Abstract
OBJECTIVE
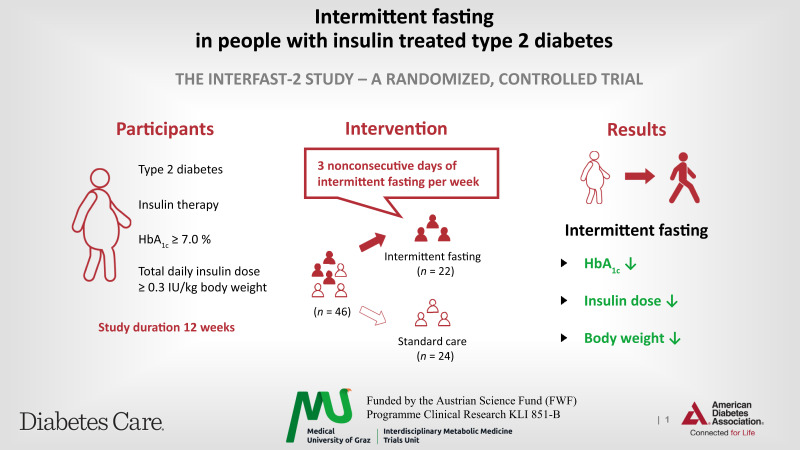
To investigate the safety and feasibility of 3 nonconsecutive days of intermittent fasting (IF) per week over 12 weeks in participants with insulin-treated type 2 diabetes.
RESEARCH DESIGN AND METHODS
Forty-six people were randomized to an IF or control group. Dietary counseling and continuous glucose monitoring was provided. Coprimary end points were the change in HbA1c from baseline to 12 weeks and a composite end point (weight reduction ≥2%, insulin dose reduction ≥10%, and HbA1c reduction ≥3 mmol/mol).
RESULTS
The IF group showed a significant HbA1c reduction (−7.3 ± 12.0 mmol/mol) compared with the control group (0.1 ± 6.1 mmol/mol) over 12 weeks (P = 0.012). The coprimary end point was achieved by 8 people in the IF and none in the control group (P < 0.001). No severe hypoglycemia occurred.
CONCLUSIONS
IF is a safe and feasible dietary option to ameliorate glycemic control while reducing total daily insulin dose and body weight in insulin-treated people with type 2 diabetes.
Graphical Abstract
Introduction
With the numbers of people with type 2 diabetes rising worldwide, dietary modifications provide an essential therapeutic approach for blood glucose, weight, and cardiovascular risk-factor management (1,2). Intermittent fasting (IF) has emerged as an alternative to classic daily caloric reduction (3). The approaches to IF range from limiting food consumption to certain hours of the day to alternate-day fasting (4,5). People with insulin-treated type 2 diabetes often struggle with weight gain (6), resulting in a vicious cycle of increasing insulin doses required to overcome the insulin resistance, leading to further weight gain, and ultimately resulting in higher cardiovascular risk (7). A recent meta-analysis suggested IF as an appropriate diet strategy in people with type 2 diabetes; however, the risk of hypoglycemia during fasting states in insulin-treated individuals remains a crucial barrier to adhere to diets demanding caloric restriction and further randomized controlled trials are required to verify its feasibility and safety in this population (8).
We hypothesized that 12 weeks of IF could improve glycemic control and decrease body weight while being safe to practice in people with insulin-treated type 2 diabetes compared with a control group.
Research Design and Methods
This open, single-center, randomized, controlled trial, Intermittent fasting in subjects with insulin-treated type 2 diabetes mellitus (INTERFAST-2), was conducted at the University Hospital Graz, Graz, Austria, and approved by the ethics committee of the Medical University of Graz, Graz, Austria (EK 30-350 ex 17/18). The detailed study protocol was published previously (9). Briefly, this study included volunteers, aged between 18 and 75 years (both inclusive), with insulin-treated type 2 diabetes, an HbA1c ≥53 mmol/mol (≥7.0%), and a daily insulin dose of ≥0.3 IU/kg body wt.
