Abstract
OBJECTIVE
To investigate the association between ultra-processed food (UPF) consumption and the incidence of metabolic syndrome (MetS).
RESEARCH DESIGN AND METHODS
From 2008 to 2010, we enrolled 15,105 adults, aged 35–74 years, who were employees from six public education and research institutions to assemble the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). We used a food frequency questionnaire to assess UPF consumption (measured in grams per day) at baseline. We then assessed the outcomes of those returning to visits between 2012 and 2014 and between 2017 and 2019. We defined incident MetS by the presence of at least three of the following five abnormalities: high fasting glucose level, high triglyceride level, low HDL cholesterol level, high blood pressure, and abdominal obesity, after excluding those meeting such criteria at baseline. We also excluded those who had missing data or an implausible energy intake, leaving 8,065 participants in the study.
RESULTS
The median age was 49 years, 59% of participants were women, and the median consumption of UPFs was 366 g/day. After 8 years, there were 2,508 new cases of MetS. In robust Poisson regression, adjusting for sociodemographics, behavioral factors, and energy intake, we found a 7% (relative risk [RR] 1.07; 95% CI 1.05–1.08) higher risk of incident MetS for an increase of 150 g/day in UPF consumption. Similarly, those in the fourth quartile (compared with the first quartile) had a 33% increased risk (RR 1.33; 95% CI 1.20–1.47). Further adjustment for BMI attenuated these associations (for 150 g/day increases in UPF consumption and for the fourth quartile compared to the first one, respectively, RR = 1.04, 95% CI 1.02–1.06; RR = 1.19, 95% CI 1.07–1.32).
CONCLUSIONS
Greater consumption of UPFs is associated with an increased risk of MetS. These findings have important implications for diabetes and cardiovascular disease prevention and management.
Graphical Abstract
Introduction
Metabolic syndrome (MetS) is the simultaneous presence of several risk factors of metabolic and cardiovascular origin that share underlying causal processes (1,2). Insulin resistance, obesity, atherogenic dyslipidemia, hypertension, and hyperglycemia are critical components of the syndrome, with its severity increasing with the number of components (3). MetS is an advanced stage along the development path of cardiometabolic diseases, leading to a fivefold increase in the risk of type 2 diabetes and a twofold increase in cardiovascular disease (CVD) risk. The prevalence of MetS has increased worldwide in recent decades, most probably related to increases in obesity and sedentary occupations and lifestyles (3).
Among factors related to the increase in obesity and chronic diseases over the past few decades, the new eating pattern based on the consumption of ultra-processed foods (UPFs) stands out. UPFs are ready-to-eat, low-cost, and highly palatable products derived from multiple industrial processes and additives (4), which increasingly dominate the world’s food supplies (5). Various cross-sectional studies found positive associations between UPF consumption and MetS (6–8). Prospective studies from our cohort (9–11) and others (12,13) have found that UPF consumption predicts the development of four conditions related to the MetS: hypertension, dyslipidemia, diabetes, and larger waist circumference. However, to our knowledge, no longitudinal study has evaluated the role of UPFs in the risk of MetS, which is defined by lower cutoffs of most of these conditions, thus representing an earlier stage in the natural history of the corresponding diseases. Therefore, we aimed to assess the association of UPFs and beverages consumption with the incidence of MetS and its components in adults participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) study, taking into account multiple potential confounders.
Research Design and Methods
Study Design and Participants
The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil; in Portuguese, Estudo Longitudinal de Saúde do Adulto) is a multicenter, prospective, occupational cohort of 15,105 adults with which researchers are addressing risk factors for developing and progressing chronic conditions, particularly CVDs and diabetes (14). Participants are active or retired, nonpregnant civil servants aged 35–74 years from public higher education and research institutions in six Brazilian capital cities (Salvador, Belo Horizonte, Rio de Janeiro, São Paulo, Vitória, and Porto Alegre). Recruitment took place between August 2008 and December 2010 in study center facilities. We invited participants to return for two follow-up visits between 2012 and 2014 and between 2017 and 2019.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the ethics committee of all the institutions involved (Fundação Oswaldo Cruz, Universidade Federal da Bahia, Universidade Federal do Espírito Santo, Universidade Federal de Minas Gerais, Universidade Federal do Rio Grande do Sul, and Universidade de São Paulo). Written informed consent was obtained from all participants.
Baseline Measurements
With standardized questionnaires, we interviewed participants to ascertain characteristics such as age, sex, self-reported race or color, educational achievement, family income, medical history, smoking (current and previous), alcohol consumption, and physical activity, using the International Physical Activity Questionnaire section on leisure time activity and transport. We also obtained anthropometric measures such as weight, height, and waist circumference, following internationally standardized protocols, and we calculated BMI as weight (in kilograms) divided by height (in meters squared). In addition, we measured blood pressure three times with an interval of 1 min after 5 min of rest, using an automatic oscillometric sphygmomanometer. Intraclass correlation coefficients for blood pressure and waist circumference were 88% and 98%, respectively (15).
