Abstract
Differentiation of multipotent mesenchymal stem cells (MSCs) into bone-forming osteoblasts requires strict coordination of transcriptional pathways. Aryl hydrocarbon receptor ligands, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), have been shown to alter osteoblast differentiation in vitro and bone formation in multiple developmental in vivo models. The goal of the present study was to establish a global transcriptomic landscape during early, intermediate, and apical stages of osteogenic differentiation in vitro in response to TCDD exposure. Human bone-derived mesenchymal stem cells (hBMSCs) were cultured in growth media (GM), osteogenic differentiation media (ODM), or ODM containing 10 nM TCDD (ODM + TCDD), thus enabling a comparison of the transcriptomic profiles of undifferentiated, differentiated, and differentiated-TCDD-exposed hBMSCs, respectively. In this test system, exposure to TCDD attenuated the differentiation of hBMSCs into osteoblasts as evidenced by reduced alkaline phosphatase activity and mineralization. At various timepoints, we observed altered expression of genes that play a role in the Wnt, fibroblast growth factor, bone morphogenetic protein/transforming growth factor beta developmental pathways, as well as pathways related to extracellular matrix organization and deposition. Reconstruction of gene regulatory networks with the interactive dynamic regulatory event miner (iDREM) analysis revealed modulation of transcription factors (TFs) including POLR3G, NR4A1, RDBP, GTF2B, POU2F2, and ZEB1, which may putatively influence osteoblast differentiation and the requisite deposition and mineralization of bone extracellular matrix. We demonstrate that the combination of RNA-Seq data in conjunction with the iDREM regulatory model captures the transcriptional dynamics underlying MSC differentiation under different conditions in vitro. Model predictions are consistent with existing knowledge and provide a new tool to identify novel pathways and TFs that may facilitate a better understanding of the osteoblast differentiation process, perturbation by exogenous agents, and potential intervention strategies targeting those specific pathways.
Keywords: mesenchymal stem cell, AhR, cell differentiation
Mesenchymal stem cells (MSCs) have proliferative, self-renewal properties and possess the ability to differentiate into cells of the osteo- (bone), chondro- (cartilage), or adipogenic (fat) lineages. Thus, multipotent MSCs represent a popular source for cell-based regenerative therapies to treat bone diseases, such as osteoporosis, osteoarthritis, and rheumatoid arthritis (Abdallah and Kassem, 2008). These same characteristics, however, make MSCs potentially sensitive to environmental exposures which may alter the commitment and/or differentiation of multipotent cells to a defined cellular lineage or phenotype. A growing body of evidence suggests that exposure to various chemical agents may dysregulate MSC regulatory pathways governing cellular events responsible for stem cell commitment, differentiation, and/or maturation of osteoblasts essential for proper skeletal development (Alexander et al., 2016; Watson et al., 2019).
Several developmental pathways including Wnt, fibroblast growth factor (FGF), bone morphogenetic protein (BMP), transforming growth factor beta (TGF-β), Hedgehog, and Notch signaling play important roles in the differentiation of MSCs into osteoblasts (Baldridge et al., 2010). Signaling mediators comprising these pathways may promote osteogenesis via enhancing transcription of a number of osteogenic regulators, such as Runt-related transcription factor 2 (RUNX2) (Schroeder et al., 2005), Osterix (OSX) (Nakashima et al., 2002), and/or distal-less homeobox 5 (DLX5) (Samee et al., 2008), which in turn activate transcription of gene sets that govern MSC differentiation and encode for bone-specific extracellular matrix (ECM) components. In addition, extracellular mediators within these pathways may serve as negative feedback loops to prevent aberrant differentiation. Another putative target for disrupting osteogenic differentiation is through influencing the activity or transcription of genes associated with maintaining the self-renewal properties of stem cells. “Stemness” genes encoding Nanog homeobox (NANOG), SRY-Box 2 (SOX2), and POU Class 5 Homeobox 1 (POU5F1/OCT4), and others have been shown to play key roles in embryonic and MSC differentiation (Ko and Puga, 2017). Further complexity arises when considering the dynamic changes in the differentiation of multipotent MSCs to preosteoblasts and mature osteoblasts.
Thus, several targets exist whereby xenobiotic agents may potentially alter the transcriptional mediators governing osteogenic differentiation and maturation. Aryl hydrocarbon receptor (AhR) ligands represent one such class of environmental agents shown to alter events responsible for proliferation and differentiation in multiple cell and tissue lineages (Abel and Haarmann-Stemmann, 2010). Numerous studies suggest that exposure to AhR ligands, including benzo[a]pyrene (BaP), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and polychlorinated biphenyls (PCBs), alter osteo-, chondro-, and adipogenic differentiation in cells of mesenchymal origin (Gadupudi et al., 2015; Ryan et al., 2007; Watson et al., 2019). More recent studies suggest that the AhR plays a critical role in maintaining pluripotency of cardiac, hematopoietic, and embryonic stem cells (Ko et al., 2016; Wang et al., 2016). Prior work from our laboratory using human bone-derived mesenchymal stem cells (hBMSCs) has also demonstrated that AhR activation with TCDD greatly attenuates MSC osteoinduction through modulation of stemness/multipotency genes, including POU5F1, NANOG, SALL4, and SOX2 (Watson et al., 2019).
The present study aims to identify the global transcriptomic landscape of AhR-mediated inhibition of MSC osteogenic specification and differentiation. We hypothesize that TCDD, a potent AhR ligand, inhibits MSC differentiation by perturbing pathways involved in maintaining self-renewal capacity while also dysregulating the expression of key inputs (activators and inhibitors) of osteoinduction and differentiation. To confirm this and future hypotheses, we establish a nonbiased global transcription landscape of hBMSCs throughout the osteogenic differentiation process. Subsequently, we modeled dynamic regulatory networks using an interactive dynamic regulatory event miner (iDREM) on our datasets to identify key genes and transcriptional regulators driving osteoinduction and dysregulation of osteogenesis following TCDD exposure. This study provides a global understanding of how a prototypic AhR ligand alters the transcription of gene regulatory networks associated with the differentiation of MSCs into bone-forming osteoblasts.
