Abstract
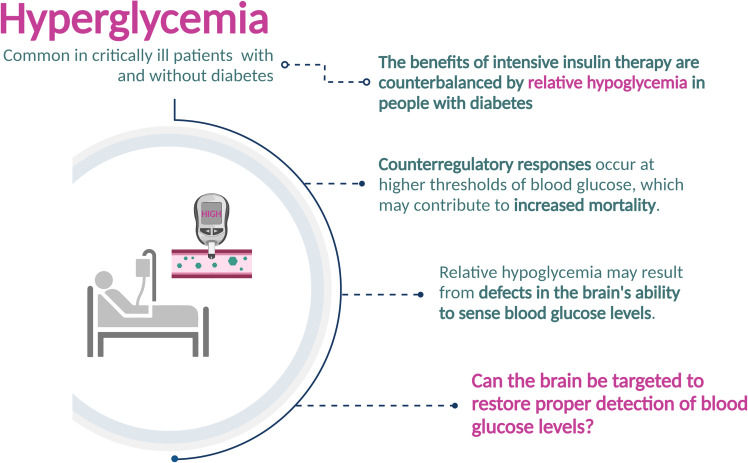
“Relative hypoglycemia” is an often-overlooked complication of diabetes characterized by an increase in the glycemic threshold for detecting and responding to hypoglycemia. The clinical relevance of this problem is linked to growing evidence that among patients with critical illness, higher blood glucose in the intensive care unit is associated with higher mortality among patients without diabetes but lower mortality in patients with preexisting diabetes and an elevated prehospitalization HbA1c. Although additional studies are needed, the cardiovascular stress associated with hypoglycemia perception, which can occur at normal or even elevated glucose levels in patients with diabetes, offers a plausible explanation for this difference in outcomes. Little is known, however, regarding how hypoglycemia is normally detected by the brain, much less how relative hypoglycemia develops in patients with diabetes. In this article, we explore the role in hypoglycemia detection played by glucose-responsive sensory neurons supplying peripheral vascular beds and/or circumventricular organs. These observations support a model wherein relative hypoglycemia results from diabetes-associated impairment of this neuronal glucose-sensing process. By raising the glycemic threshold for hypoglycemia perception, this impairment may contribute to the increased mortality risk associated with standard glycemic management of critically ill patients with diabetes.
Graphical Abstract
Although other causes exist, hypoglycemia is most often caused by insulin or other glucose-lowering medications taken by patients with diabetes. When this occurs, powerful counterregulatory responses (CRRs) are mounted that act collectively to return the blood glucose level to normal. Included among these CRRs is a robust sympathetic nervous system discharge and the secretion of the counterregulatory hormones epinephrine, norepinephrine, growth hormone, cortisol, and glucagon (1). While these responses are crucial to the recovery of euglycemia, they also constitute a significant physiological stress (2) that increases the risk of cardiovascular events and death (3,4). Evidence also suggests that a single bout of iatrogenic hypoglycemia is sufficient to activate the immune system and induce systemic inflammatory markers for at least a week (5). Avoiding hypoglycemia and associated CRRs is therefore of paramount importance in the management of patients with diabetes.
This article focuses on a phenomenon known as “relative hypoglycemia,” wherein the blood glucose threshold for perceiving and responding to hypoglycemia is increased. In normal human subjects, the glycemic threshold ranges from ∼50 mg/dL to 60 mg/dL (2.8 mmol/L to 3.4 mmol/L) (6), but in patients with diabetes, this threshold value can be much higher. Consequently, CRRs are mounted at normal or even elevated blood glucose levels in patients with relative hypoglycemia (6–9), posing a serious risk to patients with underlying critical illness. As discussed in greater detail below, relative hypoglycemia has emerged as a major clinical concern because the standard glycemic target recommended for patients in the ICU (100–180 mg/dL) is associated with increased mortality risk among some critically ill patients with diabetes (10–15). Although mechanisms underlying this increased risk remain to be established, the stress of relative hypoglycemia and associated CRRs is likely to contribute.
The goal of this article is to offer a conceptual framework within which to understand the problem of relative hypoglycemia from both clinical and basic science perspectives. After briefly reviewing the impact of diabetes on the relationship between mortality risk and glycemic management in the intensive care unit (ICU), we examine what is known and what is not known about how the brain senses hypoglycemia and how diabetes affects this process.
Not addressed in this article is the problem of hypoglycemia unawareness (formally known as hypoglycemia-associated autonomic failure), a second disorder of hypoglycemia detection. This disorder is seen primarily in insulin-treated patients with diabetes and results from repeated bouts of hypoglycemia. For reviews of this topic, see Martín-Timón and Del Cañizo-Gómez (16) and Sanchez-Rangel et al. (17).
Glycemia and Mortality Risk in the ICU
Hyperglycemia is common among critically ill patients, both those with and without diabetes, owing to complex interactions between stress hormones (catecholamines, glucagon, growth hormone, and cortisol) and cytokines. These responses raise the blood glucose level by increasing hepatic glucose production while also reducing both insulin secretion and tissue insulin sensitivity (18,19). Glucose-raising medications (such as corticosteroids) and certain forms of nutritional support can also predispose to hyperglycemia in the critical care setting.
