabetes. The National Clinical Care Commission (NCCC) was a federal advisory committee charged with evaluating and making recommendations to improve federal programs related to the prevention of diabetes and its complications. In this article, we describe the recommendations of an NCCC subcommittee that focused primarily on prevention of type 2 diabetes in people with prediabetes. These recommendations aim to improve current federal diabetes prevention activities by 1) increasing awareness of and diagnosis of prediabetes on a population basis; 2) increasing the availability of, referral to, and insurance coverage for the National Diabetes Prevention Program and the Medicare Diabetes Prevention Program; 3) facilitating Food and Drug Administration review and approval of metformin for diabetes prevention; and 4) supporting research to enhance the effectiveness of diabetes prevention. Cognizant of the burden of type 1 diabetes, the recommendations also highlight the importance of research to advance our understanding of the etiology of and opportunities for prevention of type 1 diabetes.
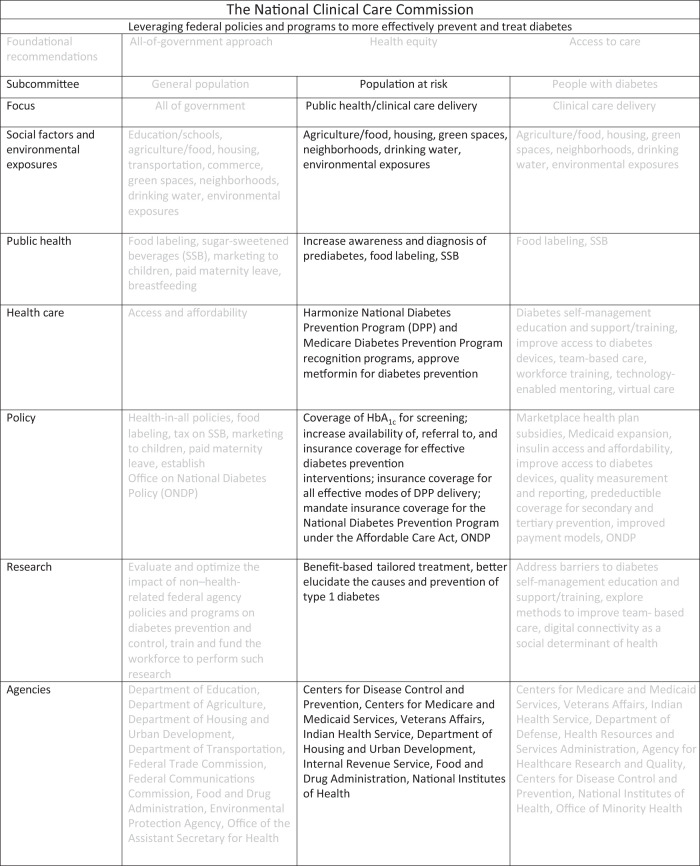
Graphical Abstract
Introduction
Prediabetes is a condition that increases the risk for type 2 diabetes and cardiovascular disease (CVD) (1). In this article, the National Clinical Care Commission (NCCC) addresses targeted diabetes prevention in people at high risk for diabetes, specifically those with prediabetes, with recommendations focused on increasing awareness of prediabetes, the referral of people with prediabetes to effective lifestyle change programs, and supporting Food and Drug Administration (FDA) approval of metformin for diabetes prevention (2). The commission’s recommendations also highlight the need for research to enhance and advance strategies to prevent type 2 diabetes and to advance the current state of our knowledge about the pathogenesis and prevention of type 1 diabetes. The recommendations included in this article complement those of the accompanying articles. Implementing recommendations such as making positive changes in food and agricultural policies, enhancing nutritional assistance programs, improving the built environment, reducing environmental exposures, and improving access to health care will benefit all Americans, including those with prediabetes. As type 2 diabetes accounts for 90–95% of diagnosed diabetes in the U.S., preventing or delaying progression to type 2 diabetes among people with prediabetes will have substantial clinical and public health benefits.
Background
Prediabetes and Risk of Diabetes
Prediabetes is a metabolic disorder in which blood glucose levels are elevated but not high enough to be classified as diabetes. The American Diabetes Association (ADA) defines prediabetes as a fasting plasma glucose of 100–125 mg/dL (impaired fasting glucose [IFG]), a plasma glucose level 2 h after a 75-g glucose challenge of 140–199 mg/dL (impaired glucose tolerance [IGT]), or a hemoglobin A1c (HbA1c) level of 5.7% to 6.4% (1).
Prediabetes is prevalent in the U.S. The Centers for Disease Control and Prevention (CDC) estimated that 96 million adults (3), or about 37% of the U.S. population over 18 years of age, and 18% of teenagers, have prediabetes (4,5). Most people with prediabetes are unaware that they have this condition; only about 19% of people with prediabetes report being told by a health professional that they have prediabetes (4). Overweight and obesity are strong risk factors for prediabetes. The prevalence of prediabetes also increases with age (4).
People with prediabetes are at higher risk of developing type 2 diabetes. Risk of progression to type 2 diabetes varies depending on population characteristics and prediabetes definitions (6,7). The rate of progression is higher in those with both IFG and IGT compared with people with only IFG or IGT (8–11). A study of over 77,000 people with prediabetes found that the risk of developing type 2 diabetes increases with higher HbA1c levels and with higher BMI (12).
Diabetes Prevention in People with Prediabetes
Applied clinical research has shown that various interventions are effective in delaying or preventing the progression from prediabetes to type 2 diabetes. The Diabetes Prevention Program (DPP) clinical trial demonstrated that an intensive lifestyle intervention that focused on a healthy diet, physical activity, and approximately 7% weight loss reduced the incidence of type 2 diabetes in people with prediabetes by 58%. Metformin reduced the incidence of type 2 diabetes by 31% over 2.8 years (13). Results of this study indicated that to prevent one case of diabetes during a period of 3 years, 6.9 people would have to participate in the lifestyle intervention and 13.9 would need to be treated with metformin (13). The DPP enrolled high-risk participants who had both IGT and IFG. Those who have only IGT or IFG have a lower rate of progression to type 2 diabetes. Of note, the number of people who need to participate in a lifestyle intervention to prevent one case of diabetes (i.e., number needed to treat) would be higher for those at lower risk of progression. One meta-analysis of 19 studies conducted in adults with prediabetes, defined by either IGT, IFG, or both, and testing a variety of lifestyle interventions (e.g., differing intensity and duration; focusing on diet, physical activity, or both) found a relative risk reduction of 39% and a number needed to treat of 25 to prevent 1 case of diabetes (14). A second meta-analysis of 23 studies testing different interventions in similar populations found that lifestyle interventions were associated with a 22% reduction in the incidence of diabetes (15).
Subsequent analysis of the DPP clinical trial found that the lifestyle intervention was effective in all people with prediabetes, regardless of age, BMI, or baseline risk of progression to type 2 diabetes (13,16). While the absolute benefit of the lifestyle intervention varies based on risk of progression, this analysis demonstrates that those at lower baseline risk of progression also benefit from the lifestyle intervention. In contrast, metformin was more effective in younger individuals, those with higher BMIs, women with histories of gestational diabetes, and people at higher baseline risk of progression to type 2 diabetes. It was not as effective in those over 60 years of age (13,16,17). Using this knowledge to inform benefit-based tailored treatment can reduce overtreatment and make prevention of diabetes more efficient, effective, and patient centered (16,18). The effectiveness of lifestyle interventions and metformin has been confirmed by several other studies in people with prediabetes defined by different criteria (people with IFG or IGT) (19–22).