We obtained an overnight fasting blood sample soon after each participant’s arrival at the clinic, and we followed standardized protocols and regular quality control assessments. We measured plasma glucose using the hexokinase method (enzymatic) and HDL cholesterol and triglyceride levels using specific enzymatic methods, with intraclass correlation coefficients ≥97% (16).
Dietary Assessment
We evaluated food and beverage consumption at baseline via a previously validated semiquantitative food frequency questionnaire (FFQ) with 114 food items (17). For each item, we obtained the frequency of consumption in the past year (with eight response options, ranging from never/almost never to more than three times per day) and the number of portions consumed, using standardized portion sizes. Next, we calculated the amount of each food item in grams per day, multiplying the number of portions by the grams per portion and the frequency of consumption. We used the University of Minnesota Nutrition Data System for Research software to estimate foods’ nutritional composition and energy. For each food item, we imputed the respective 99th percentile of consumption (in grams) for all participants above this percentile.
Following the NOVA classification, we summed food items into three groups (Supplementary Table 1), according to the extent and purpose of their industrial processing: 1) non- or minimally processed foods and culinary ingredients; 2) processed foods; and 3) UPFs (18). We aggregated the first two categories into one group because the FFQ generally did not separate minimally processed foods and culinary ingredients.
Outcomes
We defined MetS by the presence of at least three of the five following components: high fasting glucose level (≥100 mg/dL or use of hypoglycemic medication), high triglyceride levels (≥150 mg/dL or use of fibrates and/or nicotinic acid), low HDL cholesterol level (<40 mg/dL for men and <50 mg/dL for women, or use of fibrates and/or nicotinic acid), high blood pressure (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg or confirmed use of antihypertensive medication), and abdominal obesity (waist circumference ≥94 cm for men and ≥80 cm for women) (3).
After excluding those with MetS at baseline, we ascertained new cases in the second follow-up visit. When this information was unavailable (for 1,029 participants), we used data from the first follow-up visit.
Statistical Analysis
We describe participant characteristics and outcomes with absolute and relative frequencies for categorical variables and with median and 25th–75th percentiles for continuous variables. We expressed UPF consumption at baseline in two forms: first, as an absolute increase of 150 g/day (a range of approximately 10% of the UPF consumption); second, categorized in quartiles of grams consumed per day. We express UPF consumption in grams per day rather than in energy consumed per day mainly because artificially sweetened beverages have no energy content.
We analyzed UPF intake associations with the incidence of MetS, using Poisson regression with robust variance. Progressively adjusted models included age (in years); sex (male or female); self-reported race/color (White, Brown, Black, Asian, or Indigenous); research center (São Paulo, Rio de Janeiro, Minas Gerais, Espírito Santo, Rio Grande do Sul, or Bahia); school achievement (less than elementary, elementary, secondary, or college/university); and per capita family income (in Brazilian reais) in model 2; plus smoking (never, former, or current), physical activity (in MET minutes per week), and alcohol (in grams per week) in model 3; plus energy intake (in kcal/day) in model 4; plus BMI at baseline in model 5. In additional models, we evaluated the effect of additional dietary factors and weight gain. In model 6a, we added saturated fat, fiber, and sugar intake (in g/day) to model 5. In model 6b, we added minimally processed foods and culinary ingredients consumption (in g/day) to model 5. And in model 6c, we added weight gain since baseline (in kg) to model 5. Cox regression was unsuitable for our data analysis because the proportional hazards assumption was not met.
We used Poisson regression with robust variance with restricted cubic splines to assess the associations between the consumption of UPFs, expressed continuously, and incident outcomes. Using multiple linear regression with restricted cubic splines, we explored UPF associations with the individual MetS components (i.e., waist circumference; plasma glucose, triglycerides, and HDL cholesterol levels; systolic and diastolic blood pressures), expressed as differences between baseline and last visit measurements (19). For these latter analyses, we excluded those using medications for the treatment of each specific component. We assessed multicollinearity between exposure variables using the variance inflation factor. Additionally, we tested the interaction of UPF consumption with age (continuous), sex (male or female), and BMI (<30 kg/m2 or ≥30 kg/m2), and the incidence of MetS. We estimated population attributable fraction directly from the Poisson regression analysis.
Finally, we performed the following sensitivity analyses: 1) excluding participants who underwent bariatric surgery; 2) expressing UPF as a proportion of the diet’s weight (i.e., relative to total grams daily); 3) removing natural drinks (e.g., natural juice and coffee or tea) with sweetener from the non- or minimally processed foods and culinary ingredients and including them in the UPF group; 4) including patients with incident MetS who died between visits and, therefore, were not present for one or more of the follow-up visits in our analytic sample; 5) defining glucose abnormality not only as impaired fasting glucose but as impaired glucose tolerance (≥140 at 2 h) for the definition of the outcome; 6) performing multiple imputation on the missing data using the fully conditional specification method; and 7) adding the family history of diabetes as a covariate to the model. We conducted all analyses with the statistical software package SAS Studio (SAS OnDemand for Academics) and estimated the population attributable fraction with STATA, version 12.