The IF group practiced IF 3 days a week, reducing their calories on these days by 75% (i.e., consuming only 25% of the recommended caloric intake). Ingestion was only allowed at breakfast and/or lunch to maintain an 18-h period of fasting. Participants were asked to keep a food diary to monitor adherence. On the remaining 4 days, participants of the IF group had no caloric restriction. There was no restriction on macronutrient composition or on the consumption of water, unsweetened coffee, and tea without milk. On eating days, participants were allowed to consume any type of food or drink without any caloric restriction. Both groups had a comparable number of interactions with the study staff.
All participants were switched to the same basal insulin (insulin glargine U300) prior to the randomization. The basal insulin was administered in the morning. For fasting days, participants were instructed to reduce basal insulin by 20% and prandial insulin was only administered for glucose correctional reasons. To reduce the risk of hypoglycemia during the IF days, participants were given an insulin dose adjustment protocol for fasting days (Supplementary Table 2). Oral nonsulfonylurea medication was continued on fasting days (9).
All participants used a FreeStyle Libre continuous glucose monitoring system (CGM; Abbott Diabetes Care, Alameda, CA) device for the 12 weeks of the study and the insulin switch phase. Data were collected using LibreView software (www.libreview.com). Lipoproteins and serum metabolites were analyzed using nuclear magnetic resonance spectroscopy (10).