Materials and methods
hBMSC isolation and characterization
hBMSCs used for transcriptomic assessment were obtained from a 25-year-old, non-osteoporotic female, donor at the University of North Carolina-Chapel Hill hospitals (IRB exemption protocol: 10-0201). Cells were isolated and characterized by the following method: Bone fragments were washed in phosphate-buffered saline (PBS) containing 100 U/ml penicillin and 100 mg/ml streptomycin (Corning Inc.). Bone fragments were subsequently minced into approximately 1 mm3 pieces and digested in a 3 mg/ml collagenase XI solution (Sigma-Aldrich) for 3 h at 37°C on an orbital shaker. The digest solution was filtered through a 100-μm cell strainer, centrifuged at 500 × g for 5 min and pelleted cells were resuspended in growth media (GM) consisting of minimal essential media, α-modification (α-MEM, GE Healthcare) supplemented with 10% fetal bovine serum (FBS, Rocky Mountain Biologicals), 2 mM l-glutamine (Genesee Scientific), 100 U/ml penicillin, and 100 μg/ml streptomycin (Genesee Scientific). Cell suspensions were plated and incubated overnight at 37°C and 5% CO2 in a humidified incubator. After 24 h, non-adherent cells were removed by washing with PBS, and media was replaced with GM. Adherent cells were subsequently cultured until reaching 80% confluency, at which point they were trypsinized and frozen down at passage 0. For hBMSC characterization, passage 1 cells were plated and incubated overnight at 37°C at 5% CO2 in a humidified incubator. After 24 h, plates were rinsed with PBS to again wash out the nonadherent cell population, and the media was replaced with fresh GM and cells propagated for an additional 48 h. Next, a subset of cells was characterized via immunohistochemical staining to determine the presence or absence of select MSC cell surface markers (Rojewski et al., 2008). Cells that were CD34−/CD45−/CD73+/CD106−/CD166+ were determined to be MSC-positive cells. Cells were then assessed for multipotency by assessing adipogenic or osteogenic differentiation. Passage 1 hBMSCs were plated in 6-well tissue culture-treated plates (Genesee Scientific) at a density of 0.6 × 104 cells/cm2 in GM. Cells were allowed to proliferate in GM for 24 h followed by a media change to adipogenic differentiation media consisting of α-MEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, 1 µM dexamethasone (Sigma-Aldrich), 5 μg/ml h-insulin (Sigma-Aldrich), 100 μM indomethacin (Sigma-Aldrich), and 500 μM isobutylmethylxanthine (IBMX, Sigma-Aldrich), or osteogenic differentiation media (ODM) comprised of α-MEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, 50 μM ascorbic acid (Sigma-Aldrich), 0.1 μM dexamethasone (Sigma-Aldrich), and 10 mM β-glycerophosphate (Sigma-Aldrich). Media was changed every 3–4 days and cells were assessed for mineralization via alizarin red staining at day 14, or lipid accumulation via Oil Red O at day 21, as markers of terminal differentiation. Isolated hBMSCs that exhibited the proper immunophenotype and multipotency were further propagated in GM to passage 2.
For studies with TCDD, passage 2 hBMSCs were seeded into 12-well tissue culture-treated plates (Genesee Scientific) at a density of 1 × 104 cells/cm2 in GM. After 24 h, GM was replaced with ODM consisting of α-MEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, 50 μM ascorbic acid (Sigma-Aldrich), 0.1 μM dexamethasone (Sigma-Aldrich), and 10 mM β-glycerophosphate (Sigma-Aldrich) to induce differentiation (day 0). Beginning on day 0, cells were cultured for up to 17 days in ODM containing 0.1% dimethyl sulfoxide (DMSO, vehicle control) (ODM) or 10 nM TCDD (Cambridge Isotopes Laboratory) (ODM + TCDD). An osteogenic negative control consisted of hBMSCs cultured in GM with 0.1% DMSO (GM) to provide an undifferentiated reference cell population. Media was replaced every 3–4 days, and cells were cultured under standard culture conditions until the appropriate timepoint for RNA isolation.
Alizarin red S histological staining and alkaline phosphatase activity
hBMSCs, cells were expanded to passage 2, plated, and cultured under GM, ODM, or ODM + TCDD conditions as described above. Cells were assessed on days 3, 7, and 17 for the formation of mineralized bone ECM using Alizarin Red S. Cells were rinsed twice with PBS, fixed for 15 min in 10% neutral buffered formalin, and rinsed twice with PBS. Next, monolayers were stained with 40 mM Alizarin Red S for 20 min and were rinsed with PBS to remove the excess stain prior to imaging.
Alkaline phosphatase (ALP) activity was measured on day 7 using the Sensolyte pNPP Alkaline Phosphatase Assay Kit (Anaspec). Cells were rinsed in assay buffer, scraped, and incubated under agitation for 10 min at 4°C. Samples were centrifuged at 3000 × g for 10 min and supernatants were collected. In a 96-well plate, 30 µl of p-nitrophenyl phosphate was added to each sample or standard (ran in triplicate) and incubated for 30 min at 37°C. Absorbance was measured at 405 nm and ALP activity was derived from a standard curve from known ALP concentrations. For each sample, ALP activity was normalized to total cell protein concentration quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific). Data obtained from the ALP assay is reported as mean ± SEM. Statistical analyses were performed using a 2-tailed Student’s t-test (for comparison of 2 treatment groups) or a 1-way analysis of variance (ANOVA) (for comparison of 2 or more treatment groups) with p-values <.05 deemed statistically significant.
RNA isolation
Cells were harvested for total RNA from each treatment (GM-DMSO, ODM-DMSO, or ODM + TCDD) at 3 h (3H), 24 h (D1), 3 days (D3), 7 days (D7), and 17 days (D17) postosteoinduction in ODM for a total of n = 4 individual samples/treatment/timepoint. Briefly, cells were washed twice in PBS and lysed in TRI Reagent (Ambion, Life Technologies) and total RNA was isolated according to the manufacturer’s instructions. Total RNA was quantified using Agilent 2100 Bioanalyzer and 2100 Expert Software package (Agilent Technologies). RNAs with RNA Integrity numbers (RINs) lower than 9 were excluded from subsequent RNA-Seq experiments.
