Abstract
EFSA assessed the relevance of seaweed and halophyte consumption to the dietary exposure to heavy metals (arsenic, cadmium, lead and mercury) and the iodine intake in the European population. Based on sampling years 2011–2021, there were 2,093 analytical data available on cadmium, 1,988 on lead, 1,934 on total arsenic, 920 on inorganic arsenic (iAs), 1,499 on total mercury and 1,002 on iodine. A total of 697 eating occasions on halophytes, seaweeds and seaweed‐related products were identified in the EFSA Comprehensive European Food Consumption Database (468 subjects, 19 European countries). From seaweed consumption, exposure estimates for cadmium in adult ‘consumers only’ are within the range of previous exposure estimates considering the whole diet, while for iAs and lead the exposure estimates represent between 10% and 30% of previous exposures from the whole diet for the adult population. Seaweeds were also identified as important sources of total arsenic that mainly refers, with some exceptions, to organic arsenic. As regards iodine, from seaweed consumption, mean intakes above 20 μg/kg body weight per day were identified among ‘consumers only’ of Kombu and Laver algae. The impact of a future increase in seaweed consumption (‘per capita’) on the dietary exposure to heavy metals and on iodine intake will strongly depend on the seaweeds consumed. The exposure estimates of heavy metals and iodine intakes in ‘consumers only’ of seaweeds were similar to those estimated in a replacement scenario with selected seaweed‐based foods in the whole population. These results underline the relevance of the current consumption of seaweeds in the overall exposure to different heavy metals and in the intake of iodine. Recommendations are provided for further work needed on different areas to better understand the relationship between seaweed consumption and exposure to heavy metals and iodine intake.
Keywords: seaweed, halophyte, dietary exposure, arsenic, lead, mercury, cadmium, intake, iodine
Summary
Following an official request from the European Commission in January 2022, EFSA assessed the relevance of the consumption of seaweeds and halophytes in relation to the dietary exposure to heavy metals (arsenic, cadmium, lead and mercury) and to the iodine intake in the European population.
Based on the sampling years from 2011 to 2021, a total of 9,715 analytical results on food were available in the final data set (2,965 samples); the final data set also contained 254 analytical results on feed (76 samples). Food samples were collected in 22 European countries and feed samples in seven. For food, there were 2,093 analytical data on cadmium, 1,988 on lead, 1,934 on total arsenic (tAs), 920 on inorganic arsenic (iAs), 1,499 on total mercury (tHg) and 1,002 on iodine. In addition, some other few analytical results were reported for different species of these compounds (e.g. methylmercury, arsenobetaine).
The highest mean occurrence levels were reported for iodine. Among the heavy metals, the highest mean was for arsenic, in particular for tAs but also in few samples for iAs, and cadmium. Mean concentrations of mercury in seaweeds were the lowest. A relatively high variation in heavy metals and iodine levels were found across seaweed samples even within species. Overall, the highest mean levels were reported for brown seaweeds, followed by red and green seaweeds.
A total of 697 eating occasions on halophytes, seaweeds and products based on or containing seaweed were identified in the EFSA Comprehensive European Food Consumption Database (EFSA Comprehensive Database), belonging to 468 subjects from 19 European countries. Most of the eating occasions referred to ‘Algae and prokaryotes organisms’ (~63%), of which about 2/3 were reported as the red alga Laver. A relatively high number of eating occasions was also reported for ‘Algae based formulations (e.g., Spirulina, Chlorella)’ (~21%) while for all halophytes only around 7% were available.
A general dietary exposure scenario was conducted considering the consumption data available in the EFSA Comprehensive Database and the occurrence data reported to EFSA on halophytes, seaweeds and seaweed‐related products. In the whole population, the limited numbers of seaweed consumers led to rather low estimates for the different compounds since their exposure was diluted among all subjects. In this general scenario, dietary exposure was also estimated for ‘consumers only’ of halophytes, seaweed and seaweed‐related products across the different dietary surveys.
The relevance of halophytes, seaweeds and seaweed‐related products for the exposure to arsenic, cadmium, mercury and lead, and on the intake of iodine, was mainly assessed comparing the estimated exposure in ‘consumers only’ with previous EFSA assessments considering the whole diet. For cadmium and tAs, the highest mean exposure from the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ was similar to the estimates reported in previous exposure assessments considering the whole diet. In the case of cadmium, the high exposure was linked to the relatively high levels reported in the dried red algae Laver (1,675–1,676 μg/kg; LB–UB). For lead and iAs, the dietary exposure estimates from the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ were considered as non‐negligible since they represented between 10 and 30% of the total exposure in previous EFSA assessments considering the whole diet. For mercury, under the assumption that all tHg in seaweeds and halophytes were methylmercury (as considered for fish), the highest mean exposure from the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ could also represent around 10% of the highest mean dietary exposure to methylmercury in ‘Adults’ via the whole diet. Overall, in those dietary surveys with the highest estimates for the different heavy metals, seaweeds were the main and/or only contributors. For iodine, the highest mean intake from the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ was 155 μg/kg body weight (bw) per day estimated in ‘Adults’. This value would represent more than 10,000 μg per day in an adult of 70 kg. The main contributor to the highest estimate was the group ‘Algae and prokaryotes organisms’ unspecified. However, in few other dietary surveys, relatively high iodine intakes (above 20 μg/kg bw per day) were also identified following the consumption of different types of seaweed (Kombu, Laver). Therefore, although highest iodine levels are typically linked to some brown algae, a frequent intake of other type of algae such as the red algae Laver might also deserve attention. Although a comparison with previous assessments could not be done for iodine, the magnitude of iodine intake via the consumption of certain seaweeds can be put in context by looking at the Tolerable Upper Intake Level (UL) of 600 μg/day set for the adult population.
The impact of a future increase in seaweed consumption (‘per capita’) on the dietary exposure to heavy metals and on iodine intake will strongly depend on the type of seaweeds consumed. The replacement of few selected conventional foods by seaweed‐based foods showed that the European population would be exposed to iAs, lead and cadmium at levels within the range of prior exposure estimates from the whole diet. It is important to note that the replacement scenario, although being overly conservative, led to dietary exposures to heavy metals and iodine intakes similar to those estimated for the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ using the current available consumption data. This underlines again the relevance of seaweed consumers and how the current consumption of certain seaweeds can contribute to the overall exposure to different heavy metals and iodine intake.
When interpreting the results, several uncertainties associated to the assessment should be considered. As an example, processing/home preparation could reduce the amount of heavy metals and iodine initially present in edible seaweeds; this was of minimal relevance in the general dietary exposure scenario considering the occurrence and consumption data used. Main uncertainties linked to the consumption data are related to their representativity due to the low number of consumers, but also to the reporting of several eating occasions as just ‘Algae and prokaryotes organisms, unspecified’ without further details. Since the levels of iodine and heavy metals in seaweeds seem to be strongly affected by many different factors, there is uncertainty also on the representativity of the occurrence data used in the assessment. There is also uncertainty linked to the FoodEx codification provided for the seaweed samples (consumption and occurrence data) since the taxonomy of seaweed species is particularly complex. In the replacement scenario, together with the uncertainties related to data representativity and taxonomy, the main uncertainty refers to its overly conservative nature (overestimation). Additionally, in this scenario, home processing could have an impact on the exposure estimations as seaweed pasta will be boiled before consumption.
Further work is still needed to better understand the relationship between consumption of seaweed and related products and the exposure to heavy metals and iodine intake. Data collection on occurrence levels should continue since heavy metals and iodine levels in seaweed can show a high variation depending on the seaweed and are affected by many different factors. Since processing has a relevant impact on the levels of heavy metals and iodine in seaweed, monitoring should be extended to processed products; in parallel, further investigation is also needed on the effect of processing on the bioavailability of these compounds. In the case of mercury and arsenic, speciation analysis is desirable since the toxicity varies greatly with species. In the area of consumption, efforts by EFSA and Member States to collect consumption data should continue, allowing regular updates to identify possible trends. This should also help to better understand whether seaweeds and seaweed products will remain in Europe as a niche product only being consumed sporadically or, instead, they will continue gaining acceptance becoming a food consumed more often and in higher amounts. The data collection should contain comprehensive information on the seaweeds analysed/consumed, starting with an appropriate taxonomic classification at the level of species, but also details on whether the seaweeds underwent any processing/home preparation step before their analysis/consumption. Specifically for the occurrence data, information should be provided, at least, on the expression of results (whole weight/dry weight basis) and the moisture content. If known, for seaweed‐based products information on the content and possibly on the type of seaweed should also be collected. For halophytes, the lack of data is more evident than for seaweeds and seaweed‐related products. In order to adequately assess the relevance these plants might have on the exposure to heavy metals and iodine intake, more data are needed, both consumption and occurrence.
1. Introduction
In the last few years, the consumption of algae and alga‐related foods in Europe is rapidly increasing as consumers' interest in more sustainable and healthy foods is also growing (Roohinejad et al., 2017; Vellinga et al., 2021; Mendes et al., 2022). The Mintel's Global New Products Database (GNPD)1 shows that during the period 2012–2021 more than 250 seaweed products were launched each year, on average, to the EU market. Prepared meals (e.g. sushi, poke), plant‐based drinks, seaweed snacks and condiments seem to be among the seaweed products with the highest prevalence on the market, although the inclusion of seaweed into pasta is also gaining relevance in the last years as a mean to improve its nutritional and health properties (e.g. increase protein content) (Ścieszka and Klewicka, 2019). Moreover, algae are used to extract phycocolloids (alginates, agars and carrageenans), compounds used in many different food applications as texturing agents, emulsifiers and stabilisers in products such as ice cream, yoghurt and sausage (FAO and WHO, 2022).
There are two types of algae: microalgae (microscopic) and macroalgae (macroscopic). Microalgae are unicellular algae that comprise several thousands of species, with Chlorella and Arthrospira (Spirulina, a gram‐negative cyanobacterium) being probably the most used in food applications, mainly as food supplements but not only (Caporgno and Mathys, 2018; Torres‐Tiji et al., 2020). Macroalgae, also known as seaweeds, are multicellular plant‐like organisms that generally live attached to rock or other hard substrata in coastal areas. They are classified into three groups on the basis of their thallus colour, corresponding to phylum Chlorophyta (green algae), Ochrophyta (brown algae) and Rhodophyta (red algae) (Leandro et al., 2019).
Edible seaweeds are seen as nutritious and sustainable alternatives to animal‐based proteins. The consumption of seaweeds has been associated to different health benefits mainly based on their content of different macro and micronutrients (proteins, omega‐3 and ‐6 fatty acids, well‐balanced essential amino acids, minerals, vitamins, etc.) (Peña‐Rodríguez et al., 2011). Moreover, seaweeds seem to also be a good source of diverse bioactive compounds, for instance peptides with antioxidant, antimicrobial and antiviral activities, among others (Holdt and Kraan, 2011; Pandey et al., 2020; Lomartire and Gonçalves, 2022).
In Europe, there is evidence for the consumption of more than 150 edible species of algae, of which 14% are considered microalgae/cyanobacteria and 86% seaweeds (Mendes et al., 2022). In Europe, algae are considered novel foods. As today, there are around 30 species included in the EU Novel Food Catalogue.2 All these species are placed in the market as ‘not novel’ since they have been consumed to a significant degree in at least one Member State of the European Union before 15 May 1997 (not subject to the Novel Food Regulation (EU) 2015/2283). Two microalga are additionally authorised as novel food, Odontella aurita and Tetraselmis chuii, as well as diverse components extracted from and produced by specific microalgae (e.g. oil from Schizochytrium sp.).3 Among the microalgae/cyanobacteria, the most consumed is the cyanobacterium Spirulina, mainly as food supplements but also as ingredient in different food products (e.g. pasta). Consumption of seaweed in Europe is dominated by brown and red algae, with red algae from Porphyra and Pyropia genera (Laver/Nori) representing 60% of the total (Mendes et al., 2022). Other seaweeds typically consumed in Europe are the brown algae Wakame (Undaria pinnatifida) and Kombu (Laminaria sp.), as well as the red alga Dulse (Palmaria palmata). Norway, France and Ireland are the main producers in Europe, although in 2019 Asia alone covered 97.3% of the global production (FAO and WHO, 2022).
In parallel, the increased consumption of seaweeds also raises some safety concerns. Several hazards have been identified in seaweeds: chemical hazards such as heavy metals, persistent organic pollutants (e.g. dioxins and polychlorinated biphenyls), radionuclides and pesticide residues; microbiological hazards (e.g. Salmonella spp., Bacillus spp. and norovirus); physical hazards (e.g. metal pieces, glass splinters, crustacean shells, micro‐ and nanoplastics) and allergens (FAO and WHO, 2022). Several of these hazards were already pointed out by the European Food Safety Authority (EFSA) as part of its work on emerging risks (EFSA, 2017).
Seaweeds are well known to bioaccumulate metals and, therefore, seaweed and seaweed‐containing/based foods might become important contributors to the dietary exposure to several heavy metals such as arsenic, cadmium, lead and mercury (Zeraatkar et al., 2016; Luo et al., 2020). The risk assessments conducted in the past by EFSA identified different health concerns linked to long‐term exposure to these four compounds (EFSA CONTAM Panel, 2009a, 2009b, 2010, 2012). EFSA identified seaweeds as one of the food commodities with the highest concentration of cadmium although no specific concerns were identified (EFSA CONTAM Panel, 2009b). Likewise, seaweeds also possess very high levels of arsenic although primarily found in the form of arsenosugars; however, in some brown seaweeds (e.g. Hijiki, Oarweed), most of the arsenic is present as inorganic arsenic (EFSA CONTAM Panel, 2009b; Duinker et al., 2020; EFSA, 2021). In fact, different national authorities alerted in the past on possible risks linked to the seaweed Hijiki advising consumers to avoid or to limit its consumption (FSA, 2004; FSAI, 2015; SHC, 2015).
Additionally, certain brown seaweed species, in particular from Laminaria and Saccharina genus, contain very high levels of iodine (Aakre et al., 2021). Although iodine is an essential compound for humans and its deficiency can be a major public health concern, excessive intake can also provoke diverse harmful effects such as goitre, increased risk of thyroid cancer, etc. (EFSA NDA Panel, 2014). The Scientific Committee on Food (SCF) and EFSA already alerted that iodine‐rich products such as (dried) seaweed ‘can result in dangerously excessive iodine intakes’ (SCF, 2002; EFSA, 2006).
Although less popular than seaweeds, halophytes are also gaining relevance in the human diet (Barreira et al., 2017; Petropoulos et al., 2018). Halophytes are plants adapted to live in a saline environment, be it seawater, a salt‐water marsh, or a salt‐desert (Flowers et al., 1986). In Europe, the most relevant halophytes seem to be different species from Salicornia genus, commercialised with the name ‘Samphire’ or ‘Sea asparagus’, and Portulaca oleracea species (common purslane). Both halophytes are included in the EU Novel Food Catalogue and placed in the market as ‘not novel’ (consumed to a significant degree in at least one Member State of the European Union before 15 May 1997). Although not many studies are available on the chemical composition of halophytes, they are presented as foods with a high nutritional value and numerous bioactive compounds (Barreira et al., 2017; Petropoulos et al., 2018; Agudelo et al., 2021). However, together with the particularly high sodium content that is distinctive of these plants, several studies have shown that halophytes could also accumulate high levels of heavy metals (El‐Said and El‐Sikaily, 2013; Yang et al., 2020).
In 2018, the European Commission asked Member States to monitor the levels of arsenic, cadmium, lead, mercury and iodine in seaweed, halophytes and products based on seaweed.4 The aim was to obtain a better knowledge on their levels in seaweed, with the ultimate objective of assessing the contribution of seaweed to the dietary exposure to these compounds and the potential need of risk management actions (e.g. setting or modifying existing maximum levels (MLs)). As today, no MLs are established for arsenic, cadmium, lead and mercury in seaweed and halophytes under Commission Regulation (EC) No 1881/2006,5 except for the ML for cadmium for food supplements consisting exclusively or mainly of seaweed or products derived from seaweed. For mercury, a maximum residue level (MRL) for algae and prokaryotic organisms is established at the default level of 0.01 mg/kg by Regulation (EC) No 396/20056. Within Europe and at country level, only France has defined MLs for heavy metals in seaweeds (CEVA, 2014). For iodine, the SCF adopted in 2002 the value of 600 μg/day as a Tolerable Upper Intake Level (UL) for adults including pregnant and lactating women; the ULs for children were derived by adjustment of the adult UL on the basis of metabolic weight (body weight0.75) (SCF, 2002). A ML of 2,000 mg/kg dry weight for iodine is recommended in France for all species of edible seaweed (ANSES, 2018), while Germany recommends a maximum concentration of 20 mg/kg of iodine in fresh seaweed products and a maximum daily uptake of 500 μg/day (BfR, 2004). For feed, Directive 2002/32/EC7 provides ML of undesirable substances in animal feed; for seaweed meal and feed materials derived from seaweed, ML are only set for arsenic.
This scientific report aims at providing an overview of the levels of arsenic, cadmium, lead, mercury and iodine in seaweeds, seaweed‐related products and halophytes. Based on these data and the current consumption data reported in the EFSA Comprehensive European Food Consumption Database, preliminary information is shown on the relevance of these food commodities regarding the dietary exposure to heavy metals and iodine intake. A replacement scenario with selected seaweed commodities is also provided to anticipate the impact of a future increase in the consumption of seaweeds and seaweed‐related products.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
For arsenic, cadmium and lead, maximum levels (MLs) for various foodstuffs are established under Commission Regulation (EC) No 1881/2006. 5 However, currently no MLs are established for these substances in seaweed and halophytes, except for the MLs established under this Regulation for food supplements consisting exclusively or mainly of seaweed or products derived from seaweed. For mercury currently under Regulation (EC) No 396/2005 6 a maximum residue level (MRL) for algae and prokaryotic organisms is established at the default level of 0.01 mg/kg.
In view of the occurrence of these heavy metals in seaweed and halophytes and in view of the various health risks, which were identified by EFSA for these metals, by means of Recommendation (EU) 2018/464 4 the Commission recommended Member States to analyse in 2018, 2019 and 2020 arsenic, cadmium, lead and mercury in a wide range of seaweed and halophyte species and products and food additives containing seaweed and halophytes.
In 2006 the Scientific Committee for food established an upper limit for iodine intake of 600 μg/day for adults and of 200 μg a day for children of 1–3 years.8 It indicated that the ingestion of iodine‐rich algal products, particularly dried products, can lead to dangerously excessive iodine intakes, if such products contain more than 20 mg iodine/kg dry matter and the exposed population lives in an area of endemic iodine deficiency. Therefore, also the monitoring of iodine was included within the scope of Recommendation (EU) 2018/464.
Seaweed and halophytes form an increasingly important contribution to the consumption patterns of certain EU consumers. Therefore, it is necessary to assess whether the contribution of arsenic, cadmium, iodine, lead and mercury from seaweed and halophytes to the total exposure of these substances, would necessitate the establishment of MLs for these commodities or the amendment of the MRL for mercury for algae and prokaryotic organisms or any action to be taken related to the exposure to iodine from these products. The newly available occurrence data would enable such consumer exposure assessment for arsenic, cadmium, iodine (in case sufficient data are available for iodine), lead and mercury. In case the consumption data for seaweed and halophytes would appear to be limited, an estimate should be made of the exposure when specific components of the diet would be replaced by seaweed to a certain extent. As certain processing/ food preparation steps such as soaking or cooking can result in a reduction of the exposure compared to a consumption of the seaweed as such or e.g., in soup, the effects of processing/ food preparation on the exposure should also be discussed.