We analyzed the coprimary outcomes of 1) difference in the change in HbA1c from baseline to week 12 and 2) difference in the number of participants achieving a combined end point (weight reduction ≥2%, insulin dose reduction ≥10%, and HbA1c reduction ≥3 mmol/mol) for the study in a hierarchical order (change in HbA1c first) using an intention-to-treat approach. Continuous primary and secondary outcomes were analyzed using both unpaired t tests and linear mixed-effect models. A list of the predefined secondary outcomes are provided in Supplementary Table 3.
Data and Resource Availability
The data set generated during and analyzed in the study is available upon reasonable request from the corresponding author.
Results
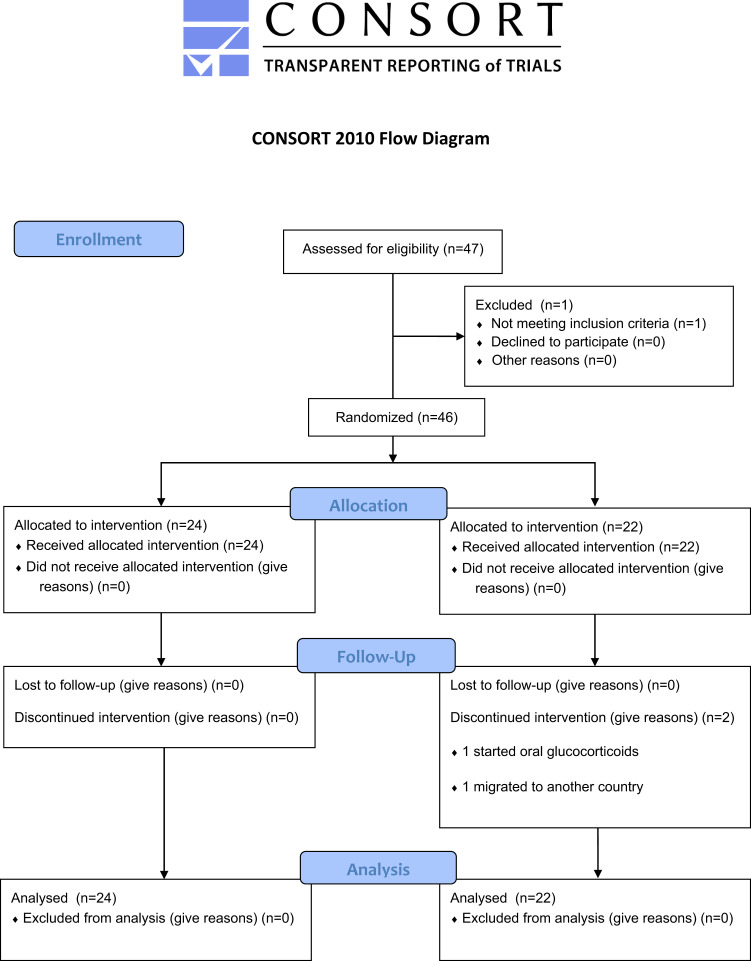
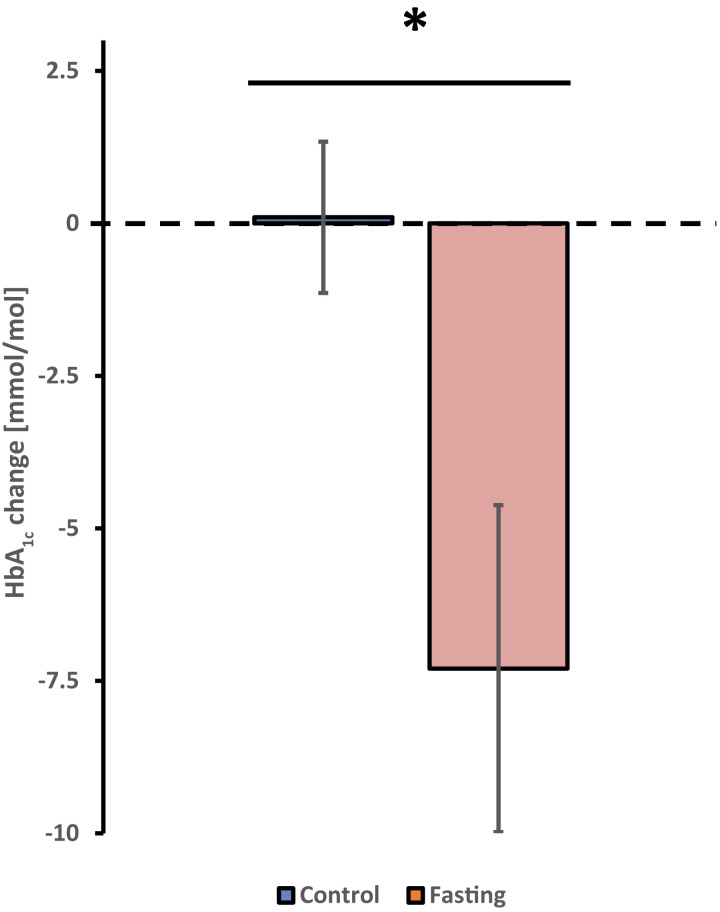
We screened 47 subjects, of whom 46 participants (22 women and 24 men) were randomized to the IF group (n = 22) or the control group (n = 24). Two participants of the IF group did not complete the intervention (Fig. 1). The mean age was 63 ± 7 years, diabetes duration was 21 ± 9 years, BMI was 34.3 ± 4.5 kg/m2, HbA1c was 67 ± 11 mmol/mol (8.3 ± 1.1%), and the mean total daily insulin dose was 56 ± 27 IU. The full details of the baseline characteristics are given in Table 1. After 12 weeks, HbA1c in the IF group decreased by 7.3 ± 12.0 mmol/mol compared with an increase in the control group by 0.1 ± 6.1 mmol/mol (P = 0.012) (Fig. 2). The difference in the change in HbA1c between the control and IF group remained statistically significant (P = 0.008) after adjusting for age, sex, diabetes duration, and baseline HbA1c. The mean time above range over the entire 12 weeks was significantly lower in the IF group (30.4 ± 20.9%) than in the control group (42.1 ± 16.1%, P = 0.029). The mean time in range was significantly higher in the IF group (68.0 ± 20.2%) compared with the control group (56.6 ± 16.0%, P = 0.031), while the mean time below range over the 12 weeks was similar in the IF (1.6 ± 2.0%) and the control group (1.3 ± 2.2%, P = 0.334) (Supplementary Fig. 1).