RNA-sequencing
Total RNA samples were submitted to the North Carolina State Genomic Sciences Laboratory (Raleigh, North Carolina) for Illumina RNA library construction and sequencing. Prior to library construction, RNA integrity, purity, and concentration were assessed using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano Chip (Agilent Technologies). Purification of messenger RNA (mRNA) was performed using the oligo-dT beads provided in the NEBNext Poly(A) mRNA Magnetic Isolation Module (New England Biolabs). Complementary DNA (cDNA) libraries for Illumina sequencing were constructed using the NEBNext Ultra Directional RNA Library Prep Kit (NEB) and NEBNext Mulitplex Oligos for Illumina (NEB) using the manufacturer-specified protocol. Briefly, the mRNA was chemically fragmented and primed with random oligos for first-strand cDNA synthesis. Second-strand cDNA synthesis was then performed with dUTPs to preserve strand orientation information. The double-stranded cDNA was then purified, end-repaired and “a-tailed” for adaptor ligation. Following ligation, the samples were selected for a final library size (adapters included) of 400–550 bp using sequential AMPure XP bead isolation (Beckman Coulter). Library enrichment was performed and specific indexes for each sample were added during the protocol-specified PCR amplification. The amplified library fragments were purified and checked for quality and final concentration using an Agilent 2200 Tapestation with a High Sensitivity DNA chip (Agilent Technologies) and a Qubit fluorometer (ThermoFisher). The final quantified libraries were pooled in equimolar amounts for clustering and sequencing on an Illumina HiSeq 2500 DNA sequencer utilizing a 125 bp single-end sequencing reagent kit (Illumina). The software package Real-Time Analysis (RTA) was used to generate raw bcl, or base call files, which were then de-multiplexed by sample into fastq files for data submission. All RNA-Seq data have been deposited in GEO (GSE217334).
RNA seq data analysis
Data analysis was performed in consultation with Bioinformatics Core at NCSU Center for Human Health and the Environment. We have generated on average approximately 28 million single-end raw RNA-Seq reads with a length of 125 bp for each replicate. The sequence data quality was assessed using FastQC command line application and 12 poor quality bases were trimmed from the 5′-end. The remaining high-quality reads were aligned to the Human reference genome (hg38) using the STAR (Dobin et al., 2013) aligner. Per-gene counts for each replicate were calculated using the htseq-count script from the HTSEQ python package (version 0.11.1). Count data were imported to R statistical computing environment (R Core Team, 2020). Genes with less than 0.5 counts per million in at least 3 samples within each experimental condition were deemed non-expressors and removed from downstream analyses. A principal component analysis (PCA) was performed on the filtered count matrix using the function prcomp (R Core Team, 2020). One outlier sample within the ODM + TCDD treatment was detected on the PCA and removed from the data set (Data not shown). Data normalization and analysis were conducted using the DESeq2 Bioconductor package (Love et al., 2014). Data were fitted using a linear model with media, treatment and timepoints as experimental conditions. Differentially expressed genes (DEGs) were identified after applying Benjamini-Hochberg multiple testing correction (padj < .05), (Benjamini and Hochberg, 1995; Love et al., 2014). The differential expression analysis consisted of 3 sets of contrasts: GM versus ODM, ODM versus ODM + TCDD, and GM versus ODM + TCDD for each time point separately. Enrichment tests of Gene Ontology terms (BP_DIRECT, CC_DIRECT, MF_DIRECT), KEGG pathways, Reactome pathways, and protein domains (INTERPRO) were performed for each set of DEGs using the Functional Annotation Tool from DAVID Bioinformatics Resources (version 6.8) (Huang et al., 2009). A term was enriched if it had a false discovery rate-corrected p-value ≤.05 Heatmaps of selected genes and pathways were generated with the R package heatmap (version 1.0.12) after the transformation and normalization of the gene expression data through the normTransform function of the R package DESeq2 (version 1.26.0) (Love et al., 2014).
Reconstruct gene regulatory networks with the iDREM analysis
To develop a model of regulator tracks for MSC proliferation (GM), osteogenic differentiation (ODM), and dysregulation of osteogenic differentiation (ODM + TCDD), we applied the iDREM (Ding et al., 2018) to our datasets. iDREM uses an Input-Output Hidden Markov Model that integrates transcription factor (TF) binding interactions with time series gene expression data to establish dynamic regulatory networks. These networks branch where previously co-expressed sets of genes begin to diverge. iDREM annotates these branching points with transition probabilities and assigns TFs predicted to regulate genes in upward and downward tracks. iDREM assigns each gene to a most probable path, each represents a unique gene co-expression pattern, through the model based on its temporal expression and the annotated TFs associated with its regulation. Conversely, a TF is assigned to a path at a branching point if the hypergeometric distribution coordinates the TF with genes expressed at the branch point of the track. This process enables the assessment of temporal gene expression changes in conjunction with TF-DNA interaction data. We utilized the human Encode 1000 TF database for all TF interactions. The exact parameters used to generate each model are described in Supplementary Table 1.
Similarity/difference index heatmaps
To further contrast the 3 models produced by the iDREM approach, we assessed how similar the sets of genes assigned to the different paths was across the GM, ODM, and ODM + TCDD models. We computed the overlap coefficient (Simpson, 1943), a similarity index, as follows: intersection or number of genes in common between a pair of paths a and b divided by the size (defined as number of genes) of the smallest path between paths a and b. The resulting values were used to produce heatmaps using the R package Complex Heatmap (Gu et al., 2016) to compare the GM, ODM, and ODM + TCDD models visually. We also calculated the percentage of genes in each path that were not shared (difference index) by any of the paths of another model; we refer to these values as the “percentages of remaining genes.” In addition, we produced another set of heatmaps with the same methodology but using the TFs associated with each of the leaf nodes of the GM, ODM, and ODM + TCDD models. The TFs used to generate the heatmaps were extracted from iDREM and included the top 50 (p < .05) transcriptional regulators for each leaf node (both expressed in our transcriptomics data set and putative predictions from iDREM).