Significantly, the effectiveness of lifestyle interventions to decrease risk of progression to type 2 diabetes is sustained over several to many years, though attenuated. In long-term follow-up of the DPP cohort, diabetes incidence was reduced by 27% in the lifestyle intervention group over 15 years (23), and a meta-analysis of 19 studies of lifestyle interventions found that participants with prediabetes had a 28% lower risk of diabetes after mean follow-up of 7.2 years (14).
A recent study assessed the population health impact of the National Health System (NHS) DPP on the incidence of type 2 diabetes in England. Although published in August 2022, after the NCCC submitted its report to the Congress, this report provides a valuable perspective on the impact of a targeted diabetes prevention intervention on population health in a real-world setting. This 9- to 12-month intervention for adults ≥18 years of age with HbA1c levels of 6.0% to 6.4% or fasting glucose levels of 100–125 mg/dL involved attending at least 13 group-based behavior change sessions incorporating structured education on nutrition, physical activity, and weight loss. The NHS DPP was rolled out in three waves beginning in June 2016. Approximately 50% of English general practices were enrolled in the first wave, and a further 25% were enrolled in the second wave starting in April 2017. The DPP became available to all general practices beginning in April 2018. By April 2020, NHS DPP providers had received 513,312 participant referrals, of whom 271,208 (52.8% of the total) had attended an initial assessment and 101,175 (19.7% of total) had attended at least 60% of program sessions. Using data from the National Diabetes Audit, which records all individuals across England who have been diagnosed with type 2 diabetes, and a difference-in-differences methodology, the authors demonstrated that the incidence of type 2 diabetes in wave 1 and wave 2 practices was significantly lower than would have been expected in the absence of the NHS DPP (difference-in-differences incidence rate ratio of 0.938 [95% CI, 0.905–0.972] and difference-in-differences incidence rate ratio of 0.927 [95% CI, 0.885–0.972] in waves 1 and 2, respectively). Whereas the U.S. DPP clinical trial demonstrated that the incidence of type 2 diabetes was reduced by 58% during the study period and by 34% during observational follow-up among randomized participants, the evaluation of the NHS DPP demonstrated that it reduced the population incidence of type 2 diabetes by 6.2% and 7.3%. This evaluation is a proof of concept that a targeted diabetes prevention intervention with broad reach and high uptake can impact the entire population, not just program participants, in a real-world setting. These findings support efforts to improve and expand the National DPP and the Medicare Diabetes Prevention Program (MDPP) in the United States (24).
Analysis of DPP clinical trial data, most relevant to people who have both IFG and IGT, has demonstrated that both the DPP lifestyle intervention and metformin are cost-effective in preventing or delaying progression to type 2 diabetes. In the U.S., interventions that cost less than $100,000 per quality-adjusted life-year (QALY) gained are generally considered to be cost-effective (25,26). It has been estimated that over 3 years (the length of the DPP clinical trial) the DPP lifestyle intervention implemented in a small-group format (with 10 participants per group) costs $13,200 per case of type 2 diabetes delayed or prevented and $27,100 per QALY gained, and metformin costs $14,300 per case of type 2 diabetes delayed or prevented and $35,000 per QALY gained (27,28). Research shows that if the effects of the DPP lifestyle intervention are extended beyond the timeframe of the intervention (23), the cost per QALY gained would further decrease. It has been estimated that over 10 years, the DPP lifestyle intervention implemented in a group format would cost $8,412 per QALY gained; metformin use is associated with a small cost saving (28). For comparison, intensive blood glucose control for patients with newly diagnosed type 2 diabetes costs approximately $41,000 per QALY gained over a lifetime (29). Since the cost-effectiveness of an intervention is dependent on participants’ risk of progression to type 2 diabetes and the effectiveness of the intervention, if the lifestyle intervention were implemented in a population at lower risk of progression or the intervention was less effective than observed in the clinical trial, the cost to prevent a case of diabetes or gain a QALY would be higher and the intervention relatively less cost-effective.
There are other compelling reasons for people with prediabetes to participate in lifestyle programs focused on diet, physical activity, and weight loss aside from prevention of type 2 diabetes. People with prediabetes are at increased risk of CVD, chronic kidney disease, and death from any cause (30–32). Preventing CVD and other adverse health outcomes is therefore an important goal of diabetes prevention interventions. In the DPP clinical trial, the lifestyle intervention improved CVD risk factors (lower blood pressure, lower triglycerides, and higher HDL cholesterol) compared with placebo and metformin therapy (33). Longer-term follow-up of the DPP study cohort has shown a 39% lower CVD end point among participants who did not develop diabetes (34). Similarly, a meta-analysis of translation and effectiveness studies implementing the DPP in non–research settings found improvements in CVD risk factors (systolic and diastolic blood pressure, HDL, and total cholesterol) in program participants (35), highlighting that these additional benefits are realized in real-world settings.
Translation of the DPP Into Real-World Settings
Every year, approximately 1.5 million American adults are diagnosed with type 2 diabetes. Many of these cases could be prevented with earlier intervention (4). The initial DPP clinical trial and subsequent translation studies served as the model for the National DPP, a partnership of public and private organizations working to build the infrastructure necessary to support delivery of this lifestyle intervention throughout the U.S. The CDC provides support to National DPP delivery organizations and ensures program quality, setting specific requirements for data collection and reporting across all CDC-recognized program delivery organizations. Currently, the National DPP has over 2,000 CDC-recognized program delivery organizations across 50 states, the District of Columbia, Puerto Rico, the Virgin Islands, and several U.S. territories.
Of the approximately 14.6 million U.S. adults with diagnosed prediabetes and elevated BMI, ∼300,000 (2%) reported having been referred to a type 2 diabetes prevention program in 2016–2017. Potential barriers to uptake include low rates of screening and diagnosis of prediabetes, inadequate health care professional health care communication with at-risk patients, confusion as to who should be screened and referred, lack of CDC-recognized programs, and insufficient insurance coverage (36).
Currently, there are differences among the ADA, U.S. Preventive Services Task Force (USPSTF), and American Medical Association (AMA) recommendations for screening for prediabetes. In its 2022 Standards of Medical Care in Diabetes (37), the ADA recommends that screening for prediabetes and diabetes begin at age 35 years for all people. It also recommends that “testing for prediabetes and/or type 2 diabetes in asymptomatic people should be considered in adults of any age with overweight or obesity who have one or more risk factors” (37). In August 2021, the USPSTF updated its 2015 recommendation on screening for prediabetes and type 2 diabetes to recommend “screening for prediabetes and type 2 diabetes in adults aged 35–70 years who have overweight or obesity” (38). The AMA Prediabetes Quality Measures were published in 2018 and recommended that screening for abnormal blood glucose be assessed as “the percentage of patients aged 40 years and older with BMI ≥25 . . . who are screened for abnormal blood glucose at least once in the last three years” (39). The AMA’s recommendations were taken verbatim from the USPSTF 2015 recommendation that called for screening for abnormal blood glucose in adults 40–70 years of age who are overweight or obese. The AMA recognized the difference between the ADA and USPSTF recommendations and reconciled them by not having an upper age limit cutoff for the measure, essentially aligning with the ADA recommendation. Because participants with prediabetes over the age of 70 were shown to benefit from the lifestyle intervention in the DPP clinical trial, the NCCC adopted this pragmatic approach and recommended screening for prediabetes and type 2 diabetes in people 35 years of age and older who have overweight or obesity.