For food additives obtained from seaweed, specifications are laid down in the annexes to Regulation (EU) No 231/2012.9 For some of these additives, EFSA recommended that the limits for the impurities of toxic elements should be revised in order to ensure that the use of these additives will not form a significant source of exposure to those toxic elements in particular for infants and young children.10 Therefore the occurrence of arsenic, cadmium, iodine, lead and mercury in food additives based on seaweed should be summarised, to assess the need to a further data collection, with a view of an exposure assessment at a later stage.
In view of the increasing trend of seaweed consumption, a prospective chapter should be included in the report to estimate the possible impact of a future increased seaweed consumption on the consumer exposure to arsenic, cadmium, iodine, lead and mercury.
In addition, a detailed overview of the available occurrence data for arsenic, cadmium, lead, mercury and iodine in seaweed and halophytes and products (food, food additives and feed) containing seaweed and halophytes should be provided, in order to consider the possible need for further regulatory activities on these substances. For this purpose, the data for fresh seaweed and dried seaweed should be clearly separated, where possible.
1.1.2. Terms of Reference
In accordance with Art. 31 of Regulation (EC) No 178/2002 the Commission asks EFSA
for a consumer exposure assessment for arsenic, cadmium, lead, mercury and iodine (in case sufficient data are available for iodine) in seaweeds and halophytes and products containing seaweed and halophytes, submitted to EFSA in the past 10 years.
an overview of the available occurrence data on arsenic (total and inorganic), cadmium, lead, mercury (methylmercury and total mercury) and iodine in seaweed and halophytes and products containing seaweed and halophytes. Separate overviews should be provided for food, food additives and feed.
2. Data and Methodologies
2.1. Occurrence data
2.1.1. Data collection and validation
Occurrence data for the heavy metals under assessment (arsenic, cadmium, lead and mercury) and iodine were collected as part of the annual call for collection of chemical contaminants occurrence data in food and feed, in the framework of Articles 23 and 33 of Regulation (EC) No 178/2002.11 The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description (SSD) for Food and Feed (EFSA, 2010a).
Data on heavy metals and iodine can be submitted to EFSA in different ways:
-
○
For arsenic as arsenic, arsenic derivatives, total arsenic (tAs), inorganic arsenic (iAs), As(V), As(III), organic arsenic, methylarsonic acid (MA), dimethylarsinic acid (DMA) and arsenobetaine (AB).
-
○
For mercury as mercury derivatives, mercury compounds, total mercury (tHg), methylmercury and inorganic mercury.
-
○
For cadmium as cadmium derivatives and cadmium.
-
○
For lead as lead derivatives and lead.
-
○
For iodine as iodine derivatives, iodine and iodate.
For food, the extraction covered analytical data in seaweeds, halophytes and products based or containing seaweed. In agreement with the European Commission, the following halophytes were considered: purslanes (Portulaca oleracea L.), agretti (Salsola soda Weinm), glassworts/samphires/sea asparagus (Salicornia europaea L.), rock samphires/sea fennel (Crithmum maritimum L.), sea aster (Aster tripolium L.), sea lavender (Limonium vulgare), hottentot fig (Carpobrotus edulis L.), goosefoot (Chenopodium spp.). Green alga, red alga, brown alga, microalga and ‘Algae based formulations’ were considered when extracting the data on seaweeds; the use of adequate facets (see Section 2.3) allows the extraction of different products based on or containing seaweed (e.g. seaweed tea, seaweed snacks). Additionally, analytical data on food additives were also extracted to identify those based on seaweed.12
For feed, the extraction covered analytical data in the following feed materials: ‘Algae’, ‘Dried algae’, ‘Algae meal’, ‘Algal oil’, ‘Algae extract [Algae fraction]’ and ‘Seaweed meal’. 13
Analytical data were extracted from the EFSA Data Warehouse on 3 February 2022.
2.1.2. Data cleaning and analysis
To ensure the appropriate quality of the occurrence data used for the dietary exposure estimations, different data cleaning and data validation steps were followed according to EFSA SOPs.14 Together with identifying duplicate samples, attention was paid to the information provided on analytical methods and their sensitivity, FoodEx classification, expression of the results, etc. Data providers were contacted when needed to confirm the information provided or to ask for additional information (e.g. inaccurate classification, reported levels initially identified as potential outliers).
The left‐censored data were treated by the substitution method using the lower bound (LB) and upper bound (UB) approach (WHO/IPCS, 2009; EFSA, 2010b). Applying the LB approach, results below the limit of detection (LOD)/limit of quantification (LOQ) were replaced by zero; for the UB approach, the results below the LOD were replaced by the value reported as the LOD; results below the LOQ and above the LOD were replaced by the value reported as the LOQ.
2.2. Food consumption data
The EFSA Comprehensive European Food Consumption Database (EFSA Comprehensive Database) provides a compilation of national information on food consumption at individual level and was first built in 2010 (EFSA, 2011a). Details on how the EFSA Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a).
Food consumption data were retrieved from the EFSA Comprehensive Database in March 2022. The latest version of the EFSA Comprehensive Database, updated in July 2021, contains results from a total of 72 dietary surveys carried out in 24 European countries covering 137,165 individuals. Detailed information on the dietary surveys can be found on the dedicated page of the EFSA website.15 The following age classes were considered:
Infants: < 12 months old;
Toddlers: ≥ 12 months to < 36 months old;
Other children: ≥ 36 months to < 10 years old;
Adolescents: ≥ 10 years to < 18 years old;
Adults: ≥ 18 years to < 65 years old;
Elderly: ≥ 65 years to < 75 years old;
Very elderly: ≥ 75 years old.
Nine additional surveys provided information on specific population groups: six on ‘Pregnant women’ (Austria: ≥ 19 years to ≤ 48 years old, Cyprus: ≥ 17 years to ≤ 43 years old; Latvia: ≥ 15 years to ≤ 45 years old, Romania: ≥ 19 years to ≤ 49 years old, Spain: ≥ 21 years to ≤ 46 years old, Portugal: 17 years old to 46 years old), two on ‘Lactating women’ (Greece: ≥ 28 years to ≤ 39 years old, Estonia: 18 years old to 45 years old) and one on ‘Vegetarians’ (Romania: ≥ 12 years to ≤ 74 years old).
When two dietary surveys were available for a country and age class, only the most recent one was used. Only dietary surveys with more than one day per subject were used to estimate the chronic dietary exposure, following the recommendations of the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a). This resulted in a total of 47 dietary surveys (86,117 subjects) carried out in 22 European countries, used for the chronic dietary exposure assessment (Annex B). Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.3. Food and feed classification
Food consumption and occurrence data were both codified according to the FoodEx2 classification system. Feed samples were classified according to the Catalogue of feed materials as described in Commission Regulation (EU) 2017/101716 and recorded following the feed hierarchy as described in FoodEx2 system.
FoodEx was developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances (EFSA, 2011b). Following its first publication, a testing phase was carried out in order to highlight strengths and weaknesses, and to identify possible issues and needs for refinement. Based on the outcome of the testing phase, EFSA published in 2015 the FoodEx2 revision 2 (EFSA, 2015).
The FoodEx2 catalogue hosts several hierarchies used for different data collections, e.g. ‘Reporting hierarchy’ for the collection of food and feed occurrence data and ‘Exposure hierarchy’ for the collection of food consumption data. FoodEx2 allows the further description of food and feed items with facets. Facets are descriptors providing additional information for a particular aspect of a food or feed, and are divided into implicit facets that are those integrated in the catalogue, and explicit facets, which are added by users while coding a food or feed item.
2.4. Methodology for dietary exposure estimations
2.4.1. General dietary exposure/intake scenario
The general dietary exposure/intake scenario only used the occurrence data on heavy metals and iodine available for halophytes, seaweeds and seaweed‐related products.
For the whole population, dietary chronic exposure to heavy metals and dietary intake to iodine was assessed at individual level by multiplying the average daily consumption for each food with the corresponding mean occurrence estimate for each compound (LB and UB) in halophytes, seaweeds and seaweed‐related products. The respective estimates throughout the diet were summed up and the results divided by the individual's body weight. For each dietary survey, the mean and 95th percentile dietary exposure/intake were estimated from the distribution of the individual exposure/intake results.
Dietary exposure/intake from the consumption of halophytes, seaweeds and seaweed‐related products was also estimated in ‘consumers only’ across the different dietary surveys; these estimations should be considered with care due to the relatively low number of consumers. Mean estimates were only considered in the main text of the report when a minimum of five consumers was available by dietary survey and age class.
In accordance with the specifications of the EFSA Guidance on the use of the Comprehensive Database, only percentiles considered as statistically robust were estimated (e.g. 75th percentile with at least 11 observations, 90th percentile with at least 29 observations, 95th percentile with at least 59 observations) (EFSA, 2011a).
Before doing the linkage between consumption and occurrence data, special attention was given to whether the samples analysed/consumed referred to dried samples or fresh samples. When this information was not available, data providers were contacted asking for additional information. When analytical results were expressed in ‘Dry matter’, the moisture content was used to convert the results into whole weight. From the final data set available for each compound and to best estimate dietary exposure, only the occurrence data that allowed an adequate linkage with the consumption data were used (e.g. both reported as fresh or dried products).
2.4.2. Replacement dietary exposure scenario
Together with the general exposure scenario, a replacement exposure scenario was carried out to complement the assessment. The replacement scenario is based on the assumption that the consumption of some conventional foods will be replaced, to a different extent, by selected foods based on or containing seaweed. The anticipated dietary exposure was estimated by using the consumption data of the conventional foods supposed to be replaced.
As commented in the Introduction section, the number of food products in the market that contain seaweed extracts or whole seaweeds is rapidly increasing. In order to identify the most relevant seaweed derived foods present in the European market, different sources of information were consulted. Together with scientific papers that could provide information on the latest developments on seaweed foods and the occurrence data submitted to EFSA on these commodities, the Mintel's Global New Products Database (GNPD) 1 was also used. At the end, four different foods were selected among the most relevant seaweed products currently present in the European market: seaweed snacks, seaweed condiments (relishes), seaweed pasta and seaweed salad.
For seaweed pasta, an average composition of 5% dried algae was assumed based on GNPD and literature data (Hasanah et al., 2021). Although looking at the GNPD, the microalga Spirulina seems to be the main one used as ingredient, seaweeds such as the brown alga Kombu could also be used (Fradinho et al., 2019). In this report, the concentrations of the different compounds assigned to seaweed pasta were derived from the average occurrence data reported for all algae samples (dried) excluding Hijiki alga. Hijiki alga (Sargassum fusiforme, synonym Hizikia fusiformis) is typically associated to relatively high levels of iAs and different food safety agencies warned consumers in the past not to eat or to decrease its consumption (FSA, 2004; FSAI, 2015; SHC, 2015). In addition, to prevent the impact of having samples of Hijiki alga among the samples reported as unspecified ‘Brown alga’, unspecified ‘Algae and prokaryotes organisms’ and ‘Other algae’, these three food groups were also excluded.
As seaweed salad present in the market seems to be mostly made of brown algae, in particular Wakame and/or Kombu, the average occurrence data reported for the different compounds in all fresh/unprocessed brown algae were used. As for seaweed pasta, Hijiki alga and the unspecified ‘Brown alga’, ‘Other algae’ and unspecified ‘Algae and prokaryotes organisms’ were not considered. The derived concentrations from fresh/unprocessed brown algae were directly used for ‘Lettuce and salad plants’ and for ‘Watercress and similar’. For ‘Mixed green salad’ and ‘Mixed vegetable salad’, it was assumed that only 20% of the ingredients would be seaweed and, therefore, the concentrations were accordingly adjusted. This percentage refers to the amount of leaf vegetables considered to be present in ‘Prepared green salad’ (EFSA, 2019).
For seaweed snacks and seaweed condiments, the levels of the different compounds under assessment were derived from the available occurrence data set. It is important to note that the few samples codified under seaweed snacks and seaweed condiments might comprise a rather heterogeneous mix of products containing different types and amounts of seaweeds in their composition. This situation undoubtedly adds uncertainty to the levels assigned to these commodities and to their linkage with the consumption data. For seaweed condiments, there were no occurrence data reported on iAs. In this case, the concentration was derived from the occurrence data on iAs reported for all fresh brown algae, as they seem to be the seaweeds mostly used in the preparation of seaweed condiments. Also here, the data on Hijiki algae and unspecified ‘Brown alga’ were excluded.
Further details on the concentration data assigned to each of the seaweed‐related foods for the compounds under assessment are shown in the corresponding sections covering the replacement scenario. Two different assumptions were considered when using the consumption data in the replacement scenario. On one hand, for seaweed snacks, seaweed condiments (relishes) and seaweed pasta it was considered that the conventional foods could be fully replaced by the seaweed‐related foods. Therefore, for all subjects in the EFSA Comprehensive Database, 100% of the reported consumption data was assumed to be seaweed‐related products. This scenario is conservative and based on a consumption pattern that could make consumers to be committed to repeatedly purchase a specific product, in this case the seaweed‐related foods. On the other hand, for seaweed salad, it is not expected that consumers of different types of salads will completely shift towards the consumption of seaweed salad only. For this reason, an arbitrary 10% replacement was assumed in all subjects for the reported consumption of different types of salads (see Table 1).
Table 1.
Selected seaweed‐related products for the dietary exposure replacement scenario
| Food commodities to be replaced in the EFSA Comprehensive Database (FoodEx2 hierarchy) | Replacement scenario | |
|---|---|---|
| Seaweed salad (a) |
‘Lettuce and salad plants’ (FoodEx L3) ‘Watercress and similar’ (FoodEx L3) ‘Mixed green salad’ and ‘Mixed vegetable salad’ (both FoodEx L4) |
For all subjects, 10% of the reported consumption of these conventional commodities will be assumed to be seaweed‐related products |
| Seaweed snacks | ‘Chips, crisps, fries and dough‐based analogues’ and ‘Snacks other than chips and similar’ (both FoodEx L3) | For all subjects, 100% of the reported consumption of these conventional commodities will be assumed to be seaweed‐related products |
| Seaweed condiments (relishes) | ‘Relishes’ (FoodEx L3) | |
| Seaweed pasta (b) | ‘Pasta, plain (not stuffed), uncooked’ (FoodEx L4) |
For ‘Mixed green salad’ and ‘Mixed vegetable salad’ it was considered that only approximately 20% of their composition are ingredients (lettuce, watercress, etc.) that will be replaced by seaweed.
For seaweed pasta an average content of 5% seaweed was assumed when deriving the occurrence values for arsenic, cadmium, lead, mercury and iodine.
3. Assessment
3.1. Occurrence data
Based on the sampling years from 2011 to 2021, a total of 10,513 analytical results on food (3,338 samples) were extracted; for feed, only 254 analytical results (76 samples) were available. Both analytical data sets are shown in Annex C.
Preliminary assessment of the data led to the exclusion of 798 analytical results on food, in many cases food additives part of the initial data set but then confirmed by data providers as not based on seaweed. Analytical data reported as ‘Arsenic’ were considered as ‘Total arsenic’ if no additional information was provided, and those provided as derivatives were excluded from the final data set. Following these first steps, a total of 9,715 analytical results on food were available (2,965 samples). No analytical data on feed were excluded; therefore, the final data set contained 254 analytical results (76 samples).
Table 2 shows the distribution of the analytical results among the compounds under assessment.
Table 2.
Analytical results on arsenic, mercury, cadmium, lead and iodine in seaweeds, halophytes and products based or containing seaweed (sampling years 2011–2021, food and feed)
| Number of analytical results | ||
|---|---|---|
| Food | Feed | |
| Cadmium | 2,093 | 55 |
| Lead | 1,988 | 54 |
| Total Arsenic (tAs) | 1,934 | 67 |
| Inorganic Arsenic (iAs) | 920 | 7 |
| Methylarsonic acid (MA) | 12 | – |
| Dimethylarsinic acid (DMA) | 125 | – |
| Arsenate ‐ As(V) | 29 | – |
| Arsenite ‐ As(III) | 29 | – |
| Arsenobetaine | 25 | – |
| Total mercury (tHg) | 1,499 | 67 |
| Methylmercury | 54 | 4 |
| Inorganic mercury | 5 | – |
| Iodine | 1,002 | – |
In the below sections describing the food occurrence data for the compounds under assessment, concentrations are reported in whole weight. Unfortunately, it was not possible to show all concentrations in ‘Dry matter’ since in most of the cases the moisture content was not reported. Most of the samples were reported to EFSA as ‘Whole weight’ even though the product was described as ‘dried’ (e.g. Nori sheets); for these samples, in few cases, the moisture content was provided (typically 5–10%). There were few samples with results expressed in ‘Dry matter’ and providing moisture content; the analytical results were converted into ‘Whole weight’ and classified as dried/fresh product depending on the information and the moisture content reported.
A total of 22 different sampling countries were reported for food samples; around 75% of the analytical results were collected in five countries: Norway (~20%), Belgium, Germany and Ireland (each of them ~14%), and the Netherlands (~12%). Feed samples were collected in seven different countries, with Belgium, the Netherlands, Denmark and France as main sampling countries. Regarding the sampling year, Figure 1 shows the different years when the food and feed samples were collected since 2011.
Figure 1.
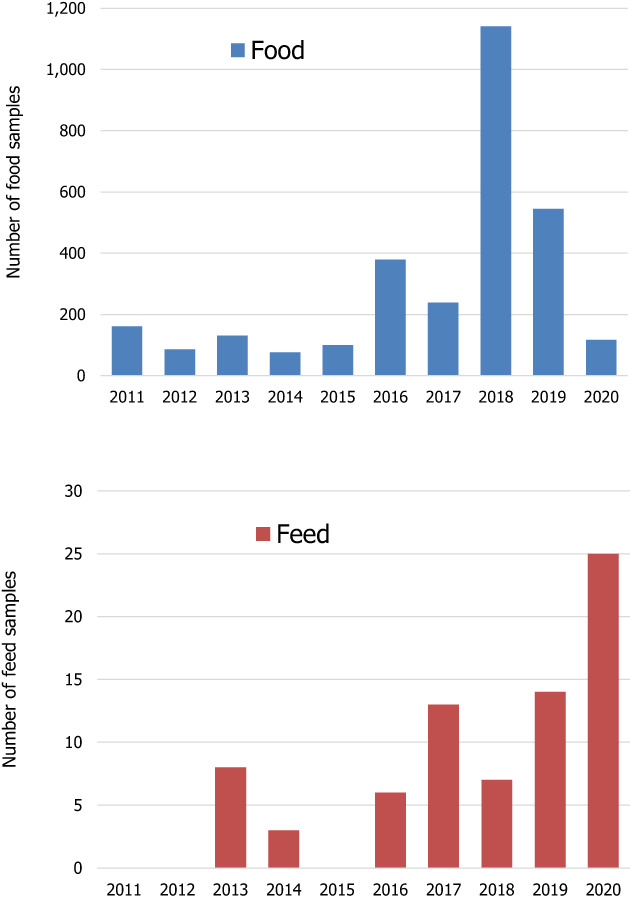
Number of food and feed samples by sampling year
Among the analytical methods reported, the preferred detection method was inductively coupled plasma mass spectrometry (ICP‐MS) regardless the compound analysed. Diverse spectroscopic methods were also reported, in particular atomic absorption spectrometry (AAS), atomic emission spectrometry (AES) and atomic fluorescence spectrometry (AFS). Overall, the highest sensitivities were for ICP‐MS: the lowest LOQs ranged between 0.0233 μg/kg for cadmium and 20 μg/kg in the analysis of different arsenic species [DMA, As(III), As(V)].
3.1.1. Food occurrence data on cadmium
A total of 2,093 analytical data on cadmium were available in the final data set. Detailed summary statistics of these data are shown in Annex D, also covering few food additives based on seaweed (n = 41). From this data set, a total of 1,459 samples (see Table 3) were used in the general and in the replacement exposure scenario based on the methodology described in Section 2.4.
Table 3.