Results
TCDD attenuates osteogenic differentiation of hBMSCs
hBMSCs were first assessed to confirm the inhibitory action of TCDD treatment on osteogenic differentiation. Following an initial proliferative phase in GM, hBMSCs were cultured in either GM, ODM, or ODM containing 10 nM TCDD (ODM + TCDD) for up to 17 days and evaluated for ALP activity and mineralization of the cell monolayers. Robust mineralization was observed in ODM hBMSCs on days 7 and 17, which was markedly reduced with the addition of 10 nM TCDD in ODM + TCDD hBMSCs and absent in undifferentiated GM hBMSCs (Figure 1A). A similar pattern was noted in ALP activity, where on day 7, ALP activity was nearly 2-fold higher in ODM relative to the GM negative controls but was significantly lower in ODM + TCDD hBMSCs when compared with hBMSCs cultured in ODM alone (Figure 1B).
Figure 1.
Apical assessment of human bone-derived mesenchymal stem cells (hBMSCs). hBMSCs were cultured in growth media (GM), osteogenic differentiation media (ODM), and ODM + TCDD (10 nM) TCDD for 17 days. A, Alizarin red staining was performed on days 3, 7, and 17. B, Alkaline phosphatase (ALP) activity was measured on day 7. Different letters in (B) indicate groups with significantly different ALP activity (p < .05; 1-way ANOVA with a Tukey’s post hoc analysis). N = 4 individual samples/treatment. Abbreviation: TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
hBMSCs undergo global gene expression changes driven by media, time, and treatment
To identify alterations in the transcriptional landscape during osteogenic differentiation, RNA-Seq was performed on hBMSCs cultured under the 3 conditions (GM, ODM, or ODM + TCDD) at 5-time points (3H, D1, D3, D7, and D17). Based on clustering patterns from PCA, time and media type (GM versus ODM) appear to be primary drivers (see PC1–PC4) for the observed differences in gene expression profiles. Treatment with TCDD also seemed to drive variability (observable in PC1–PC4) between ODM and ODM + TCDD beginning as early as 24 h postosteoinduction (eg, the addition of ODM + TCDD on time zero) (Figure 2A). The number of DEGs between GM versus ODM and ODM versus ODM + TCDD and GM versus ODM + TCDD were conducted to examine the influence of these conditions on MSC differentiation. For each comparison, we observed an increase in total DEGs (both up- and downregulated) with time as hBMSCs initiated and underwent osteogenic differentiation. Moreover, at each timepoint, we identified select biological and/or functional enrichments within KEGG pathways and GO between our 2 comparisons (Figure 2B, Supplemental Figure 1).
Figure 2.
Principal component analysis (PCA) of differentially expressed genes (DEGs) in hBMSCs during osteogenic differentiation. RNA-Seq was performed on total RNA collected from hBMSCs at 3 h, 24 h, 3 days, 7 days, and 17 days when cultured in growth media (GM), osteogenic differentiation media (ODM), and ODM + TCDD (10 nM). Shown in (A) are 2 PCA renderings exhibiting the distribution of data replicates between PC1-2 and PC3-4 with time and treatment as covariates. B, Summary of the number of differentially expressed genes, whose expression is either increased or decreased (adjusted p-value < .05), based on GM versus ODM or ODM versus ODM + TCDD. Number of GO and KEGG assigned to DEGs at each time point of the study. N = 4 individual samples/treatment/timepoint. Abbreviation: hBMSCs, human bone-derived mesenchymal stem cells; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Comparisons of (GM vs ODM) identified several pathways associated with osteoblast and/or bone development (Supplementary Table 2). Across several time points, pathways involving cell cycle arrest, DNA replication, cell shape, and actin cytoskeletal reorganization were altered with the addition of ODM. Within 3 h, transient changes were observed in pathways associated with stem cell potency, Wnt, TGF-β, and other signaling pathways known to influence osteoblast differentiation and skeletal development. Changes at later time points were associated with processes such as ECM organization, ECM-receptor interaction, and autophagy that characterize phenotypically mature osteoblasts. Together, these patterns indicate that ODM promotes early commitment and subsequent differentiation of hBMSCs into osteoblasts.
Comparisons of (ODM vs ODM + TCDD) identified similar pathways involving stem cell potency, TGF-β, Wnt, BMP, and insulin receptor signaling that were differentially regulated in the presence of TCDD (Supplementary Table 2). Pathways associated with terminal osteoblast differentiation, such as ECM organization, collagen fibril organization, vesicle-mediated transport, and autophagy were also influenced by exposure to TCDD, supporting our finding of reduced mineralization with TCDD treatment. Lastly, comparisons of GM and ODM + TCDD demonstrated dysregulation of pathways associated with mitogenesis and DNA repair including, regulation of cell cycle, sister chromatin exchange, and positive and negative regulation of transcription (Supplementary Table 2).
TCDD alters the expression of bone-specific ECM genes
Several trends were identified within pathways driving osteoblast differentiation and ECM organization. In general, genes that encode TFs and/or upstream regulators demonstrated lower relative expression than genes encoding more highly expressed ECM components (eg, collagens). Within the osteoblast differentiation pathway (GO:0001649), LRRC17 demonstrated differential expression as early as day 3 with expression following the profile GM > ODM > ODM + TCDD. Several genes were significantly altered with the addition of TCDD including ALPL, IGFBP3/5, FGF9, PENK, and TMEM119, which were most apparent at the later stages of differentiation on day 7 and/or day 17 (Figure 3A). With the addition of TCDD (ODM + TCDD), several ECM genes (GO:003019) showed altered expression as early as 24 h, with notable changes present by day 3 for POSTN, CCDC80, ELN, DCN, COL8A1, COMP, and PECAM1. Other ECM genes demonstrated altered expression later (day 7 or 17) in the differentiation process such as COL1A1/2, COL5A1/2/3, SPARC, FBN1, LUM, VCAN, TNC, FBLN1, ITGA8, SMOC2, and EGFL6 (Figure 3B).
Figure 3.