Results
Among the 15,105 participants enrolled, we excluded those with prevalent MetS at baseline (n = 5,975), who died (n = 248), who did not attend the second visit (n = 313), who had missing data on variables of interest (n = 402), or an implausible daily energy intake (<600 kcal/day or >6,000 kcal/day; n = 102). The final analytic sample consisted of 8,065 participants (Supplementary Figure 1).
As described in Table 1, the median age and BMI were 49.0 years and 24.8 kg/m2, respectively. Most participants were women (58.7%), with a college or university degree (58.8%), and who never smoked (62.0%). Median UPF consumption was 366 g/day. Those in the highest quartile (>552 g/day) of UPF consumption, compared with those in the lowest quartile (<234 g/day), had higher total energy intake and higher weight gain since baseline but lower age and lower levels of physical activity. Those in the highest quartile were also more frequently White and were less often women.
Table 1.
Characteristics of the study sample according to quartile of UPF consumption; ELSA-Brasil, 2008–2010 (n = 8,065)
| Characteristic | Quartile 1* (n = 2,016) | Quartile 2* (n = 2,016) | Quartile 3* (n = 2,017) | Quartile 4* (n = 2,016) | Total (n = 8,065) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median or N | P25–P75 or % | Median or N | P25–P75 or % | Median or N | P25–P75 or % | Median or N | P25–P75 or % | Median or N | P25–P75 or % | |
| UPFs (g/day) | 166 | 118–201 | 299 | 266–332 | 443 | 403–489 | 735 | 627–912 | 366 | 234–552 |
| Age (years) | 52 | 46–59 | 50 | 44–56 | 48 | 43–55 | 47 | 42–54 | 49 | 44–56 |
| Female sex | 1,217 | 60.4 | 1,259 | 62.5 | 1,230 | 61 | 1,026 | 50.9 | 4,732 | 58.7 |
| Race/color | ||||||||||
| Black | 332 | 16.5 | 262 | 13 | 254 | 12.6 | 300 | 14.9 | 1,148 | 14.2 |
| Brown | 589 | 29.2 | 581 | 28.8 | 560 | 27.8 | 475 | 23.6 | 2,205 | 27.3 |
| White | 992 | 49.2 | 1,109 | 55 | 1,139 | 56.5 | 1,179 | 58.5 | 4,419 | 54.8 |
| Asian | 81 | 4 | 49 | 2.4 | 51 | 2.5 | 43 | 2.1 | 224 | 2.8 |
| Indigenous | 22 | 1.1 | 15 | 0.7 | 13 | 0.6 | 19 | 0.9 | 69 | 0.9 |
| Income (Brazilian reais)† | 1,452 | 7,467–2,352 | 1,522 | 913–2,352 | 1,522 | 889–2,352 | 1,452 | 726–2,352 | 1,452 | 747–2,352 |
| Education level | ||||||||||
| Less than elementary school | 106 | 5.3 | 63 | 3.1 | 45 | 2.2 | 80 | 4 | 294 | 3.7 |
| Elementary school | 105 | 5.2 | 96 | 4.8 | 80 | 4 | 117 | 5.8 | 398 | 4.9 |
| Secondary school | 642 | 31.9 | 641 | 31.8 | 675 | 33.5 | 672 | 33.3 | 2,630 | 32.6 |
| College/university | 1,163 | 57.7 | 1,216 | 60.3 | 1,217 | 60.3 | 1,147 | 56.9 | 4,743 | 58.8 |
| Smoking | ||||||||||
| Never | 1,214 | 60.2 | 1,276 | 63.3 | 1,274 | 63.2 | 1,232 | 61.1 | 4,996 | 62 |
| Former | 537 | 26.6 | 526 | 26.1 | 513 | 25.4 | 517 | 25.6 | 2,093 | 26 |
| Current | 265 | 13.1 | 214 | 10.6 | 230 | 11.4 | 267 | 13.2 | 976 | 12 |
| Physical activity (MET min/week) | 396 | 0–1,074 | 396 | 0–1,188 | 297 | 0–1,000 | 264 | 0–990 | 330 | 0–1,074 |
| Alcohol (g/week) | 0 | 0–64.8 | 0 | 0–55.9 | 0 | 0–51.8 | 0 | 0–64.8 | 0 | 0–59.5 |
| Energy intake (kcal/day) | 1,973 | 1,614–2,439 | 2,241 | 1,861–2,768 | 2,547 | 2,111–3,099 | 3,013 | 2405–3,773 | 2,413 | 1,927–3,054 |
| BMI (kg/m2) | 24.4 | 22.4–26.7 | 24.6 | 22.6–27 | 24.9 | 22.7–27.4 | 25.4 | 23–28.4 | 24.8 | 22.6–27.3 |
| Saturated fat (g/day) | 20.9 | 15.8–27.5 | 25.8 | 20.1–32.5 | 29.8 | 23.1–38.1 | 36.2 | 27.3–47.6 | 27.6 | 20.5–36.6 |
| Sugar (g/day) | 84.7 | 64.1–112 | 103 | 79.4–131 | 120 | 93.7–153 | 153 | 115–198 | 112 | 82.9–152 |
| Fiber (g/day) | 25.2 | 19.3–33.3 | 27.1 | 20.4–35.4 | 29.1 | 22.3–38.2 | 31.7 | 23.6–42.1 | 28.1 | 21.1–37.3 |
| Minimally processed foods and culinary ingredients (g/day) | 1,603 | 1,252–2,042 | 1,669 | 1,283–2,098 | 1,717 | 1,352–2,197 | 1,835 | 1,439–2,341 | 1,703 | 1,323–2,176 |
| Weight gain since baseline (kg)‡ | 2 | −1 to 5 | 2.3 | −0.5 to 5.4 | 2.6 | −0.4 to 5.7 | 2.8 | −0.3 to 6.3 | 2.4 | −0.5 to 5.6 |
UPF quartiles: first, <234 g/day; second, between 234 g/day and 365 g/day; third, between 366 g/day and 552 g/day; and fourth, >552 g/day.
