Abstract
OBJECTIVE
Ischemia with nonobstructive coronary arteries (INOCA) is a prevailing finding in patients with angina. However, the main factors underlying the risk of being rehospitalized for chest pain in patients with INOCA remain mostly unknown.
RESEARCH DESIGN AND METHODS
We evaluated INOCA patients referred to the “Casa di Cura Montevergine” in Mercogliano (Avellino), Italy, from January 2016 to January 2021 for percutaneous coronary intervention (PCI). In these subjects, we assessed the impact of the stress hyperglycemia ratio (SHR), defined as the ratio of mmol/L blood glucose and % HbA1c, on the risk of rehospitalization for chest pain.
RESULTS
A total of 2,874 patients with INOCA successfully completed the study. At the 1-year follow-up, the risk of rehospitalization for chest pain was significantly higher (P < 0.001) in INOCA patients with SHR >1 compared to patients with SHR ≤1. These findings were confirmed by multivariable analyses (adjusting for potential confounders, including age, BMI, blood pressure, heart rate, chronic kidney disease, and cholesterol), propensity score matching, and inverse probability of treatment weighting.
CONCLUSIONS
Our data indicate, to our knowledge for the first time, that SHR on hospital admission significantly and independently increases the risk of rehospitalization for chest pain in INOCA patients.
Graphical Abstract
Introduction
Ischemia with nonobstructive coronary arteries (INOCA) is often diagnosed in patients with angina (1,2). INOCA has been associated with an increased risk of death, myocardial infarction, and stroke, particularly in symptomatic subjects (1). Although ∼40% of patients admitted for coronary angiography due to angina and/or positive stress test may have nonobstructive coronary artery disease, the pathophysiology of INOCA is still not fully understood, and its management remains very debated (3–9). Coronary microvascular dysfunction, triggering structural remodeling and vasomotor disorders of coronary arterioles, is currently one of the leading hypotheses to explain the pathogenesis of INOCA.
Previous investigations have evidenced the key role of poor glycemic control and diabetes in coronary microvascular dysfunction (10–13), and our group has recently evidenced the independent role of admission hyperglycemia on the risk of restenosis in ST-elevation myocardial infarction (STEMI), with or without diabetes (14). Equally important, a nonoptimal metabolic control has been proposed as a predictor of cardiovascular events in patients with diabetes, independent of other cardiovascular risk factors (15). However, the exact role of hyperglycemia in INOCA has not been investigated at this time.
Research Design and Methods
Study Design and Participants
We evaluated INOCA patients referred to the “Casa di Cura Montevergine” in Mercogliano, Avellino, Italy, from January 2016 to January 2021 for percutaneous coronary intervention (PCI). All patients were followed up for 1 year.
INOCA was defined by the presence of the following criteria:
Symptoms of myocardial ischemia
Nonobstructive coronary artery stenosis, defined as <50% diameter reduction and/or fractional flow reserve >0.80
Objective evidence of myocardial ischemia
Impaired coronary microvascular function, defined as impaired coronary flow reserve (≤2.0), abnormal index of coronary microvascular resistance, coronary microvascular spasm, endothelial dysfunction with ≥20% luminal constriction during acetylcholine infusion, and/or coronary slow flow phenomenon.
The study was designed and conducted according to the principles outlined in the later amendments of the Declaration of Helsinki. The Campania Nord Institutional Review Board approved the protocol. All patients or their legal representatives signed an informed consent.
Inclusion criteria were age ≥18 years, diagnosis of INOCA, and availability to participate in the study. Exclusion criteria were cancer, atrial fibrillation or left bundle branch block, previous acute coronary syndrome (ACS) or cardiac revascularization, pregnancy, or severe heart valve disease.
Data Collection and Definitions
We calculated the stress hyperglycemia ratio (SHR) as the ratio of the glucose value (expressed in mmol/L) recorded upon hospital admission and the chronic glycemic burden in the prior 8–12 weeks estimated from HbA1c (expressed in %). We defined chronic kidney disease as an estimated glomerular filtration rate <60 mL/min/1.73 m2 persisting for at least 3 months (16,17).