Heatmaps of broad select KEGG/GO terms associated with osteoblast differentiation and extracellular matrix organization. A, Heatmap of osteoblast differentiation (GO:0001649). B, Heatmap of extracellular matrix organization (GO:0030198). Dotted boxes and/or gray triangles highlight selected genes whose expression patterns appear to be influenced by TCDD treatment. N = 4 individual samples/treatment/timepoint. Abbreviation: TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
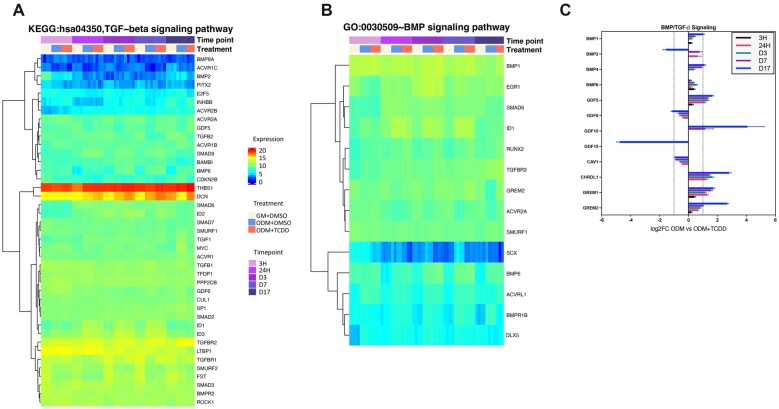
TCDD alters Wnt, BMP/TGF-β, and FGF pathways
Genes associated with specific signaling pathways (eg, Wnt, BMP, TGF-β, insulin receptor signaling) were also examined. Analysis of the BMP/TGF-β pathway revealed several genes whose expression appeared to be altered by TCDD (Figs. 4A and 4B). Looking specifically at expression changes between ODM versus ODM + TCDD, BMP2/4/6 appeared to increase in the presence of TCDD, except for BMP2 at D17, which was decreased more than 2-fold. GDF5 and GDF6 expression increased and decreased, with time in the presence of TCDD. A similar pattern was observed with GDF10 (increased) and GDF15 (decreased); however, these differences occurred at later time points. BMP inhibitors CHRDL1, GREM1, and GREM2 demonstrated robust increases with time with a 2-fold increased expression by D7 for all 3 genes (Figure 4C).
Figure 4.
Heatmaps and relative expression of select KEGG/GO terms associated with TGF-β and BMP signaling. A, Heatmap of genes associated with TGF-β (hsa04350). B, Heatmap of BMP (GO:0030501) pathways. C, Relative expression levels comparing ODM versus ODM + TCDD for BMP signaling pathway. All genes shown in are significantly different from the ODM controls (padj < .05); dotted line (---) represents 2-fold increase or decrease expression. N = 4 individual samples/treatment/timepoint. Abbreviations: ODM, osteogenic differentiation media; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TGF-β, transforming growth factor beta.
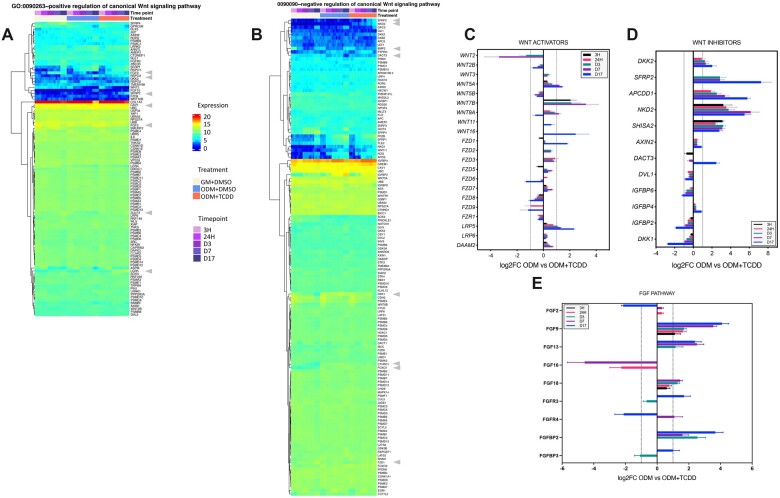
TCDD treatment significantly impacted select genes known to activate or repress Wnt pathway activity (Figure 5). Within the Wnt activators, several Frizzled and Wnt genes were significantly altered. WNT2/2B/5 were lower at various time points; however, other Wnt genes such as WNT3/5A/7B/11/16 demonstrated increased expression in TCDD. The family of Frizzled genes also demonstrated mixed responses in TCDD (Figs. 5A and 5C). LRP5 expression increased significantly in a time-dependent manner in TCDD-treated hBMSCs. Wnt inhibitors displayed the most robust response across multiple time points. DKK2, SFRP2, APCDD1, NKD2, and SHISA2 were significantly upregulated, in some cases as early as 3 h for SHISA2 and NKD2, and upregulation was maintained through D17. Conversely, the expression of other inhibitors such as IGFBP2, DKK1, IGFBP6, and DVL1 was downregulated by as much as 4-fold by D17 (Figs. 5B and 5D). The FGF pathway, another developmental pathway overlapping with WNT also appeared to be significantly altered with TCDD treatment (Figure 5E). FGF9/13/18 and FGFBP2 were increased considerably with the addition of TCDD. At the same time, other FGF mediators (FGF2, FGFR3/4, and FGFBP3) exhibited differential responses depending on the timepoint (increased then decreased, or vice versa) with the addition of TCDD.
Figure 5.
Heatmaps and relative expression of select GO terms associated with Wnt and fibroblast growth factor signaling. Heatmaps of genes associated with (A) positive (GO:00902263) and (B) negative regulation (GO:0090090) of the Wnt pathways. Gray triangles highlight selected genes whose expression patterns appear to be influenced by TCDD treatment. Relative expression levels comparing ODM versus ODM + TCDD for (C) Wnt activators, (D) Wnt inhibitors, and (E) fibroblast growth factor pathway. All genes shown in C-E are significantly different from the ODM controls (padj < 0.05); dotted line (---) represents 2-fold increase or decrease expression. N = 4 individual samples/treatment/timepoint. Abbreviations: ODM, osteogenic differentiation media; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Reconstruction of gene regulatory networks with the iDREM analysis
We next developed dynamic regulatory network models for each tested condition including MSC proliferation (GM), osteogenic differentiation (ODM), and dysregulation of osteogenic differentiation (ODM + TCDD) using the iDREM program (Ding et al., 2018). Each of our 3 models (Figs. 6A–C) exhibited distinctive characteristics in degree of branching and number of primary, secondary and tertiary nodes, ranked gene expression, and slope of gene expression change over time. In the MSC proliferation (GM) model (Figure 6A), we identified 10 gene expression tracks that differed in their gene expression between 3 h and day 17 timepoints. Initial divergence in gene expression occurred between 3 and 24 h with 3 primary nodes, 1 upregulated (node 1, Figure 6A) and 2 downregulated (nodes 2 and 3, Figure 6A).