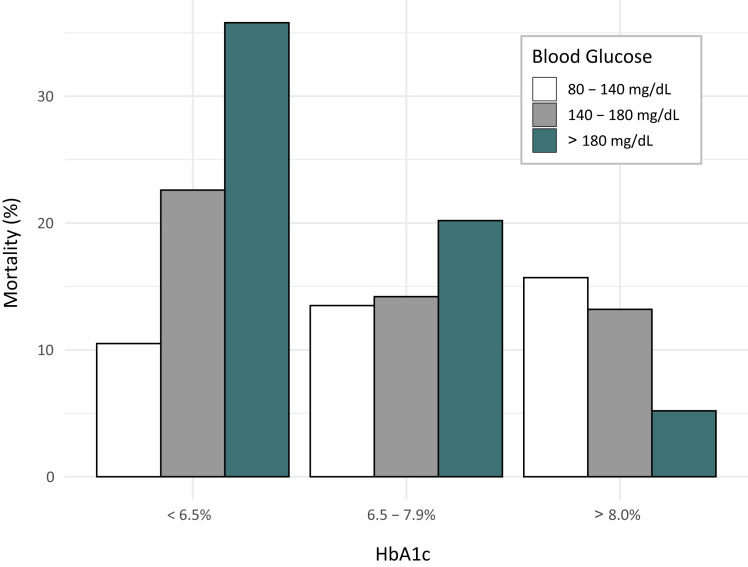
While this hyperglycemia requires treatment, the optimal glycemic target remains controversial after more than 2 decades of investigation. At issue is that randomized controlled trials (RCTs) investigating outcomes of intensive insulin therapy in the ICU have yet to distinguish between patients with and those without diabetes (20–24). Most patients included in these trials did not have preexisting diabetes, as acute-onset stress hyperglycemia is relatively common among critically ill patients. However, growing evidence indicates that whereas higher ICU blood glucose is strongly associated with higher mortality in patients with stress hyperglycemia who do not have diabetes, higher ICU blood glucose is associated with lower mortality in patients with preexisting diabetes and high HbA1c (e.g., ≥8.0%) (Fig. 1) (10–15). While the specific mechanism of injury remains to be established, relative hypoglycemia and the adverse impact of CRRs on patient outcomes are leading candidates.
Figure 1.
Impact of prehospitalization blood glucose level (assessed by HbA1c level) on the relationship between glycemic control in the ICU and mortality risk. Note that, whereas higher ICU blood glucose is associated with higher mortality in patients with stress hyperglycemia that do not have diabetes, higher ICU blood glucose is associated with lower mortality in patients with preexisting diabetes and high HbA1c. Data were analyzed using a multivariable analysis model that incorporated mean ICU blood glucose and the APACHE IV prediction of mortality. Modified with permission from Krinsley et al. (10).
In the ICU setting, relative hypoglycemia is defined as having occurred following either a ≥30% decrease from estimated preadmission glycemia (11,12) or any drop into the blood glucose range of 70–110 mg/dL (or 3.9–6.1 mmol/L) for patients with preadmission HbA1c ≥8.0% (13). Evidence that mortality risk increases significantly among ICU patients meeting these criteria (10–15) has prompted calls for an individualized approach that takes the preadmission, time-averaged glucose level into account (based on HbA1c levels) (15). This possibility awaits a well-designed RCT that achieves adequate separation of blood glucose levels between intervention and control groups while stratifying by HbA1c level and minimizing hypoglycemic events. These observations also highlight the need for an improved understanding of mechanisms underlying relative hypoglycemia, about which little is currently known.
Impact of Diabetes on the Glycemic Threshold for Hypoglycemia Detection
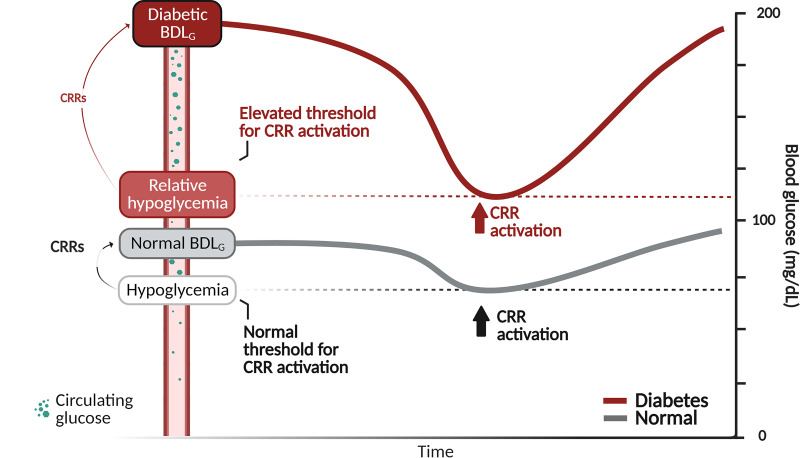
The glycemic threshold for activating CRRs in normal individuals was first established by measuring the blood glucose level at which various CRRs are first detected during a graded hypoglycemic clamp (6,7). Emblematic of this work is a seminal article showing that in normal subjects, this glycemic threshold ranges between 51 mg/dL (2.8 mM) and 61 mg/dL (3.4 mM), depending on the specific response measured (6). In the same article, the glycemic threshold for activating most (but not all) of these responses was shown to be increased by ∼45% in patients with type 1 diabetes. Years later, two separate groups (8,9) used the same graded hypoglycemic clamp method to document a comparable increase (∼40–50%) in the glycemic threshold in patients with type 2 diabetes. These findings were interpreted to suggest that sustained hyperglycemia raises the glycemic threshold for detecting and reacting to hypoglycemia, a phenomenon subsequently termed relative hypoglycemia (Fig. 2).