Better harnessing of the capabilities of electronic medical records to facilitate prediabetes case findings and referrals, increasing payment for the National DPP lifestyle change program to avoid supply distortions, and extending coverage and broadening access to diabetes prevention interventions have also been recommended to address the issues of inadequate professional health care communication with at-risk patients and referrals (40). Currently, the National DPP is offered in-person, online, via distance learning, and through a combination of these delivery modes to provide populations at high-risk greater access to the intervention. In response to data indicating lower retention rates for some participants in the National DPP (e.g., younger participants and some racial/ethnic groups) (41), the CDC, along with many of its partners, is also working to improve both participant engagement and retention in the program and has developed many resources to assist delivery organizations with this important effort.
In March 2016, the Secretary of Health and Human Services announced that the National DPP lifestyle change program met statutory eligibility criteria for expansion into Medicare as the MDPP. The decision of the Centers for Medicare and Medicaid Services (CMS) to cover the MDPP was based on an analysis that assessed the impact of the YMCA DPP on Medicare spending and utilization (42). The YMCA of the USA received a Healthcare Innovation Award from CMS to provide the DPP to Medicare beneficiaries with prediabetes in 17 regional networks of participating YMCA groups nationwide. Using claims data to compute total medical costs for fee-for-service and Medicare Advantage participants and a matched comparison group of nonparticipants, the investigators found that the overall weighted average savings per member per quarter during the first 3 years of the intervention was $278. The MDPP was approved for Medicare Part B or C beneficiaries who have prediabetes and also meet BMI and other program eligibility criteria. Prediabetes is defined as a fasting plasma glucose of 110–125 mg/dL, a plasma glucose level 2 h after a 75-g glucose challenge of 140–199 mg/dL, or an HbA1c of 5.7–6.4%. Beneficiaries who do not meet these criteria are not eligible to participate. Like the National DPP, the MDPP consists of a minimum of 16 intensive “core” sessions of a CDC-approved curriculum delivered over 6 months in a group-based, classroom-style setting, with monthly follow-up meetings thereafter, for a total of 12 months. Virtual (telehealth) and online programs are not included in the MDPP, although during the coronavirus disease 2019 (COVID-19) pandemic CMS allowed beneficiaries to participate virtually (43).
An evaluation of the MDPP published in March 2021 demonstrated that of the 2,248 beneficiaries served by the MDPP, beneficiaries attended 16 sessions on average and lost 5.1% of their initial body weight. Forty-nine percent of beneficiaries met the 5% weight loss goal (44). As of January 2022, only 315 organizations have been approved as MDPP suppliers, a small proportion of the 1,210 MDPP-eligible organizations with preliminary or full recognition by the CDC at that time (45). Based on feedback from program delivery organizations participating in the CDC’s Diabetes Prevention Recognition Program, the MDPP reimbursement rate is a barrier to MDPP program availability and sustainability, and payments that are dependent on participants achieving at least 5% weight loss may be problematic. Currently, the cost of delivering the National DPP lifestyle change program may outweigh Medicare reimbursement amounts, especially in large urban health systems serving diverse populations. Starting in 2022, CMS reimbursement for participants who meet all performance benchmarks increased 56%, from $450 to $705 per person (46). It is too early to know whether this change in payment will increase MDPP supply. A pay-for-performance funding model may also have a detrimental impact on health equity. Non-Hispanic White adults are most likely to be retained in the MDPP and to achieve the 5% weight loss goal linked to MDPP pay-for-performance reimbursement. Pay for performance might lead providers to offer the MDPP in affluentWhite neighborhoods, leading to higher participation by White individuals and an increased gap in diabetes prevalence between non-Hispanic White adults and other groups (41).
As of April 2022, over 600,000 adults at high risk for type 2 diabetes had enrolled in the National DPP lifestyle change program. In 2016–2017, median retention was 28 weeks and the median number of sessions attended was 16. Sixty-three percent of participants were retained in the program through the 18th week, and 32% of participants were retained through the entire program. Retention was associated with older age, non-Hispanic White race, and success in the program as assessed by early weight loss and greater reported physical activity (41). While people with prediabetes and their clinicians may choose to individualize the decision to participate in a lifestyle change program based on factors such as degree of glycemia, BMI, and presence of other medical conditions and priorities, the vast majority of people with prediabetes have not participated in lifestyle change programs or been prescribed metformin, despite the proven efficacy and cost-effectiveness of these interventions (47). To date, the FDA has not approved metformin for type 2 diabetes prevention. Prescribing metformin for people with prediabetes is therefore off-label, which may contribute to a lack of patient and clinician awareness of the benefits of metformin. Additionally, clinicians may presume that patients would prefer not to take a medication for type 2 diabetes prevention (48).
When framing type 2 diabetes prevention efforts through the lens of health equity, which is achieved when every person has the opportunity to attain their full health potential and no one is disadvantaged from achieving this potential because of social position or other socially determined circumstances, the current lack of access to lifestyle programming represents a missed opportunity. The U.S. Department of Health and Human Services defined the “elimination of health disparities and achievement of health equity” as one of their “most critical public health goals” for the 2020–2030 national plan (49). As has been well documented, type 2 diabetes disproportionately affects American Indian/Alaska Native, Black, Hispanic, and Asian people in the U.S. (4), and social determinants of health strongly affect type 2 diabetes prevalence in communities. To help address these disparities, we must ensure that preventative interventions are accessible to all people with prediabetes and are equitably implemented in all populations. Achieving these goals requires the sustainment and enhancement of federal programs and activities related to diabetes prevention, including targeted outreach, evidence-based interventions, and research support.
Methods
The methods employed by the NCCC have been described previously (50). Briefly, the 23 members of the NCCC formed three subcommittees that gathered information from federal agencies, stakeholders, key informants, and the public as well as a systematic search and review of the scientific literature. Through an iterative process, the NCCC developed broad recommendations for the Secretary of Health and Human Services and Congress regarding diabetes prevention and treatment. In this report, we describe the recommendations for diabetes prevention among people at high risk for developing diabetes, including those with prediabetes.
Results
Increase Awareness of Prediabetes and the Diagnosis of Prediabetes
Expand Support for the CDC’s National Public Service Campaign
Since 2016, the CDC has collaborated with the Ad Council on a national public service campaign to raise awareness of prediabetes. Since the start of the campaign, approximately 4 million individuals visited the Prediabetes Awareness Campaign website and completed the prediabetes risk test. Although these initial numbers are promising, support is still needed to continue these outreach efforts to reach the intended audience. Most Americans with prediabetes are still unaware of their condition and have not enrolled in the National DPP lifestyle intervention (48,51). In fact, only 19% of adults with prediabetes have been informed by a health care professional that they have prediabetes, with numbers especially low for young and early-middle-aged adults, men, and individuals of Asian or Hispanic ancestry (4). In addition, only 4.9% of adults diagnosed by a physician with prediabetes were advised to participate in a diabetes prevention program (51). This underscores the need to improve awareness of prediabetes and the National DPP among both patients and clinicians.
Recommendation
The NCCC recommends increasing support to the CDC for its campaign to raise awareness of prediabetes and the National DPP lifestyle change program. This includes the following steps:
The CDC should utilize various marketing methods, including social media, to increase awareness of prediabetes for populations disproportionately affected by type 2 diabetes.
The CDC should continue tracking visits to the “Do I Have Prediabetes?” campaign webpage and completions of the prediabetes risk test. An expanded focus on the degree to which populations at increased risk are being reached would help to reduce disparities in awareness and could increase engagement in interventions.