Mean cadmium concentrations (μg/kg, whole weight) in halophytes, seaweeds and products based on or containing seaweed as used to estimate dietary exposure in the general and replacement scenario
| Additional information | N | LC (%) | Mean concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| LB | UB | |||||
| Halophytes | Purslanes and similar | Fresh product | 7 | 57 | 350.1 | 367.1 |
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | Dried product | 142 | 0.7 | 1,325.4 | 1,325.7 |
| Algae and prokaryotes organisms, unspecified | Fresh product | 30 | 43 | 223.8 | 304.1 | |
| Green algae, unspecified | Dried product (a) | 3 | – | 226.0 | 226.0 | |
| Green algae, unspecified | Fresh product | 18 | 17 | 108.6 | 110.8 | |
| Sea lettuce | Dried product (a) | 15 | – | 997.5 | 997.5 | |
| Sea lettuce | Fresh product | 18 | 11 | 270.5 | 275.3 | |
| Red alga, unspecified | Dried product | 15 | – | 1,172.4 | 1,172.4 | |
| Laver | Dried product | 212 | 5 | 1,675.0 | 1,676.4 | |
| Laver | Fresh product | 19 | 21 | 850.4 | 857.4 | |
| Carrageen mosses | Dried product (a) | 37 | 14 | 767.7 | 769.4 | |
| Carrageen mosses | Fresh product | 73 | 25 | 300.2 | 339.3 | |
| Dulse | Dried product (a) | 14 | 21 | 432.3 | 463.3 | |
| Dulse | Unspecified | 7 | 71 | 27.3 | 146.3 | |
| Brown algae, unspecified | Dried product | 77 | – | 1,089.0 | 1,089.0 | |
| Kombu | Dried product | 70 | 4 | 457.8 | 461.2 | |
| Kombu | Fresh product (a) | 129 | 7 | 191.6 | 204.2 | |
| Sea spaghetti | Dried product (a) | 18 | – | 588.4 | 588.4 | |
| Sea spaghetti | Fresh product(a) | 5 | – | 261.0 | 261.0 | |
| Wakame | Dried product | 83 | 1 | 1,276.4 | 1,276.5 | |
| Wakame | Fresh product | 16 | – | 817.5 | 817.5 | |
| Rockweed | Dried product(a) | 5 | – | 586.0 | 586.0 | |
| Rockweed | Fresh product(a) | 18 | – | 137.1 | 137.1 | |
| Spirulina | Dried product | 14 | 64 | 20.3 | 49.0 | |
| Products for non‐standard diets, food imitates and food supplements | Algae‐based formulations (e.g. Spirulina, chlorella) | Dried product | 382 | 34 | 297.8 | 310.4 |
| Fried or extruded cereal, seed or root‐based products | Snacks | With/containing seaweed | 16 | – | 536.2 | 536.2 |
| Hot drinks and similar (coffee, cocoa, tea and herbal infusions) | Seaweed tea | Seaweed tea | 4 | 25 | 3.2 | 3.3 |
| Seasoning, sauces and condiments | Relishes | With/containing seaweed | 12 | 17 | 104.9 | 108.5 |
Occurrence data exclusively used in the replacement scenario; %LC: percentage of left‐censored data.
The highest number of samples were for ‘Algae based formulations (e.g., Spirulina, Chlorella)’ (n = 382), followed by dried samples of Laver (n = 212) and ‘Algae and prokaryotes organisms, unspecified’ (n = 141). Overall, the highest mean levels of cadmium were reported for red algae in particular for the alga Laver (dried) with LB–UB average concentration of 1,675–1,676 μg/kg, although also for brown algae (e.g. dried Wakame, LB–UB = 1,276–1,276 μg/kg) relatively high mean values were reported. Among seaweeds the lowest mean value was reported for green algae (see Table 3). This is in line with data reported in the literature where levels of cadmium in red alga seems to be highest (Topçuoğlu et al., 2001; Chen et al., 2018; Banach et al., 2020; Duinker et al., 2020). As compared to the 2012 EFSA scientific report, mean levels of cadmium (LB–UB) seem to be lower for ‘Algae based formulations (e.g. Spirulina, Chlorella)’ (1,514–1,515 μg/kg in 2012 and 297.8–310.2 μg/kg in this report); most of the current samples were described as being Spirulina tablets/powder. In the same 2012 report, the mean levels reported for seaweed (1,122 μg/kg, LB=UB) were similar to those reported now for ‘Algae and prokaryotes organisms, unspecified’ (~1,325, LB=UB), and for some of the seaweeds (Table 3).
As concerns the occurrence data used for the replacement scenario (see Section 2.4 for the methodology followed), for seaweed condiments and seaweed snacks, the mean reported occurrence data for these commodities were used, 104.9–108.5 μg/kg (LB–UB, n = 12) for the first and 536.2 μg/kg (LB=UB, n = 16) for the latter. For seaweed pasta, a concentration of 59.1–59.3 μg/kg (LB–UB) was assigned, considering a 5% content of dried algae in the seaweed pasta and an initial cadmium concentration of 1,182–1,185 μg/kg (n = 486, LB–UB) derived from the average occurrence data reported for different types of dried algae samples as described in Section 2.4. For seaweed salad, an initial value of 247–257 μg/kg (n = 168, LB–UB) was derived from all the reported samples of different types of fresh ‘Brown algae’ as explained in Section 2.4. This concentration was directly assigned to ‘Lettuce and salad plants’ and ‘Watercress and similar’. Instead, for ‘Mixed green salad’ and ‘Mixed vegetable salad’ a concentration value of 49.5–51.4 μg/kg (LB–UB) was used, assuming that only 20% of the ingredients would be seaweed (Section 2.4).
3.1.2. Food occurrence data on lead
A total of 1,988 analytical data on lead were available in the final data set. Detailed summary statistics of these data are shown in Annex D, also covering few food additives based on seaweed (n = 40). From this data set, a total of 1,379 samples (see Table 4) were used in the general and in the replacement exposure scenario based on the methodology described in Section 2.4.
Table 4.
Mean lead concentrations (μg/kg) in halophytes, seaweeds and products based on or containing seaweed as used to estimate dietary exposure in the general and replacement scenario
| Additional information | N | LC (%) | Mean concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| LB | UB | |||||
| Halophytes | Purslanes and similar | Fresh product | 7 | 57 | 63.7 | 82.8 |
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | Dried product | 123 | 6 | 496.2 | 499.4 |
| Algae and prokaryotes organisms, unspecified | Fresh product | 29 | 45 | 285.1 | 374.8 | |
| Green algae, unspecified | Dried product (a) | 3 | – | 890.0 | 890.0 | |
| Green algae, unspecified | Fresh product | 18 | 11 | 94.9 | 103.1 | |
| Sea lettuce | Dried product (a) | 15 | – | 817.0 | 817.0 | |
| Sea lettuce | Fresh product | 18 | 22 | 193.3 | 265.5 | |
| Red alga, unspecified | Dried product | 15 | 47 | 328.4 | 1261.8 | |
| Laver | Dried product | 205 | 8 | 193.5 | 197.0 | |
| Laver | Fresh product | 19 | 26 | 119.3 | 162.1 | |
| Carrageen mosses | Dried product (a) | 27 | 11 | 360.9 | 367.2 | |
| Carrageen mosses | Fresh product | 71 | 38 | 192.1 | 257.4 | |
| Dulse | Dried product (a) | 14 | 14 | 590.7 | 607.7 | |
| Dulse | Unspecified | 7 | 43 | 137.1 | 222.9 | |
| Brown algae, unspecified | Dried product | 76 | 25 | 681.7 | 1088.3 | |
| Kombu | Dried product | 70 | 19 | 209.5 | 369.4 | |
| Kombu | Fresh product (a) | 129 | 62 | 63.2 | 241.7 | |
| Sea spaghetti | Dried product (a) | 18 | 22 | 142.0 | 377.5 | |
| Sea spaghetti | Fresh product (a) | 5 | 60 | 17.2 | 124.8 | |
| Wakame | Dried product | 83 | 7 | 477.9 | 502.3 | |
| Wakame | Fresh product | 14 | 29 | 301.7 | 344.9 | |
| Rockweed | Dried product (a) | 5 | 20 | 292.0 | 317.6 | |
| Rockweed | Fresh product (a) | 18 | 39 | 77.6 | 288.7 | |
| Spirulina | Dried product | 14 | 36 | 273.1 | 301.1 | |
| Products for non‐standard diets, food imitates and food supplements | Algae‐based formulations (e.g. Spirulina, chlorella) | Dried product | 344 | 26 | 268.6 | 305.8 |
| Fried or extruded cereal, seed or root‐based products | Snacks | With/containing seaweed | 16 | 25 | 384.4 | 393.9 |
| Hot drinks and similar (coffee, cocoa, tea and herbal infusions) | Seaweed tea | Seaweed tea | 4 | – | 4.4 | 4.4 |
| Seasoning, sauces and condiments | Relishes | With/containing seaweed | 12 | 8 | 228.8 | 231.9 |
Occurrence data exclusively used in the replacement scenario; %LC: percentage of left‐censored data.
The highest number of samples were reported for ‘Algae based formulations (e.g., Spirulina, Chlorella)’ (n = 344), and dried samples of Laver (n = 205) and the brown alga Kombu (n = 129). The highest mean concentrations were reported for ‘Green algae, unspecified’ (dried) with LB=UB lead levels of 890 μg/kg. Overall and as described in the literature, the lead levels in seaweeds were relatively low as compared to cadmium (Duinker et al., 2020).
For the occurrence data used in the replacement scenario (see Section 2.4 for the methodology followed), for seaweed condiments and seaweed snacks, the mean reported occurrence data for these commodities were used, 229–232 μg/kg (LB–UB, n = 12) for the first and 384–394 μg/kg (LB–UB, n = 16) for the latter. For seaweed pasta, a concentration of 14.9–18.4 μg/kg (LB–UB) was assigned, considering a 5% content of dried alga in the seaweed pasta and an initial lead concentration of 298–368 μg/kg (n = 469, LB–UB) derived from the average occurrence data reported for different types of dried algae samples as described in Section 2.4. For seaweed salad, an initial value of 83.5–252 μg/kg (n = 166, LB–UB) was derived from all the reported samples of different types of fresh ‘Brown algae’ as explained in Section 2.4. This concentration was directly assigned to ‘Lettuce and salad plants’ and ‘Watercress and similar’. Instead, for ‘Mixed green salad’ and ‘Mixed vegetable salad’ a concentration value of 16.7–50.5 μg/kg (LB–UB) was used, assuming that only 20% of the ingredients would be seaweed (Section 2.4).
3.1.3. Food occurrence data on total arsenic
A total of 1,934 analytical data on tAs were available in the final data set. Detailed summary statistics of these data are shown in Annex D, also covering few food additives based on seaweed (n = 39). From this data set, a total of 1,286 samples (see Table 5) were used in the general and in the replacement exposure scenario based on the methodology described in Section 2.4.
Table 5.
Mean tAs concentrations (μg/kg) in halophytes, seaweeds and products based on or containing seaweed as used to estimate dietary exposure in the general and replacement scenario
| Additional information | N | LC (%) | Mean concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| LB | UB | |||||
| Halophytes | Purslanes and similar | Fresh product | 7 | 57 | 41 | 59 |
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | Dried product | 149 | – | 28,480 | 28,480 |
| Algae and prokaryotes organisms, unspecified | Fresh product | 27 | – | 19,183 | 19,183 | |
| Green algae, unspecified | Dried product (a) | 3 | – | 4,333 | 4,333 | |
| Green algae, unspecified | Fresh product | 13 | 15 | 1,734 | 1,767 | |
| Sea lettuce | Dried product (a) | 14 | – | 18,456 | 18,456 | |
| Sea lettuce | Fresh product | 18 | – | 4,048 | 4,048 | |
| Red alga, unspecified | Dried product | 15 | – | 18,951 | 18,951 | |
| Laver | Dried product | 208 | 0.5 | 21,179 | 21,179 | |
| Laver | Fresh product | 17 | – | 8,219 | 8,219 | |
| Carrageen mosses | Dried product (a) | 35 | 6 | 22,842 | 22,850 | |
| Carrageen mosses | Fresh product | 73 | 7 | 10,983 | 10,988 | |
| Dulse | Dried product (a) | 15 | – | 11,285 | 11,285 | |
| Dulse | Unspecified | 7 | – | 4,737 | 4,737 | |
| Brown algae, unspecified | Dried product | 77 | – | 45,300 | 45,300 | |
| Kombu | Dried product | 72 | 1 | 54,757 | 54,757 | |
| Kombu | Fresh product (a) | 128 | – | 18,162 | 18,162 | |
| Sea spaghetti | Dried product (a) | 14 | – | 26,645 | 26,645 | |
| Sea spaghetti | Fresh product (a) | 6 | – | 13,089 | 13,089 | |
| Wakame | Dried product | 79 | 3 | 30,772 | 30,773 | |
| Wakame | Fresh product | 16 | – | 19,037 | 19,037 | |
| Rockweed | Dried product (a) | 3 | – | 26,633 | 26,633 | |
| Rockweed | Fresh product (a) | 18 | – | 15,639 | 15,639 | |
| Spirulina | Dried product | 9 | 22 | 792 | 840 | |
| Products for non‐standard diets, food imitates and food supplements | Algae‐based formulations (e.g. Spirulina, chlorella) | Dried product | 231 | 20 | 9,558 | 9,625 |
| Fried or extruded cereal, seed or root‐based products | Snacks | With/containing seaweed | 16 | 6 | 9,482 | 9,482 |
| Hot drinks and similar (coffee, cocoa, tea and herbal infusions) | Seaweed tea | Seaweed tea | 4 | – | 190 | 190 |
| Seasoning, sauces and condiments | Relishes | With/containing seaweed | 12 | – | 4,602 | 4,602 |
Occurrence data exclusively used in the replacement scenario; %LC: percentage of left‐censored data.
The highest number of available samples were for ‘Algae based formulations (e.g., Spirulina, chlorella)’ (n = 231), and dried samples of Laver (n = 208) and ‘Algae and prokaryotes organisms, unspecified’ (n = 149). Seaweeds are well known to possess high levels of tAs with the predominant species being, overall, organic (mainly arsenosugars, but also methylated arsenicals and arsenolipids) (Taylor et al., 2017). The highest mean levels of tAs were reported for the dried brown alga Kombu (LB=UB = 54,757 μg/kg) (see Table 5).
As regards the occurrence data used for the replacement scenario (see Section 2.4 for the methodology followed), for seaweed condiments and seaweed snacks, the mean reported occurrence data for these commodities were used, 4,602 μg/kg (LB=UB, n = 12) for the first and 9,482 μg/kg (LB=UB, n = 16) for the latter. For seaweed pasta, a concentration of 1,366–1,367 μg/kg (LB–UB) was assigned, considering a 5% content of dried alga in the seaweed pasta and an initial tAs concentration of 27,330–27,332 μg/kg (n = 467, LB–UB) derived from the average occurrence data reported for different types of dried algae samples as described in Section 2.4. For seaweed salad, an initial value of 17,794 μg/kg (n = 168, LB=UB) was derived from all the reported samples of different types of fresh ‘Brown algae’ as explained in Section 2.4. This concentration was directly assigned to ‘Lettuce and salad plants’ and ‘Watercress and similar’. Instead, for ‘Mixed green salad’ and ‘Mixed vegetable salad’ a concentration value of 3,559 μg/kg (LB=UB) was used, assuming that only 20% of the ingredients would be seaweed (Section 2.4).
3.1.4. Food occurrence data on inorganic arsenic
A total of 920 analytical data on iAs were available in the final data set. A detailed summary of these data is shown in Annex D, including iAs levels in two food additives based on seaweed. From this data set, a total of 601 samples (see Table 6) were used in the general and in the replacement exposure scenario based on the methodology described in Section 2.4.
Table 6.
Mean iAs concentrations (μg/kg) in seaweeds and products based on or containing seaweed as used to estimate dietary exposure in the general and replacement scenario
| Additional information | N | LC (%) | Mean concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| LB | UB | |||||
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | Dried product | 123 | 50% | 1,613 | 1,669 |
| Algae and prokaryotes organisms, unspecified | Fresh product | 2 | 100 | 0.0 | 65.0 | |
| Green algae, unspecified | Dried product (a) | 3 | – | 303.3 | 303.3 | |
| Green algae, unspecified | Fresh product | 7 | 29 | 136.3 | 189.7 | |
| Sea lettuce | Dried product (a) | 8 | 38 | 308.8 | 337.5 | |
| Sea lettuce | Fresh product | 13 | 8 | 104.3 | 112.0 | |
| Red alga, unspecified | Dried product | 12 | – | 123.1 | 123.1 | |
| Laver | Dried product | 106 | 60 | 83.3 | 174.2 | |
| Laver | Fresh product | 5 | – | 47.9 | 47.9 | |
| Carrageen mosses | Dried product (a) | 15 | 73 | 645.8 | 841.4 | |
| Carrageen mosses | Fresh product | 6 | 33 | 69.3 | 89.3 | |
| Dulse | Dried product (a) | 2 | 50 | 149.5 | 158.0 | |
| Brown algae, unspecified | Dried product | 62 | 8 | 3,402 | 3,404 | |
| Kombu | Dried product | 37 | 51 | 2,723 | 2,758 | |
| Kombu | Fresh product (a) | 40 | 20 | 490.6 | 509.4 | |
| Sea spaghetti | Dried product (a) | 8 | 25 | 167.9 | 194.1 | |
| Sea spaghetti | Fresh product (a) | 1 | – | 5.0 | 5.0 | |
| Wakame | Dried product | 57 | 47 | 136.1 | 191.4 | |
| Wakame | Fresh product | 8 | 100 | 0.0 | 78.8 | |
| Rockweed | Dried product (a) | 2 | – | 52.5 | 52.5 | |
| Rockweed | Fresh product (a) | 15 | 27 | 28.4 | 29.1 | |
| Spirulina | Dried product | 3 | 100 | 0.0 | 83.0 | |
| Products for non‐standard diets, food imitates and food supplements | Algae‐based formulations (e.g. Spirulina, chlorella) | Dried product | 62 | 37 | 240.9 | 263.2 |
| Fried or extruded cereal, seed or root‐based products | Snacks | With/containing seaweed | 4 | 75 | 7.5 | 90.0 |
Occurrence data exclusively used in the replacement scenario; %LC: percentage of left‐censored data.
The most important seaweed and seaweed‐related products in terms of consumption (i.e. alga Laver and ‘Algae based formulations’, see Table 10) were covered with the available occurrence data on iAs. However, as compared to the other compounds under assessment, few eating occasions were not covered (e.g. halophytes, relishes, seaweed tea) since data on iAs were not available on the corresponding commodities.
Table 10.
Eating occasions on halophytes, seaweeds and products based on or containing seaweed identified in the EFSA Comprehensive Database
| FoodEx2 | Eating occasions | |
|---|---|---|
| Halophytes | Purslanes | 17 |
| Winter purslanes | 16 | |
| Agretti | 5 | |
| Glassworts | 6 | |
| Sea lavanders | 1 | |
| Rock samphires | 3 |
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | 73 | |
| Green algae | Green algae, unspecified | 1 | |
| Sea lettuce | 6 | ||
| Red algae | Carrageen mosses | 1 | |
| Laver | 293 | ||
| Dulse | 1 | ||
| Brown algae | Wakame | 16 | |
| Kombu | 40 | ||
| Other brown algae | 2 | ||
| Micro‐phyte | Spirulina | 3 | |
| Miscellaneous supplements/nutraceuticals | Algae‐based formulations (e.g. Spirulina, chlorella) | 191 | |
| Herbal formulations and plant extracts | 11 | ||
| Other products based on or containing seaweed | Pickled/marinated vegetables | 2 |
| Gelling agents | 2 | |
| Non‐fermented tea, infusion | 1 | |
| Herbal and other non‐tea infusions | 1 | |
| Canned/jarred vegetables | 1 | |
| Cheese | 1 | |
| Rice based dishes, cooked | 1 | |
| Snacks other than chips and similar | 1 | |
| Mashed vegetable puree | 1 |
The highest number of samples were for dried ‘Algae and prokaryotes organisms, unspecified’ (n = 123) and dried Laver (n = 106). Although as commented above, most of the arsenic present in seaweeds is overall organic, certain seaweeds have been reported to contain from moderate to very high levels of iAs, in particular brown algae such as Hijiki and, in some cases, Kombu (Taylor et al., 2017; Cherry et al., 2019; EFSA, 2021). In fact, the highest mean levels of iAs were reported for the dried alga Kombu (LB–UB = 2,723–2,758 μg/kg).