Figure 2.
Schematic depiction of the phenomenon of relative hypoglycemia. CRRs are mounted when blood glucose levels are lowered below their biologically defended value, regardless of whether this value is normal (gray line) or elevated, as is common among patients with diabetes (red line). Relative hypoglycemia results when the blood glucose threshold for detecting hypoglycemia and activating CRRs is elevated into the normal or even hyperglycemic range. BDLG, biologically defended level of glycemia.
Defining the Relationship Between Glucose Levels in Blood and Brain
Having established the glycemic threshold for hypoglycemia detection in humans, investigators commenced studies that employed magnetic resonance spectroscopy (MRS) to investigate the effect of clamped hyperglycemia on glucose levels in the brain. The MRS method involves placing the subject’s head in a powerful magnet and quantifying the size of the glucose MRS spectral peak in a small, well-defined brain area (typically in the occipital cortex). By simultaneously monitoring blood and brain glucose levels while changing the level of glucose in the bloodstream, investigators were able to show that blood glucose levels are at least fivefold higher than levels in brain at baseline and that brain levels increase only modestly in response to a sustained increase of the plasma glucose level (25).
These studies also established that diabetes and obesity blunt the effect of clamped hyperglycemia to raise brain glucose levels. Data from Hwang et al. (25) show that when the blood glucose level is raised during a hyperglycemic clamp, the corresponding increase of brain glucose is 20% lower in obese individuals and 50% lower in patients with type 2 diabetes compared with healthy controls. A separate study from the same group (26) showed a similar (∼30%) reduction in the fractional increase of brain glucose levels during clamped hyperglycemia among type 1 diabetes patients (mean HbA1c of 7.6%). More recently it was shown (27) that following a 12-week period of tight control to lower the HbA1c level in a cohort of type 2 diabetes patients (from 9.9% to 7.7%), the extent to which clamped hyperglycemia increases the brain glucose level was raised by 20% compared with baseline. This evidence that preexisting hyperglycemia reduces glucose transport from blood to brain offers a potential explanation for relative hypoglycemia. The validity of this explanation, however, rests on the notion that a low level of glucose in the brain is the primary mechanism underlying detection of hypoglycemia.
The Brain Glucose-Sensing Model
For convenience, we refer to this mechanism of hypoglycemia detection as the “brain glucose-sensing model.” This theory posits that brain glucose sensing is mediated by specialized glucose-responsive neurons located within brain parenchyma and that hypoglycemia is detected when the glucose level in brain interstitial fluid (ISF) surrounding these neurons drops below a critical threshold. Support for the brain glucose-sensing model includes evidence that glucose-responsive neurons are concentrated in brain areas involved in glucose homeostasis (28) and that the activity of these neurons is sensitive to changes in local glucose concentration (29). Further, CRRs can be triggered in experimental animals by central administration of glucopenic agents that block cellular glucose metabolism (e.g., 2-deoxy-d-glucose) (30). Interfering with brain glucose metabolism is therefore sufficient to activate counterregulatory responses normally engaged by hypoglycemia. As detailed in the following sections, however, several fundamental aspects of the response to hypoglycemia cannot be explained by this type of mechanism. An alternative mechanism must therefore exist.
Problems With the Brain Glucose-Sensing Model
One concern about this model is that it relies on brain levels of the regulated variable as the primary source of afferent information. This is an inherently inefficient means by which to promote homeostasis because it ensures that the brain level must change before adaptive responses are mounted. Consequently, this type of arrangement requires that brain glucose levels change more or less continuously. In addition to being maladaptive, studies that have monitored brain glucose levels over time suggest that this is not the case (25).
The thermoregulatory system offers a useful example for how we might think about brain glucose sensing. In this system, afferent information is provided to hypothalamic thermoregulatory centers by sensory fibers innervating the skin and other peripheral tissues rather than by detecting the temperature within the brain (31). Consequently, placing an animal in a cold environment elicits rapid and robust adaptive responses that protect core body temperature despite the fact that the brain’s temperature does not change (32). This is the expected outcome if the goal of homeostasis is to ensure stability of the brain temperature, and it would not be possible if the brain temperature had to change for adaptive responses to be mounted. On teleological grounds, therefore, the brain glucose-sensing model seems maladapted to the goal of ensuring stability in the brain’s fuel supply.