Statistical Analysis
Data are expressed as means ± SD, means ± SE, or number and percentage, as indicated. We calculated Kaplan-Meier product limits for the cumulative ratio of reaching the end point, and we applied the log-rank test. To further confirm our results, we performed a multivariable analysis with Cox regression to adjust for potential confounders including age, BMI, blood pressure, heart rate, chronic kidney disease, and cholesterol. To further ensure the best possible balance in comparing our groups, we also performed a propensity score matching, and we applied a propensity score-adjusted analysis using inverse probability of treatment weighting (IPTW), as previously described (14,18). Matching was performed within a maximum caliper set to 0.15 SD of the propensity score using the nearest neighbor method without replacement. Covariate balance was assessed using standardized mean differences between the groups: balance was achieved if standardized mean differences were <0.1 and the P value for differences between the two groups was >0.05 (14). We established significance at a P value of <0.01. All calculations were performed using the open statistical software jamovi (version 2.3.16) and SPSS 26 (IBM, Armonk, NY).
Results
Baseline Characteristics of INOCA Patients
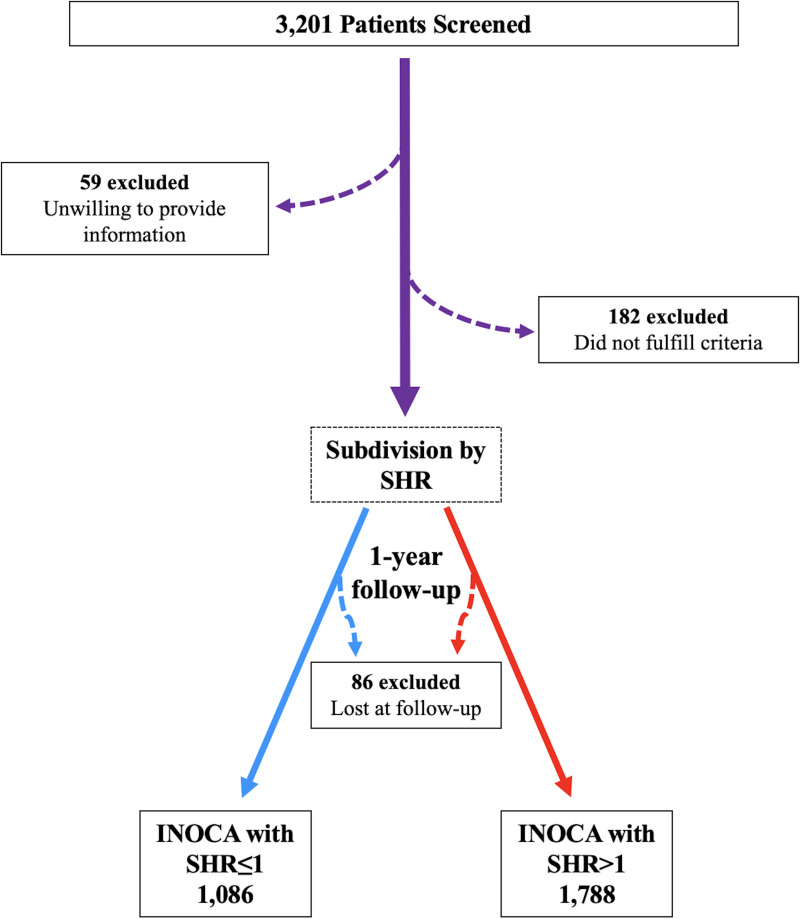
A total of 2,874 patients with a confirmed diagnosis of INOCA successfully completed the study (Fig. 1). Admission hyperglycemia could reflect metabolic disturbances and unrecognized diabetes, yet it can also occur on the background of a normal glucose homeostasis that is abruptly perturbed by an acute challenge. In order to control for background glycemia, we assessed the impact of SHR as a biomarker of critical illness more reliable than absolute hyperglycemia. Thus, we divided our population according to the SHR value, obtaining 1,086 patients with SHR ≤1 and 1,788 patients with SHR >1. The main characteristics of the two groups at baseline are reported in Table 1.