Figure 6.
Interactive dynamic regulatory event miner (iDREM) models. Schematic representation of gene expression tracks and bifurcation nodes for hBMSC treatments model including (A) GM, (B) ODM, and (C) ODM + TCDD. iDREM analysis was conducted according to Ding et al. (2018). A track in the graph represents a group of genes sharing similar gene expression patterns (letters). Each of the tracks comprises a list of consecutive nodes (numbers) across time points, in which each node denotes a regulatory state (junction) where regulators and gene expression divergence can possibly happen. All tracks are anchored as a single node at 3 h postosteoinduction. Computational parameters are listed in Supplementary Table 1. Abbreviations: GM, growth media; hBMSC, human bone-derived mesenchymal stem cell; ODM, osteogenic differentiation media; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Between 24 h and D3, the 3 primary nodes (1–3) split into 7 secondary nodes (nodes 4–10, Figure 6A) indicative a significant transcriptional event between these time points. At this stage, 4 tracks were characterized by an increase in gene expression tracks A, B/C, D, E/F (Figure 6A) and 3 tracks characterized by a decrease in expression G/H, I, J (Figure 6A). Little change in the regulation of gene expression was observed between days 3 and 7 indicated by only one bifurcation occurring within gene tracts of node 5 likely indicative of minor functional differences in cell activities during this time. Between days 7 and 17, 2 tertiary nodes (nodes 15 and 16, Figure 6A) underwent a final divergence suggesting a late-stage change in gene expression events as cells aged or became quiescent.
In the ODM model (Figure 6B), we identified 9 gene expression tracks that differed in their gene expression between the 3 h and day 17 timepoints. The ODM model differed significantly from the proliferation GM model between 3 and 24 h, with the initial divergence forming 2 distinct primary nodes representing large clusters of both up (node 1, Figure 6B) and down (node 2, Figure 6B) regulated genes. By 3 days postosteoinduction, each primary node diverged again, forming 4 secondary nodes including 2 nodes for upregulated genes (nodes 3 and 4, Figure 6B) and 2 nodes for downregulated genes (nodes 5 and 6, Figure 6B) indicative of expansive gene regulation between these time points. Between days 3 and 7, 2 secondary nodes (nodes 3 and 6, Figure 6B) underwent an additional bifurcation resulting in a total of 6 tertiary nodes (nodes 7–12, Figure 6B) suggestive of functional differences in osteogenic cell activities during this time. Between days 7 and 17, 3 of the tertiary tracks (nodes 9–11, Figure 6B) diverged again, indicative of late-stage modulations in gene expression events as cells undergo terminal differentiation and deposition of osteogenic ECM.
Compared with the ODM model, the addition of TCDD to ODM media during differentiation significantly altered gene expression patterns between 3 h and day 17 (Figure 6C). In this model, we identified a total of 10 gene expression tracks that differed in their gene expression throughout the study. Initial divergence in gene expression occurred between 3 and 24 h with 3 primary nodes, 1 upregulated (node 1, Figure 6C) and 2 downregulated (nodes 2 and 3, Figure 6C) similar to that observed in the GM model. At 3 days postosteoinduction, 2 of the 3 primary nodes (nodes1 and 2, Figure 6C) underwent a secondary bifurcation resulting in a total of 6 unique tracks (nodes 4–9, Figure 6C), indicative of a significant shift in gene expression/regulation between these time points. At this stage, 3 tracks were characterized by an increase in gene expression (tracks A, B, C/D, E/F, Figure 6C) and 3 tracks characterized by a decrease in expressions (tracks G/H, I, J, Figure 6C). Consistent with both the GM and ODM models, there was little change in the regulation of gene expression between days 3 and 7 except for, a single bifurcation in secondary node 4 suggesting that some cellular functionalities were modulated during this time. Between days 7 and 17, however, 3 of the secondary tracks split at nodes 12, 13, and 14 suggesting a late-stage change in gene expression events as cells either aged, became quiescent or produced limited ECM as indicated in Figure 1A.
In each model, the slope of gene expression exhibited a temporal pattern. In all 3 models, the slope of ranked gene expression was steepest between 3 and 24 h indicating that early regulatory events were driving dynamic gene expression associated with proliferation (GM) or differentiation (ODM), or modulated differentiation (ODM + TCDD). However, significant regulatory events continued to drive abundant gene expression changes between 24 h and 3 days at a lower slope. These data are consistent with our PCA plots demonstrating robust separation between conditions (GM, ODM, and ODM + TCDD) between 3 and 24 h, and 24 h and day 3. Little change in gene expression is evident (flat slope) between days 3 and 7 followed by slight changes (increased slope) in regulatory events between select tracks in each condition between days 7 and 17.
We next compared genes assigned to individual tracts of each model to identify distinguishing gene features associated with osteogenesis (GM vs ODM) and modulation of osteogenesis (ODM vs ODM + TCDD) (Figure 7, Supplementary Figs. 2A and 2B). Using a similarity/dissimilarity index (as described in Materials and methods), we established a list of similar or unique genes between individual tracks of each model (GM vs ODM, ODM vs ODM + TCDD, GM vs ODM + TCDD). As demonstrated in Figure 7, comparisons of gene tracts between ODM and ODM + TCDD demonstrate significant variation in common gene sets from no similarity (0.0) to significantly similar (0.6). The same approach was taken to compare GM versus ODM and GM versus ODM + TCDD (Supplementary Figs. 2A and 2B). To identify putative drivers within each model we additionally established a gene dissimilarity index indicated by the colored tiles adjacent to each tract (Figure 7). We subsequently compiled lists of dissimilar genes for each condition and conducted enrichment analysis using ToppGene to assign GO terms, pathways, and/or disease terms. Comparisons between ODM and ODM + TCDD data revealed enrichments unique to the ODM + TCDD treatment on osteogenesis including: an intrinsic component of the plasma membrane, bone mineral density, Ehlers-Danlos syndrome, osteogenesis imperfecta, regulation of receptor binding, negative regulation of growth, ECM organization, ECM organization, skeletal system development, and others indicating significant modulation of gene sets involved in select skeletal biogenesis and diseases (Figure 7 and Supplementary Table 3). A similar comparison was made between GM and ODM revealing significant enrichments GO terms and disease models associated with osteogenesis including skeletal system development, Wnt signaling pathway, ECM organization, Osteogenesis Imperfecta (Supplementary Table 4), reflective of osteoblast differentiation, and mineral matrix secretion. A similar analysis for GM and ODM + TCDD is provided in Supplementary Table 5 for reference.