Beyond such theoretical concerns is a series of more substantive problems with this model. The first is that changes in the brain glucose level elicited by a corresponding change in the plasma level are both dampened and delayed, as noted above. Specifically, Hwang et al. (25) reported that when the blood glucose level is rapidly doubled (from 6 mmol/L to 12 mmol/L) during a hyperglycemic clamp, the brain glucose level rises only very slowly to a plateau value that is only ∼1.2 mmol/L over baseline, a value fivefold lower than the peak value in blood. This amounts to a very dampened response. Could this blunted brain response result instead from an effect of hyperglycemia to increase the rate of brain glucose utilization? Were this to occur, it would give the appearance of relatively reduced glucose entry into the brain. However, a study undertaken to investigate this hypothesis shows that, in human subjects, the rate of brain glucose utilization is not substantially altered by experimental hyperglycemia (33). Moreover, the rate at which the brain glucose level increases in response to a clamped increase in the blood setting is quite slow: the maximal rate of increase is ∼0.3 mmol/L per 10 min, reaching steady state only after 90 min of clamped hyperglycemia.
Based on these collective findings, we infer that a change in the blood glucose level must be both pronounced and prolonged to elicit a change of brain glucose detectable to most glucose-sensing neurons. The fact that the brain glucose level remains relatively stable over time, even in the face of pronounced changes of glycemia, is consistent with a homeostatic system that evolved to protect the brain against changes in glucose availability.
Another challenge to the brain glucose-sensing model stems from evidence that the effect of a hypoglycemic clamp on brain glucose levels does not differ between humans with and without diabetes. This small study (34) shows that as predicted, brain glucose levels in normal human subjects are lower on average during hypoglycemia than at their normoglycemic baseline. However, the magnitude of this effect was quite variable, with estimated brain glucose levels ranging by fourfold (between 0.3–1.2 µmol/g) after the blood glucose level had been dropped from ∼6 mmol/L to 3 mmol/L. This degree of variability is far greater than the glycemic threshold for activating CRRs (6). Moreover, this study also showed that the decrease of brain glucose levels between subjects with and without type 1 diabetes (mean HbA1c 7.7% ± 1.4%) did not differ in response to the same degree of clamped hypoglycemia. Although more work is needed, these findings highlight an apparent lack of a relationship between the brain glucose level and the threshold for CRR activation.
Perhaps the most compelling evidence against the brain glucose-sensing model is that hypoglycemia elicits CRRs much more rapidly than can be accounted for by the corresponding change in brain glucose level. As noted above (25), it takes tens of minutes for the brain glucose level to change substantially in response to a sustained, robust change in the blood glucose level. In contrast, the adrenomedullary response to hypoglycemia (i.e., epinephrine secretion) is virtually instantaneous (35).
Taken together, these findings leave little doubt that afferent information relevant to the circulating glucose level is conveyed to autonomic centers much more rapidly than can be accounted for by a change in the level of glucose in brain ISF. Alternative explanations for hypoglycemia detection by the brain must therefore exist.
The Blood Glucose-Sensing Model
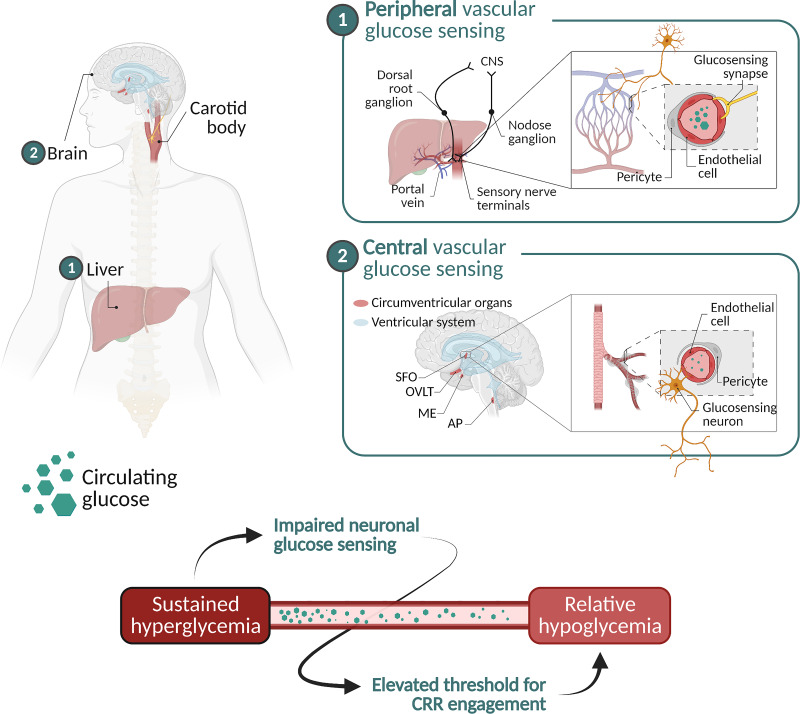
One possibility is referred to here as the blood glucose-sensing model. This model proposes that the level of glucose in the circulation is detected by glucose-responsive sensory neurons innervating the vasculature, and this afferent glucose-sensing information is then conveyed to glucoregulatory centers in the brain. According to this theory, CRRs are mounted when the level of glucose in the bloodstream (rather than in the brain) drops to a level incompatible with proper brain function (36,37) (Fig. 3 and Table 1).
Figure 3.