In the baseline period of the study, US$1 was equivalent to R$1.6–1.9 in Brazilian currency.
Weight gain was calculated as the difference between the baseline and second follow-up visit; for participants not attending the second follow-up, we used weight obtained at the first follow-up.
After 7.9 ± 1.3 years (±SD) of follow-up, 2,508 participants (31.1%) had developed MetS. Table 2 (left column) shows the association between UPF intake and MetS when expressed for a difference of 150 g/day in UPF consumption in progressively adjusted models. When adjusting for sociodemographic and behavioral characteristics (model 3), the estimated increment in the risk of MetS for every 150 g/day increase in UPF consumption was 5% (relative risk [RR] 1.05; 95% CI 1.03–1.06). When adding total energy intake (model 4), the RR increased slightly (RR 1.07; 95% CI 1.05–1.08). The addition of BMI (model 5) diminished the association, although it remained statistically significant (RR 1.04; 95% CI 1.02–1.06). The additional inclusion of dietary factors and weight gain did not further alter the association (models 6a–c).
Table 2.
Association of UPF consumption when expressed continuously (150 g/day) and categorically (quartiles) with MetS incidence (n = 8,065)
| Models* | 150 g/increment | Quartile 2† | Quartile 3† | Quartile 4† | P for trend | ||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| 1 | 1.05 | 1.03–1.07 | 0.95 | 0.86–1.05 | 1.11 | 1.01–1.21 | 1.23 | 1.12–1.34 | <0.0001 |
| 2 | 1.05 | 1.03–1.06 | 0.98 | 0.88–1.08 | 1.15 | 1.04–1.26 | 1.24 | 1.13–1.36 | <0.0001 |
| 3 | 1.05 | 1.0–31.06 | 0.98 | 0.89–1.08 | 1.15 | 1.04–1.26 | 1.24 | 1.13–1.36 | <0.0001 |
| 4 | 1.07 | 1.05–1.08 | 1.00 | 0.91–1.11 | 1.19 | 1.08–1.32 | 1.33 | 1.20–1.47 | <0.0001 |
| 5 | 1.04 | 1.02–1.06 | 0.98 | 0.89–1.08 | 1.14 | 1.04–1.25 | 1.19 | 1.07–1.32 | <0.0001 |
| 6a | 1.04 | 1.02–1.06 | 0.98 | 0.89–1.08 | 1.14 | 1.03–1.25 | 1.18 | 1.06–1.32 | 0.0002 |
| 6b | 1.04 | 1.02–1.06 | 0.98 | 0.89–1.08 | 1.14 | 1.04–1.26 | 1.20 | 1.07–1.33 | <0.0001 |
| 6c | 1.04 | 1.02–1.06 | 0.98 | 0.89–1.08 | 1.13 | 1.03–1.25 | 1.19 | 1.07–1.31 | <0.0001 |
Models developed in Poisson regression with robust variance were 1) adjustment models, as follows: model 1: nonadjusted; model 2: model 1 plus age, sex, center, race or color, income, school achievement; model 3: model 2 plus smoking, physical activity, alcohol; model 4: model 3 plus energy intake; and model 5: model 4 plus BMI. 2) Additional models were: model 6a: model 5 plus saturated fat, sugar, fiber; model 6b: model 5 plus minimally processed foods and culinary ingredients; and model 6c: model 5 plus weight gain since baseline.
UPF quartiles: quartile 1, <234 g/day; quartile 2, between 234 g/day and 365 g/day; quartile 3, between 366 g/day and 552 g/day; and quartile 4, >552 g/day of UPF consumption. Results for quartiles 2–4 use the first quartile as the reference.