Figure 1.
Trial flowchart.
Table 1.
General characteristics at baseline
| All (n = 46) | Control (n = 24) | Fasting (n = 22) | |
|---|---|---|---|
| Age (years) | 63 ± 7 | 61 ± 7 | 65 ± 6* |
| Male sex | 24 (52) | 14 (58) | 10 (46) |
| Duration of diabetes (years) | 21 ± 9 | 18 ± 7 | 24 ± 10* |
| Weight (kg) | 100 ± 15 | 104 ± 13 | 96 ± 16 |
| Height (m) | 1.71 ± 0.09 | 1.72 ± 0.09 | 1.70 ± 0.07 |
| BMI (kg/m2) | 34.3 ± 4.5 | 35.0 ± 4.3 | 33.5 ± 4.7 |
| HbA1c (%) | 8.3 ± 1.1 | 8.2 ± 1.0 | 8.5 ± 1.2 |
| HbA1c (mmol/mol) | 67 ± 11 | 66 ± 10 | 69 ± 12 |
| Total daily insulin dose (IU) | 56 ± 27 | 59 ± 33 | 52 ± 19 |
| Fasting glucose (mg/dL) | 174 ± 44 | 176 ± 41 | 173 ± 47 |
| Total cholesterol (mg/dL) | 163 ± 45 | 164 ± 41 | 162 ± 51 |
| HDL cholesterol (mg/dL) | 48 ± 17 | 42 ± 15 | 57 ± 17* |
| LDL cholesterol (mg/dL) | 81 ± 35 | 81 ± 32 | 80 ± 39 |
| Blood pressure systolic (mmHg) | 141 ± 22 | 145 ± 23 | 136 ± 19 |
| Blood pressure diastolic (mmHg) | 82 ± 10 | 85 ± 10 | 80 ± 10 |
| Other glucose-lowering medication | |||
| GLP-1RA | 20 (43) | 11 (46) | 9 (41) |
| SGLT-2 inhibitors | 20 (43) | 9 (38) | 11 (50) |
| DPP4-inhibitors | 14 (30) | 5 (21) | 9 (41) |
| Metformin | 34 (74) | 17 (71) | 17 (77) |
| Comorbidities | |||
| Hypertension | 39 (85) | 18 (75) | 21 (96) |
| Heart failure | 5 (11) | 1 (4) | 4 (18) |
| Coronary artery disease | 12 (26) | 6 (25) | 6 (27) |
| History of myocardial infarction | 10 (22) | 3 (13) | 7 (32) |
| History of stroke | 2 (4) | 2 (8) | 0 (0) |
| Retinopathy | 10 (22) | 5 (21) | 5 (23) |
| Polyneuropathy | 18 (39) | 11 (46) | 7 (32) |
| Amputation | 2 (4) | 1 (4) | 1 (5) |
| Hyperlipidemia | 41 (89) | 21 (88) | 20 (91) |
Categorical data are presented as n (%), and continuous variables are presented as mean ± SD. DPP4, dipeptidyl peptidase 4; GLP1-RA, glucagon-like peptide 1 receptor agonist; SGLT-2, sodium–glucose linked transporter 2.
P < 0.05.
Figure 2.
Change in HbA1c from baseline to 12 weeks in control and IF group. Data are displayed as mean ± SEM. *P = 0.012.