Provide CMS Coverage for HbA1c as a Screening Test for Prediabetes
The USPSTF and the 2022 ADA Standards of Medical Care in Diabetes both recommend FPG, oral glucose tolerance tests (OGTTs), and HbA1c as appropriate tests for clinicians to use in screening for and diagnosing prediabetes and diabetes (37,38). However, Medicare does not cover HbA1c testing for prediabetes screening, potentially contributing to low rates of screening among Medicare beneficiaries. The two tests that are covered (FPG and OGTTs) may present logistical barriers (fasting for FPG and extended visits for OGTTs) to identifying patients with prediabetes. These logistical issues do not apply to HbA1c testing.
Recommendation
The NCCC recommends that CMS provide coverage for HbA1c testing when used to screen for prediabetes.
Adopt AMA Proposed Clinical Quality Measures for Prediabetes Screening, Intervention, and Follow-up
In 2019, a technical expert panel convened by the AMA proposed three electronic clinical quality measures for review by the National Quality Forum to monitor and improve the quality of care for patients with prediabetes. The proposed measures, as recently revised, are the following:
Screen patients aged ≥35 years with a BMI ≥25 kg/m2 for abnormal blood glucose at least once in the previous 3 years
- Provide one of the following interventions for patients with prediabetes during the 12 months following determination of abnormal blood glucose:
- ∘ Referral to a CDC-recognized diabetes prevention program
- ∘ Referral to medical nutrition therapy with a registered dietitian
- ∘ Prescription of metformin
Retest patients’ glycemia in the year after they were identified with prediabetes (the measurement of glycemia is currently under revision as a potential quality outcome measure, i.e., the percentage of patients who do not progress to type 2 diabetes during a defined time period)
Recent studies found marked variation in levels of screening for prediabetes and frequent failure to document a diagnosis of prediabetes when the diagnostic criteria are met. In addition, significant gaps in awareness of CDC-recognized organizations offering the National DPP were also noted (52–54). These findings emphasize the salience of the proposed quality measures to monitor and improve the timely diagnosis of prediabetes and implementation of preventive measures.
The opportunity to identify and intervene for patients at risk for type 2 diabetes may be missed during acute or routine medical visits because of competing priorities or incomplete information available at the time. Registries of patients at high risk or already meeting the criteria for prediabetes (that is, on the basis of BMI, history of hypertension, and glucose or HbA1c results) could help prompt clinic staff to contact patients to discuss prediabetes, offer definitive diagnostic testing, and offer referrals to the National DPP or MDPP lifestyle change programs. Projects that have systematically retrieved results from medical records to identify and report patients with prediabetes have shown improvement in referrals to the National DPP lifestyle change program (55,56).
Recommendation
The NCCC recommends that all federal agencies that directly deliver or influence the delivery of medical care implement the revised 2019 AMA proposed prediabetes quality measures related to screening for abnormal glycemia, intervention for prediabetes, and retesting of abnormal glycemia in patients with prediabetes. These agencies should implement a process for systematically using administrative and clinical data to identify patients at risk for or already meeting criteria for prediabetes and to ensure appropriate referral and follow-up.
To support implementation of the revised 2019 AMA proposed prediabetes quality measures related to screening and interventions for abnormal glycemia, quality improvement programs should be introduced to improve performance and reduce disparities.
Increasing the Availability of, Referral to, and Insurance Coverage for Effective Diabetes Prevention Interventions
Simplify and Harmonize National DPP and MDPP Rules for Program Recognition and Payment and Increase Payment Rates to Ensure Program Sustainability
In response to the growing prevalence of type 2 diabetes in the U.S., Congress authorized the CDC to establish the National DPP in 2010 (57). In 2017, the Physician Fee Schedule final rule enabled National DPP program delivery organizations with full or preliminary CDC recognition to enroll as MDPP suppliers (58). However, some National DPP providers in rural and underserved areas may experience challenges in achieving full CDC recognition and applying to become MDPP suppliers due to increased administrative burden. Differences also exist between the MDPP and National DPP structures, including blood glucose eligibility criteria and allowable service delivery modalities, which may make it difficult for a provider organization to deliver both the National DPP lifestyle change program and MDPP.
Recommendation
The NCCC recommends that the CDC continues to streamline the National DPP recognition process while maintaining quality and that CMS coordinates with the CDC to harmonize MDPP processes. Differences in program eligibility and delivery modalities between the National DPP (led by the CDC) and the MDPP (led by CMS) should also be eliminated or, at minimum, reduced.
Ensure Reimbursement for All Proven Effective Modes of Diabetes Prevention Program Delivery (in Person, Telehealth, and Online)
Federal agencies use a variety of methods (e.g., in person, online, and distance learning [telehealth]) to deliver evidence-based interventions to delay or prevent type 2 diabetes (57). Other diabetes-related interventions, such as the Department of Defense Diabetes Center of Excellence Virtual Diabetes Self-Management Education Program, have also been implemented successfully in a fully virtual platform for military health system beneficiaries. However, payer coverage for these different delivery methods in the general population varies and is often nonexistent. Promoting and improving coverage for evidence-based type 2 diabetes prevention interventions through a variety of delivery methods could improve access.
Recommendation
The NCCC recommends that Congress promote coverage for all proven effective methods of delivery for evidence-based interventions that produce successful participant outcomes that meet or exceed those of the National DPP quality standards.
Mandate Private Insurance Coverage for the National DPP Lifestyle Change Program Under the Provisions of the Affordable Care Act
Section 2713 of the Affordable Care Act requires private health plans to cover certain evidence-based preventive services and to eliminate cost sharing for preventive care, including preventive services recommended by the USPSTF (59). The current USPSTF recommendation on screening for type 2 diabetes includes the following recommendation: “Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” noting that “lifestyle interventions that focus on diet, physical activity, or both and metformin have demonstrated efficacy in preventing or delaying progression to diabetes in people with prediabetes” (38). Private insurers are not consistently providing coverage for the National DPP lifestyle change program, a proven effective diabetes prevention intervention. This may contribute to underutilization and fewer cases of type 2 diabetes being prevented.
Recommendation
The NCCC recommends, consistent with provisions of the Patient Protection and Affordable Care Act, that all insurers be required to provide coverage for participation in and completion of a CDC-recognized diabetes prevention program for those who are eligible.
Expand MDPP Access and Sustainability by Eliminating Barriers
The MDPP was approved as a model expansion service in 2016. It is an innovative service delivery model based on the National DPP. The MDPP expanded model is currently being evaluated based on factors such as quality of care delivered, patient outcomes, and costs (44). MDPP services are covered services under the model expansion, pending results of the evaluation (58); however, the original lifestyle intervention has already been studied extensively and has substantial evidence supporting its effectiveness across settings and populations.
Additionally, full virtual delivery of the MDPP is not currently included under the expanded model. This likely affected MDPP uptake and completion during the COVID-19 pandemic and is inconsistent with the National DPP, which allows virtual delivery and requires virtual delivery organizations to meet the same CDC national quality standards and achieve the same participant outcomes as in-person delivery organizations. During the COVID-19 pandemic, CMS allowed for virtual delivery of MDPP services; however, it is unclear whether this option will remain in place after the pandemic.
Finally, there is a once-in-a-lifetime limit on the MDPP service (60). However, people may not be able to fully engage in or complete the program, which may necessitate them repeating the program or re-enrolling at a future date. Currently, it is not possible to do this.
Recommendation
The NCCC recommends that the Medicare DPP be approved as a permanent covered benefit (not only a model expansion service) and that coverage of the MDPP be expanded to include virtual delivery. Furthermore, the once-in-a-lifetime limit on participation in the MDPP should be removed.
Update the MDPP Payment Model
The calendar year 2017 and 2018 Physician Fee Schedule final rules (58,61) established the benefit structure and payment rates for the MDPP based on a diabetes prevention program model test conducted by the YMCA of the USA from 2013 to 2015. The current MDPP payment model offers reimbursement only when participants reach certain attendance and weight loss benchmarks.