Figure 1.
Flowchart of the study.
Table 1.
Baseline clinical characteristics of our population divided according to SHR
| SHR ≤1n = 1,086 | SHR >1n = 1,788 | |
|---|---|---|
| Sex | ||
| Male, n | 494 (45.5) | 801 (44.8) |
| Female, n | 592 (54.5) | 987 (55.2) |
| Age (years) | 55.5 ± 10.3 | 61.2 ± 13.4* |
| BMI (kg/m2) | 28.4 ± 3.5 | 28.3 ± 3.4 |
| Blood pressure | ||
| Systolic (mmHg) | 122.2 ± 10.7 | 122.6 ± 10.8 |
| Diastolic (mmHg) | 79.3 ± 8.2 | 79.9 ± 7.8* |
| Heart rate (bpm) | 75.1 ± 10.3 | 75.3 ± 11.0 |
| Comorbidities | ||
| Dyslipidemia | 353 (32.5) | 640 (35.8) |
| Hypertension | 420 (38.7) | 711 (39.8) |
| Diabetes | 287 (26.4) | 594 (33.2)* |
| Chronic kidney disease | 44 (4.05) | 75 (4.19) |
| Smoking | 328 (30.2) | 536 (30.0) |
| Medications | ||
| ACE inhibitor/ARB | 386 (35.54) | 654 (36.57) |
| Calcium channel blocker | 123 (11.32) | 206 (11.52) |
| β-Blockers | 105 (9.67) | 179 (10.01) |
| Diuretics | 113 (10.4) | 188 (10.5) |
| Statins | 312 (28.73) | 519 (29.02) |
| Oral hypoglycemic agents | 271 (24.9) | 468 (26.17) |
| Insulin | 25 (2.3) | 45 (2.52) |
| Laboratory analyses | ||
| Plasma glucose (mmol/L) | 5.2 ± 0.9 | 9.7 ± 2.8* |
| HbA1c (%) | 6.1 ± 0.7 | 6.2 ± 1.0* |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Total cholesterol (mg/dL) | 182.1 ± 30.2 | 182.3 ± 30.3 |
| HDL cholesterol (mg/dL) | 51.6 ± 15.9 | 52.0 ± 16.0 |
| LDL cholesterol (mg/dL) | 129.3 ± 23.1 | 129.0 ± 23.2 |
Data are means ± SD or n (%). ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
P < 0.01.
Effects of Admission SHR on the Risk of Rehospitalization for Chest Pain
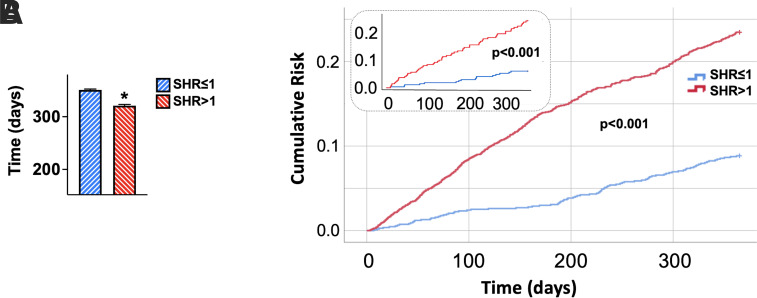
INOCA patients with SHR >1 on hospital admission had a significantly shorter time to rehospitalization (Fig. 2A) and an increased risk of rehospitalization (Fig. 2B) for chest pain compared to patients with SHR ≤1. This finding was validated by multivariable analysis adjusting for potential confounders (Table 2) and after propensity score matching, which yielded 234 patients with SHR ≤1 and 234 patients with SHR >1, showing no significant differences in clinical characteristics between the two groups (Fig. 2B, inset; Supplementary Tables 1 and 2). The increased risk of rehospitalization conferred by admission SHR >1 was further confirmed by the propensity score-adjusted analysis using IPTW (hazard ratio 2.820; 95% CI 2.196–3.622; P < 0.001).