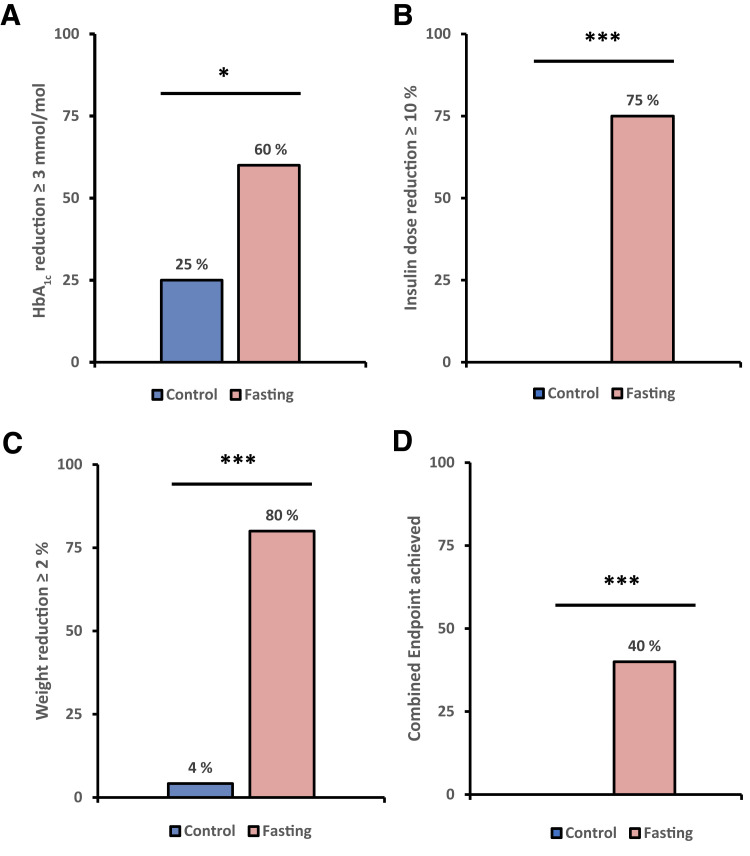
After 12 weeks, 8 participants (40%) in the IF group achieved the composite coprimary end point compared with none of the participants in the control group (P < 0.001) (Fig. 3). The same number of participants in the IF group (n = 8 [40%]) achieved the combined end point when higher thresholds were applied (at least 3% weight loss, at least 0.5% HbA1c reduction, and at least 10% insulin dose reduction).
Figure 3.
Coprimary end point; percentage of participants achieving each individual aspect and the combined coprimary end point. *P < 0.05, ***P < 0.001.
After 12 weeks of intervention, the IF group showed a significant reduction in weight (4.77 ± 4.99 kg) compared with the control group (+0.27 ± 1.34 kg, P < 0.001) and in fat mass (3.5 ± 3.3 kg in the IF group and +0.1 ± 1.3 kg in the control group, P < 0.001). There was no statistically significant difference in the change in lean mass or bone mass between the two groups according to the DXA measurements.
The resting metabolic rate (RMR) was not different between the IF and control group, both at baseline (IF: 2,286 ± 357 kcal, control: 2,439 ± 375 kcal) and after 12 weeks (IF: 2,248 ± 331 kcal, control: 2,429 ± 398 kcal). No difference was observed in the change of the RMR from baseline to 12 weeks between the two groups (P = 0.735). Likewise, no difference was observed in the change of the physical activity levels between the groups (P = 0.541). The mean total daily dose of insulin at baseline was 52 ± 19 IU in the IF group and 59 ± 33 IU in the control group. At 12 weeks, the IF group had an insulin dose of 45 ± 19 IU while the control group had an insulin dose of 63 ± 35 IU, resulting in a total daily insulin dose reduction in the IF group over 12 weeks by 9 ± 10 IU as opposed to the control group with an increase by 4 ± 10 IU (P = 0.008). A significant difference in the change of perceived health (EuroQol-5D visual analog scale) between the IF (from 70 ± 20 to 74 ± 21) and control group (from 70 ± 20 to 65 ± 23) was observed (P = 0.043).