Under this model, program delivery organizations assume a level of risk and may be under-resourced to cover the upfront costs associated with program certification, marketing, and participant engagement and enrollment. Current reimbursement rates may not fully incentivize program delivery organizations to apply to become MDPP suppliers, as only a limited number of eligible organizations with CDC preliminary or full recognition have applied to become MDPP suppliers. This limits availability of the MDPP for Medicare beneficiaries with prediabetes and may also have a disproportionate impact on smaller and rural programs that often serve populations at increased risk.
Recommendation
The NCCC recommends that funding be provided to support the testing of new payment models that allow for greater upfront payments and more equitable risk-sharing between CMS and MDPP program delivery organizations. In addition, there should be an increase in payment levels to MDPP program delivery organizations to make MDPP programs financially sustainable. The NCCC notes that the CMS calendar year 2022 Physician Fee Schedule final rule may better align the duration of the MDPP and National DPP and will increase MDPP payment for participants who attend at least 9 sessions.
Provide Incentives for State Medicaid Programs to Cover Proven Effective Diabetes Prevention Programs
Medicaid coverage for the National DPP lifestyle change program is a state-level decision. Since 2012, 20 states have enacted varying levels of Medicaid coverage for the National DPP lifestyle change program (62). There are variations across states in 1) whether the National DPP lifestyle change program is a benefit covered by Medicaid; 2) delivery modes covered (i.e., in person, online, distance learning, and telehealth); and 3) the level of reimbursement. Additionally, risk for type 2 diabetes is higher in Medicaid beneficiaries, a population that is vulnerable to financial barriers to services. Using information from non–disability-based adult Medicaid beneficiaries 19–64 years of age at high risk for type 2 diabetes and a decision analytic simulation model, Laxy et al. (63) assessed the incremental cost-effectiveness ratios of covering versus not covering lifestyle interventions for prevention of type 2 diabetes in the Medicaid population. From a health care system perspective, they found that an initial program investment of $800 per person would be offset after 13 years and subsequently translate into cost savings. Minorities and low-income groups would benefit most from the intervention if it were offered by Medicaid (63).
Recommendation
The NCCC recommends that financial incentives be provided for state Medicaid programs to cover the National DPP lifestyle change program for Medicaid beneficiaries with prediabetes. Coverage should include all proven methods of delivery (i.e., in person, online, and distance learning or telehealth) that produce successful participant outcomes.
Support Additional Federal Programs Focusing on Diabetes Prevention
American Indian and Alaska Native individuals have the highest prevalence of diabetes of any racial and ethnic group (4). In response, the Special Diabetes Program for Indians (SDPI) was established by Congress in 1997 to support diabetes prevention and treatment among American Indian and Alaska Native communities. The SDPI is coordinated by the Indian Health Service (IHS) Division of Diabetes Treatment and Prevention with guidance from the Tribal Leaders Diabetes Committee. It provides funds for diabetes treatment and prevention to IHS, tribal, and urban Indian health programs (64). By maintaining a focus on diabetes prevention and leveraging SDPI funds, IHS and tribes implemented programs and services that contributed to lowering prevalence of diabetes in American Indian and Alaska Native adults over 4 years, from 15.4% in 2013 to 14.6% in 2017 (65,66). However, funding for this program has not increased since 2004.
There are also geographic disparities in diabetes prevalence. Alabama has the highest prevalence of diabetes (13.2%) among all U.S. states. The U.S. regions with the highest diabetes prevalence are in the Southeast and Appalachia; rural areas have a higher diabetes prevalence and generally have less medical infrastructure than urban areas (67–69). The Health Resources and Services Administration (HRSA) Delta States Rural Development Network Grant Program provides grants to the eight states in the Mississippi Delta for network and rural health infrastructure development (70). Grantees are required to focus on diabetes, CVD, and obesity but not specifically on type 2 diabetes prevention. Given the higher burden of type 2 diabetes in the Southern U.S. and the proven effectiveness of diabetes prevention interventions, providing additional resources to the HRSA Delta States Rural Development Network Grant Program would allow the program to include type 2 diabetes prevention as a focus while not detracting from the program’s, or HRSA’s, other important aims.
Recommendations
Funding for the SDPI should be made in 5-year increments so that evidence-based tribal diabetes prevention programs have the resources to 1) sustain the effort to combat diabetes and its complications; 2) develop additional culturally appropriate, high-impact type 2 diabetes prevention interventions; and 3) evaluate outcomes.
Increase funding for SDPI to address inflation costs, which have consumed more than 34% of the program’s resources since 2004, the last year Congress increased funding for the program. In the future, annual increases in funding should, at a minimum, address the costs of inflation.
Increase funding to HRSA’s Delta States Rural Development Network Grant Program to allow the program to include type 2 diabetes prevention as a focus.
Facilitate an Application to the FDA for Approval of Metformin for Diabetes Prevention
Metformin was approved by the FDA in 1995 for treatment of type 2 diabetes, and rigorous scientific evidence supports its safety and effectiveness in delaying the onset of type 2 diabetes in individuals at high risk with prediabetes (71). However, because metformin is not FDA approved for this purpose, prescribing it for prediabetes is an off-label use, and therefore metformin use in prediabetes treatment may be less frequent.
The DPP Research Group demonstrated that during the 3-year clinical trial, metformin, compared with the placebo intervention, reduced the incidence of diabetes by 34%, and during 15 years of follow-up it reduced diabetes incidence by 18% compared with placebo. After only 2 weeks of treatment withdrawal, the benefit of metformin therapy for diabetes prevention was attenuated (72). A systematic review and meta-analysis of randomized clinical trials demonstrated attenuation of all medication effects for diabetes prevention at the end of the washout period (14). The fact that no medication trials to date have shown a persistent benefit on diabetes prevention after medication withdrawal has indeed been a stumbling block for FDA approval of metformin for type 2 diabetes prevention. Requiring that a pharmacologic therapy for diabetes prevention alter the natural history of prediabetes and type 2 diabetes does not seem to be reasonable, since the complications of diabetes arise as a result of the degree and duration of hyperglycemia. Interventions to delay or prevent the onset of hyperglycemia are beneficial, just as those for hypertension and dyslipidemia are beneficial, without necessarily changing untreated blood pressure levels or lipid profiles if treatment is withdrawn.
Although there were no overall differences in the aggregate microvascular outcome in the metformin and placebo groups in the DPP, those who did not progress to diabetes had a 28% lower prevalence of microvascular complications than those who progressed. At the time the NCCC Report was submitted to Congress, there were no published data on the long-term effects of metformin on cardiovascular outcomes. In May 2022, the DPP Research Group published data describing the long-term effects of metformin on cardiovascular events (73). Over a 21-year median follow-up, the first occurrence of nonfatal myocardial infarction, stroke, or cardiovascular death did not differ between the metformin and placebo groups. Risk factor adjustment did not change these results, and no effect was apparent when a broader cardiovascular outcome was assessed. Thus, despite decreasing diabetes development, metformin did not reduce major adverse cardiovascular events compared with placebo. These results must be viewed in the context of the modest progression of hyperglycemia, extensive out-of-study use of lipid-lowering and antihypertensive medications, provision of a lifestyle intervention to all Diabetes Prevention Program Outcomes Study participants, and increased out-of-study metformin use over time, which may have limited the apparent effects of the intervention.