Schematic illustration of detection of the circulating glucose level by glucose-responsive neurons supplying the vasculature, either peripheral vascular beds (such as the hepatic portal vein) or circumventricular organs (such as the median eminence [ME] or area postrema [AP]). The bottom image depicts the role played by impaired neuronal glucose sensing in the mechanism linking sustained hyperglycemia to relative hypoglycemia. Circumventricular organs include the following: subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), ME, and AP. CNS, central nervous system.
Table 1.
Comparison of brain glucose-sensing and blood glucose-sensing models
| Model | Sensing mechanism | Hypoglycemia detection | Explanation for relative hypoglycemia |
|---|---|---|---|
| Brain glucose sensing | Detection of glucose in brain ISF by glucose-sensing neurons in hypothalamus and elsewhere. | The level of glucose in brain ISF drops below a critical threshold. | Sustained hyperglycemia reduces glucose transport across the BBB and into brain ISF, thereby lowing the brain glucose level for any blood glucose level. |
| Blood glucose sensing | Brain areas involved in glucose homeostasis receive afferent input regarding the circulating glucose level from glucose-sensing neurons that supply the vasculature and brain areas that lack a BBB. | The blood glucose level drops below a critical threshold. | Sustained hyperglycemia impairs glucose sensing by afferent neurons supplying the vasculature, such that the brain perceives the blood glucose level to be lower than it truly is. |
In support of this model is evidence that specific vascular beds are innervated by glucose-sensing neurons, including the hepatic portal vein (38–40) and carotid body (41). Watts and Donavan have shown further that responsiveness to hypoglycemia is dependent on afferent signals generated by sensory neurons innervating the hepatic portal vein and adjacent vascular beds (39,40).
In addition to sensory neurons that innervate peripheral vasculature, glucose-sensing neurons are concentrated in brain areas known as circumventricular organs. As these brain areas lack a properly formed blood–brain barrier (BBB), neuronal processes are exposed directly to the circulation and, thus, are well positioned to detect the circulating glucose level. Among these brain areas is the median eminence, located on the floor of the mediobasal hypothalamus, adjacent to the third cerebral ventricle. This brain area is specialized to detect and respond to circulating hormones and nutrient-related signals and to convey this afferent information to neurocircuits located behind the BBB (42,43), and it is richly supplied with neurons that express glucokinase (44), a glycolytic enzyme whose activity varies according to the glucose concentration. Like pancreatic β-cells, these neurons are specialized to sense glucose. In addition, tanycytes, astrocytes, and other glial cells implicated in glucose homeostasis are concentrated in this brain area (43,45–48).
Glucose-responsive neurons are also found in the area postrema (49), a circumventricular organ located at the base of the hindbrain (50). This structure is situated adjacent to both the nucleus of the solitary tract and dorsal motor nucleus of the vagus, nuclei crucial to afferent and efferent functions of the parasympathetic nervous system, respectively. This brain area is therefore well placed to rapidly transduce glucose-sensing information into adaptive autonomic responses. While the roles played by the median eminence, area postrema, and other circumventricular organs in hypoglycemia detection require additional study, we presume that a distributed system exists for detecting glucose levels in the bloodstream and conveying this information to glucoregulatory neurocircuits located behind the BBB.
Implications for Relative Hypoglycemia
As noted above, the brain glucose-sensing model views relative hypoglycemia as the result of a deleterious effect of sustained hyperglycemia on the efficiency of glucose transport from blood into the brain, such that the brain glucose level is lower than expected for a given plasma level. The validity of this theory is challenged by the rapidity with which CRRs are mounted in response to hypoglycemia and several other serious flaws.
In comparison, data implicating impaired neuronal glucose sensing in the pathogenesis of relative hypoglycemia are much stronger. In this context, the glucoregulatory phenotype of patients with maturity-onset diabetes of the young type 2 (MODY2) (9) is of strong interest. MODY2 is a form of diabetes caused by a partial loss-of-function mutation of the gene encoding glucokinase, the enzyme that phosphorylates glucose specifically in glucose-sensing cells. It is expressed in some neurons as well as in the liver and pancreatic β-cells, and it mediates the initial, rate-limiting step in cellular glucose sensing. It therefore follows that glucose sensing by both neurons and β-cells is impaired in MODY2 patients.
The question addressed by Chakera et al. (9) is whether defective neuronal glucose sensing is sufficient to cause relative hypoglycemia. Consistent with this hypothesis, they found not only that the glycemic threshold for CRRs is increased by ∼50% in MODY2 patients but also that crossing this threshold elicits an exaggerated increase of epinephrine secretion, a response driven solely by the brain. Moreover, this exaggerated epinephrine response cannot be attributed to preexisting hyperglycemia, since HbA1c levels in MODY2 patients were only marginally above those of healthy control values.
Based on these findings, we infer that the mechanism linking sustained hyperglycemia to relative hypoglycemia involves impaired neuronal glucose sensing (Fig. 3). Extending this logic, we suggest further that the effect of sustained hyperglycemia to reduce brain glucose transport is not required for relative hypoglycemia to develop. Instead, we favor the explanation offered by the blood glucose-sensing model that hyperglycemia impairs the function of glucose-responsive neurons exposed to glucose in the circulation, and this blunted glucose responsiveness leads in turn to relative hypoglycemia.