Results of nuclear magnetic resonance–based metabolomics analysis showed the metabolites most contributing to the difference between fasting and control subjects included acetic acid, dimethylsulfone, and some ketone bodies (acetoacetic acid, 3-hydroxybutyric acid, and acetone). Of all 35 metabolites investigated, only acetic acid (probably derived from fatty acid metabolism) significantly increased in fasting individuals (32 ± 10 µmol/L vs. 19 ± 8 µmol/L, fold-change, 1.68; Padj = 0.002) (Supplementary Fig. 2).
Of the 22 participants in the IF group, 20 (91%) achieved >75% adherence to the given fasting protocol.
During the study period, five serious adverse events leading to hospitalization were reported, two in the IF group and three in the control group. None of the serious adverse events were considered to be related to the study intervention.
Conclusions
We demonstrated that 3 days of nonconsecutive IF per week over the duration of 12 weeks improved HbA1c, reduced body weight, and led to a total daily insulin dose reduction in people with insulin-treated type 2 diabetes.
Our data are in line with previous studies showing that IF was effective in HbA1c reduction in people with type 2 diabetes (11). Li et al. (12) also reported data from a 7-day fasting program with a maximum intake of 300 kcal to reduce body weight in participants with type 2 diabetes; however, participants with intensified insulin treatment were excluded. Hence, our study extends previous beneficial effects on body weight and glycemic control to people with type 2 diabetes treated with insulin. Recent meta-analyses demonstrated similar HbA1c-reducing potential of IF compared with continuous calorie restriction in people with type 2 diabetes, while the weight loss appeared to be more pronounced (8,13) with IF. Mechanistic studies suggest that prolonged fasting might have additional beneficial metabolic effects, independent of weight loss, by switching the metabolism to fatty acid mobilization, β-oxidation, and enhanced ketone body production or inducing autophagy (14).
From a clinical perspective, for some individuals, IF appears to be an easy to apply dietary intervention without the need for continuous caloric reductions, ultimately leading to reduced caloric intake through the time-restricted eating pattern without vigorous documentation or calorie counting (15,16). As demonstrated in our study, the risk of hypoglycemia during IF can be mitigated by reducing the insulin dose on fasting days and using a CGM system, as previously observed during Ramadan fasting (17). However, it appears critical that participants and health care personnel are instructed on insulin dose adjustments during IF.
One of the limitations of dietary studies on glycemic parameters in insulin-treated individuals with type 2 diabetes is that glucose control, body weight reduction, and insulin dose are interrelated and that changes in the insulin dose can alter the observed HbA1c. For this reason, we chose a coprimary outcome besides HbA1c to investigate changes in HbA1c along with weight and insulin dose. A limitation of our study is the intermittently scanned (is)CGM was introduced in 16 participants in the control group and in 13 in the IF group at study start and that the isCGM use was not blinded to the participants, which might have influenced the eating behavior of the participants in both groups. Finally, participants were allowed to eat up to 25% of the recommended daily caloric intake on the fasting days as breakfast and/or lunch, which was originally introduced to reduce hypoglycemic risk and increase adherence to the study protocol in people with insulin-treated type 2 diabetes. With our study data we would feel confident to omit this caloric intake in further studies.
Strengths of the study include its randomized controlled design in people with type 2 diabetes using a basal bolus insulin regimen with a reproducible insulin dosing adjustment algorithm together with isCGM data and metabolomics analysis of IF induced changes. We also monitored the RMR and physical activity throughout the study, which remained unchanged.
Our data demonstrate that IF over 12 weeks in insulin-treated people with type 2 diabetes is safe, reduces HbA1c, body weight, and total daily insulin dose, while RMR and the physical activity levels remained unaltered.
Article Information
Acknowledgments. The authors are very grateful to the study participants for their cooperation, Angela Jacan (Center for Biomarker Research in Medicine, CBmed, Graz, Austria) for her contribution to the development of the earliest version of the study protocol, Dr. Regina Riedl (Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, Graz, Austria) for her help with the sample size calculation, and the team of the Endocrinology Lab Platform (Medical University of Graz, Graz, Austria), especially Cornelia Missbrenner and Dorrit Münzer-Ornik.