Although other pharmacologic treatments have been shown to be effective for type 2 diabetes prevention, including weight loss medications (orlistat and phentermine-topiramate), thiazolidinediones, α-glucosidase inhibitors, and even insulin glargine, the NCCC did not recommend them for diabetes prevention. A more recent study has also highlighted the potential role of glucagon-like peptide 1-receptor agonists for type 2 diabetes prevention (74). Because every kilogram of weight lost is associated with an additional 7% decrease in risk of progression to diabetes (14), newer treatments, including once-weekly glucagon-like peptide 1-receptor agonists and new dual receptor agonists such as tirzepatide, have great potential to reduce weight and prevent type 2 diabetes if rigorously demonstrated to be safe and effective for this indication.
Because there is no comprehensive synthesis of available data, pursuing FDA approval of metformin would require the applicant to collect, analyze, and organize data to show the safety and effectiveness of metformin in patients with prediabetes. As multiple generic versions of metformin exist, pharmaceutical companies have little incentive to do this. While data could be submitted to the FDA for review through other means, i.e., a Citizen’s Petition, the costs and amount of work involved with filing a Citizen’s Petition are high (75).
Recommendation
The NCCC recommends that funding be provided to the National Institutes of Health to fund a third party to collect, analyze, and summarize the available data from the Diabetes Prevention Program clinical trial describing the effectiveness and safety of metformin for type 2 diabetes delay or prevention in patients with prediabetes, including subpopulations most likely to benefit. Such a summary (with safety and efficacy data) should then be used to inform an appropriate submitter’s request for the FDA to review and consider an indication for the use of metformin in high-risk patients with prediabetes.
Support Research to Enhance the Effectiveness of Interventions for Type 2 Diabetes Prevention and Improve Our Understanding of the Etiology and Opportunities for Prevention of Type 1 Diabetes
Support Research to Understand Who Is Most Likely to Benefit From Participation in Diabetes Prevention Lifestyle Interventions and From Metformin to Better Target These Interventions to Those at Risk for Type 2 Diabetes
Despite the remarkable outcomes of the DPP, most people with prediabetes have not participated in a diabetes prevention program such as the National DPP lifestyle change program and are not taking metformin (47). The reasons for not using metformin for prediabetes vary greatly and, as referenced previously, may include 1) physicians not wanting to use medication to treat people with prediabetes; 2) physicians’ and patients’ lack of awareness of the benefit of using metformin; 3) concerns about possible side effects of metformin; 4) concerns about lack of FDA approval for use of metformin in treating prediabetes; or 5) a combination of these reasons. These factors emphasize the importance of further studies on metformin uptake and alternative medication choices to treat prediabetes.
People with prediabetes are a heterogeneous group. In addition to social, geographic, financial, or cultural barriers, individuals have different physiologic characteristics that contribute to dysglycemia. As a result, some people with prediabetes may develop type 2 diabetes and other complications (e.g. CVD and kidney failure) more quickly than others (76). More research could improve our understanding of how these parameters affect specific risk factors and lead to the development of individually tailored screening and both lifestyle and pharmacologic interventions to maximize effectiveness. Solutions, strategies, and policies need to be developed that can be implemented and sustained at scale. Research to assess the performance of screening tests and efficacy of interventions across racial and ethnicity populations is also needed (77).
Recommendation
- The NCCC recommends funding type 2 diabetes prevention research to discover how to ensure that all individuals at high risk of developing type 2 diabetes can lower their risk for diabetes and its complications. Examples of areas for further research include the following:
- ∘ What impediments prevent participation in effective diabetes prevention programs for communities with the greatest needs?
- ∘ Are programs that combine both lifestyle intervention and metformin to prevent diabetes more effective than programs with either lifestyle change or metformin alone?
- ∘ What is the best number, frequency, duration, and content of lifestyle intervention sessions to successfully prevent diabetes in the long term?
- ∘ What are the barriers and solutions to long-term maintenance of weight loss for those people who successfully complete a diabetes prevention program?
- ∘ What are the barriers and solutions at the health system, provider, and patient levels to implementation, and how can in-person and virtual diabetes prevention programs be more effectively implemented?
Support Research to Elucidate the Causes and Prevention of Type 1 Diabetes
Scientifically, it is still not well understood why people develop type 1 diabetes or how it can be best prevented (78). Approximately 30% of patients with new-onset type 1 diabetes present with diabetic ketoacidosis (DKA) (79,80), a serious yet avoidable acute metabolic complication. Some interventions (such as immune modulators and monoclonal antibodies) may be able to delay or prevent type 1 diabetes (81). A better understanding of the causes of type 1 diabetes can help identify those at high risk before they develop type 1 diabetes complications such as diabetic ketoacidosis.
In 1998 Congress passed the Special Statutory Funding Program for Type 1 Diabetes Research, also known as the Special Diabetes Program (SDP). This program has resulted in substantial progress in type 1 diabetes research and development of innovative collaborative research consortia and clinical trials networks. The SDP has funded research studies such as The Environmental Determinants of Diabetes in the Young (TEDDY) and the Type 1 Diabetes TrialNet, both of which have improved our understanding of the basic biological mechanisms of type 1 diabetes and are making strides to discover new treatment and prevention modalities (81,82). Additional research is needed to leverage emerging data from TEDDY and TrialNet to 1) develop screening programs to identify people at high risk for type 1 diabetes who might benefit from interventions; 2) develop efficient and cost-effective screening methods for type 1 diabetes in the general population; and 3) advance research to prevent type 1 diabetes.
The SDP was originally funded for 5-year intervals, but the program most recently has been funded for shorter intervals, sometimes on an annual basis. This change to short-term funding inhibits opportunities for research progress because it limits planning and initiation of long-term research projects. Sustained multiyear funding could help use federal dollars more effectively, maximize research opportunities for long-term studies such as TEDDY and TrialNet, and pursue new promising treatment and prevention studies and trials. Additionally, the SDP funding for type 1 diabetes research has been level at $150 million since 2004, without increases to account for inflation.
Recommendations
The NCCC recommends funding the SDP in 5-year increments so that new, innovative research can be developed effectively.
An increase in SDP program funding is needed to address inflation costs. Inflation costs have consumed more than 34% of the program’s resources since 2004, the last year Congress increased funding for the SDP. In the future, annual increases in funding should, at minimum, address the costs of inflation.
Summary and Conclusions
Reducing the incidence and prevalence of diabetes in the U.S. is a public health priority. Moreover, ensuring that type 2 diabetes prevention interventions are accessible, available, and equitably implemented is crucial to addressing this priority. With this objective in mind, the NCCC established a subcommittee to evaluate federal policies and programs for the prevention of type 2 diabetes and its complications in targeted, high-risk populations, namely, those with prediabetes. Through a 3-year process of key informant and stakeholder interviews, review of federal agency documents, and examination of relevant scientific literature, the subcommittee developed 13 recommendations for Congress and the U.S. Department of Health and Human Services to address diabetes prevention in these targeted populations. Evidence-based recommendations included the following:
Increased awareness and advocacy for the National DPP
Expanded coverage for prediabetes/type 2 diabetes screening and diagnostic testing
Adoption and promotion of clinical quality standards
Support for the use of metformin in prediabetes
Requirements for insurance coverage and permanent benefit status for prevention programs and various delivery modalities
Sustainable funding and support for new payment models
Streamlining and harmonizing the National DPP and the MDPP
Enhancement of state Medicaid coverage for the National DPP lifestyle change program
Support for special federal programs dedicated to American Indians and Alaskan Native communities and networks of rural Mississippi Delta communities
Lastly, the NCCC subcommittee made recommendations on applied research of essential diabetes prevention programs, including ways to optimize intervention program effectiveness, and research on prevention of type 1 diabetes.