In the replacement scenario for iAs, for seaweed snacks, only four samples codified as ‘Fried or extruded cereal, seed or root‐based products’ were available (7.5–90 μg/kg, LB–UB). These iAs levels are consistent with those reported in roasted seaweed snacks (14–15 μg/kg) in a recent study on arsenic species in seaweed and seaweed products (Wolle et al., 2021). For seaweed pasta, a concentration of 26.4–29.9 μg/kg (LB–UB) was assigned, considering a 5% content of dried alga in the seaweed pasta and an initial iAs concentration of 528–598 μg/kg (n = 253, LB–UB) derived from the average occurrence data reported for different types of dried algae samples as described in Section 2.4. For seaweed salad, an initial value of 313–335 μg/kg (n = 64, LB–UB) was derived from all the reported samples of different types of fresh ‘Brown algae’ as explained in Section 2.4. This concentration was directly assigned to ‘Lettuce and salad plants’ and ‘Watercress and similar’. Instead, for ‘Mixed green salad’ and ‘Mixed vegetable salad’ a concentration value of 62.7–67.0 μg/kg (LB–UB) was used, assuming that only 20% of the ingredients would be seaweed. For seaweed condiments no data were reported on iAs. The iAs concentration for these commodities was derived using the mean iAs levels reported for different types of fresh ‘Brown algae’ as explained in Section 2.4 (96.4–118 μg/kg) (n = 63, LB=UB).
Some food commodities (fresh) where the few samples available were all left‐censored data were kept in the data set used for the exposure estimations since iAs was quantified in the corresponding dried samples (‘Algae and prokaryotes organisms, unspecified’, Wakame, Spirulina).
3.1.5. Food occurrence data on total mercury
A total of 1,499 analytical data on tHg were available in the final data set. A detailed summary of these data is shown in Annex D, also covering few food additives based on seaweed (n = 39). From this data set, a total of 1,066 samples (see Table 7) were used in the general and in the replacement exposure scenario based on the methodology described in Section 2.4.
Table 7.
Mean total mercury concentrations (μg/kg) in halophytes, seaweeds and products based on or containing seaweed as used to estimate dietary exposure in the general and replacement scenario
| Additional information | N | LC (%) | Mean concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| LB | UB | |||||
| Halophytes | Purslanes and similar | Fresh product | 6 | 67 | 8.3 | 11.3 |
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | Dried product | 101 | 58 | 12.0 | 18.1 |
| Algae and prokaryotes organisms, unspecified | Fresh product | 28 | 89 | 8.6 | 150.4 | |
| Green algae, unspecified | Dried product (a) | 3 | 100 | 0.0 | 500.0 | |
| Green algae, unspecified | Fresh product | 14 | 93 | 0.1 | 20.4 | |
| Sea lettuce | Dried product (a) | 14 | 50 | 11.5 | 15.5 | |
| Sea lettuce | Fresh product | 16 | 63 | 2.3 | 32.1 | |
| Red alga, unspecified | Dried product | 15 | 87 | 2.2 | 382.4 | |
| Laver | Dried product | 107 | 51 | 12.1 | 16.3 | |
| Laver | Fresh product | 14 | 71 | 3.7 | 28.0 | |
| Carrageen mosses | Dried product (a) | 22 | 55 | 32.7 | 47.5 | |
| Carrageen mosses | Fresh product | 28 | 79 | 12.6 | 47.6 | |
| Dulse | Dried product (a) | 11 | 36 | 61.6 | 62.2 | |
| Dulse | Unspecified | 6 | 33 | 75.7 | 93.3 | |
| Brown algae, unspecified | Dried product | 73 | 36 | 12.3 | 107.1 | |
| Kombu | Dried product | 56 | 25 | 24.3 | 82.0 | |
| Kombu | Fresh product (a) | 118 | 80 | 5.9 | 54.3 | |
| Sea spaghetti | Dried product (a) | 16 | 50 | 20.6 | 85.0 | |
| Sea spaghetti | Fresh product (a) | 6 | 83 | 2.9 | 37.3 | |
| Wakame | Dried product | 59 | 32 | 20.0 | 65.4 | |
| Wakame | Fresh product | 11 | 64 | 5.2 | 24.8 | |
| Rockweed | Dried product (a) | 5 | 20 | 123.0 | 127.0 | |
| Rockweed | Fresh product (a) | 18 | 61 | 12.6 | 85.9 | |
| Spirulina | Dried product | 7 | – | 50.1 | 50.1 | |
| Products for non‐standard diets, food imitates and food supplements | Algae‐based formulations (e.g. Spirulina, chlorella) | Dried product | 282 | 67 | 5.4 | 12.2 |
| Fried or extruded cereal, seed or root‐based products | Snacks | With/containing seaweed | 15 | 67 | 7.0 | 10.6 |
| Hot drinks and similar (coffee, cocoa, tea and herbal infusions) | Seaweed tea | Seaweed tea | 4 | 25 | 0.2 | 0.3 |
| Seasoning, sauces and condiments | Relishes | With/containing seaweed | 11 | 82 | 7.4 | 9.0 |
Occurrence data exclusively used in the replacement scenario; %LC: percentage of left‐censored data.
The highest number of samples were for ‘Algae based formulations (e.g., Spirulina, Chlorella)’ (n = 282), and dried samples of Kombu (n = 117) and Laver (n = 107). As compared to other heavy metals, tHg levels in seaweeds were low, in line with the levels of tHg and its different species reported in literature (Duinker et al., 2020). In a recent study conducted in Spain, the Agencia Catalana de Seguridad Alimentaria analysed different types of seaweed; highest levels of tHg were found in the dried alga Hijiki (60 μg/kg) with no methylmercury detected in any of the samples analysed (LOD = 2 μg/kg) (Timoner‐Alonso et al., 2020). The highest mean levels of tHg were reported for the dried brown alga Rockweed (LB–UB = 123–127 μg/kg), with only five samples available (see Table 7), and one sample greatly impacting the mean values (540 μg/kg).
For the occurrence data used for the replacement scenario (see Section 2.4 for the methodology followed), for seaweed condiments and seaweed snacks, the mean reported occurrence data for these commodities were used, 7.4–9.0 μg/kg (LB–UB, n = 11) for the first and 7.0–10.6 μg/kg (LB–UB, n = 15) for the latter. For seaweed pasta, a concentration of 1.1–3.2 μg/kg (LB–UB) was assigned, considering a 5% content of dried alga in the seaweed pasta and an initial tHg concentration of 21.6–64.8 μg/kg (n = 312, LB–UB) derived from the average occurrence data reported for different types of dried algae samples as described in Section 2.4. Three samples of ‘Green algae, unspecified’ all reporting left‐censored data and with relatively high LOQ (see Table 7) were excluded when deriving the values. For seaweed salad, an initial value of 6.6–55.2 μg/kg (n = 153, LB–UB) was derived from all the reported samples of different types of fresh ‘Brown algae’ as explained in Section 2.4. This concentration was directly assigned to ‘Lettuce and salad plants’ and ‘Watercress and similar’. Instead, for ‘Mixed green salad’ and ‘Mixed vegetable salad’ a concentration value of 1.3–11.1 μg/kg (LB–UB) was used, assuming that only 20% of the ingredients would be seaweed (Section 2.4).
3.1.6. Food occurrence data on iodine
A total of 1,002 analytical data on iodine were available in the final data set. A detailed summary of these data is shown in Annex D, also covering few food additives based on seaweed (n = 10). From this data set, a total of 699 samples (see Table 8) were used in the general and in the replacement exposure scenario based on the methodology described in Section 2.4.
Table 8.
Mean iodine concentrations (μg/kg) in halophytes, seaweed and products based on or containing seaweed as used to estimate dietary exposure in the general and replacement scenario
| Additional information | N | LC (%) | Mean concentration (μg/kg) | |||
|---|---|---|---|---|---|---|
| LB | UB | |||||
| Halophytes | Purslanes and similar | Fresh product | 3 | 100 | 0 | 3,266 |
| Algae and prokaryotes organisms | Algae and prokaryotes organisms, unspecified | Dried product | 96 | 2 | 999,433 | 999,471 |
| Algae and prokaryotes organisms, unspecified | Fresh product | 3 | – | 262,473 | 262,473 | |
| Green algae, unspecified | Dried product (a) | 3 | – | 70,667 | 70,667 | |
| Green algae, unspecified | Fresh product | 7 | – | 10,959 | 10,959 | |
| Sea lettuce | Dried product (a) | 10 | – | 348,322 | 348,322 | |
| Sea lettuce | Fresh product | 15 | 7 | 23,553 | 26,886 | |
| Red alga, unspecified | Dried product | 15 | – | 101,616 | 101,616 | |
| Laver | Dried product | 148 | 1 | 84,786 | 85,146 | |
| Laver | Fresh product | 7 | 14 | 9,743 | 16,886 | |
| Carrageen mosses | Dried product (a) | 4 | – | 185,613 | 185,613 | |
| Carrageen mosses | Fresh product | 9 | 33 | 32,354 | 32,688 | |
| Dulse | Dried product (a) | 7 | – | 248,860 | 248,860 | |
| Dulse | Unspecified | 3 | – | 156,067 | 156,067 | |
| Brown algae, unspecified | Dried product | 72 | – | 1,571,762 | 1,571,762 | |
| Kombu | Dried product | 54 | – | 3,528,862 | 3,528,862 | |
| Kombu | Fresh product (a) | 99 | – | 750,596 | 750,596 | |
| Sea spaghetti | Dried product (a) | 14 | – | 79,737 | 79,737 | |
| Sea spaghetti | Fresh product (a) | 3 | – | 27,540 | 27,540 | |
| Wakame | Dried product | 45 | – | 197,560 | 197,560 | |
| Wakame | Fresh product | 8 | – | 113,555 | 113,555 | |
| Rockweed | Dried product (a) | 2 | – | 565,000 | 565,000 | |
| Rockweed | Fresh product (a) | 17 | – | 229,353 | 229,353 | |
| Spirulina | Dried product | 4 | 75 | 34 | 12,536 | |
| Products for non‐standard diets, food imitates and food supplements | Algae‐based formulations (e.g. Spirulina, chlorella) | Dried product | 29 | 28 | 338,228 | 350,319 |
| Fried or extruded cereal, seed or root‐based products | Snacks | With/containing seaweed | 10 | 10 | 221,229 | 221,556 |
| Hot drinks and similar (coffee, cocoa, tea and herbal infusions) | Seaweed tea | Seaweed tea | 2 | – | 1,455 | 1,455 |
| Seasoning, sauces and condiments | Relishes | With/containing seaweed | 10 | – | 64,259 | 64,259 |
Occurrence data exclusively used in the replacement scenario; %LC: percentage of left‐censored data.
The highest number of analytical results was reported for dried samples of Laver (n = 148), followed by fresh samples of Kombu (n = 99) and dried samples of ‘Algae and prokaryotes organisms, unspecified’ (n = 96). A large variation in iodine levels was observed between but also within species across the different groups of macroalgae as also reported in the literature. Iodine levels in seaweed seems to be affected by a number of factors such as geographical origin, environment (season, salinity of the water, etc.) but also the part of the seaweed used, age of the seaweed and post‐harvest storage conditions among others (Teas et al., 2004). Overall, mean levels of iodine were highest in brown algae as compared to red and green alga. The highest mean concentrations were reported for dried Kombu (3,529 mg/kg, LB=UB). Several brown algae with food applications are well known for being rich sources of iodine, in particular in the Laminaria and Saccharina genus (e.g. Kombu, Sugar kelp), with iodine levels that can go, in certain occasions, above 10,000 mg/kg dw (Duinker et al., 2016; Duinker et al., 2020; Aakre et al., 2021; Blikra et al., 2022). To also mention the relatively low mean levels of iodine reported in dried samples of the red alga Laver (84.8–85.1 mg/kg, LB–UB), the most reported alga in the EFSA Comprehensive Database, typically consumed as sushi and part of other Japanese dishes. The relatively high values reported for ‘Algae based formulations (e.g., Spirulina, Chlorella)’ seem an indication that several samples contain specific brown alga species rich in iodine since Spirulina and Chlorella, two typical algae used as food supplement, are well known to contain much lower or almost no iodine at all. Very limited data on iodine were available for halophytes (n = 3); much lower iodine levels were reported as compared to seaweeds.
As concerns the occurrence data used for the replacement scenario (see Section 2.4 for the methodology followed), for seaweed condiments and seaweed snacks, the mean reported occurrence data for these commodities were used, 64,259 μg/kg (LB=UB, n = 10) for the first and 221,229–221,556 μg/kg (LB–UB, n = 10) for the latter. For seaweed pasta, a concentration of 36,266–36,282 μg/kg (LB–UB) was assigned, considering a 5% content of dried alga in the seaweed pasta and an initial iodine concentration of 725,318–725,656 μg/kg (n = 306, LB–UB) derived from the average occurrence data reported for different types of dried algae samples as described in Section 2.4. For seaweed salad, an initial value of 623,615 μg/kg (n = 127, LB=UB) was derived from all the reported samples of different types of fresh ‘Brown algae’ as explained in Section 2.4. This concentration was directly assigned to ‘Lettuce and salad plants’ and ‘Watercress and similar’. Instead, for ‘Mixed green salad’ and ‘Mixed vegetable salad’ a concentration value of 124,723 μg/kg (LB=UB) was used, assuming that only 20% of the ingredients would be seaweed (Section 2.4).
3.1.7. Feed occurrence data
Only 254 analytical results in feed (for a total of 76 different samples) were available on the different compounds under assessment. Table 9 shows an overview of the analytical data for different heavy metals; no data on iodine were available. For the different heavy metals, the most reported feed commodity was in general ‘Seaweed meal’. Detailed occurrence data by compound and feed commodity can be found in Annex E.
Table 9.
Distribution of analytical results in feed samples among the compounds under assessment
| Number of analytical results/samples | |
|---|---|
| Cadmium | 55 |
| Lead | 67 |
| Total Arsenic (tAs) | 54 |
| Inorganic Arsenic (iAs) | 67 |
| Total mercury (tHg) | 7 |
| Methylmercury | 4 |
3.2. Consumption data
Currently the amount of consumption data on seaweeds and halophytes in the EFSA Comprehensive Database is limited. Despite the recent increase in consumption of these commodities in Europe, this increase might not yet be properly reflected in the EFSA Comprehensive Database. Following the same strategy as described in Section 2.1.1 to extract the occurrence data, a total of 697 eating occasions on seaweed, halophytes and products based on or containing seaweed were identified. Table 10 shows a compilation of the different eating occasions; these eating occasions were provided by 468 subjects from 19 European countries. It can be seen that around 63% of the eating occasions corresponded to ‘Algae and prokaryotes organisms’, of which about 2/3 were reported as the red alga Laver. A total of 191 eating occasions referred to ‘Algae based formulations (e.g., Spirulina, Chlorella)’, around 50 to halophytes, and few ones were identified as food products with seaweeds in their composition. For the reported eating occasions of the dried red alga Laver (n = 245), the mean amount was 9.2 g (4.6 g/day), with a 95th percentile of 37.5 g (18.8 g/day). In the case of the brown alga Kombu, the mean amount among the reported eating occasions (dried alga, n = 40) was 5.8 g (2.4 g/day). The eating occasions of ‘Algae based formulations (e.g., Spirulina, Chlorella)’ mostly referred to pills/tablets or powder (n = 191). The mean amount among the eating occasions was 1.5 g (0.7 g/day), with a 95th percentile of 6 g (3 g/day).
To allow an adequate and accurate linkage with the occurrence data, information was sought on how the different seaweeds, halophytes and products based or containing seaweed were consumed, in particular if the reported amounts referred to dried or fresh products. For the few eating occasions on halophytes, this information was overall missing, although it is assumed that in most of the cases halophytes are consumed fresh. For seaweed, information on this was available in most of the cases. As an example, for the red algae Laver most the eating occasions were reported as dried, the same as for the brown alga Kombu.
3.3. Dietary exposure assessment
This section describes the dietary exposure to cadmium, lead, tAs, iAs and tHg, and the dietary intake of iodine; a general and a replacement exposure scenario were conducted for each of the compounds under assessment (see Section 2.4).
The general dietary exposure/intake scenario only used the occurrence data on heavy metals and iodine available for halophytes, seaweeds and seaweed‐related products, and the available consumption data in Comprehensive Database. When considering the whole population, in all but one dietary survey there was less than 5% seaweed consumers. This led to 95th percentile exposure estimates equal to zero in all dietary surveys except for one dietary survey for pregnant women. When considering ‘consumers only’ of halophytes, seaweeds and seaweed‐related products, mean exposure was estimated in those population groups with at least five consumers; 75th and 90th percentiles were provided when at least 11 and 29 consumers, respectively, were available.
In the replacement scenario, dietary exposure was estimated in the whole population considering the four seaweed products described in Section 2.4. [seaweed snacks, seaweed condiments (relishes), seaweed pasta and seaweed salad]. As explained in that section, for seaweed snacks, seaweed condiments (relishes) and seaweed pasta, the 100% of the reported consumption data on the selected conventional commodities (see Table 1) was replaced by these seaweed‐related products. For seaweed salad, only 10% of the reported consumption of the different types of salads was replaced.
3.3.1. Dietary exposure assessment to cadmium
Previous work from EFSA on dietary exposure to cadmium goes back to 2012. At that time, mean exposure estimates in the European population via the whole diet varied between 1.15 and 7.84 μg/kg bw per week (minimum LB–maximum UB) and between 2.01 and 12.1 μg/kg bw per week for the 95th percentile. The highest dietary exposure was estimated for ‘Toddlers’ (EFSA, 2012).
3.3.1.1. General dietary exposure scenario
In the whole population, the highest average exposure (LB=UB) via the consumption of halophytes, seaweeds and seaweed‐related products was 0.04 μg/kg bw per week in ‘Adults’. For Pregnant women, the 95th percentile exposure was 0.009 μg/kg bw per week (LB=UB).
From the consumption of halophytes, seaweeds and seaweed‐related products, the highest average dietary exposure in ‘consumers only’ (n = 458) was estimated in ‘Adults’ (3.1 μg/kg bw per week, n = 15, LB=UB) via the consumption of the red alga Laver (see Table 11). Overall, in those dietary surveys with the highest estimates, seaweeds were the main and/or only contributors. The maximum highest reliable percentile was 4.4 μg/kg bw per week, also in ‘Adults’ (90th percentile, LB=UB). Exposure estimates across the different dietary surveys are shown in Annexes F and G.
Table 11.