We observed a similar pattern of association when expressing UPF consumption in quartiles (Table 2, right columns). Considering model 4, the risk of MetS among the third and fourth (versus first) quartile consumers was 19% (RR 1.19; 95% CI 1.08–1.32) and 33% (RR 1.33; 95% CI 1.20–1.47) higher, respectively. After adding BMI (model 5), these increases in RR were 14% (RR 1.14; 95% CI 1.04–1.25) and 19% (RR 1.19; 95% CI 1.07–1.32), respectively. Including dietary factors and weight gain in further models minimally changed the associations (models 6a–c). The estimated population attributable fraction for the consumption of UPF above the first quartile was 11.4% (95% CI 5.5–17.0) and 6.6% (95% CI 0.5–12.3), considering models 4 and 5, respectively.
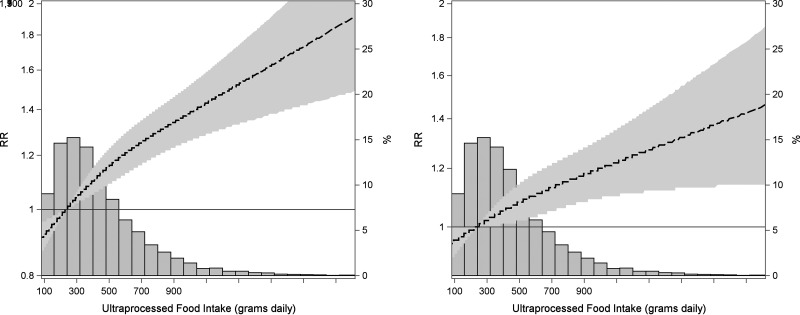
As presented through estimates from restricted cubic spline regression (Fig. 1), the association between UPF consumption and the development of MetS increased steadily across the entire range of UPF values (P < 0.001) in a linear fashion (P for nonlinearity = 0.18). This graded increase in risk was slightly stronger when obesity was not included in the adjustment (model 4; left).
Figure 1.
Association of UPF consumption with the incidence of MetS, as estimated through Poisson regression with robust variance using restricted cubic splines and adjusting for age, sex, center, race or color, income, school achievement, smoking, physical activity, alcohol, energy intake (model 4; left), and additionally for BMI (model 5; right). The dashed line shows the point RR estimates along the spectrum of UPF consumption, and the stippled area indicates the 95% confidence zone. The accompanying histogram shows the distribution of UPF consumption (percentage of study sample; right vertical axis).
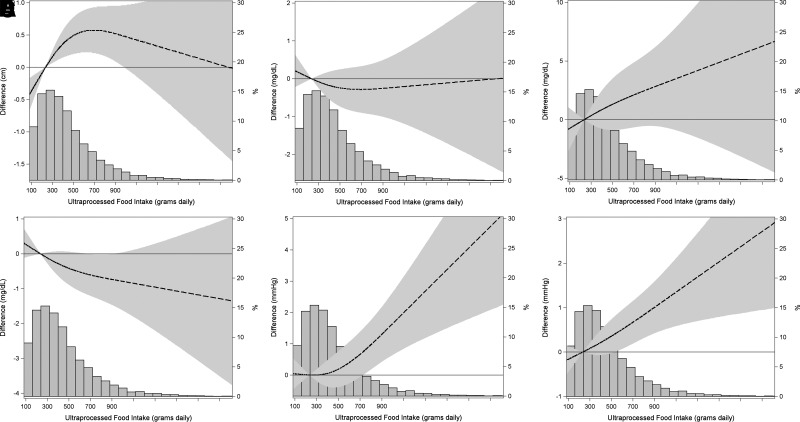
Examining individual MetS components (Fig. 2), associations (model 4) were also linear (P for nonlinearity > 0.11), except for waist circumference (P for nonlinearity = 0.01). For waist circumference, we observed a steady increase (P = 0.003), producing a difference in waist of 0.7 cm at consumption of UPF of approximately 700 g/day (near the 75th percentile) in relation to the reference consumption of 234 g/day. For triglycerides (P = 0.26), we noted a steady increase that was 2 mg/dL greater at a consumption level of 700 g/day; similarly, for HDL cholesterol (P = 0.13), we noted a decrease of 0.6 mg/dL, although associations were not statistically significant. For systolic pressure (P = 0.002) and diastolic pressure (P = 0.003), we observed increases of 0.6 mmHg. The association of UPFs with plasma glucose levels did not indicate an increased risk (P = 0.62). Supplementary Figure 2 presents the same plots but adjusted additionally for BMI (model 5), showing similar results.
Figure 2.
Association of UPF consumption with difference between visits in the components of MetS: waist circumference (A), plasma glucose level (B), triglyceride levels (C), HDL cholesterol level (D), and systolic (E) and diastolic (F) blood pressures, as estimated through Poisson regression with robust variance using restricted cubic splines and adjusting for age, sex, center, race or color, income, school achievement, smoking, physical activity, alcohol, and energy intake (model 4). Dashed lines are point estimates of change along the spectrum of UPF consumption, and the stippled area indicates the 95% confidence zone. The accompanying histograms show the distribution of UPF consumption (percentage of study sample; right vertical axis).