In theory, the deleterious effect of hyperglycemia on neuronal glucose sensing could involve the same mechanisms that impair glucose sensing in the pancreatic β-cells in patients with type 2 diabetes (51,52). Consistent with this notion, the mechanism underlying defective cellular glucokinase activity in β-cells from patients with type 2 diabetes appears to involve an effect of hyperglycemia to reduce glycolysis and to overwhelm cellular mitochondrial metabolism (51). Interventions that limit excessive β-cell glucose flux appear to reverse the defect (53). Further evidence suggests that intact glucokinase activity depends on β-cell insulin signal transduction, which is defective secondary to insulin resistance in obesity and type 2 diabetes (54,55). Taken together, these considerations suggest that reduced neuronal glucokinase activity contributes to the pathogenesis of relative hypoglycemia not only in MODY2 but also in type 2 diabetes.
Glucoregulatory Neurocircuits and Glucose Homeostasis
Growing evidence suggests that the mechanism underlying the counterregulatory response to hypoglycemia involves activation of glucoregulatory neurocircuits in the hypothalamic ventromedial nucleus (VMN), a brain area known for its role in glucose homeostasis (56–58). Interestingly, activation of a specific subset of VMN neurons is implicated not only in the response to hypoglycemia but also in the pathogenesis of hyperglycemia. This assertion is based on studies in which these VMN neurons were selectively inactivated in a mouse model of uncontrolled insulin-deficient diabetes induced by experimental destruction of insulin-secreting β-cells. Remarkably, this intervention resulted in nearly complete normalization of their hyperglycemia (56). In mice, therefore, neurons that are normally engaged in response to hypoglycemia become drivers of hyperglycemia in mice with insulin-deficient diabetes.
One interpretation of these data is that as diabetes develops, the brain’s ability to sense the circulating glucose level becomes impaired, owing perhaps to impaired glucokinase activity in glucose-responsive neurons. This defect in turn may activate neurocircuits normally engaged in the response to hypoglycemia, creating a vicious cycle (59,60). This type of pathogenic sequence offers an interesting alternative explanation for how relative hypoglycemia and hyperglycemia are linked in individuals with diabetes. Based on these considerations, therapeutic reversal of diabetes-associated impairment of neuronal glucose sensing may have salutary effects on both relative hypoglycemia and hyperglycemia.
Can Relative Hypoglycemia Be Averted?
Growing evidence indicates that in rodent models of diabetes, the brain can be targeted to return the biologically defended blood glucose level to normal (59,61). Exemplifying this phenomenon are studies in which the peptide fibroblast growth factor 1 (FGF1) was administered directly into the brain in rodent models of type 2 diabetes. This work shows not only that diabetic hyperglycemia is ameliorated but also that the effect is sustained for weeks or months following a single intracerebroventricular (icv) injection of FGF1 in both rat and mouse models of type 2 diabetes (62).
Interestingly, this profound and sustained glucose-lowering effect appears to occur without eliciting relative hypoglycemia. This assertion is based on a study (63) conducted in two groups of Zucker diabetic fatty rats (a model of type 2 diabetes), one of which received an icv FGF1 injection while the other received icv vehicle. Three weeks later (after blood glucose levels had normalized in the former group), a 3-h intravenous insulin infusion was performed with the goal of lowering the blood glucose level of vehicle-treated controls to match those of the FGF1-treated animals. To assess the stress associated with glucose lowering in the vehicle group, plasma levels of corticosterone (the rodent equivalent of cortisol) were measured at both the beginning and the end of the study. Whereas corticosterone remained unchanged over the course of the study in FGF1-treated, normoglycemic rats, a threefold rise was observed in vehicle-treated rats after their blood glucose levels were precisely matched to those receiving icv FGF1 injection (63). Stated differently, the use of insulin to rapidly normalize the elevated blood glucose level of Zucker diabetic fatty rats induced what appears to be relative hypoglycemia, but this response was not evident when the same blood glucose levels were achieved via the central action of FGF1. These findings raise the possibility that FGF1 action in the brain normalizes diabetic glycemia in part by correcting an underlying neuronal glucose-sensing defect.
Conclusions
Recent literature suggests that relative hypoglycemia is strongly associated with higher mortality risk in critically ill patients with diabetes. Although the underlying mechanisms remain uncertain, we favor the hypothesis that sustained exposure to hyperglycemia impairs glucose sensing by neurons as well as pancreatic β-cells. This combination of defects creates a vicious cycle that elevates the defended blood glucose level while also impairing insulin secretion, with relative hypoglycemia arising as a clinically relevant downstream consequence. Based on this synthesis, we anticipate that future strategies aimed at reversing the underlying sensory defect can ameliorate or even eliminate the problem of relative hypoglycemia in patients with diabetes. To achieve this goal will require an improved understanding of how brain glucose sensing works in normal individuals and how it becomes impaired in patients with diabetes.
Article Information
Funding. This work was supported by National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK101997 and R01DK083042 (M.W.S.) and F32-DK130535 (C.L.F.) and by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases–funded Nutrition Obesity Research Center (P30DK035816) and Diabetes Research Center (P30DK017047) at the University of Washington.