Dietary exposure to cadmium in ‘consumers only’ of halophytes, seaweeds and seaweed‐related products across different dietary surveys (μg/kg bw per week)
| Number of surveys (a) | Number of consumers (range) | Mean exposure (μg/kg bw per week) | Highest reliable percentile (μg/kg bw per week) | |||
|---|---|---|---|---|---|---|
| Min LB | Max UB | Min LB | Max UB | |||
| Other children | 1 | 9 | 0.4 | 0.4 | ||
| Adolescents | 4 | 6–13 | 0.03 | 0.7 | 0.2 (b) | 0.2 (b) |
| Adults | 12 | 5–54 | 0.01 | 3.1 | 0.6 (c) | 4.4 (c) |
| Elderly | 2 | 6–10 | 0.1 | 0.5 | ||
| Very elderly | 1 | 8 | 0.8 | 0.9 | ||
| Pregnant women | 1 | 13 | 0.04 | 0.05 | 0.02 (b) | 0.02 (b) |
| Lactating women | 1 | 7 | 0.3 | 0.3 | ||
Number of dietary surveys with at least five consumers.
75th percentile.
90th percentile.
3.3.1.2. Replacement scenario
Table 12 shows the dietary exposure to cadmium in the whole population across age classes under the replacement scenario. The highest mean and 95th percentile dietary exposure considering the four seaweed products described in Section 2.4.2 were estimated for ‘Toddlers’ with 1.6 μg/kg bw per week (LB=UB) and 7.8 μg/kg bw per week (LB=UB), respectively. In the age classes with the highest estimates, either seaweed pasta and/or seaweed snacks would be the food categories contributing the most to the dietary exposure to cadmium.
Table 12.
Anticipated cadmium exposure estimates (μg/kg bw per week, LB–UB) under the replacement scenario
| N | Dietary exposure to cadmium (μg/kg bw per week) | ||||
|---|---|---|---|---|---|
| Mean exposure (LB–UB) | 95th percentile exposure (LB–UB) | ||||
| Min | Max | Min | Max | ||
| Infants | 12 | 0.0–0.0 | 0.45–0.45 | 0.0–0.0 | 2.1–2.1 |
| Toddlers | 15 | 0.12–0.12 | 1.6–1.6 | 0.56–0.56 | 7.8–7.8 |
| Other children | 19 | 0.54–0.54 | 1.3–1.3 | 2.1–2.1 | 6.4–6.4 |
| Adolescents | 21 | 0.28–0.28 | 1.1–1.1 | 1.2–1.2 | 4.1–4.1 |
| Adults | 22 | 0.10–0.10 | 0.72–0.72 | 0.25–0.25 | 3.6–3.6 |
| Elderly | 19 | 0.04–0.04 | 0.39–0.39 | 0.15–0.16 | 1.3–1.3 |
| Very elderly | 14 | 0.0–0.0 | 0.40–0.40 | 0.13–0.13 | 0.82–0.83 |
| Pregnant women | 6 | 0.16–0.16 | 0.27–0.27 | 0.64–0.65 | 1.6–1.6 |
| Lactating women | 2 | 0.15–0.15 | 0.20–0.20 | 0.68–0.68 | 0.87–0.87 |
| Vegetarians | 1 | 0.23–0.23 | 2.0–2.0 | ||
3.3.2. Dietary exposure assessment to lead
In 2010, EFSA conducted a full risk assessment on lead in food (EFSA CONTAM Panel, 2010). The highest mean dietary exposure via the whole diet was estimated for children aged 1 to 3 years and ranged from 1.10 μg/kg bw per day to 3.10 μg/kg bw per day (minimum LB–maximum UB); in the same age class, the 95th percentile exposure was 1.71–5.51 μg/kg bw per day. Mean dietary exposure for adults ranged from 0.36 to 1.24 μg/kg bw per day (minimum LB–maximum UB) and from 0.73 to 2.43 μg/kg bw per day for the 95th percentile exposure.
3.3.2.1. General dietary exposure scenario
In the whole population, the highest average exposure (LB–UB) via the consumption of halophytes, seaweeds and seaweed‐related products was 0.0013–0.0015 μg/kg bw per day in ‘Pregnant women’. The 95th percentile exposure was 0.00034–0.00035 μg/kg bw per day (LB–UB) in ‘Pregnant women’. From the consumption of halophytes, seaweeds and seaweed‐related products, the highest average dietary exposure in ‘consumers only’ (n = 458) was estimated in ‘Adults’ (0.093–0.10 μg/kg bw per day, n = 5, LB–UB) via the consumption of unspecified ‘Algae and prokaryotes organisms’ (see Table 13). Overall, in those dietary surveys with the highest estimates, seaweeds were the main and/or only contributors. The maximum highest reliable percentile was 0.072–0.073 μg/kg bw per day also in ‘Adults’ (90th percentile, LB–UB). Exposure estimates across the different dietary surveys are shown in Annexes F and G.
Table 13.
Dietary exposure to lead in ‘consumers only’ of halophytes, seaweeds, and seaweed‐related products across different dietary surveys (μg/kg bw per day)
| Number of surveys (a) | Number of consumers (range) | Mean exposure (μg/kg bw per day) | Highest reliable percentile (μg/kg bw per day) | |||
|---|---|---|---|---|---|---|
| Min LB | Max UB | Min LB | Max UB | |||
| Other children | 1 | 9 | 0.013 | 0.013 | ||
| Adolescents | 4 | 6–13 | 0.002 | 0.022 | 0.003 (b) | 0.003 (b) |
| Adults | 12 | 5–54 | 0.001 | 0.10 | 0.015 (c) | 0.073 (c) |
| Elderly | 2 | 6–10 | 0.012 | 0.015 | ||
| Very elderly | 1 | 8 | 0.023 | 0.029 | ||
| Pregnant women | 1 | 13 | 0.006 | 0.007 | 0.002 (b) | 0.003 (b) |
| Lactating women | 1 | 7 | 0.004 | 0.004 | ||
Number of dietary surveys with at least five consumers.
75th percentile.
90th percentile.
3.3.2.2. Replacement scenario
Table 14 shows the dietary exposure to lead in the whole population across age classes under the replacement scenario. The highest mean and 95th percentile dietary exposure considering the four seaweed products described in Section 2.4.2 were estimated for ‘Toddlers’ with 0.15–0.16 μg/kg bw per day (LB–UB) and 0.80–0.82 μg/kg bw per day (LB–UB), respectively. In the age classes with the highest estimates, either seaweed pasta and/or seaweed snacks would be the food categories contributing the most to the dietary exposure to lead.
Table 14.
Anticipated lead exposure estimates (μg/kg bw per day, LB–UB) under the replacement scenario
| N | Dietary exposure to lead (μg/kg bw per day) | ||||
|---|---|---|---|---|---|
| Mean exposure (LB–UB) | 95th percentile exposure (LB–UB) | ||||
| Min | Max | Min | Max | ||
| Infants | 12 | 0.0–0.0 | 0.029–0.030 | 0.0–0.0 | 0.081–0.10 |
| Toddlers | 15 | 0.005–0.006 | 0.15–0.16 | 0.021–0.027 | 0.80–0.82 |
| Other children | 19 | 0.027–0.032 | 0.13–0.14 | 0.087–0.11 | 0.66–0.68 |
| Adolescents | 21 | 0.011–0.014 | 0.10–0.11 | 0.046–0.058 | 0.42–0.43 |
| Adults | 22 | 0.006–0.008 | 0.064–0.069 | 0.009–0.018 | 0.37–0.78 |
| Elderly | 19 | 0.002–0.003 | 0.020–0.025 | 0.007–0.012 | 0.13–0.13 |
| Very elderly | 14 | 0.0–0.0 | 0.015–0.025 | 0.005–0.012 | 0.050–0.060 |
| Pregnant women | 6 | 0.012–0.014 | 0.023–0.027 | 0.051–0.071 | 0.16–0.16 |
| Lactating women | 2 | 0.009–0.012 | 0.012–0.015 | 0.035–0.048 | 0.070–0.072 |
| Vegetarians | 1 | 0.020–0.023 | 0.19–0.19 | ||
3.3.3. Dietary exposure assessment to total arsenic
In the last few years, dietary exposure assessments on arsenic have been mainly focused on iAs rather than on tAs. The last time EFSA estimated dietary exposure to tAs was in its 2009 scientific opinion (EFSA CONTAM Panel, 2009a). Using the Concise European Food Consumption Database, mean tAs dietary exposure (LB–UB) via the whole diet ranged from 0.45–0.65 to 4.31–4.6 μg/kg bw per day and the 95th percentile dietary exposure (LB–UB) between 1.75–1.97 and 10.96–11.2 μg/kg bw per day.
3.3.3.1. General dietary exposure scenario
In the whole population, the highest average exposure (LB=UB) via the consumption of halophytes, seaweeds and seaweed‐related products was 0.079 μg/kg bw per day in ‘Adults’. The 95th percentile exposure was 0.038 μg/kg bw per day (LB=UB) in ‘Pregnant women’. From the consumption of halophytes, seaweeds and seaweed‐related products, the highest average dietary exposure in ‘consumers only’ (n = 458) was estimated in ‘Adults’ (5.5 μg/kg bw per day, n = 5, LB=UB) via the consumption of unspecified ‘Algae and prokaryotes organisms’ (see Table 15). Overall, in those dietary surveys with the highest estimates, seaweeds were the main and/or only contributors. The maximum highest reliable percentile was 7.9 μg/kg bw per day, also in ‘Adults’ (90th percentile, LB=UB). Exposure estimates across the different dietary surveys are shown in Annexes F and G.
Table 15.
Dietary exposure to total arsenic in ‘consumers only’ of halophytes, seaweeds and seaweed‐related products across different dietary surveys (μg/kg bw per day)
| Number of surveys (a) | Number of consumers (range) | Mean exposure (μg/kg bw per day) | Highest reliable percentile (μg/kg bw per day) | |||
|---|---|---|---|---|---|---|
| Min LB | Max UB | Min LB | Max UB | |||
| Other children | 1 | 9 | 0.77 | 0.77 | ||
| Adolescents | 4 | 6–13 | 0.20 | 0.40 | 0.32 (b) | 0.32 (b) |
| Adults | 12 | 5–54 | 0.16 | 5.5 | 0.99 (c) | 7.9 (c) |
| Elderly | 2 | 6–10 | 0.1 | 0.44 | ||
| Very elderly | 1 | 8 | 0.08 | 0.08 | ||
| Pregnant women | 1 | 13 | 0.32 | 0.32 | 0.08 (b) | 0.08 (b) |
| Lactating women | 1 | 7 | 0.46 | 0.46 | ||
Number of dietary surveys with at least five consumers.
75th percentile.
90th percentile.
3.3.3.2. Replacement scenario
Table 16 shows the dietary exposure to tAs in the whole population across age classes under the replacement scenario. The highest mean and 95th percentile dietary exposure considering the four seaweed products described in Section 2.4.2 were estimated for ‘Toddlers’ with 5.1–5.2 μg/kg bw per day (LB–UB) and 19.8 μg/kg bw per day (LB=UB), respectively. In the age classes with the highest estimates, either seaweed pasta and/or seaweed snacks would be the food categories contributing the most to the dietary exposure to tAs.
Table 16.
Anticipated tAs exposure estimates (μg/kg bw per day, LB–UB) under the replacement scenario
| N | Dietary exposure to tAs (μg/kg bw per day) | ||||
|---|---|---|---|---|---|
| Mean exposure (LB–UB) | 95th percentile exposure (LB–UB) | ||||
| Min | Max | Min | Max | ||
| Infants | 12 | 0.0–0.0 | 1.5–1.5 | 0.0–0.0 | 7.2–7.2 |
| Toddlers | 15 | 0.4–0.4 | 5.1–5.2 | 1.8–1.8 | 19.8–19.8 |
| Other children | 19 | 1.6–1.6 | 4.3–4.3 | 6.0–6.0 | 17.3–17.3 |
| Adolescents | 21 | 1.0–1.0 | 3.0–3.0 | 4.1–4.1 | 11.2–11.2 |
| Adults | 22 | 0.3–0.3 | 2.1–2.1 | 1.2–1.2 | 9.2–9.2 |
| Elderly | 19 | 0.2–0.2 | 1.8–1.8 | 0.7–0.7 | 3.9–3.9 |
| Very elderly | 14 | 0.0–0.0 | 1.8–1.8 | 0.6–0.6 | 4.0–4.0 |
| Pregnant women | 6 | 0.6–0.6 | 1.2–1.2 | 2.7–2.7 | 4.8–4.8 |
| Lactating women | 2 | 0.6–0.6 | 0.7–0.7 | 2.5–2.5 | 3.1–3.1 |
| Vegetarians | 1 | 0.8–0.8 | 5.1–5.1 | ||
3.3.4. Dietary exposure assessment to inorganic arsenic
EFSA carried out its last dietary exposure assessment to iAs in 2021; only reported data on iAs were used in the assessment (EFSA, 2021). The highest mean dietary exposure estimates at the LB via the whole diet were in ‘Toddlers’ (0.30 μg /kg bw per day) and 0.61 μg /kg bw per day (UB) in both ‘Infants’ and ‘Toddlers’. At the 95th percentile, the highest exposure estimates (LB–UB) were 0.58 and 1.20 μg/kg bw per day in ‘Toddlers’ and ‘Infants’, respectively.
3.3.4.1. General dietary exposure scenario
In the whole population, the highest average exposure to iAs (LB–UB) via the consumption of halophytes, seaweeds and seaweed‐related products was 0.0039–0.0040 μg/kg bw per day in ‘Adults’. The 95th percentile exposure was 0–0.00031 μg/kg bw per day (LB–UB) in ‘Pregnant women’. From the consumption of halophytes, seaweeds and seaweed‐related products, the highest average dietary exposure in ‘consumers only’ (n = 413) was estimated in ‘Adults’ (0.21–0.22 μg/kg bw per day, n = 5, LB–UB) via the consumption of unspecified ‘Algae and prokaryotes organisms’ (see Table 17). Overall, in those dietary surveys with the highest estimates, seaweeds were the main and/or only contributors. When interpreting this result, together with the low number of consumers in the dietary survey, it should be taken into account that the reported iAs levels for ‘Algae and prokaryotes organisms, unspecified’ (LB–UB = 1,617–1,660 μg/kg) are likely impacted by the presence of either Kombu or Hijiki alga among the samples analysed. By excluding the dietary survey with the highest estimate, the exposure among ‘Adults’ ranged between 0.002 and 0.045 μg/kg bw per day (min LB–maximum UB). The maximum highest reliable percentile was 0.032–0.067 μg/kg bw per day also in ‘Adults’ (90th percentile, LB–UB). Exposure estimates across the different dietary surveys are shown in Annexes F and G.
Table 17.
Dietary exposure to inorganic arsenic in ‘consumers only’ of halophytes, seaweeds and seaweed‐related products across different dietary surveys (μg/kg bw per day)
| Number of surveys (a) | Number of consumers (range) | Mean exposure (μg/kg bw per day) | Highest reliable percentile (μg/kg bw per day) | |||
|---|---|---|---|---|---|---|
| Min LB | Max UB | Min LB | Max UB | |||
| Other children | 1 | 9 | 0.005 | 0.009 | ||
| Adolescents | 4 | 5–13 | 0.0 | 0.006 | 0.001 (b) | 0.003 (b) |
| Adults | 11 | 5–54 | 0.0 | 0.22 | 0.006 (c) | 0.067 (c) |
| Elderly | 1 | 6 | 0.011 | 0.012 | ||
| Very elderly | 1 | 6 | 0.002 | 0.002 | ||
| Pregnant women | 1 | 13 | 0.002 | 0.003 | 0.002 (b) | 0.002 (b) |
| Lactating women | 1 | 7 | 0.002 | 0.004 | ||
Number of dietary surveys with at least five consumers.
75th percentile.
90th percentile.
3.3.4.2. Replacement scenario
Table 18 shows the dietary exposure to iAs in the whole population across age classes under the replacement scenario. The highest mean and 95th percentile dietary exposure considering the four seaweed products described in Section 2.4.2 were estimated for ‘Toddlers’ with 0.090–0.11 μg/kg bw per day (LB–UB) and 0.15–0.23 μg/kg bw per day (LB–UB), respectively. In the age classes with the highest estimates, it was mainly seaweed pasta the food category contributing the most to the dietary exposure to iAs.
Table 18.
Anticipated iAs exposure estimates (μg/kg bw per day, LB–UB) under the replacement scenario
| N | Dietary exposure to iAs (μg/kg bw per day) | ||||
|---|---|---|---|---|---|
| Mean exposure (LB–UB) | 95th percentile exposure (LB–UB) | ||||
| Min | Max | Min | Max | ||
| Infants | 12 | 0.0–0.0 | 0.029–0.033 | 0.0–0.0 | 0.070–0.13 |
| Toddlers | 15 | 0.004–0.008 | 0.090–0.11 | 0.014–0.039 | 0.15–0.23 |
| Other children | 19 | 0.004–0.017 | 0.072–0.086 | 0.017–0.067 | 0.16–0.20 |
| Adolescents | 21 | 0.002–0.013 | 0.039–0.048 | 0.012–0.048 | 0.11–0.14 |
| Adults | 22 | 0.002–0.005 | 0.033–0.037 | 0.010–0.022 | 0.067–0.10 |
| Elderly | 19 | 0.002–0.003 | 0.033–0.037 | 0.008–0.010 | 0.070–0.077 |
| Very elderly | 14 | 0.000–0.000 | 0.033–0.037 | 0.011–0.012 | 0.074–0.081 |
| Pregnant women | 6 | 0.004–0.008 | 0.019–0.022 | 0.018–0.031 | 0.061–0.069 |
| Lactating women | 2 | 0.006–0.009 | 0.014–0.016 | 0.028–0.043 | 0.058–0.065 |
| Vegetarians | 1 | 0.007–0.012 | 0.029–0.059 | ||
3.3.5. Dietary exposure assessment to total mercury
The previous dietary exposure assessment conducted by EFSA on mercury was focused on inorganic mercury and methylmercury (EFSA CONTAM Panel, 2012). At that time, the reported tHg (> 98% of the data) was converted into methylmercury and inorganic mercury by applying conversion factors based on the methylmercury/ tHg proportion from literature data (EFSA CONTAM Panel, 2012). The highest mean and 95th percentile methylmercury dietary exposure estimates via the whole diet were 1.57–5.05 μg/kg bw per week in toddlers and adolescents, respectively. For inorganic mercury, maximum mean and 95th percentile exposure estimates were 2.16–4.06 μg/kg bw per week, both in toddlers considering the whole diet.
3.3.5.1. General dietary exposure scenario
Dietary exposure to tHg was very low as compared to that estimated for other heavy metals. In the whole population, the highest average exposure to tHg (LB–UB) via the consumption of halophytes, seaweeds and seaweed‐related products was 0.00018–0.0031 μg/kg bw per week in ‘Toddlers’. The 95th percentile exposure was 0.00015–0.00020 μg/kg bw per week (LB–UB) in ‘Pregnant women’.
From the consumption of halophytes, seaweeds and seaweed‐related products, the highest average dietary exposure in ‘consumers only’ (n = 458) was estimated in ‘Adults’ (0.017–0.12 μg/kg bw per week, n = 5, LB–UB) via the consumption of unspecified ‘Algae and prokaryotes organisms’ (see Table 19). Overall, in those dietary surveys with the highest estimates, seaweeds were the main and/or only contributors. The maximum highest reliable percentile was 0.032–0.054 μg/kg bw per week also in ‘Adults’ (90th percentile, LB–UB). Exposure estimates across the different dietary surveys are shown in Annexes F and G.
Table 19.
Dietary exposure to total mercury in ‘consumers only’ of halophytes, seaweeds, and seaweed‐related products across different dietary surveys (μg/kg bw per week)
| Number of surveys (a) | Number of consumers (range) | Mean exposure (μg/kg bw per week) | Highest reliable percentile (μg/kg bw per week) | |||
|---|---|---|---|---|---|---|
| Min LB | Max UB | Min LB | Max UB | |||
| Other children | 1 | 9 | 0.003 | 0.005 | ||
| Adolescents | 4 | 6–13 | 0.001 | 0.022 | 0.0013 (b) | 0.0017 (b) |
| Adults | 12 | 5–54 | 0.0005 | 0.12 | 0.0050 (c) | 0.054 (c) |
| Elderly | 2 | 6–10 | 0.002 | 0.014 | ||
| Very elderly | 1 | 8 | 0.019 | 0.026 | ||
| Pregnant women | 1 | 13 | 0.001 | 0.015 | 0.0003 (b) | 0.0008 (b) |
| Lactating women | 1 | 7 | 0.002 | 0.003 | ||
Number of dietary surveys with at least five consumers.