Figure 7.
Similarity/dissimilarity index for ODM versus ODM + TCDD genes. Heatmap of gene similarities across individual tracks for iDREM models ODM versus ODM + TCDD. The values represent similarity of gene composition between individual tracks of each model using an overlap coefficient similarity index (1 = full overlap; 0 = no overlap). The order of the rows and columns of the heatmaps are based on the order of the paths in each model. The more similar 2 models are, the higher the values in the diagonal region of the heatmap. The side bars of the heatmap depict the proportion of genes in a path that is dissimilar between 2 individual tracks. The row and column labels show the names of the tracks and the number of dissimilar genes is represented in parenthesis. The table shows select GO, KEGG, and other terms enrichment results for genes sets within particular paths. Abbreviations: iDREM, interactive dynamic regulatory event miner; ODM, osteogenic differentiation media; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
We next investigated the transcriptional regulators associated with each iDREM model (GM, ODM, and ODM + TCDD). iDREM enables the extraction of transcriptional regulators (present in our transcriptomics dataset and putative predictions from iDREM) for gene expression tracks at each bifurcation/node. This analysis extracted the top 50 TFs from each path for GM, ODM, and ODM + TCDD models. TFs were then assessed across time and condition using a similarity/difference equation to identify regulators impacting MSC functions, including (proliferation GM, osteogenic differentiation ODM, and disruption of osteogenic differentiation ODM + TCDD). Using this approach, we found that select regulators were consistent across all conditions (GM, ODM, and ODM + TCDD) including YY1, POL2, GATA2, STAT3, CTCF, SMC3, MAFK, E2F6, EP300, JUND, SPI1, TBP, RAD21, EGR1, CEBPB, ELF1, SP1, MYC, USF1, TAF1, JUN, MAX, POLR2A, FOXA1. Of these, we note that several have well-established roles in promoting osteogenesis (CTCF, E2F6, EGR1, ELF1, EP300, GATA2, JUND, MAFK, MYC, SP1, STAT3, USF1, YY1, NANOG), stem cell pluri/multipotency (CTCF, E2F6, EGR1, ELF1, EP300, GATA2, JUN, JUND, MYC, POL2, RAD21, STAT3, YY1, SP1), and either directly or indirectly modulate Wnt signaling (E2F6, EGR1, JUN, JUND, MYC, POL2, MAFK, EP300, RAD21, SP1, STAT3, YY1). Moreover, we identified 6 unique regulators that were not shared across all conditions, POLR3G, NR4A1, RDBP, GTF2B, POU2F2, and ZEB1, and likely impact MSC functions. In bimodal comparisons between GM tracts A-J and ODM tracts A-I, osteoinduction (replacement of GM with ODM) promotes the utilization of RDBP, a member of the Negative Elongation Factor Complex. Conversely, GTF2B, POU2F2, and ZEB1 appear during cell proliferation in GM. Interestingly, comparisons of ODM tracks A-I with ODM + TCDD tracts A-J, indicate that POLR3G and NR4A1 function in ODM, whereas GTF2B, POU2F2, and ZEB1 function in ODM + TCDD similar to that observed in GM. Lastly, comparisons between GM tracks A-J and ODM + TCDD tracks A-J indicate that RDBP is prominent in ODM + TCDD, similar to ODM, whereas POLR3G and NR4A1 are prominent in GM (Figure 8, Supplementary Figure 3).
Figure 8.
Similarity/dissimilarity index for ODM versus ODM + TCDD transcription factors. Heatmap of transcription factor (TF) similarities across individual tracks for iDREM models ODM versus ODM + TCDD. The values represent similarity of TF composition between individual tracks of each model using an overlap coefficient similarity index (1 = full overlap; 0 = no overlap). Given the high similarity of TF composition across models, the rows and columns of the heatmap were rearranged through hierarchical clustering to highlight the differences. The side bars of the heatmap depict the proportion of TFs in a path that is dissimilar between 2 individual tracks. The row and column labels show the names of the tracks, and the number of dissimilar TFs is represented in parenthesis. The table denotes unique TFs assigned to individual tracks across models. Abbreviations: iDREM, interactive dynamic regulatory event miner; ODM, osteogenic differentiation media; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Lastly, we examined the average expression of gene sets regulated by POLR3G, NR4A1, GTF2B, POU2F2, ZEB1, and AhR, as an iDREM output based upon their putative roles in the GM, ODM, and ODM + TCDD models as described above (Figure 9). Under GM conditions, the gene regulatory role of GTF2B, POU2F2, ZEB1, and AhR is relatively minor until day 7 when there appears to be a surge of activity and an increase in average gene expression. Genes regulated by POLR3G, exhibit a steady increase in activity from 24 h through day 17, and genes regulated by NR4A1 remain relatively constant across all time points. By comparison, the average expression of gene sets regulated by, AhR, GTF2B, POU2F2, and ZEB1 appear to be upregulated as early as 24 h following osteoinduction up to days 3/7, followed by a decline in average gene expression events often below baseline through the remainder of the 17-day experiment. Genes regulated by POLR3G follow a similar induction pattern but remain higher over the course of the experiment.
Figure 9.
Average expression of transcription factor-regulated genes. Based on iDREM output data, average expression of select transcription factors enriched in tested condition including GM (circle), ODM (square), and ODM + TCDD (triangle) were established for select enriched transcription factors identified in Figure 8. Including average expression of (A) AhR, (B) POU2F2, (C) GTF2B, (D) ZEB1, (E) POLR3G, and (F) NR4A1-related genes. Data derived are generated through the iDREM program as a calculated average and thus no error statistics are reported. Abbreviations: iDREM, interactive dynamic regulatory event miner; ODM, osteogenic differentiation media; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Conversely, gene sets regulated by NR4A1 exhibit a steady decline following osteoinduction for the duration of the experiment. The addition of TCDD to ODM media during osteoinduction resulted in a steady elevation in AhR, GTF2B, POU2F2, and ZEB1-regulated genes between 24 h and day 7, followed by a slight decline between 7 and 17 days, indicative of a strong inductive effect of early AhR activation. Interestingly, gene sets regulated by POLR3G exhibit a significantly higher degree of expression at 24 h and remain steady through day 7, followed by a decline on day 17. By comparison, NR4A1-regulated genes are downregulated with TCDD for the duration of the experiment.