Figure 2.
Impact of admission SHR on the risk of hospitalization for chest pain in INOCA patients. A: INOCA patients with SHR >1 exhibited a significantly shorter time to rehospitalization compared to patients with SHR ≤1. Data are means ± SE. *P < 0.001. B: Kaplan-Meier curves showing that INOCA patients with SHR >1 had a significantly higher risk of hospitalization compared to patients with SHR ≤1. Inset: Kaplan-Meier curves in the propensity score-matched groups.
Table 2.
Multivariable analysis with Cox regression
| HR | 95% CI for HR | P | ||
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Age | 1.010 | 1.002 | 1.018 | 0.011 |
| BMI | 1.021 | 0.994 | 1.048 | 0.132 |
| Blood pressure | ||||
| Systolic | 1.008 | 0.997 | 1.018 | 0.143 |
| Diastolic | 0.996 | 0.982 | 1.010 | 0.596 |
| Heart rate | 1.009 | 0.999 | 1.018 | 0.055 |
| HDL cholesterol | 0.995 | 0.989 | 1.002 | 0.142 |
| LDL cholesterol | 1.001 | 0.997 | 1.006 | 0.544 |
| Chronic kidney disease | 0.904 | 0.533 | 1.533 | 0.708 |
| SHR | 2.719 | 2.124 | 3.481 | <0.001 |
The bold P value is statistically significant (P < 0.01).
Conclusions
The management of INOCA represents a challenging conundrum: diabetes, hypertension, dyslipidemia, and aging may all be linked to the onset of INOCA (1,4). In this scenario, our data highlight the significant contribution of a fundamental factor, such as hyperglycemia, to the risk of rehospitalization for chest pain. Hyperglycemia is known to impair endothelium-dependent and independent coronary vasodilatory properties in individuals with diabetes (19,20). Equally important, by evoking inflammation, atherosclerosis, and oxidative stress, hyperglycemia can increase the risk of microvascular dysfunction, no reflow, and death in acute coronary syndromes and after PCI (14,21–24).
In our study, we show that admission SHR drives adverse events in INOCA patients; indeed, in our population, we observed a significant and independent effect of SHR in determining the risk of rehospitalization for chest pain. Our multivariable analyses with Cox regression validated the independent association of SHR with the risk of rehospitalization for chest pain, irrespective of the diabetes status. Strikingly, our findings were also corroborated by propensity score matching and IPTW adjustment, substantially reducing potential bias and ensuring better quality matches.
Consistent with our results, previous investigations found an association between disturbances in glucose homeostasis and the outcome of myocardial infarction (25–27), including death (28), no-reflow phenomenon (29), and myocardial salvage (30) following successful revascularization, new-onset atrial fibrillation (31), or acute kidney injury (32). More recently, an association between admission blood glucose and 2-year mortality in patients with myocardial infarction has been demonstrated (33).
By analyzing the data from a multicenter international registry of patients with acute myocardial infarction, our group has shown that hyperglycemia on hospital admission is less prevalent in subjects receiving sodium–glucose cotransporter 2 inhibitors (34). Intriguingly, admission hyperglycemia has also been linked to a worse outcome in COVID-19, probably by aggravating respiratory deterioration (35–40). In INOCA patients, hyperglycemia might be the consequence of marked inflammation resulting from the stress of myocardial ischemia, both chronic and acute, and as such, it may represent just the tip of the iceberg (26).
This report is the first to provide solid evidence that SHR is an independent risk factor for hospitalization in INOCA patients, with or without diabetes. We speculate that hyperglycemia may trigger coronary microvascular dysfunction, driving worse outcomes in these patients; in this context, a proper glycemic control may be a crucial goal to be achieved in this population. Further studies in larger populations and with a longer follow-up are warranted to ratify our findings.