We found no interaction by age (P = 0.42) or sex (P = 0.23), but we found an interaction present with levels of obesity (P = 0.03): for an increase of 150 g/day of UPFs, the risk of MetS was 6% higher (RR 1.06; 95% CI 1.04–1.08) among participants without obesity but only 3% higher (RR 1.03; 95% CI 1.00–1.06) among participants with obesity.
To evaluate the robustness of the associations found, we performed sensitivity analyses (Supplementary Table 2) using models 4 and 5. Results were virtually unchanged with two exceptions. First (see sensitivity analysis c in Supplementary Table 2), when we moved natural juice and coffee or tea with sweetener into the UPF group, the associations increased in quartile analyses. Second (see sensitivity analysis f in Supplementary Table 2), when we imputed values for covariates, associations decreased slightly.
Finally, we also evaluated the association of UPFs with plasma glucose levels after moving natural juice and coffee or tea with sweetener into the UPF group. Estimates from restricted cubic spline plots (model 4) now showed a direction indicative of risk, although it still was not statistically significant (Supplementary Figure 3).
Conclusions
Findings from this Brazilian adult cohort study show that greater consumption of UPFs and beverages is independently associated with a greater risk of developing MetS during approximately 8 years of follow-up. Greater UPF consumption (>552 g/day) compared with less consumption (<234 g/day) increased the risk of MetS by 19%, which could represent 6.6% of incident cases of MetS above the first quartile of its distribution being attributable to the consumption of UPF. To our knowledge, this is the first longitudinal assessment to document the association of UPF with the development of MetS, an early stage for various cardiometabolic diseases, when intervention is more likely to improve the disease course.
Public health authorities in countries such as Brazil, Canada, and Uruguay (20–22) have recommended diminishing or avoiding UPFs and, in their place, favor the consumption of fresh, natural, or minimally processed foods. Indeed, UPF consumption has been related to incident chronic diseases such as type 2 diabetes (12,13), CVDs (23), and cancer (24), as well as all-cause mortality (25). Additionally, UPF consumption has been related to risk factors such as obesity (9), hypertension (10), and dyslipidemia (11).
Moreover, three cross-sectional studies found positive associations between UPF intake and MetS. The Nituuchischaayihititaau Aschii Environment-and-Health Study (2005–2009) evaluated 811 adults from a Canadian indigenous Eeyouch population. The participants had an elevated average consumption of UPFs (52%) and also had a heavy burden of MetS (57%) and obesity (70%). In this context, a UPF consumption greater than 72.6% of total daily energy intake, when compared with lower than 30.2%, was associated with 90% higher odds of having MetS (odds ratio 1.90; 95% CI 1.14–3.17) (7). The CAMELIA project examined 210 adolescents from Brazil and found that the consumption of UPFs at greater than 1,245 g/day was associated with a 150% greater prevalence of MetS (6). The National Health and Nutrition Examination Survey conducted in the United States between 2009 and 2014 (8) found that among 6,385 adults, the average UPF consumption was 55.5% of the total daily energy intake, with a 10% increase in the consumption of UPF being associated with a 4% greater prevalence of MetS (prevalence ratio 1.04; 95% CI 1.02–1.07). A consumption of UPFs greater than 71% of the total energy intake was associated with a 28% higher prevalence of MetS compared with consumption below 40% (prevalence ratio 1.28; 95% CI 1.09–1.50). The association was strongest in young adults and decreased with age. The main limitation of these three studies is their cross-sectional design, which raises issues of reverse causality.
Our prospective study thus strengthens evidence from previous work. For every increment of 150 g of UPFs consumed daily (∼10% of the consumption range), we found increases of 4% and 7% in the incidence of MetS in middle-aged and older adults, with and without adjustment for BMI, respectively. When UPF consumption was expressed categorically, the risk of MetS increased 14% to 19% with daily consumption of 366 g to 552 g, and 19% to 33% with a consumption >552 g, compared with consumptions of <234 g, with and without adjustment for BMI, respectively. Of note, compared with the National Health and Nutrition Examination Survey findings, our sample had a lower average consumption of UPFs in relation to the total energy intake (25.2% vs. 55.5%), highlighting that increased risk is present even in populations with lower UPF consumption.
The association between UPF consumption and the development of MetS increased linearly across the range of UPF values. Trends of individual MetS components were generally consistent with these overall results, although they were not statistically significant. Interestingly, a slight decrease in plasma glucose level with greater consumption of UPFs was seen. However, this inverse trend was not seen in sensitivity analyses where natural juice and coffee or tea with artificial sweeteners were included within the UPF group. In Brazil, artificial sweeteners are frequently added to coffee and taken with sodas. Previous findings from this cohort suggested that this practice may be related to glucose abnormalities in individuals without obesity (26). Thus, our results support considering these artificially sweetened drinks as UPFs.