Duality of Interest. M.W.S. has received research support from Novo Nordisk. J.S.K. is a consultant for Dexcom. I.B.H. has research grants or contracts with Dexcom, Beta Bionics, Omnipod, and Medtronic Diabetes and receives consulting fees from Abbott Diabetes Care, Roche, LifeScan, and GWave. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air.
References
- 1. Seaquist ER, Anderson J, Childs B, et al.; American Diabetes Association; Endocrine Society . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab 2013;98:1845–1859 [DOI] [PubMed] [Google Scholar]
- 2. Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care 2012;35:1814–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seaquist ER, Miller ME, Bonds DE, et al.; ACCORD Investigators . The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care 2012;35:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 5. Verhulst CEM, van Heck JIP, Fabricius TW, et al. Sustained proinflammatory effects of hypoglycemia in people with type 2 diabetes and in people without diabetes. Diabetes 2022;71:2716–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cryer PE, Gerich JE. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med 1985;313:232–241 [DOI] [PubMed] [Google Scholar]
- 7. Amiel SA, Tamborlane WV, Saccà L, Sherwin RS. Hypoglycemia and glucose counterregulation in normal and insulin-dependent diabetic subjects. Diabetes Metab Rev 1988;4:71–89 [DOI] [PubMed] [Google Scholar]
- 8. Spyer G, Hattersley AT, MacDonald IA, Amiel S, MacLeod KM. Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type-2 diabetes. Lancet 2000;356:1970–1974 [DOI] [PubMed] [Google Scholar]
- 9. Chakera AJ, Hurst PS, Spyer G, et al. Molecular reductions in glucokinase activity increase counter-regulatory responses to hypoglycemia in mice and humans with diabetes. Mol Metab 2018;17:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krinsley JS, Rule P, Pappy L, Ahmed A, Heluey-Rodrigues C, Preiser JC. The interaction of acute and chronic glycemia on the relationship of hyperglycemia, hypoglycemia and glucose variability to mortality in the critically ill. Crit Care Med 2020;48:1744–1751 [DOI] [PubMed] [Google Scholar]
- 11. Kwan TN, Zwakman-Hessels L, Marhoon N, et al. Relative hypoglycemia in diabetic patients with critical illness. Crit Care Med 2020;48:e233–e240 [DOI] [PubMed] [Google Scholar]
- 12. Kwan TN, Marhoon N, Young M, Holmes N, Bellomo R. Insulin therapy associated relative hypoglycemia during critical illness. J Crit Care 2022;70:154018. [DOI] [PubMed] [Google Scholar]
- 13. Krinsley JS, Rule PR, Roberts GW, et al. Relative hypoglycemia and lower hemoglobin A1c-adjusted time in band are strongly associated with increased mortality in critically ill patients. Crit Care Med 2022;50:e664–e673 [DOI] [PubMed] [Google Scholar]
- 14. Fadini GP. Perturbation of glucose homeostasis during acute illness: stress hyperglycemia and relative hypoglycemia. Diabetes Care 2022;45:769–771 [DOI] [PubMed] [Google Scholar]
- 15. Krinsley JS, Brownlee M, Schwartz MW, et al. Blood glucose targets in the critically ill: is one size fits all still appropriate? Lancet Diab Endocrinol 2022;10:555–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes 2015;6:912–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanchez-Rangel E, Deajon-Jackson J, Hwang JJ. Pathophysiology and management of hypoglycemia in diabetes. Ann N Y Acad Sci. 6 October 2022 [Epub ahead of print]. DOI: 10.1111/nyas.14904 [DOI] [PubMed] [Google Scholar]
- 18. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009;373:1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003;78:1471–1478 [DOI] [PubMed] [Google Scholar]
- 20. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 21. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 22. Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35:1738–1748 [DOI] [PubMed] [Google Scholar]
- 23. Brunkhorst FM, Engel C, Bloos F, et al.; German Competence Network Sepsis (SepNet) . Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–139 [DOI] [PubMed] [Google Scholar]
- 24. NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 25. Hwang JJ, Jiang L, Hamza M, et al. Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2017;2:e95913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang JJ, Jiang L, Sanchez Rangel E, et al. Glycemic variability and brain glucose levels in type 1 diabetes. Diabetes 2019;68:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchez-Rangel E, Gunawan F, Jiang L, et al. Reversibility of brain glucose kinetics in type 2 diabetes mellitus. Diabetologia 2022;65:895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang L, Dunn-Meynell AA, Routh VH, et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 2006;55:412–420 [DOI] [PubMed] [Google Scholar]
- 29. Thorens B. Sensing of glucose in the brain. Handb Exp Pharmacol 2012;209:277–294 [DOI] [PubMed] [Google Scholar]
- 30. Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol 2007;292:R1792–R1798 [DOI] [PubMed] [Google Scholar]
- 31. Morrison SF, Nakamura K. Central mechanisms for thermoregulation. Annu Rev Physiol 2019;81:285–308 [DOI] [PubMed] [Google Scholar]
- 32. Bratincsák A, Palkovits M. Evidence that peripheral rather than intracranial thermal signals induce thermoregulation. Neuroscience 2005;135:525–532 [DOI] [PubMed] [Google Scholar]
- 33. Hasselbalch SG, Knudsen GM, Capaldo B, Postiglione A, Paulson OB. Blood-brain barrier transport and brain metabolism of glucose during acute hyperglycemia in humans. J Clin Endocrinol Metab 2001;86:1986–1990 [DOI] [PubMed] [Google Scholar]