Article Information
Funding. The Santulli Laboratory is supported in part by the National Institutes of Health: National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK123259, R01-DK033823), National Heart, Lung, and Blood Institute (R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), the National Center for Advancing Translational Sciences (UL1TR002556-06) to G.S., the Diabetes Action Research and Education Foundation (to G.S.), and the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST995561). S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.M., T.T., and G.S. designed the study, drafted the manuscript, approved its final version, and made the decision to submit and publish the manuscript. A.L., A.P., and X.W. analyzed data, revised the manuscript’s intellectual content, and approved the final version. L.S., A.C., G.P., and T.T. acquired the data, revised the manuscript’s intellectual content, and approved the final version. F.V., A.P., S.S.J., I.F., and R.A. curated data and figures, edited the manuscript, and approved its final version. G.S. was responsible for conceptualization, methodology, project administration, and editing the manuscript. P.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Scientific Sessions of the American Heart Association 2022, Chicago, IL, 5–7 November 2022.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21498396.
References
- 1. Reynolds HR, Picard MH, Spertus JA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO-ISCHEMIA study. Circulation 2021;144:1008–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen B, Holtzman JN, Juszczynski C, et al. Ischemia with no obstructive arteries (INOCA): a review of the prevalence, diagnosis and management. Curr Probl Cardiol 2022;48:101420. [DOI] [PubMed] [Google Scholar]
- 3. Bonanni A, d’Aiello A, Pedicino D, et al. Molecular hallmarks of ischemia with non-obstructive coronary arteries: the “INOCA versus Obstructive CCS” challenge. J Clin Med 2022;11:1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beltrame JF, Tavella R, Jones D, Zeitz C. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ 2021;375:e060602. [DOI] [PubMed] [Google Scholar]
- 5. Reynolds HR, Diaz A, Cyr DD, et al.; ISCHEMIA Research Group . Ischemia with nonobstructive coronary arteries: insights from the ISCHEMIA Trial. JACC Cardiovasc Imaging. 13 September 2022 [Epub ahead of print]. DOI: 10.1016/j.jcmg.2022.06.015 [Google Scholar]
- 6. Gulati M, Khan N, George M, et al. Ischemia with no obstructive coronary artery disease (INOCA): a patient self-report quality of life survey from INOCA international. Int J Cardiol. 23 September 2022 [Epub ahead of print]. DOI: 10.1016/j.ijcard.2022.09.047 [DOI] [PubMed] [Google Scholar]
- 7. Schumann CL, Mathew RC, Dean JL, et al. Functional and economic impact of INOCA and influence of coronary microvascular dysfunction. JACC Cardiovasc Imaging 2021;14:1369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishimiya K, Suda A, Fukui K, et al. Prognostic links between OCT-delineated coronary morphologies and coronary functional abnormalities in patients with INOCA. JACC Cardiovasc Interv 2021;14:606–618 [DOI] [PubMed] [Google Scholar]
- 9. Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN. Ischemia and no obstructive coronary artery disease (INOCA): what is the risk? J Am Heart Assoc 2018;7:e008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng C, Abdu FA, Mohammed AQ, et al. Prognostic impact of coronary microvascular dysfunction assessed by caIMR in overweight with chronic coronary syndrome patients. Front Endocrinol (Lausanne) 2022;13:922264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen S, Shen Y, Liu YH, et al. Impact of glycemic control on the association of endothelial dysfunction and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2021;20:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sara JD, Taher R, Kolluri N, Vella A, Lerman LO, Lerman A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc Diabetol 2019;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jankauskas SS, Kansakar U, Varzideh F, et al. Heart failure in diabetes. Metabolism 2021;125:154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mone P, Gambardella J, Minicucci F, Lombardi A, Mauro C, Santulli G. Hyperglycemia drives stent restenosis in STEMI patients. Diabetes Care 2021;44:e192–e193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ricks J, Molnar MZ, Kovesdy CP, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes 2012;61:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trimarco V, Izzo R, Mone P, et al. Therapeutic concordance improves blood pressure control in patients with resistant hypertension. Pharmacol Res. 2022;187:106557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson S, Mone P, Jankauskas SS, Gambardella J, Santulli G. Chronic kidney disease: definition, updated epidemiology, staging, and mechanisms of increased cardiovascular risk. J Clin Hypertens (Greenwich) 2021;23:831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ding D, Qiu J, Li X, et al. Hyperglycemia and mortality among patients with coronary artery disease. Diabetes Care 2014;37:546–554 [DOI] [PubMed] [Google Scholar]