Some mechanisms can be hypothesized to explain associations between UPF and MetS. Greater energy intake from the consumption of UPF products can lead to weight gain, as previously seen in longitudinal studies (9,27,28) and a randomized clinical trial (29). In addition, nutritional aspects of UPFs such as trans and saturated fats, sugar, sodium, and their high glycemic index may also contribute to the development of MetS (30). However, the association remained statistically significant after additional adjustments, including energy intake and BMI, as well as additional dietary factors such as saturated fat, sugar, fiber, and weight gain, which suggests that UPF products contribute to MetS in ways other than weight gain and these nutritional factors.
The high consumption of UPF replaces fresh or minimally processed foods such as legumes, whole grains, vegetables, fruits, and oilseeds, which are foods shown to prevent MetS and type 2 diabetes (30,31). However, we also performed additional adjustments for the minimally processed foods group consumption, and the association remained significant. Other components of UPF products may explain the associations, but evidence on potential biological mechanisms is still limited. Emulsifiers and sweeteners have been implicated in changes in the gut microbiota, which can lead to inflammation and consequent metabolic changes (32–35). Packaging contact materials, such as bisphenol A and phthalates, are involved in endocrine disruption and insulin resistance (36,37), and some components formed during industrial processes also seem to lead to insulin resistance (38).
Our results show an interaction with BMI, with the association between UPFs and MetS being larger and statistically significant only among participants without obesity. Individuals with obesity may be already at a stage of metabolic and inflammatory disturbance (39), upon which additional effects derived from UPFs might be minimal.
Our study has some limitations. First, our FFQ was not explicitly designed to evaluate the NOVA classification groups, which may have led to an underestimation of the size of the associations reported. Although errors in classification could be present, the frequency of UPF consumption based on the ELSA-Brasil cohort questionnaire at baseline was similar to what was found in a nationally representative survey (40). Second, our follow-up of approximately 8 years may be short to evaluate the contribution of UPF consumption to the development of MetS. Third, although we made statistical adjustments for multiple potential confounders, residual confounding cannot be completely ruled out. However, our findings have biological plausibility, and results remained unchanged after additional analyses adjusting for lifestyle and dietary confounders and were only slightly attenuated when we imputed missing values for covariates.
Some strengths should also be considered. First, ELSA-Brasil is a large, contemporary cohort study with minor losses to follow-up. Second, we performed highly standardized measurements with strict quality control (15). Third, although prospective studies have shown associations of UPFs with the individual cardiometabolic phenotypes of the MetS (9–13), the novelty of our findings is showing that UPF consumption predicts the development of MetS, a conjoint entity defined by lower cutoffs than diabetes and hypertension, and thus representing an earlier stage of the natural history of cardiometabolic disease. In addition, our spline analyses permitted a detailed assessment of the change in risk across the continuum of UPF distribution. Finally, given the complexity of the association here investigated, our sequential modeling permits the evaluation of the independent effects of energy intake, BMI, and various nutritional factors in the associations.
In conclusion, we found a positive association between UPF consumption and the development of MetS. These findings add to the growing evidence for the role of UPFs in several diet-related noncommunicable diseases and help inform public policy for diabetes and CVD prevention and management.
Article Information
Acknowledgments. The authors thank the staff and participants of ELSA-Brasil for their essential contributions.
Funding. This study was supported by the Brazilian Ministry of Health (Department of Science and Technology) and Ministry of Science, Technology, and Innovation (Financiadora de Estudos e Projetos; grants 01 06 0010.00, 01 06 0212.00, 01 06 0300.00, 01 06 0278.00, 01 06 0115.00, and 01 06 0071.00) and the National Council for Scientific and Technological Development. S.L.C. also received a fellowship from Fundação de Desenvolvimento da Pesquisa.
Researchers were independent of funders. Funders had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.L.C. performed the statistical analysis, wrote the manuscript, and had primary responsibility for the final content; A.V. helped with statistical analyses and reviewed the manuscript; V.C.L., S.M.A.M., L.G., and R.B.L. reviewed the manuscript; B.B.D. and M.I.S. designed the research, wrote and reviewed the manuscript, and had primary responsibility for the final content; M.d.C.M. and S.B. designed the study and reviewed the manuscript. S.L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21578916.