Funding. The study was funded by the Austrian Science Fund (FWF) Programme Clinical Research grants KLI 851-B and KLI-1076 to H.S. and by grants P28854, I3792, DK-MCD W1226, and DOC-130 to T.M. T.M. was supported by Austrian Research Promotion Agency (FFG) grants 864690 and 870454, the Integrative Metabolism Research Center Graz, Austrian Infrastructure Program 2016/2017, the Styrian Government (Zukunftsfonds, doc.funds program), the City of Graz, and BioTechMed-Graz (Flagship project DYNIMO).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.Ob. wrote the final manuscript. A.Ob. and N.J.T. contributed to efficient project management and day-to-day operation. A.Ob., N.J.T., P.N.P., H.K., F.Az., A.M., F.Ab., C.S., T.P., B.O.-P., and V.S. contributed to collection, analysis, and interpretation of data. A.Ob., F.Az., and A.Ou. contributed to the statistical analysis. N.J.T. and H.S. significantly contributed to development of the study protocol. N.J.T. and H.S. contributed to acquiring ethical approval for the trial. F.Az., A.M., and H.S. had access to and verified the raw data. M.S. is the dietitian of the trial. H.H. and T.M. performed the metabolomics analysis. H.S. conceived the trial. All authors reviewed and contributed to the final manuscript. A.Ob. and H.S are the guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 58th European Association for the Study of Diabetes (EASD) Annual Meeting, Stockholm, Sweden, 19–23 September 2022.
Footnotes
Clinical trial reg. no. DRKS00018070, Deutschen Register Klinischer Studien (DRKS)
This article contains supplementary material online at https://doi.org/10.2337/figshare.21563463.
References
- 1. Zhou B, Lu Y, Hajifathalian K, et al. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res 2014;164:302–311 [DOI] [PubMed] [Google Scholar]
- 4. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol 2022;18:309–321 [DOI] [PubMed] [Google Scholar]
- 6. Balkau B, Home PD, Vincent M, Marre M, Freemantle N. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care 2014;37:2108–2113 [DOI] [PubMed] [Google Scholar]
- 7. Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017;60:422–434 [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Li Q, Liu Y, Jiang H, Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2021;179:109003. [DOI] [PubMed] [Google Scholar]
- 9. Obermayer A, Tripolt NJ, Pferschy PN, et al. INTERmittent FASTing in people with insulin-treated type 2 diabetes mellitus—the INTERFAST-2 study protocol. Diabet Med 2022;39:e14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reisinger AC, Posch F, Hackl G, et al. Branched-chain amino acids can predict mortality in ICU sepsis patients. Nutrients 2021;13:3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open 2018;1:e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Sadraie B, Steckhan N, et al. Effects of a one-week fasting therapy in patients with type-2 diabetes mellitus and metabolic syndrome—a randomized controlled explorative study. Exp Clin Endocrinol Diabetes 2017;125:618–624 [DOI] [PubMed] [Google Scholar]
- 13. Borgundvaag E, Mak J, Kramer CK. Metabolic impact of intermittent fasting in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of interventional studies. J Clin Endocrinol Metab 2021;106:902–911 [DOI] [PubMed] [Google Scholar]
- 14. Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring) 2018;26:254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cienfuegos S, Gabel K, Kalam F, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab 2020;32:366–378.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 2018;4:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elhadd T, Bashir M, Baager KA, et al.; PROFAST Ramadan Study Group . Mitigation of hypoglycemia during Ramadan using the flash glucose monitoring system following dose adjustment of insulin and sulphonylurea in patients taking multiple glucose-lowering therapies (The PROFAST-IT Study). Diabetes Res Clin Pract 2021;172:108589. [DOI] [PubMed] [Google Scholar]