- 20. Mone P, Gambardella J, Pansini A, et al. Cognitive impairment in frail hypertensive elderly patients: role of hyperglycemia. Cells 2021;10:2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gambardella J, Wang X, Mone P, Khondkar W, Santulli G. Genetics of adrenergic signaling drives coronary artery calcification. Atherosclerosis 2020;310:88–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwakura K, Ito H, Ikushima M, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol 2003;41:1–7 [DOI] [PubMed] [Google Scholar]
- 24. Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res 2020;116:787–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu W, Yang YM, Zhu J, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol 2022;21:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Zheng Y, Li C, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care 2022;45:947–956 [DOI] [PubMed] [Google Scholar]
- 27. Kojima T, Hikoso S, Nakatani D, et al.; Osaka Acute Coronary Insufficiency Study (OACIS) Group . Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2020;125:851–859 [DOI] [PubMed] [Google Scholar]
- 28. Schmitz T, Freuer D, Harmel E, et al. Prognostic value of stress hyperglycemia ratio on short- and long-term mortality after acute myocardial infarction. Acta Diabetol 2022;59:1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalfallah M, Maria DA, Allaithy A. Impact of stress hyperglycemia on no-reflow phenomenon in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Glob Heart 2022;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teraguchi I, Imanishi T, Ozaki Y, et al. Impact of stress hyperglycemia on myocardial salvage following successfully recanalized primary acute myocardial infarction. Circ J 2012;76:2690–2696 [DOI] [PubMed] [Google Scholar]
- 31. Koracevic GP, Petrovic S, Damjanovic M, Stanojlovic T. Association of stress hyperglycemia and atrial fibrillation in myocardial infarction. Wien Klin Wochenschr 2008;120:409–413 [DOI] [PubMed] [Google Scholar]
- 32. Gao S, Liu Q, Chen H, Yu M, Li H. Predictive value of stress hyperglycemia ratio for the occurrence of acute kidney injury in acute myocardial infarction patients with diabetes. BMC Cardiovasc Disord 2021;21:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cui K, Fu R, Yang J, et al. Admission blood glucose and 2-year mortality after acute myocardial infarction in patients with different glucose metabolism status: a prospective, nationwide, and multicenter registry. Front Endocrinol (Lausanne) 2022;13:898384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paolisso P, Bergamaschi L, Santulli G, et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol 2022;21:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lombardi A, Agarwal S, Schechter C, Tomer Y. In-hospital hyperglycemia is associated with worse outcomes in patients admitted with COVID-19. Diabetes Care 2022;45:2683–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonora BM, Fogar P, Zuin J, et al. Hyperglycemia, reduced hematopoietic stem cells, and outcome of COVID-19. Diabetes 2022;71:788–794 [DOI] [PubMed] [Google Scholar]
- 37. Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care 2021;44:2645–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ceriello A, Standl E, Catrinoiu D, et al.; “Diabetes and Cardiovascular Disease (D&CVD)” Study Group of the European Association for the Study of Diabetes (EASD) . Issues for the management of people with diabetes and COVID-19 in ICU. Cardiovasc Diabetol 2020;19:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calvisi SL, Ramirez GA, Scavini M, et al. Thromboembolism risk among patients with diabetes/stress hyperglycemia and COVID-19. Metabolism 2021;123:154845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mondal S, DasGupta R, Lodh M, et al. Stress hyperglycemia ratio, rather than admission blood glucose, predicts in-hospital mortality and adverse outcomes in moderate-to severe COVID-19 patients, irrespective of pre-existing glycemic status. Diabetes Res Clin Pract 2022;190:109974. [DOI] [PMC free article] [PubMed] [Google Scholar]