References
- 1. Grundy SM, Cleeman JI, Daniels SR, et al.; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 2. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberti KGMM, Eckel RH, Grundy SM, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 4. Monteiro CA, Cannon G, Levy RB, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr 2019;22:936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moodie R, Stuckler D, Monteiro C, et al.; Lancet NCD Action Group . Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 2013;381:670–679 [DOI] [PubMed] [Google Scholar]
- 6. Tavares LF, Fonseca SC, Garcia Rosa ML, Yokoo EM. Relationship between ultra-processed foods and metabolic syndrome in adolescents from a Brazilian family doctor program. Public Health Nutr 2012;15:82–87 [DOI] [PubMed] [Google Scholar]
- 7. Lavigne-Robichaud M, Moubarac J-C, Lantagne-Lopez S, et al. Diet quality indices in relation to metabolic syndrome in an indigenous Cree (Eeyouch) population in northern Québec, Canada. Public Health Nutr 2018;21:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martínez Steele E, Juul F, Neri D, Rauber F, Monteiro CA. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev Med 2019;125:40–48 [DOI] [PubMed] [Google Scholar]
- 9. Canhada SL, Luft VC, Giatti L, et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr 2020;23:1076–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scaranni PODS, Cardoso LO, Chor D, et al. Ultra-processed foods, changes in blood pressure and incidence of hypertension: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr 2021;24:3352–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scaranni P, De Oliveira Cardoso L, Griep RH, Lotufo PA, Barreto SM, Fonseca M. Consumption of ultra-processed foods and incidence of dyslipidemias: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Br J Nutr 2022;1–9. [DOI] [PubMed] [Google Scholar]
- 12. Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé Prospective Cohort. JAMA Intern Med 2020;180:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy RB, Rauber F, Chang K, et al. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clin Nutr 2021;40:3608–3614 [DOI] [PubMed] [Google Scholar]
- 14. Schmidt MI, Duncan BB, Mill JG, et al. Cohort profile: longitudinal study of adult health (ELSA-Brasil). Int J Epidemiol 2015;44:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt MI, Griep RH, Passos VM, et al. [Strategies and development of quality assurance and control in the ELSA-Brasil]. Rev Saude Publica 2013;47(Suppl. 2):105–112 [DOI] [PubMed] [Google Scholar]
- 16. Ladwig R, Vigo A, Fedeli LMG, et al. Variability in baseline laboratory measurements of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Braz J Med Biol Res 2016;49:e5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molina MdelC, Benseñor IM, Cardoso L de O, et al. [Reproducibility and relative validity of the food frequency questionnaire used in the ELSA-Brasil]. Cad Saude Publica 2013;29:379–389 [PubMed] [Google Scholar]
- 18. Monteiro CA, Cannon G, Levy RB. NOVA. The star shines bright. World Nutr J. 2016;7:28–38 [Google Scholar]
- 19. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–1057 [DOI] [PubMed] [Google Scholar]
- 20. Ministério da Saúde, Secretaria de Atenção à Saúde . Guia Alimentar para a População Brasileira. 2014. Accessed 23 November 2022. Available from https://bvsms.saude.gov.br/bvs/publicacoes/guia_alimentar_populacao_brasileira_2ed.pdf
- 21. Food and Agriculture Organization of the United Nations . Food-based dietary guidelines – Uruguay. 2016. Accessed 1 March 2022. Available from https://www.fao.org/nutrition/education/food-based-dietary-guidelines/regions/countries/uruguay/en/
- 22. Health Canada . Canada’s Dietary Guidelines. 2019. Accessed 1 March 2022. Available from https://food-guide.canada.ca/en/healthy-eating-recommendations/limit-highly-processed-foods/
- 23. Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 2019;365:l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fiolet T, Srour B, Sellem L, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 2018;360:k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019;365:l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yarmolinsky J, Duncan BB, Chambless LE, et al. Artificially sweetened beverage consumption is positively associated with newly diagnosed diabetes in normal-weight but not in overweight or obese Brazilian adults. J Nutr 2016;146:290–297 [DOI] [PubMed] [Google Scholar]
- 27. Rauber F, Chang K, Vamos EP, et al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr 2021;60:2169–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendonça RD, Pimenta AM, Gea A, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 2016;104:1433–1440 [DOI] [PubMed] [Google Scholar]
- 29. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 2021;30:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab 2007;32:46–60 [DOI] [PubMed] [Google Scholar]
- 31. Li J, Glenn AJ, Yang Q, et al. Dietary protein sources, mediating biomarkers, and incidence of type 2 diabetes: findings from the Women’s Health Initiative and the UK Biobank. Diabetes Care 2022;45:1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–186 [DOI] [PubMed] [Google Scholar]
- 33. Régnier M, Van Hul M, Knauf C, Cani PD. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J Endocrinol 2021;248:R67–R82 [DOI] [PubMed] [Google Scholar]
- 34. de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut 2022;71:1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol 2011;7:346–353 [DOI] [PubMed] [Google Scholar]
- 37. Stojanoska MM, Milosevic N, Milic N, Abenavoli L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017;55:666–681 [DOI] [PubMed] [Google Scholar]
- 38. Feroe AG, Attanasio R, Scinicariello F. Acrolein metabolites, diabetes and insulin resistance. Environ Res 2016;148:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saad MJA, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 2016;31:283–293 [DOI] [PubMed] [Google Scholar]
- 40. Instituto Brasileiro de Geografia e Estatística . Pesquisa de Orçamentos Familiares 2017–2018: Análise de Consumo Alimentar Pessoal no Brasil. Rio de Janeiro, Brazil: Instituto Brasileiro de Geografia e Estatística, 2020 [Google Scholar]