Article Information
Acknowledgments. The NCCC acknowledges Alicia A. Livinski and Nancy L. Terry, biomedical librarians from the National Institutes of Health Library, Division of Library Services, Office of Research Services, who performed the literature searches. The NCCC also thanks Yanni Wang (International Biomedical Communications) and Heather Stites (University of Michigan) for their editorial assistance.
Funding. The NCCC was supported through a Joint Funding Agreement among eight federal agencies: the Agency for Healthcare Research and Quality (AHRQ), the Centers for Disease Control and Prevention (CDC), the Centers for Medicare and Medicaid Services (CMS), the Food and Drug Administration (FDA), the Health Resources and Services Administration (HRSA), the Indian Health Service (IHS), the National Institutes of Health (NIH), and the Office of Minority Health (OMH). The Office of the Assistant Secretary for Health (OASH), the Office of Disease Prevention and Health Promotion (ODPHP), and the Office on Women’s Health (OWH) provided management staff and contractor support.
The funders had no role in the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Health and Human Services or other departments and agencies of the federal government.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this study were presented at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3–7 June 2022.
Footnotes
C.P. was the Designated Federal Officer for the National Clinical Care Commission. All other authors were members of the National Clinical Care Commission.
J.M.B. and H.T. made equal contributions as first authors.
This article is part of a special article collection available at https://diabetesjournals.org/collection/1586/The-Clinical-Care-Commission-Report-to-Congress.
References
- 1. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 2. National Clinical Care Commission . Report to Congress on Leveraging Federal Programs to Prevent and Control Diabetes and Its Complications, 2021. Accessed 10 August 2022. Available from https://health.gov/about-odphp/committees-workgroups/national-clinical-care-commission/report-congress
- 3. Centers for Disease Control and Prevention . Prediabetes–Your Chance to Prevent Type 2 Diabetes, 2021. Accessed 24 February 2022. Available from https://www.cdc.gov/diabetes/basics/prediabetes.html
- 4. Centers for Disease Control and Prevention . National Diabetes Statistics Report. Accessed 12 August 2022. Available from https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 5. Centers for Disease Control and Prevention . One in 5 adolescents and 1 in 4 young adults now living with prediabetes. Accessed 15 December 2022. Available from https://www.cdc.gov/media/releases/2019/p1202-diabetes.html
- 6. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan DM, Davidson MB, DeFronzo RA, et al.; American Diabetes Association . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 8. Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–312 [DOI] [PubMed] [Google Scholar]
- 9. Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . Ten-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2011;58:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heianza Y, Hara S, Arase Y, et al. HbA1c 5.7-6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011;378:147–155 [DOI] [PubMed] [Google Scholar]
- 12. Glauber H, Vollmer WM, Nichols GA. A simple model for predicting two-year risk of diabetes development in individuals with prediabetes. Perm J 2018;22:17–050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2017;177:1808–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jonas D, Crotty K, Yun JD, et al. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: An Evidence Review for the U.S. Preventive Services Task Force. Evidence synthesis no. 207. AHRQ publication 21-05276-EF-1. Rockville, MD, Agency for Healthcare Research and Quality, 2021 [PubMed] [Google Scholar]
- 16. Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ 2015;350:h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diabetes Prevention Program Research Group . Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 2019;42:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herman WH, Pan Q, Edelstein SL, et al.; Diabetes Prevention Program Research Group . Impact of lifestyle and metformin interventions on the risk of progression to diabetes and regression to normal glucose regulation in overweight or obese people with impaired glucose regulation. Diabetes Care 2017;40:1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saito T, Watanabe M, Nishida J, et al.; Zensharen Study for Prevention of Lifestyle Diseases Group . Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 20. Sakane N, Kotani K, Takahashi K, et al. Effects of telephone-delivered lifestyle support on the development of diabetes in participants at high risk of type 2 diabetes: J-DOIT1, a pragmatic cluster randomised trial. BMJ Open 2015;5:e007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 22. Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes prevention. Am J Prev Med 2018;55:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diabetes Prevention Program Research Group . Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McManus E, Meacock R, Parkinson B, Sutton M. Population level impact of the NHS Diabetes Prevention Programme on incidence of type 2 diabetes in England: an observational study. Lancet Reg Health Eur 2022;19:100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–797 [DOI] [PubMed] [Google Scholar]
- 26. Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann Intern Med 2021;174:25–32 [DOI] [PubMed] [Google Scholar]
- 27. Diabetes Prevention Program Research Group . Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diabetes Prevention Program Research Group . The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. CDC Diabetes Cost-effectiveness Group . Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–2551 [DOI] [PubMed] [Google Scholar]
- 30. Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ 2020;370:m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honigberg MC, Zekavat SM, Pirruccello JP, Natarajan P, Vaduganathan M. Cardiovascular and kidney outcomes across the glycemic spectrum: insights from the UK Biobank. J Am Coll Cardiol 2021;78:453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. Br Med J (Clin Res Ed) 1983;287:867–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratner R, Goldberg R, Haffner S, et al.; Diabetes Prevention Program Research Group . Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 2005;28:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nathan D, Molitch M, Goldberg RB, et al. DPPOS Shows Long-Term Diabetes Prevention with Lifestyle and Metformin Intervention; Preventing Diabetes Lowers Risk of Vascular Complications. ADA Scientific Sessions, 2020 [Google Scholar]
- 35. Mudaliar U, Zabetian A, Goodman M, et al. Cardiometabolic risk factor changes observed in diabetes prevention programs in US settings: a systematic review and meta-analysis. PLoS Med 2016;13:e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and use of diabetes prevention services in the United States, 2016-2017. JAMA Netw Open 2019;2:e193160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 38. Davidson KW, Barry MJ, Mangione CM, et al.; US Preventive Services Task Force . Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA 2021;326:736–743 [DOI] [PubMed] [Google Scholar]
- 39. American Medical Association (AMA) Prediabetes Quality Measures Technical Expert Panel . Prediabetes Quality Measures Revised: Final, 2019. Accessed 26 September 2022. Available from https://amapreventdiabetes.org/ama-publications
- 40. Alva ML, Chakkalakal RJ, Moin T, Galaviz KI. The diabetes prevention gap and opportunities to increase participation in effective interventions. Health Aff (Millwood) 2022;41:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cannon MJ, Masalovich S, Ng BP, et al. Retention among participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012–2017. Diabetes Care 2020;43:2042–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alva ML, Hoerger TJ, Jeyaraman R, Amico P, Rojas-Smith L. Impact of the YMCA of the USA diabetes prevention program on Medicare spending and utilization. Health Aff (Millwood) 2017;36:417–424 [DOI] [PubMed] [Google Scholar]
- 43. Centers for Medicare & Medicaid Services . Medicare Diabetes Prevention Program (MDPP) Expanded Model Public Health Emergency (PHE) Flexibilities. Available from https://innovation.cms.gov/media/document/mdpp-phe-flexibilities-faqs
- 44. Hoerger TJ, Jacobs S, Romaine M, et al. Evaluation of the Medicare Diabetes Prevention Program. RTI project number 0214448.001.011.000.004. RTI International, 2021 [Google Scholar]