75th percentile.
90th percentile.
3.3.5.2. Replacement scenario
Table 20 shows the dietary exposure to tHg in the whole population across age classes under the replacement scenario. The highest mean and 95th percentile dietary exposure considering the four seaweed products described in Section 2.4.2 were estimated for ‘Toddlers’ with 0.028–0.081 μg/kg bw per week (LB–UB) and 0.112–0.176 μg/kg bw per week (LB–UB), respectively. In the age classes with the highest estimates, either seaweed pasta and/or seaweed snacks would be the food categories contributing the most to the dietary exposure to tHg.
Table 20.
Anticipated total mercury exposure estimates (μg/kg bw per week, LB–UB) under the replacement scenario
| N | Dietary exposure to total mercury (μg/kg bw per week) | ||||
|---|---|---|---|---|---|
| Mean exposure (LB–UB) | 95th percentile exposure (LB–UB) | ||||
| Min | Max | Min | Max | ||
| Infants | 12 | 0.0–0.0 | 0.008–0.025 | 0.0–0.0 | 0.035–0.096 |
| Toddlers | 15 | 0.002–0.006 | 0.028–0.081 | 0.010–0.029 | 0.11–0.18 |
| Other children | 19 | 0.008–0.014 | 0.023–0.069 | 0.031–0.055 | 0.097–0.16 |
| Adolescents | 21 | 0.005–0.012 | 0.015–0.040 | 0.021–0.043 | 0.056–0.11 |
| Adults | 22 | 0.001–0.004 | 0.010–0.033 | 0.005–0.020 | 0.047–0.092 |
| Elderly | 19 | 0.001–0.003 | 0.008–0.033 | 0.003–0.010 | 0.018–0.072 |
| Very elderly | 14 | 0.0–0.0 | 0.008–0.033 | 0.002–0.011 | 0.016–0.081 |
| Pregnant women | 6 | 0.003–0.007 | 0.005–0.022 | 0.012–0.029 | 0.023–0.076 |
| Lactating women | 2 | 0.002–0.009 | 0.004–0.012 | 0.010–0.042 | 0.017–0.049 |
| Vegetarians | 1 | 0.004–0.011 | 0.026–0.052 | ||
3.3.6. Iodine dietary intake assessment
Most recent EFSA's work on iodine dates back to 2014 when the Panel on Dietetic Products, Nutrition and Allergies (NDA) derived Dietary Reference Values (DRVs) as Adequate Intake (AI) for this nutrient (EFSA NDA Panel, 2014). The scientific opinion concluded that habitual iodine intakes are typically inaccurate as classical dietary assessment methods are not adequate, and because the quality of iodine data in food composition tables is poor (e.g. no info on whether the salt used is iodised or not). Because of these limitations, urinary iodine excretion is typically used as a valuable marker of iodine intake (EFSA NDA Panel, 2014).
3.3.6.1. General dietary exposure scenario
In the whole population, the highest mean intake via the consumption of halophytes, seaweeds and seaweed‐related products was 5.1 μg/kg bw per day in ‘Adults’ (LB=UB). This estimate corresponded to a dietary survey with two consumers of the alga Kombu (9.5 g/day each) who could have an iodine intake of 465.6–632.5 μg/kg bw per day (see Table 8 for iodine concentrations). To note that in all dietary surveys for ‘Adults’ but the one with the highest value, mean iodine intakes ranged between 0.035 and 0.31 (min LB–max UB). The 95th percentile intake was 0.15 μg/kg bw per day (LB=UB) in ‘Pregnant women’. From the consumption of halophytes, seaweeds and seaweed‐related products, the highest mean intake in ‘consumers only’ (n = 458 subjects) was estimated in ‘Adults’ (155 μg/kg bw per day, n = 5, LB=UB) via the consumption of unspecified ‘Algae and prokaryotes organisms’ (see Table 21). Overall, in those dietary surveys with the highest estimates, seaweeds were the main and/or only contributors. The maximum highest reliable percentile was 32.7 μg/kg bw per day also in ‘Adults’ (90th percentile, LB=UB). Intake estimates across the different dietary surveys are shown in Annexes F and G.
Table 21.
Dietary intake to iodine in ‘consumers only’ of halophytes, seaweeds and seaweed‐related products across different dietary surveys (μg/kg bw per day)
| Number of surveys (a) | Number of consumers (range) | Mean intake (μg/kg bw per day) | Highest reliable percentile (μg/kg bw per day) | |||
|---|---|---|---|---|---|---|
| Min LB | Max UB | Min LB | Max UB | |||
| Other children | 1 | 9 | 8.9 | 9.1 | ||
| Adolescents | 4 | 6–13 | 0.9 | 5.4 | 1.3 (b) | 1.3 (b) |
| Adults | 12 | 5–54 | 2.2 | 155 | 32.7 (c) | 32.7 (c) |
| Elderly | 2 | 6–10 | 0.0 | 16 | ||
| Very elderly | 1 | 8 | 2.3 | 3.5 | ||
| Pregnant women | 1 | 13 | 5.7 | 5.7 | 3.1 (b) | 3.1 (b) |
| Lactating women | 1 | 7 | 1.9 | 1.9 | ||
Number of dietary surveys with at least five consumers.
75th percentile.
90th percentile.
3.3.6.2. Replacement scenario
Table 22 shows iodine dietary intake in the whole population across age classes under the replacement scenario. The highest mean and 95th percentile dietary intake considering the four seaweed products described in Section 2.4.2 were estimated for ‘Toddlers’ with 136 μg/kg bw per day (LB=UB) and 464–465 μg/kg bw per day (LB–UB), respectively. In the age classes with the highest estimates, either seaweed pasta and/or seaweed snacks would be the food categories contributing the most to the dietary intake of iodine.
Table 22.
Anticipated iodine intake estimates (μg/kg bw per day, LB–UB) under the replacement scenario
| N | Iodine dietary intake (μg/kg bw per day) | ||||
|---|---|---|---|---|---|
| Mean intake (LB–UB) | 95th percentile intake LB–UB) | ||||
| Min | Max | Min | Max | ||
| Infants | 12 | 0.1–0.1 | 39.8–39.8 | 0.0–0.0 | 191,191 |
| Toddlers | 15 | 10.0–10.0 | 136–136 | 46.8–46.8 | 464–465 |
| Other children | 19 | 38.4–38.5 | 118–118 | 149–149 | 396–397 |
| Adolescents | 21 | 25.9–25.9 | 73.2–73.3 | 97.9–97.9 | 272–272 |
| Adults | 22 | 8.9–8.9 | 55.0–55.0 | 36.2–36.2 | 231–232 |
| Elderly | 19 | 4.9–4.9 | 54.1–54.2 | 22.2–22.2 | 120–120 |
| Very elderly | 14 | 0.0–0.0 | 53.6–53.6 | 18.1–18.1 | 131–131 |
| Pregnant women | 6 | 14.5–14.5 | 37.9–37.9 | 70.2–70.2 | 131–131 |
| Lactating women | 2 | 16.5–16.5 | 19.9–20.0 | 78.8–78.8 | 79.4–79.5 |
| Vegetarians | 1 | 21.9–22.0 | 124–124 | ||
4. Effect of processing
The relatively high moisture content (85–90%) in fresh harvested algae can lead to a short shelf‐life due to oxidation, enzymatic activity and/or the growing of spoilage organisms (Cascais et al., 2021; Ho and Redan, 2022). This demands immediate processing or alternatively the use of preservatives such as sodium chloride after harvest. Although some types of algae can be consumed fresh (e.g. red alga Dulse, Sea spaghetti), most of the algae dedicated to human consumption in Europe are processed before being consumed.
There are different methods used to process seaweed, mainly washing, dehydration, blanching, fermentation, rehydration (soaking) and cooking (boiling) (Nitschke and Stengel, 2016; FAO and WHO, 2022), but innovative methods such as ultrasound have also been assessed (Noriega‐Fernández et al., 2021). The main processing method is drying, and the most frequently used are sun‐drying and oven‐drying due to their accessibility and relatively low operation cost (Cascais et al., 2021).
In many cases, edible seaweeds are blanched (scalded in boiling water or steam for a short time) just after being harvested. This is a process typically applied to edible brown alga (e.g. Kombu, Wakame), and apart from inactivating enzymes to extend the shelf‐life, it also induces colour changes from brown to a green colour that seems to increase consumer acceptance (Akomea‐Frempong et al., 2021; FAO and WHO, 2022). A big proportion of seaweeds reach the consumers as dried products; in many cases they can be consumed directly at home without further processing (e.g. dry sheets of Laver). However, other seaweeds might undergo further processing at home, e.g. dry algae soaked in water before consumption or boiling as it is the case for seaweed pasta.
Together with extending shelf‐life and increasing consumer acceptability, post‐harvest processing can help to reduce the content of iodine and heavy metals in seaweeds. For cadmium, mercury and lead there is not much information on the effect of processing on the initial levels. For cadmium and mercury, a recent study showed that fermentation of Sugar kelp with Lactobacillus plantarum for 48 h reduced significantly the content of both heavy metals as compared to the raw kelp (Bruhn et al., 2019). Soaking in hypersaline solution seems also to reduce the levels of cadmium in some brown algae, but the nutrient content is also affected (Stévant et al., 2018).
In contrast, numerous studies have assessed the impact of processing on arsenic and iodine levels in seaweeds. Regarding arsenic, as Hijiki alga is well known by its high levels of iAs, most of the studies have targeted this alga. One study demonstrated that the combination of washing and soaking may reduce the tAs concentration by up to 60% in this alga (Hanaoka et al., 2001). In another study, soaking and subsequently boiling, reduced the levels of tAs by 90% (Ichikawa et al., 2006). Similar combination of treatments, in this case using also Nori algae, shows a decrease of 70% in the iAs content of Hijiki and a more modest effect on Nori (6–24%), although the levels in the latter were initially relatively low (Cheyns et al., 2017). Boiling alone seems also effective to remove iAs from different types of seaweed, with reductions between 34% and 71% of the tAs (García‐Sartal et al., 2012). Park and co‐workers observed that soaking Hijiki alga in a 2% sodium chloride solution improves the elimination of iAs in a subsequent boiling step (Park et al., 2019). Overall, it is accepted that processing, including home processing, reduces the arsenic levels in seaweed. In fact, national organisations such as the Japanese Ministry of Agriculture, Forestry and Fisheries and international ones such as JECFA (FAO/WHO Joint Expert Committee on Food Additives) recommend washing and soaking seaweeds to reduce the iAs content (JECFA, 2011; WHO, 2011).
As commented above, the effect of processing on the iodine content of seaweed has also been extensively studied. In the case of iodine, the studies have mainly focused on brown seaweeds and, in particular, on the Laminariales (kelp) order as they are well known to possess the highest levels among seaweeds. Most of the iodine in brown seaweeds is present not only as inorganic forms mainly iodide (I−) but also as iodate (IO3 −), but it can also be bound to macromolecules (Blikra et al., 2022). On one hand, some authors have reported that processing such as washing and dehydration (air‐, oven‐ and freeze‐drying) hardly affects iodine levels (Nitschke and Stengel, 2016). On the other hand, different studies coincided in up to a 60% decrease of iodine content in several brown algae following rehydration/soaking (Nitschke and Stengel, 2016; Stévant et al., 2018). Soaking could have a very different impact on eliminating iodine depending on the chosen conditions, e.g. time, water temperature, salinity, etc. (Stévant et al., 2018). A combination of soaking and boiling seems to further reduce the iodine content (Nitschke and Stengel, 2016), although just a boiling treatment for two minutes in fresh water was reported to reduce iodine levels in the brown alga Sugar kelp by 70% (Lüning and Mortensen, 2015). Blanching, another typical method for seaweed preservation, has also proven to efficiently decrease iodine levels; a study with Saccharina latissima (Sugar kelp) showed that water blanching induced up to 88% reduction at optimised conditions (Nielsen et al., 2020). On the same alga, a recent study assessed the effect of different cooking methods on the content of iodine. Boiling in water for 15 min released 50–90% of iodine to the water, and of this iodine, 50% was released to air as hydrogen iodide. Frying released on average 50% of the iodine (25–80%), and drying provoked iodine losses of around 25% (Duinker et al., 2020).
Overall, processing/home preparation may help to reduce the amounts of heavy metals and iodine initially present in edible seaweeds. However, in some cases, particularly for iodine, the levels could still be high after processing. Processing may also lead to changes in the alga structure promoting the release of heavy metals and iodine from the alga matrix increasing therefore their bioaccessibility (Laparra et al., 2003; Blikra et al., 2022; Ho and Redan, 2022). Additionally, a potential conversion of certain forms of organic arsenic into iAs during seaweed processing might also deserve attention as such changes were already reported in shellfish (Liao et al., 2018).
5. Discussion
Only 697 eating occasions on halophytes, seaweeds and seaweed‐related products were available in the EFSA Comprehensive Database. This relatively low number could be explained, at least partially, by the fact that the dietary surveys used in this assessment cover a wide range of years (2001–2020) and only the most recent ones could be suitable to identify the latest years' increase in seaweed consumption. Additionally, the likely occasional consumption of these commodities might not be properly captured by the surveys included in the EFSA Comprehensive Database due to different reasons, such as the survey design, use of short‐term instruments (24 h recalls and food records) or the number of subjects. The red alga Laver represented almost 60% of the eating occasions without considering the consumption of alga‐based supplements. In few dietary surveys, the consumption of seaweeds was reported as just ‘Algae and prokaryotes organisms, unspecified’, without further information (around 10% of the total eating occasions).
The highest mean occurrence levels were reported for iodine. Among the heavy metals, the highest mean levels were for arsenic, in particular for tAs but also in few samples for iAs, and cadmium. Mean concentrations of tHg in seaweeds were the lowest, in line with data in the literature (Duinker et al., 2020). A relatively high variation in heavy metals/iodine levels were found across seaweed samples even within species; this variability is described elsewhere (FAO and WHO, 2022). The absorption and accumulation rates for minerals and trace elements in seaweeds depends on many factors, from the cell wall chemistry to environmental (availability of nutrients in the sea, oceanic currents, pH, salinity, etc.) and seasonal factors (Roleda et al., 2019; Aakre et al., 2021). Overall, the highest levels were reported for brown seaweeds, followed by red and green seaweeds. The high bioaccumulation of heavy metals in brown seaweeds seems to be related to their cell wall composition in sulfated fucans and carboxylate alginic acids (Andrade et al., 2010), while the high levels of iodine in certain brown seaweeds are related to the presence of haloperoxidases in the cell wall that facilitates its uptake, conversion, and storage (Küpper et al., 1998).
Table 23 provides information on the outcomes of the general dietary exposure scenario via the consumption of halophytes, seaweeds and seaweed‐related products (in the whole population and ‘consumers only’), and of the replacement dietary exposure scenario described in Section 2.4.2. Additionally, details are also shown on previous dietary exposure assessments conducted by EFSA on the heavy metals under assessment considering the whole diet.
Table 23.
Summary of the dietary exposure/intake estimates in the general scenario (via consumption of halophytes, seaweeds and seaweed‐related products), and the replacement scenario for total arsenic, inorganic arsenic, total mercury, cadmium, lead and iodine; information is also provided on EFSA previous dietary exposure/intake assessments considering the whole diet
| General dietary exposure/intake scenario | Dietary exposure/intake replacement scenario | EFSA previous dietary exposure/intake assessment | ||
|---|---|---|---|---|
| Whole population | Consumers only | |||
| Cadmium |
Highest mean exposure (LB=UB): 0.042 μg/kg bw per week in ‘Adults’. Highest 95th percentile exposure dietary: 0.009 μg/kg bw per week (LB=UB). |
Highest mean exposure (LB=UB): 3.1 μg/kg bw per week in ‘Adults’. Maximum highest reliable percentile (90th percentile): 4.4 μg/kg bw per week in ‘Adults’ (LB=UB). Main contributor: red Alga Laver. |
Highest mean dietary exposure: 1.59 μg/kg bw per week (LB=UB) for ‘Toddlers’. Highest 95th percentile exposure dietary: 7.82 μg/kg bw per week (LB=UB) for ‘Toddlers’. Main contributors: seaweed pasta and seaweed snacks. |
Mean exposure: 1.15–7.84 μg/kg bw per week (minimum LB−maximum UB). 95th percentile exposure: 2.01–12.1 μg/kg bw per week (minimum LB−maximum UB). (EFSA, 2012) |
| Total arsenic |
Highest mean exposure (LB=UB): 0.0793 μg/kg bw per day in ‘Adults’. Highest 95th percentile exposure dietary: 0.0381 μg/kg bw per day (LB=UB). |
Highest mean exposure (LB=UB): 5.52 μg/kg bw per day in ‘Adults’ Maximum highest reliable percentile (90th percentile): 7.87 μg/kg bw per day in ‘Adults’ (LB=UB) Main contributor: unspecified ‘Algae and prokaryotes organisms’ |
Highest mean dietary exposure: 5.1–5.2 μg/kg bw per day (LB=UB) for Toddlers' Highest 95th percentile exposure dietary: 19.8 μg/kg bw per day (LB=UB) for ‘Toddlers Main contributors: seaweed pasta and seaweed snacks |
Mean exposure: 0.45–4.6 μg/kg bw per day (minimum LB−maximum UB) 95th percentile exposure: 1.75–11.2 μg/kg bw per day (minimum LB−maximum UB). (EFSA CONTAM Panel, 2009a) |
| Inorganic arsenic |
Highest mean exposure (LB–UB): 0.0039–0.0040 μg/kg bw per day in ‘Adults’. Highest 95th percentile exposure dietary: 0–0.00031 μg/kg bw per day (LB–UB). |
Highest mean exposure (LB–UB): 0.21–0.22 μg/kg bw per day in ‘Adults’. (b) Maximum highest reliable percentile (90th percentile): 0.032–0.067 μg/kg bw per day in ‘Adults’ (LB–UB). Main contributor: unspecified ‘Algae and prokaryotes organisms’. |
Highest mean dietary exposure: 0.090–0.11 μg/kg bw per day (LB–UB) for ‘Toddlers’. Highest 95th percentile exposure dietary: 0.15–0.23 μg/kg bw per day (LB–UB) for ‘Toddlers’. Main contributors: seaweed pasta. |
Mean exposure (infants to adolescents): 0.04–0.61 μg/kg bw per day (minimum LB−maximum UB). Mean exposure (adults to very elderly): 0.03–0.15 μg/kg bw per day (minimum LB−maximum UB). 95th percentile exposure (infants to adolescents): 0.10–1.2 μg/kg bw per day (minimum LB−maximum UB). 95th percentile exposure (adults to very elderly): 0.06–0.33 μg/kg bw per day (minimum LB−maximum UB). (EFSA, 2021) |
| Lead |
Highest mean exposure (LB–UB): 0.0013–0.0015 μg/kg bw per day in ‘Pregnant women’. Highest 95th percentile exposure dietary: 0.00034–0.00035 μg/kg bw per day (LB–UB). |
Highest mean exposure (LB–UB): 0.093–0.10 μg/kg bw per day in ‘Adults’. Maximum highest reliable percentile (90th percentile): 0.072–0.073 μg/kg bw per day in ‘Adults’ (LB–UB). Main contributor: unspecified ‘Algae and prokaryotes organisms’. |
Highest mean dietary exposure: 0.15–0.16 μg/kg bw per day (LB–UB) for ‘Toddlers’. Highest 95th percentile exposure dietary: 0.81–0.82 μg/kg bw per day (LB–UB) for ‘Toddlers’. Main contributors: seaweed pasta and seaweed snacks. |
Children aged 1 to 7 years (minimum LB−maximum UB): Mean dietary exposure: 0.80–3.10 μg/kg bw per day; 95th percentile exposure: 1.30–5.51 μg/kg bw per day. Adults (minimum LB−maximum UB): Mean dietary exposure: 0.36–1.24 μg/kg bw per day; 95th percentile exposure: 0.73–2.43 μg/kg bw per day. (EFSA CONTAM Panel, 2010) |
| Total mercury |
Highest mean exposure (LB–UB): 0.00018–0.0031 μg/kg bw per week in ‘Toddlers’. Highest 95th percentile exposure dietary: 0.00015–0.00020 μg/kg bw per week (LB–UB). |
Highest mean exposure (LB–UB): 0.017–0.12 μg/kg bw per week in ‘Adults’. Maximum highest reliable percentile (90th percentile): 0.0050–0.054 μg/kg bw per week in ‘Adults’ (LB–UB). Main contributor: Unspecified ‘Algae and prokaryotes organisms.’ |
Highest mean dietary exposure: 0.028–0.081 μg/kg bw per week (LB–UB) for ‘Toddlers.’ Highest 95th percentile exposure dietary: 0.11–0.18 μg/kg bw per week (LB–UB) for ‘Toddlers’. Main contributors: seaweed pasta and seaweed snacks. |
Mean exposure to inorganic mercury: 0.13 and 2.16 μg/kg bw per week (minimum LB−maximum UB); (0.14–0.70 μg/kg bw per week in ‘Adults’). 95th percentile exposure to inorganic mercury: 0.25 and 4.06 μg/kg bw per week (minimum LB−maximum UB); (0.36–1.83 μg/kg bw per week in ‘Adults’). Mean exposure to methylmercury: 0.06 and 1.57 μg/kg bw per week (minimum LB−maximum MB); (0.07–1.08 μg/kg bw per week in ‘Adults’). 95th percentile exposure to methylmercury: 0.14 and 5.05 μg/kg bw per week (minimum LB−maximum MB); (0.51–3.04 μg/kg bw per week in ‘Adults’). (EFSA CONTAM Panel, 2012) |
| Iodine |
Highest mean intake (LB=UB): 5.1 μg/kg bw per day in ‘Adults.’ (a) Highest 95th percentile intake dietary: 0.15 μg/kg bw per day (LB=UB). |
Highest mean intake (LB=UB): 155 μg/kg bw per day μg/kg bw per day in ‘Adults’. Maximum highest reliable percentile (90th percentile): 32.7 μg/kg bw per day in ‘Adults’ (LB=UB). Main contributor: unspecified ‘Algae and prokaryotes organisms’. |
Highest mean dietary intake: 136 μg/kg bw per day (LB=UB) for ‘Toddlers’. Highest 95th percentile intake dietary: 464–465 μg/kg bw per day (LB–UB) for ‘Toddlers’. Main contributors: seaweed pasta and seaweed snacks. |
Scientific Opinion on Dietary Reference Values for iodine (EFSA NDA Panel, 2014) (c) |
Just one dietary survey with two consumers. Mean iodine intakes in all the other dietary surveys ranged between 0.035 and 0.31 (LB–UB).