- 34. van de Ven KC, van der Graaf M, Tack CJ, Heerschap A, de Galan BE. Steady-state brain glucose concentrations during hypoglycemia in healthy humans and patients with type 1 diabetes. Diabetes 2012;61:1974–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amiel SA, Simonson DC, Tamborlane WV, DeFronzo RA, Sherwin RS. Rate of glucose fall does not affect counterregulatory hormone responses to hypoglycemia in normal and diabetic humans. Diabetes 1987;36:518–522 [DOI] [PubMed] [Google Scholar]
- 36. Brown JM, Scarlett JM, Schwartz MW. Rethinking the role of the brain in glucose homeostasis and diabetes pathogenesis. J Clin Invest 2019;129:3035–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bentsen MA, Mirzadeh Z, Schwartz MW. Revisiting how the brain senses glucose and why. Cell Metab 2019;29:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mithieux G. Metabolic effects of portal vein sensing. Diabetes Obes Metab 2014;16(Suppl. 1):56–60 [DOI] [PubMed] [Google Scholar]
- 39. Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 2010;31:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab 2007;293:E96–E101 [DOI] [PubMed] [Google Scholar]
- 41. Joyner MJ, Limberg JK, Wehrwein EA, Johnson BD. Role of the carotid body chemoreceptors in glucose homeostasis and thermoregulation in humans. J Physiol 2018;596:3079–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martínez F, Cifuentes M, Tapia JC, Nualart F. The median eminence as the hypothalamic area involved in rapid transfer of glucose to the brain: functional and cellular mechanisms. J Mol Med (Berl) 2019;97:1085–1097 [DOI] [PubMed] [Google Scholar]
- 43. Kohnke S, Buller S, Nuzzaci D, et al. Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep 2021;36:109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE. Localization of glucokinase gene expression in the rat brain. Diabetes 2000;49:693–700 [DOI] [PubMed] [Google Scholar]
- 45. Yoon NA, Diano S. Hypothalamic glucose-sensing mechanisms. Diabetologia 2021;64:985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lhomme T, Clasadonte J, Imbernon M, et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J Clin Invest 2021;131:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nishio T, Toyoda Y, Hiramatsu M, Chiba T, Miwa I. Decline in glucokinase activity in the arcuate nucleus of streptozotocin-induced diabetic rats. Biol Pharm Bull 2006;29:216–219 [DOI] [PubMed] [Google Scholar]
- 48. Herrera Moro Chao D, Kirchner MK, Pham C, Foppen E, Denis RGP, Castel J, Morel C, Montalban E, Hassouna R, Bui LC, Renault J, Mouffle C, Garcia-Caceres C, Tschop MH, Li D, Martin C, Stern JE, Luquet SH. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab 2022;34:1532–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 2002;51:2056–2065 [DOI] [PubMed] [Google Scholar]
- 50. Funahashi M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation. Brain Res Bull 1993;32:531–535 [DOI] [PubMed] [Google Scholar]
- 51. Haythorne E, Rohm M, van de Bunt M, et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat Commun 2019;10:2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Merrins MJ, Corkey BE, Kibbey RG, Prentki M. Metabolic cycles and signals for insulin secretion. Cell Metab 2022;34:947–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jahan I, Corbin KL, Bogart AM, et al. Reducing glucokinase activity restores endogenous pulsatility and enhances insulin secretion in islets from db/db mice. Endocrinology 2018;159:3747–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sternisha SM, Miller BG. Molecular and cellular regulation of human glucokinase. Arch Biochem Biophys 2019;663:199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu S, Okada T, Assmann A, et al. Insulin signaling regulates mitochondrial function in pancreatic beta-cells. PLoS One 2009;4:e7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Flak JN, Goforth PB, Dell’Orco J, et al. Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin. J Clin Invest 2020;130:2943–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meek TH, Nelson JT, Matsen ME, et al. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci USA 2016;113:E2073–E2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Faber CL, Matsen ME, Velasco KR, et al. Distinct neuronal projections from the hypothalamic ventromedial nucleus mediate glycemic and behavioral effects. Diabetes 2018;67:2518–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mirzadeh Z, Faber CL, Schwartz MW. Central nervous system control of glucose homeostasis: a therapeutic target for type 2 diabetes? Annu Rev Pharmacol Toxicol 2022;62:55–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Myers MG Jr, Affinati AH, Richardson N, Schwartz MW. Central nervous system regulation of organismal energy and glucose homeostasis. Nat Metab 2021;3:737–750 [DOI] [PubMed] [Google Scholar]
- 61. Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev 2011;91:389–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scarlett JM, Rojas JM, Matsen ME, et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med 2016;22:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scarlett JM, Muta K, Brown JM, et al. Peripheral mechanisms mediating the sustained antidiabetic action of FGF1 in the brain. Diabetes 2019;68:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]