- 45. CMS Innovation Center Programs . Medicare Diabetes Prevention Program. Available from https://data.cms.gov/cms-innovation-center-programs/alternative-payments-medicare-diabetes-prevention-program/medicare-diabetes-prevention-program
- 46. Centers for Medicare & Medicaid Services (CMS), Health and Human Services (HHS) . Medicare Program; CY 2022 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Provider Enrollment Regulation Updates; and Provider and Supplier Prepayment and Post-Payment Medical Review Requirements. Federal Register 86 FR 64996, 19 November 2021. [Google Scholar]
- 47. Schmittdiel JA, Adams SR, Segal J, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care 2014;37:565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kandula NR, Moran MR, Tang JW, O’Brien MJ. Preventing diabetes in primary care: providers’ perspectives about diagnosing and treating prediabetes. Clin Diabetes 2018;36:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. U.S. Department of Health and Human Services . Healthy People 2030 Questions & Answers, 2021. Accessed 24 February 2022. Available from https://health.gov/our-work/national-health-initiatives/healthy-people/healthy-people-2030/questions-answers#q1
- 50. Herman WH, Bullock A, Boltri J, et al. The National Clinical Care Commission Report to Congress: background, methods, and foundational recommendations. Diabetes Care 2023;46:e14–e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. U.S. Food and Drug Administration . FDA Updates and Press Announcements on NDMA in >Metformin, 2021. Accessed 24 February 2022. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-metformin
- 52. Keck JW, Thomas AR, Hieronymus L, Roper KL. Prediabetes knowledge, attitudes, and practices at an academic family medicine practice. J Am Board Fam Med 2019;32:505–512 [DOI] [PubMed] [Google Scholar]
- 53. Nhim K, Khan T, Gruss SM, et al. Primary care providers’ prediabetes screening, testing, and referral behaviors. Am J Prev Med 2018;55:e39–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tseng E, Greer RC, O’Rourke P, et al. Survey of primary care providers’ knowledge of screening for, diagnosing and managing prediabetes. J Gen Intern Med 2017;32:1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holliday CS, Williams J, Salcedo V, Kandula NR. Clinical identification and referral of adults with prediabetes to a diabetes prevention program. Prev Chronic Dis 2019;16:E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keck JW, Roper KL, Hieronymus LB, et al. Primary care cluster RCT to increase diabetes prevention program referrals. Am J Prev Med 2020;59:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Centers for Disease Control and Prevention . National Diabetes Prevention Program: About the National DPP, 2018. Accessed 4 August 2021. Available from https://www.cdc.gov/diabetes/prevention/about.htm
- 58. Centers for Medicare & Medicaid Services (CMS), HHS . Medicare program; revisions to payment policies under the physician fee schedule and other revisions to Part B for CY 2017; Medicare Advantage bid pricing data release; Medicare Advantage and Part D medical loss ratio data release; Medicare Advantage provider network requirements; expansion of Medicare diabetes prevention program model; Medicare shared savings program requirements. Final rule. Fed Regist 2016;81:80170–80562 [PubMed] [Google Scholar]
- 59. Department of the Treasury, Internal Revenue Service; Department of Labor, Employee Benefits Security Administration; Department of Health and Human Services . Coverage of certain preventive services under the Affordable Care Act. Fed Regist 2015;80:41317–41347 [PubMed] [Google Scholar]
- 60. Centers for Medicare & Medicaid Services, HHS . Medicare program; revisions to payment policies under the physician fee schedule and other revisions to Part B for CY 2018; Medicare shared savings program requirements; and Medicare diabetes prevention program. Fed Regist 2017;82:53246–53248 [PubMed] [Google Scholar]
- 61. Centers for Medicare & Medicaid Services (CMS), HHS . Medicare program; revisions to payment policies under the physician fee schedule and other revisions to Part B for CY 2018; Medicare shared savings program requirements; and Medicare diabetes prevention program. Final rule. Fed Regist 2017;82:52976–53371 [PubMed] [Google Scholar]
- 62. National Association of Chronic Disease Directors, Division of Diabetes Translation at the Centers for Disease Control and Prevention . The National DPP Coverage Toolkit: Participating Payers and Employers, 2021. Accessed 3 August 2021. Available from https://coveragetoolkit.org/participating-payers/?goto=medicaid&space=-100
- 63. Laxy M, Zhang P, Ng BP, et al. Implementing lifestyle change interventions to prevent type 2 diabetes in US Medicaid programs: cost effectiveness, and cost, health, and health equity impact. Appl Health Econ Health Policy 2020;18:713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Indian Health Service . Special Diabetes Program for Indians (SDPI). Accessed 3 August 2021. Available from https://www.ihs.gov/sdpi/ [PubMed]
- 65. Bullock A, Sheff K, Hora I, et al. Prevalence of diagnosed diabetes in American Indian and Alaska Native adults, 2006-2017. BMJ Open Diabetes Res Care 2020;8:e001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Indian Health Service, Special Diabetes Program for Indians . 2020 Report to Congress: Changing the Course of Diabetes: Charting Remarkable Progress. Accessed 15 December 2022. Available from https://www.ihs.gov/sites/newsroom/themes/responsive2017/display_objects/documents/SDPI2020Report_to_Congress.pdf [PubMed]
- 67. Centers for Disease Control and Prevention . National and State Diabetes Trends. Accessed 3 August 2021. Available from https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html
- 68. Towne SD, Bolin J, Ferdinand A, Nicklett EJ, Smith ML, Ory MG. Assessing diabetes and factors associated with foregoing medical care among people with diabetes: disparities facing American Indian/Alaska Native, Black, Hispanic, low income, and southern adults in the U.S. (2011-2015). Int J Environ Res Public Health 2017;14:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boln JN, Schulze A, Helduser J, Ory MG. The burden of diabetes in rural America. In Rural Healthy People 2020. Bolin JN, Bellamy G, Ferdinand AO, Kash BA, Helduser JW, Eds. College Station, TX, Texas A&M University Health Science Center, School of Public Health, Southwest Rural Health Research Center, 2015, pp. 43–53. [Google Scholar]
- 70. Health Resources & Services Administration . Delta States Rural Development Network Grant Program. Accessed 3 August 2021. Available from https://www.hrsa.gov/grants/find-funding/hrsa-20-087
- 71. Aroda VR, Knowler WC, Crandall JP, et al.; Diabetes Prevention Program Research Group . Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017;60:1601–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Diabetes Prevention Program Research Group . Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goldberg RB, Orchard TJ, Crandall JP, et al.; Diabetes Prevention Program Research Group . Effects of long-term metformin and lifestyle interventions on cardiovascular events in the diabetes prevention program and its outcome study. Circulation 2022;145:1632–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Perreault L, Davies M, Frias JP, et al. Changes in glucose metabolism and glycemic status with once-weekly subcutaneous semaglutide 2.4 mg among participants with prediabetes in the STEP program. Diabetes Care 2022;45:2396–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. United States Code. 21 CFR Sec. 10.30 . Citizen petition, 2021. Accessed 11 August 2021. Available from https://ecfr.federalregister.gov/current/title-21/chapter-I/subchapter-A/part-10/subpart-B/section-10.30
- 76. Wagner R, Heni M, Tabák AG, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med 2021;27:49–57 [DOI] [PubMed] [Google Scholar]
- 77. Khosla L, Bhat S, Fullington LA, Horlyck-Romanovsky MF. HbA1c performance in African descent populations in the United States with normal glucose tolerance, prediabetes, or diabetes: a scoping review. Prev Chronic Dis 2021;18:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anderson RL, DiMeglio LA, Mander AP, et al. Innovative designs and logistical considerations for expedited clinical development of combination disease-modifying treatments for type 1 diabetes. Diabetes Care 2022;45:2189–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia 2020;63:1530–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Duca LM, Reboussin BA, Pihoker C, et al. Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: the SEARCH for diabetes in youth study. Pediatr Diabetes 2019;20:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rewers M, Hyöty H, Lernmark Å, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY) study: 2018 update. Curr Diab Rep 2018;18:136. [DOI] [PMC free article] [PubMed] [Google Scholar]