Apart from the highest estimate from a dietary survey with five consumers, the mean exposure estimations in the adult population among ‘consumers only’ ranged between 0.002 and 0.045 μg/kg bw per day.
Urinary Iodine (UI) excretion, a marker of iodine intake, is listed for various European countries in the EFSA Scientific Opinion on Dietary Reference Values for iodine (EFSA NDA Panel, 2014).
In the general dietary exposure scenario, for the whole population, the limited numbers of seaweed consumers led to rather low estimates for the different compounds since the exposure was diluted among all subjects. Meaningful 95th percentiles for the whole population could only be estimated for one dietary survey because for the others there was less than 5% consumers of seaweeds. The dietary exposure estimates via the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ should be interpreted with caution because of the low number of consumers in most of the dietary surveys and age classes. Additionally, some of the highest estimates referred to consumers of unspecified ‘Algae and prokaryotes organisms’; for these eating occasions the occurrence value used for the linkage could be biased due to the presence of particular seaweeds with relatively high values (e.g. iAs in Hijiki alga).
The relevance of halophytes, seaweed and seaweed‐related products for the exposure to arsenic, cadmium, mercury and lead, and iodine intake, was assessed mainly by comparing the estimated exposure via the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ with previous assessments considering the whole diet in the whole population. This provides a rough indication of the potential contribution of seaweed to the total exposure. Together with the limitations mentioned above related to the consumers' estimates, comparisons with previous assessments are challenging because some of them refer to assessments conducted more than 10 years ago.
For cadmium, from the consumption of halophytes, seaweeds and seaweed‐related products, the highest mean exposure in ‘consumers only’ was 3.1 μg/kg bw per week (LB=UB); this estimate is within the range of the mean and 95th percentile of dietary exposures estimated via the whole diet (EFSA, 2012, see also Table 23). This indicates that depending on the type of seaweed, consumers might have a relatively high exposure to cadmium. In the current assessment, the highest estimates were driven by the relatively high mean levels of cadmium reported for the dried red algae Laver (1,675–1,676 μg/kg; LB–UB).
In the case of tAs, the relatively high mean levels reported in seaweeds, including in those well represented in the EFSA Comprehensive Database (e.g. red alga Laver), led to rather high exposure estimates. In fact, from the consumption of halophytes, seaweeds and seaweed‐related products, the highest mean estimate in ‘consumers only’ (5.5 μg/kg bw per day in ‘Adults’) was higher than the mean estimate and within the range of 95th percentile exposure estimates for the whole diet (EFSA CONTAM Panel, 2009a, see also Table 23). Recently, the risk assessment on arsenic has been focused on iAs since among the different arsenic species iAs is currently acknowledged as the most toxic (‘carcinogenic to humans’ as described by the International Agency for Research on Cancer, IARC). However, new scientific information is available on the toxicity of organic arsenic and, in fact, EFSA recently received a European Commission request to conduct a risk assessment not only on inorganic but also on organic arsenic. Apart from some exceptions already commented in this report, seaweeds are well known to possess predominantly organic arsenic (Taylor et al., 2017).
Without considering the highest estimate (0.21–0.22 μg/kg bw per day, LB–UB) that corresponds to one dietary survey with just five subjects consuming unspecified ‘Algae and prokaryotes organisms’, the dietary exposure to iAs from the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ ranged in ‘Adults’ between 0.002 and 0.045 μg/kg bw per day (min LB–max UB). The highest estimate in the range represents around 30% of the highest mean dietary exposure in ‘Adults’ via the whole diet (EFSA, 2021).
For lead, from the consumption of halophytes, seaweeds and seaweed‐related products, the highest mean estimates in ‘consumers only’ (0.093–0.10 μg/kg bw per day, ‘Adults’) represent between 10 and 30% of the prior dietary exposure estimates considering the whole diet (EFSA CONTAM Panel, 2010, see also Table 23).
For mercury, the available occurrence data mainly referred to tHg. This fact hampered any accurate assessment on the relevance of the consumption of seaweeds since previous assessments were carried out separately for inorganic mercury and methylmercury (EFSA CONTAM Panel, 2012). However, if assumed that all total mercury in seaweed and halophytes was methylmercury (as considered for fish), from the consumption of halophytes, seaweeds and seaweed‐related products, the current highest mean exposure at the UB in ‘consumers only’ (0.12 μg/kg bw per week, ‘Adults’) could represent around 10% of the highest mean dietary exposure in ‘Adults’ via the whole diet (EFSA CONTAM Panel, 2012).
Overall, for the different heavy metals, the main contributors to the highest exposure estimates via the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ were seaweeds.
As concerns iodine, from the consumption of halophytes, seaweeds and seaweed‐related products, the highest mean intake in ‘consumers only’ was 155 μg/kg bw per day estimated in ‘Adults’. This value would represent more than 10,000 μg per day in an adult of 70 kg. The main contributor to the highest estimate was the group ‘Algae and prokaryotes organisms’ unspecified. However, in other dietary surveys with relatively high mean intakes (above 20 μg/kg bw per day) different types of seaweeds were identified as the main contributors (Kombu, Laver). Therefore, although highest iodine levels are typically linked to some brown algae, a frequent intake of other type of algae such as the red algae Laver might also deserve attention. To make a comparison with previously reported dietary intakes is difficult since they are typically expressed as urinary iodine (UI) excretion due to the known limitations in estimating iodine dietary intake (EFSA NDA Panel, 2014). Yet, the magnitude of iodine intake via the consumption of certain seaweeds can be put in context by looking at the UL of 600 μg/day set for the adult population (SCF, 2002).
This report also assessed the impact of a future increase in the consumption of seaweed and seaweed products on the dietary exposure to heavy metals and on iodine intake. This increase in consumption can be seen from different perspectives. There could be an increase in the number of consumers as these products could become more popular; this would not necessarily imply an increase in the current exposure estimates under the assumption that the new consumers might have similar consumption to the current consumers. Another scenario would be an increase of seaweed consumption ‘per capita’ that could occur via the incorporation of new seaweed/seaweed products to the diet or an increase in the consumption of already consumed seaweeds. An increase of 10–20% in the current exposure of seaweed consumers would further boost the already relevant contribution of seaweed to the exposure to heavy metals and to iodine intake. Very important to point out that the extent of the increase still will strongly depend on the type of seaweed/seaweed products consumed.
The impact of the future increase of seaweed consumption was assessed using a replacement scenario in the whole population. The assumption was that some conventional products (e.g. salads, pasta, snacks) might be replaced by different seaweed‐related products anticipating, therefore, a regular consumption of seaweed products. Even though the increasing consumption of seaweed in the last years, the situation represented by the replacement scenario (e.g. all snacks consumed are made of or contain seaweeds), if ever to occur, would require a steady increase of seaweed consumption over a long time period. The outcome of this replacement scenario shows that for all heavy metals except mercury, the highest mean estimates in the whole population considering the four seaweed products described in Section 2.4.2 would be within the range of exposure estimates via the whole diet. In all cases, the main contributors would be seaweed pasta and seaweed snacks. In the case of lead and iAs, the previous exposure assessments identified cereals and cereal‐based products (specifically rice for iAs) as relevant contributors to the exposure (EFSA, 2012; EFSA, 2021). A more comprehensive assessment including the whole diet should help to characterise how the replacement of some conventional foods by seaweeds and seaweed products might affect the total dietary exposure. It is important to note that the replacement scenario, although being overly conservative, led to exposure estimations similar to those estimated for ‘consumers only’ using the current available consumption data. This underlines again the relevance of seaweed consumers and how the current consumption of certain seaweeds can contribute to the overall dietary exposure to heavy metals and iodine intake. For mercury, more speciation data are needed before doing any comprehensive interpretation of the estimates, although the exposure estimates in the replacement scenario were also similar to those identified for ‘consumers only’.
6. Uncertainties
When interpreting the results, different uncertainties should be considered. As described in Section 4, processing/home preparation could reduce the amount of heavy metals and iodine initially present in edible seaweeds. Therefore, it is essential to have information on whether the samples were processed before the analysis (and what type of processing). This information should be complemented at the consumption level to understand whether the consumed seaweeds/seaweed products refer to processed samples (e.g. dried) and also whether they were further processed at home before being consumed (e.g. rehydration).
In this report, the available consumption data mainly refer to the dried red alga Laver that typically is consumed as such without further processing as part of different types of sushi preparations (e.g., maki, temaki), and to alga‐based formulations that are typically pills/tablets consumed as such. Since occurrence data were available for both dried red alga Laver and alga‐based formulations, the uncertainty linked to the effect of processing can be considered as minor in the general exposure assessment.
Main uncertainties linked to the consumption data are related to their representativity due to the low number of consumers, but also to the reporting of several eating occasions as just ‘Algae and prokaryotes organisms, unspecified’ without further details. This led to the highest exposure estimates for some of the heavy metals assessed (e.g. iAs).
Since the levels of iodine and heavy metals in seaweeds seem to be strongly affected by many different factors, there is uncertainty also on the representativity of the occurrence data used in the assessment. As the taxonomy of seaweed species is particularly complex, there is also uncertainty linked to the FoodEx codification provided for the seaweed samples (consumption and occurrence data). In the replacement scenario, together with the uncertainties related to data representativity and taxonomy, the main uncertainty refers to its overly conservative nature (overestimation). Additionally, in this scenario, home processing could have an impact on the exposure estimations as seaweed pasta will be boiled before consumption, a process that as described in Section 4 can lead to heavy metals/iodine losses (overestimation).
7. Conclusions
This scientific report shows that from the consumption of halophytes, seaweeds and seaweed‐related products, ‘consumers only’ of seaweeds are currently exposed to cadmium levels that are within the range of previous exposure estimates considering the whole diet and, therefore, the consumption of seaweed and seaweed products may significantly increase the overall exposure. This relatively high exposure to cadmium was mainly linked to the consumption of the red alga Laver. The dietary exposure to iAs and lead from the consumption of halophytes, seaweeds and seaweed‐related products in ‘consumers only’ was also noted as non‐negligible, and it could represent between 10 and 30% of previously estimated exposure from the whole diet in the adult population. This is even more relevant considering that previous EFSA risk assessments identified possible health concerns for these three heavy metals. Seaweeds were also identified as important contributors to tAs exposure when compared to estimates from previous dietary exposure assessments via the whole diet; further work on the toxicity of organic arsenic, the main arsenic present in seaweeds, is needed to better understand the relevance of this finding. As regards iodine, very high mean levels were reported for certain algae such as for dried Kombu (3,500 mg/kg); mean intakes above 20 μg/kg bw per day were identified among consumers of these algae but also of the red alga Laver.
The impact of a future increase in seaweed consumption (‘per capita’) on the dietary exposure to heavy metals or on the intake of iodine will strongly depend on the type of seaweeds consumed. The replacement of few selected conventional foods by seaweed‐based foods showed that the European population would be exposed, only from the consumption of seaweed based products, to iAs, lead and cadmium at levels within the range of prior exposure estimates via the whole diet. It is important to note that the replacement scenario, although being overly conservative, led to dietary exposures to heavy metals and iodine intakes similar to those estimated for ‘consumers only’ using the currently available consumption data. This underlines the relevance of seaweed consumers and how the current consumption of certain seaweeds can contribute to the overall dietary exposure to heavy metals and iodine intake.
For mercury, there is a need for more speciation data in seaweed to better interpret the estimates as the toxicity varies greatly with species, although the current contribution of seaweeds to mercury exposure seems overall small as compared to the other compounds under assessment.
8. Recommendations
Further work is still needed to better understand the relationship between consumption of seaweeds and related products and the exposure to heavy metals and iodine intake. This work would help to reduce, at least partially, some of the uncertainties identified in the current assessment.
As shown in this report and in line with published literature, heavy metals and iodine levels in seaweeds can show a high variation depending on the type of seaweed (brown algae & green/red algae, Laminariaceae & Fucaceae). Therefore, data collection on occurrence levels should continue to characterise these differences, also considering the relevance, among other factors, of the geographical origin, the environment (season, salinity of the water, etc.) and even the part of the seaweed used, particularly for iodine levels. Since processing has a relevant impact on the levels of heavy metals and iodine in seaweeds, monitoring should be extended to processed products; in parallel, further investigation is needed on the effect of processing on the bioavailability of these compounds. In the case of mercury and arsenic, speciation analysis is desirable since the toxicity varies greatly with species.
In the area of consumption, efforts by EFSA and Member States to collect consumption data should continue, allowing regular updates to identify possible trends. This should also help to better understand whether seaweeds and seaweed products will remain in Europe as a niche product, only consumed sporadically or, instead, they will continue gaining acceptance becoming a food consumed more often and in higher amounts. Projects such as the EFSA's EU Menu (EFSA, 2014) that aims to provide standardised information on what people eat in countries and regions across the EU will also help to collect more detailed consumption data to allow more accurate and representative dietary exposure assessments.
The data collection should contain comprehensive information on the seaweeds analysed/consumed, starting with an appropriate taxonomic classification at the level of species, but also details on whether the seaweeds underwent any processing/home preparation step before their analysis/consumption. Specifically for the occurrence data, information should be provided, at least, on the expression of results (whole weight/dry weight basis) and the moisture content. If known, for seaweed products, information on the content and possibly on the type of seaweed should also be collected. To facilitate the reporting of occurrence data, EFSA developed a guidance in 2010 (Standard Sample Description, SSD) providing standardised data elements to allow data providers to submit all the information identified as essential to conduct risk assessments (EFSA, 2010a). The last version (SSD2) was published in 2015 and incorporates FoodEx2, a food description system that allows a detailed classification and description of the samples through the use of facets (EFSA, 2015).
For halophytes the lack of data is more evident than for seaweeds and seaweed‐related products. In order to adequately assess the relevance these plants might have on the exposure to heavy metals and iodine intake, more data are needed, both on consumption and occurrence.
Abbreviations
- AAS
atomic absorption spectrometry
- AB
arsenobetaine
- AES
atomic emission spectrometry
- AFS
atomic fluorescence spectrometry
- AI
Adequate Intake
- As(III)
arsenite/arsenous acid
- As(V)
arsenate/arsenic acid
- BfR
German Institute for Risk Assessment
- bw
body weight
- CONTAM Panel
Panel on Contaminants in the Food Chain
- DMA
dimethylarsinic acid
- DWH
Data warehouse
- FAO/WHO
Food and Agriculture Organization/ World Health Organization
- FSA
Food Standards Agency (United Kingdom)
- FSAI
Food Safety Authority Ireland
- GNPD
Mintel's Global New Products Database
- iAs
inorganic arsenic
- IARC
International Agency for Research on Cancer
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- ICP
inductively coupled plasma
- LB
lower bound
- LOD
limit of detection
- LOQ
limit of quantification
- MA
methylarsonic acid
- ML
maximum level
- MRL
maximum residue level
- MS
mass spectrometry
- NDA Panel
EFSA Panel on Panel on Dietetic Products Nutrition and Allergies
- SCF
Scientific Committee on Food
- SHC
Superior Health Council
- SSD
Standard Sample Description
- tAs
total arsenic
- tHg
total mercury
- UB
upper bound
- UI
urinary iodine
- UL
Tolerable Upper Intake Level
Annex A – Protocol for the EFSA scientific report on dietary exposure to heavy metals and iodine intake via consumption of seaweeds and halophytes in the European population
Available on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Annex B – Consumption data used for chronic dietary exposure estimations
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Annex C – Raw occurrence data set for food and feed samples (2011–2021) as extracted from EFSA DWH on 3 February 2022
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Annex D – Food occurrence data on arsenic (total and inorganic), cadmium, lead, mercury (methylmercury and total mercury) and iodine
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Annex E – Feed occurrence data on arsenic (total and inorganic), cadmium, lead and mercury (methylmercury and total mercury)
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Annex F – Dietary exposure assessment to heavy metals and dietary intake to iodine (whole population)
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Annex G – Dietary exposure assessment to heavy metals and dietary intake to iodine (‘consumers only’)
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.7576613
Supporting information
Protocol for the EFSA scientific report on dietary exposure to heavy metals and iodine intake via consumption of seaweeds and halophytes in the European population
Consumption data used for chronic dietary exposure estimations
Raw occurrence data set for food and feed samples (2011–2021) as extracted from EFSA DWH on 3 February 2022
Food occurrence data on arsenic (total and inorganic), cadmium, lead, mercury (methylmercury and total mercury) and iodine
Feed occurrence data on arsenic (total and inorganic), cadmium, lead and mercury (methylmercury and total mercury)
Dietary exposure assessment to heavy metals and dietary intake to iodine (whole population)
Dietary exposure assessment to heavy metals and dietary intake to iodine (‘consumers only’)
Suggested citation EFSA (European Food Safety Authority) , Dujardin B, Ferreira de Sousa, R , Gómez Ruiz JA, 2023. Scientific Report on the dietary exposure to heavy metals and iodine intake via consumption of seaweeds and halophytes in the European population. EFSA Journal 2023;21(1):7798, 47 pp. 10.2903/j.efsa.2023.7798
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00212
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: EFSA specially thanks Jean‐Charles Leblanc and Annette Petersen for providing valuable comments during the preparation of the report and for reviewing the final version. EFSA also wishes to acknowledge all European countries that provided the consumption and occurrence data used in the preparation of this scientific output. This scientific report was endorsed by the EFSA Panel on Contaminants in the Food Chain during its 127th Plenary meeting on 23 November 2022.
Approved: 14 December 2022
Notes
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 1,000,000 are or have been available on the European food market.
The Novel Food Catalogue (NFC) is a non‐binding database that reflects the views of Member States on the novel food status of foods and that have been discussed and completed over the years. A food item listed in the Novel Food Catalogue may have one of the following status: novel, not novel, not novel in food supplements or subject to an ongoing novel food status consultation (Aráujo and Peteiro, 2021).
Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 72–201.
Commission Recommendation (EU) 2018/464 of 19 March 2018 on the monitoring of metals and iodine in seaweed, halophytes and products based on seaweed (OJ L78, 21.3.2018, p. 16).
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (OJ L 364, 20.12.2006, p. 5).
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (OJ L 70, 16.3.2005, p. 1).
Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed, OJ L 140, 0.5.2002, p. 10.
Tolerable upper intake levels for vitamins and minerals – Scientific Committee on Food – Scientific Panel on Dietetic Products, Nutrition and Allergies. February, 2006. http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council (OJL 83, 22.3.2012, p. 1).
Re‐evaluation of agar (E406) as a food additive. EFSA Journal 2016; 14(12): 4645.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
E‐400 alginic acid, E‐401 sodium alginate, E‐402 potassium alginate, E‐403 ammonium alginate, E‐404 calcium alginate, E‐405 propane‐1,2‐diol alginate, E‐406 agar, E‐407 carrageenan, E‐407a processed Eucheuma seaweed.
Commission Regulation (EU) 2017/1017 of 15 June 2017 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. OJ L 159, 21.6.2017, pp. 48–119.
Commission Regulation (EU) 2017/1017 of 15 June 2017 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. OJ L 159/48, 2161.2017, pp. 1–72.
References
- Aakre I, Solli DD, Markhus MW, Mæhre HK, Dahl L, Henjum S, Alexander J, Korneliussen PA, Madsen L and Kjellevold M, 2021. Commercially available kelp and seaweed products–valuable iodine source or risk of excess intake? Food & Nutrition Research, 2021, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo A, Carvajal M and Martinez‐Ballesta MD, 2021. Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change. Foods, 10(1), 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akomea‐Frempong S, Skonberg DI, Camire ME and Perry JJ, 2021. Impact of blanching, freezing, and fermentation on physicochemical, microbial, and sensory quality of sugar kelp (Saccharina latissima). Foods, 10(10), 2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade LR, Leal RN, Noseda M, Duarte ME, Pereira MS, Mourão PA, Farina M and Amado Filho GM, 2010. Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Marine Pollution Bulletin, 60(9), 1482–1488. [DOI] [PubMed] [Google Scholar]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety) , 2018. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the risk of excess iodine intake from the consumption of seaweed in foodstuffs. Maisons‐Alfort. Available online: https://www.anses.fr/en/system/files/NUT2017SA0086EN.pdf [Google Scholar]
- Araújo R and Peteiro C, 2021. Algae as food and food supplements in Europe. Publications Office of the European Union: Luxembourg, 2021; ISBN 978‐92‐76‐40548‐1.
- Banach JL, Hoek‐van den Hil EF and van der Fels‐Klerx HJ, 2020. Food safety hazards in the European seaweed chain. Comprehensive Reviews in Food Science and Food Safety, 19, 332–364. [DOI] [PubMed] [Google Scholar]
- Barreira L, Resek E, Rodrigues MJ, Rocha MI, Pereira H, Bandarra N, da Silva MM, Varela J and Custódio L, 2017. Halophytes: Gourmet food with nutritional health benefits? Journal of Food Composition and Analysis, 59, 35–42. [Google Scholar]
- BfR , 2004. Health risks linked to high iodine levels in dried algae. BfR Opinion No. 026/2007. Berlin: Federal Institute for Risk Assessment. Available online: https://mobil.bfr.bund.de/cm/349/health_risks_linked_to_high_iodine_levels_in_dried_algae.pdf
- Blikra MJ, Henjum S and Aakre I, 2022. Iodine from brown algae in human nutrition, with an emphasis on bioaccessibility, bioavailability, chemistry, and effects of processing: a systematic review. Comprehensive Reviews in Food Science and Food Safety, 21, 1517–1536. [DOI] [PubMed] [Google Scholar]
- Bruhn A, Brynning G, Johansen A, Lindegaard MS, Sveigaard HH, Aarup B, Fonager L, Andersen LL, Rasmussen MB, Larsen MM and Elsser‐Gravesen D, 2019. Fermentation of sugar kelp (Saccharina latissima)—effects on sensory properties, and content of minerals and metals. Journal of Applied Phycology, 31(5), 3175–3187. [Google Scholar]
- Caporgno MP and Mathys A, 2018. Trends in microalgae incorporation into innovative food products with potential health benefits. Frontiers in Nutrition, 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascais M, Monteiro P, Pacheco D, Cotas J, Pereira L, Marques JC and Gonçalves AM, 2021. Effects of heat treatment processes: health benefits and risks to the consumer. Applied Sciences, 11(18), 8740. [Google Scholar]
- CEVA , 2014. Edible seaweed and French regulation ‐ Synthesis made by CEVA (31/03/2014). CEVA, Pleubian, France. [Google Scholar]
- Chen Q, Pan XD and Huang BF, 2018. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Scientific Reports, 8, 3578. 10.1038/s41598-018-21732-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry P, O'Hara C, Magee PJ, McSorley EM and Allsopp PJ, 2019. Risks and benefits of consuming edible seaweeds. Nutrition Reviews, 77, 307–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyns K, Waegeneers N, Van de Wiele T and Ruttens A, 2017. Arsenic release from foodstuffs upon food preparation. Journal of Agricultural and Food Chemistry, 65, 2443–2453. [DOI] [PubMed] [Google Scholar]
- Duinker A, Roiha IS, Amlund H, Dahl L, Lock EJ, Kögel T, Måge A and Lunestad BT, 2016. Potential risks posed by macroalgae for application as feed and food–a Norwegian perspective. National Institute of Nutrition and Seafood Research (NIFES). 2016 Jun 17:23.
- Duinker A, Kleppe M, Fjære E, Biancarosa I, Heldal HE, Dahl L and Lunestad BT, 2020. Knowledge update on macroalgae food and feed safety‐based on data generated in the period 2014–2019 by the Institute of Marine Research, Norway. Rapport fra havforskningen. 2020.
- EFSA (European Food Safety Authority) , 2006. Statement on a request from the Commission related to iodine in seaweed. EFSA Journal 2006;4(10):1046, 2 pp. 10.2903/j.efsa.2006.1046 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011b. The food classification and description system FoodEx 2 (draft‐revision 1). Supporting Publications 2011:215, 438 pp. 10.2903/sp.efsa.2011.EN-215. [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551, 37 pp. 10.2903/j.efsa.2012.2551 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Guidance on the EU Menu methodology. EFSA Journal 2014;12(12):3944, 77 pp. 10.2903/j.efsa.2014.3944 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication, 2015:EN‐804, 90 pp. 10.2903/sp.efsa.2015.EN-804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Afonso A, García Matas R, Maggiore A, Merten C and Robinson T, 2017. Technical report on EFSA's Activities on Emerging Risks in 2016. EFSA Supporting Publication 2017;EN‐1336, 59 pp. 10.2903/sp.efsa.2017.EN-1336 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Dujardin B and Kirwa L, 2019. Technical report on the raw primary commodity (RPC) model: strengthening EFSA's capacity to assess dietary exposure at different levels of the food chain, from raw primary commodities to foods as consumed. EFSA supporting publication 2019:EN‐1532, 30 pp. 10.2903/sp.efsa.2019.EN-1532 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Arcella D, Cascio C and Gómez Ruiz JA, 2021. Scientific report on the chronic dietary exposure to inorganic arsenic. EFSA Journal 2021;19(1):6380, 50 pp. 10.2903/j.efsa.2021.6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2009a. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/sp.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2009b. Cadmium in food. EFSA Journal 2009;7(3):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2010. Scientific Opinion on Lead in Food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Panel on Dietetic Products Nutrition and Allergies) , 2014. Scientific Opinion on Dietary Reference Values for iodine. EFSA Journal 2014;12(5):3660, 57 pp. 10.2903/j.efsa.2014.3660 [DOI] [Google Scholar]
- El‐Said GF and El‐Sikaily A, 2013. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environmental Monitoring and Assessment, 185, 6089–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO and WHO , 2022. Report of the expert meeting on food safety for seaweed – Current status and future perspectives. Rome, 28–29 0ctober 2021. Food Safety and Quality Series No. 13. Rome. 10.4060/cc0846en [DOI]
- Flowers TJ, Hajibagheri MA and Clipson NJ, 1986. Halophytes. The Quarterly Review of Biology, 61, 313–337. [Google Scholar]
- Fradinho P, Raymundo A, Sousa I, Domínguez H and Torres MD, 2019. Edible brown seaweed in gluten‐free pasta: technological and nutritional evaluation. Foods, 8, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSA (Food Standard Agency) , 2004. Committee on toxicity of chemicals in food, consumer products and the environment. Urgent COT opinion on arsenic in seaweed. Available online: http://www.food.gov.uk/multimedia/pdfs/TOX-2004-35.pdf [Google Scholar]
- FSAI , 2015. Consumption of Hijiki Seaweed. Available online: https://www.fsai.ie/faq/hijiki_seaweed.html
- García‐Sartal CG, del Carmen B‐AM and Bermejo‐Barrera P, 2012. Effect of the cooking procedure on the arsenic speciation in the bioavailable (dialyzable) fraction from seaweed. Microchemical Journal, 105, 65–71. [Google Scholar]
- Hanaoka KI, Yosida K, Tamano M, Kuroiwa T, Kaise T and Maeda S, 2001. Arsenic in the prepared edible brown alga hijiki, Hizikia fusiforme. Applied Organometallic Chemistry, 15, 561–565. [Google Scholar]
- Hasanah AN, Munandar A, Surilayani D, Haryati S, Aditia RP, Sumantri MH, Pratama G and Meata BA, 2021. Characterization of dried noodles from seaweed (Kappaphycus alvarezii) as potential substitute for wheat flour. Food Science and Technology Journal, 3, 113–120. [Google Scholar]
- Ho KK and Redan BW, 2022. Impact of thermal processing on the nutrients, phytochemicals, and metal contaminants in edible algae. Critical Reviews in Food Science and Nutrition, 62, 508–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt SL and Kraan S, 2011. Bioactive compounds in seaweed: functional food applications and legislation. Journal of Applied Phycology, 23, 543–597. [Google Scholar]
- Ichikawa S, Kamoshida M, Hanaoka KI, Hamano M, Maitani T and Kaise T, 2006. Decrease of arsenic in edible brown algae Hijikia fusiforme by the cooking process. Applied Organometallic Chemistry, 20, 585–590. [Google Scholar]
- JECFA , 2011. Evaluation of certain contaminants in food: Seventy‐second report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Switzerland, World Health Organization (WHO), Geneva. [Google Scholar]
- Küpper FC, Schweigert N, Ar Gall E, Legendre JM, Vilter H and Kloareg B, 1998. Iodine uptake in Laminariales involves extracellular, haloperoxidase‐mediated oxidation of iodide. Planta, 207, 163–171. [Google Scholar]
- Laparra JM, Velez D, Montoro R, Barbera R and Farré R, 2003. Estimation of arsenic bioaccessibility in edible seaweed by an in vitro digestion method. Journal of Agricultural and Food Chemistry, 51, 6080–6085. [DOI] [PubMed] [Google Scholar]
- Leandro A, Pereira L and Gonçalves AM, 2019. Diverse applications of marine macroalgae. Marine Drugs, 18, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Wang G, Li K and Zhao W, 2018. Change of arsenic speciation in shellfish after cooking and gastrointestinal digestion. Journal of Agricultural and Food Chemistry, 66, 7805–7814. [DOI] [PubMed] [Google Scholar]
- Lomartire S and Gonçalves AM, 2022. Antiviral Activity and Mechanisms of Seaweeds Bioactive Compounds on Enveloped Viruses—A Review. Marine Drugs, 20, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüning K and Mortensen L, 2015. European aquaculture of sugar kelp (Saccharina latissima) for food industries: iodine content and epiphytic animals as major problems. Botanica Marina, 58, 449–455. [Google Scholar]
- Luo H, Wang Q, Liu Z, Wang S, Long A and Yang Y, 2020. Potential bioremediation effects of seaweed Gracilaria lemaneiformis on heavy metals in coastal sediment from a typical mariculture zone. Chemosphere, 245, 125636. [DOI] [PubMed] [Google Scholar]
- Mendes MC, Navalho S, Ferreira A, Paulino C, Figueiredo D, Silva D, Gao F, Gama F, Bombo G, Jacinto R and Aveiro SS, 2022. Algae as food in Europe: an overview of species diversity and their application. Foods, 11, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CW, Holdt SL, Sloth JJ, Marinho GS, Sæther M, Funderud J and Rustad T, 2020. Reducing the high iodine content of Saccharina latissima and improving the profile of other valuable compounds by water blanching. Foods, 9, 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke U and Stengel DB, 2016. Quantification of iodine loss in edible Irish seaweeds during processing. Journal of Applied Phycology, 28, 3527–3533. [Google Scholar]
- Noriega‐Fernández E, Sone I, Astráin‐Redín L, Prabhu L, Sivertsvik M, Álvarez I and Cebrián G, 2021. Innovative ultrasound‐assisted approaches towards reduction of heavy metals and iodine in macroalgal biomass. Foods, 10, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Chauhan OP and Semwal AD, 2020. Seaweeds—a potential source for functional foods. Defence Life Science Journal, 5, 315–322. [Google Scholar]
- Park GY, Kang DE, Davaatseren M, Shin C, Kang GJ and Chung MS, 2019. Reduction of total, organic, and inorganic arsenic content in Hizikia fusiforme (Hijiki). Food Science and Biotechnology, 28, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña‐Rodríguez A, Mawhinney TP, Ricque‐Marie D and Cruz‐Suárez LE, 2011. Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chemistry, 129, 491–498. [DOI] [PubMed] [Google Scholar]
- Petropoulos SA, Karkanis A, Martins N and Ferreira IC, 2018. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends in Food Science & Technology, 74, 69–84. [Google Scholar]
- Roleda MY, Marfaing H, Desnica N, Jónsdóttir R, Skjermo J, Rebours C and Nitschke U, 2019. Variations in polyphenol and heavy metal contents of wild‐harvested and cultivated seaweed bulk biomass: health risk assessment and implication for food applications. Food Control, 95, 121–134. [Google Scholar]
- Roohinejad S, Koubaa M, Barba FJ, Saljoughian S, Amid M and Greiner R, 2017. Application of seaweeds to develop new food products with enhanced shelf‐life, quality and health‐related beneficial properties. Food Research International, 99, 1066–1083. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food) , 2002. Opinion of the Scientific Committee on Food on the tolerable upper intake level of iodine. 15 pp. Brussels, Belgium: SCF.
- Ścieszka S and Klewicka E, 2019. Algae in food: a general review. Critical Reviews in Food Science and Nutrition, 59, 3538–3547. [DOI] [PubMed] [Google Scholar]
- SHC , 2015. Arsenic and Other Elements in Algae and Dietary Supplements Based on Algae (No. 9149). Publication of the Superior Health Council of Belgium, 50 pp.
- Stévant P, Marfaing H, Duinker A, Fleurence J, Rustad T, Sandbakken I and Chapman A, 2018. Biomass soaking treatments to reduce potentially undesirable compounds in the edible seaweeds sugar kelp (Saccharina latissima) and winged kelp (Alaria esculenta) and health risk estimation for human consumption. Journal of Applied Phycology, 30, 2047–2060. [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, Karagas MR and Francesconi KA, 2017. Human exposure to organic arsenic species from seafood. Science of the Total Environment, 580, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teas J, Pino S, Critchley A and Braverman LE, 2004. Variability of iodine content in common commercially available edible seaweeds. Thyroid, 14, 836–841. 10.1089/thy.2004.14.836 [DOI] [PubMed] [Google Scholar]
- Timoner‐Alonso I, Castell‐Garralda V, Bosch‐Collet J, Abuín S and Calderón‐Delgado J, 2020. Estudi de la presència de metalls pesants i iode en algues destinades al consum humà: avaluació del risc associat i la seva contribució a la dieta total. Agencia Catalana de Seguridad Alimentaria, 51 pp.
- Topçuoğlu S, Güven KC, Kırbaşoğlu Ç, Güngör N, Ünlü S and Yılmaz YZ, 2001. Heavy metals in marine algae from Şile in the Black Sea, 1994–1997. Bulletin of Environmental Contamination and Toxicology, 67, 288–294. [DOI] [PubMed] [Google Scholar]
- Torres‐Tiji Y, Fields FJ and Mayfield SP, 2020. Microalgae as a future food source. Biotechnology Advances, 41, 107536. [DOI] [PubMed] [Google Scholar]
- Vellinga RE, Sam M, Verhagen H, Jakobsen LS, Ravn‐Haren G, Sugimoto M, Torres D, Katagiri R, Thu BJ, Granby K and Hoekstra J, 2021. Increasing Seaweed Consumption in The Netherlands and Portugal and the Consequences for the Intake of Iodine, Sodium, and Exposure to Chemical Contaminants: A Risk‐Benefit Study. Frontiers in Nutrition, 8, 792923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) , 2011. Safety evaluation of certain contaminants in food. Addendum on arsenic. Geneva, WHO and Rome, FAO. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/document.aspx?docID=8998
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety) , 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety, Environmental Health Criteria 240. Chapter 6: Dietary Exposure Assessment of Chemicals in Food. Available online: http://www.who.int/ipcs/food/principles/en/index1.html
- Wolle MM, Todorov TI and Conklin SD, 2021. Arsenic species in seaweeds commercially available in the United States. ACS Food Science & Technology, 1, 511–523. [Google Scholar]
- Yang H, Deng Y, Wang MW, Zhu M, Liu C, Ge ZJ and Xing JC, 2020. Risk of heavy metal contamination in three seawater‐cultivated vegetables. Polish Journal of Environmental Studies, 29, 961–967. [Google Scholar]
- Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR and McHenry MP, 2016. Potential use of algae for heavy metal bioremediation, a critical review. Journal of Environmental Management, 181, 817–831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the EFSA scientific report on dietary exposure to heavy metals and iodine intake via consumption of seaweeds and halophytes in the European population
Consumption data used for chronic dietary exposure estimations
Raw occurrence data set for food and feed samples (2011–2021) as extracted from EFSA DWH on 3 February 2022
Food occurrence data on arsenic (total and inorganic), cadmium, lead, mercury (methylmercury and total mercury) and iodine
Feed occurrence data on arsenic (total and inorganic), cadmium, lead and mercury (methylmercury and total mercury)
Dietary exposure assessment to heavy metals and dietary intake to iodine (whole population)
Dietary exposure assessment to heavy metals and dietary intake to iodine (‘consumers only’)