Discussion
In the present study, we provide a global, time-course assessment of gene expression changes occurring in an hBMSC line undergoing osteogenic differentiation in the presence or absence of TCDD. To anchor our transcriptomic assessment, we demonstrate that exposure to 10 nM TCDD attenuates ALP activity and histological staining of mineralized bone matrix, 2 apical hallmarks of osteoblast maturation and function in vitro. In doing so, we corroborate previous in vitro findings by our laboratory (Watson et al., 2019) and others (Ryan et al., 2007; Yun et al., 2017) who demonstrate AhR-mediated inhibition of osteoblast differentiation. Here we expand upon these earlier studies to assess transcriptomic changes at various points across the continuum of MSC commitment and differentiation, and disruption of this process by TCDD. To visualize temporal changes in select target pathways, we generated individual heatmaps incorporating magnitude, direction, and dynamic range of gene expression. Doing so provided enhanced granularity while still anchoring genes to a given biological process or function. In addition, we performed iDREM analysis on these data to tease out specific genes and transcriptional regulators whose expression could modulate osteogenic differentiation.
TCDD exposure markedly affected ECM genes known to modulate cell-cell and cell-matrix interactions. The most highly induced genes were SERPINB2, VCAN, DCN, COL5A3, and MMP7. Alteration of ECM synthesis and/or remodeling during development appears to be a conserved mode of action following exposure to TCDD or other AhR ligands. Such effects have been reported in a number of studies examining various tissues, including the heart (Aragon et al., 2008), kidney (Abbott et al., 1987), thymus (Nottebrock et al., 2006), mammary gland (Hushka et al., 1998), and seminal vesicles (Hamm et al., 2000). Our findings in hBMSCs were most pronounced at the latter stages of differentiation (D7 and D17) when ECM deposition and matrix mineralization occurs. A study using devitalized hBMSCs demonstrates the importance of intact ECM scaffolding in promoting osteoblast differentiation (Baroncelli et al., 2018). Thus, it is plausible that by altering the ECM environment, AhR activation could inhibit apical function and/or activity needed to carry out subsequent ECM deposition, mineralization, and ossification resulting in an overall attenuation of hBMSCs osteogenic differentiation.
Given these ECM-associated changes, we sought to understand which gene regulatory pathways may be modulated in the early commitment of MSCs to an osteoblast lineage and subsequent osteoblast maturation. Global assessment of our RNA Seq data set illustrated that the Wnt pathway appeared to be a susceptible target, as several Wnt inhibitors SFRP2, DKK2, NKD2, and SHISA2 were among the most highly induced genes under ODM + TCDD conditions. Canonical Wnt signaling (eg, via β-catenin signaling) plays an essential role in skeletal formation and is an indispensable regulator of osteoblast differentiation and activity. Studies demonstrate that canonical Wnt signaling promotes osteoblast lineage commitment and inhibits MSC differentiation toward adipocytes, cartilage, and other lineages of mesenchymal origin (D’Alimonte et al., 2013). Secreted Wnt inhibitors DKK2 and SFRP2 have been shown to prevent Wnt ligands from binding target receptors LRP5/6 and the Frizzled family of receptors, respectively (Katoh and Katoh, 2007a). Interestingly, we observed a consistent reduction in DKK1 expression despite its similar role in preventing the binding of Wnt ligands to the LRP5/6 receptors. This illustrates the dynamic nature of Wnt signaling and perhaps different regulatory mechanisms between DKK1 and DKK2 in response to TCDD exposure. We also observed increased expression of the intracellular Wnt inhibitors NKD2 and SHISA2, suggesting multiple intracellular and/or extracellular targets whereby TCDD can modulate Wnt signaling.
Numerous studies identify canonical Wnt signaling as a sensitive target of activated AhR. In rodents, gestational exposure to TCDD inhibits early fetal prostate development in the ventral urogenital sinus (UGS) (Vezina et al., 2010). TCDD-mediated agenesis of the ventral prostate is driven by the inhibition of Wnt signaling as evidenced by modulation of CTNNB1, Wnt10a, Wnt16, Ror2, Wif1, and Rspo2 (Schneider et al., 2014). Inhibition of prostate bud formation has also been observed in TCDD-exposed UGS tissues ex vivo, along with reduced expression of Wnt activators Rspo2 and Rspo3 and downstream Wnt target genes (Lef1, Wif1, and Tcf1); in a co-culture where RSPO2 and RSPO3 block the inhibitory effects of TCDD (Branam et al., 2013). Similar inhibition of Wnt signaling has been observed in other tissues and cell types, including the developing palate in mice (Hu et al., 2015), rat liver progenitor cells (Procházková et al., 2011), human MCF-7 cells (Zhao et al., 2012), and human trophoblastic spheroids and endometrial epithelial cells (co-culture) (Tsang et al., 2012). Our findings of disrupted Wnt signaling in TCDD-treated hBMSCs adds to the growing body of evidence that multipotent stem cells are yet another sensitive target of AhR activation. In addition, our global time-course RNA-seq further supports the notion that Wnt signaling requires a precise balance between activation and inhibition during MSC commitment and differentiation to precisely regulate downstream developmental events.
Through our iDREM analysis, we additionally identified select TFs (POLR3G, NR4A1, GTF2B, POU2F2, ZEB1) that appear to play a role in MSC proliferation, osteogenesis, and disruption of osteogenesis by TCDD. Consistent with our observation that TCDD modulates Wnt signaling in our MSC model, several of the identified TFs are demonstrated to be involved in osteogenesis and play a role in Wnt signaling. ZEB1 is a well-described TF known for its role in epithelial-to-mesenchymal transition (Chen et al., 2022). More recently, ZEB1 is also demonstrated to play a role in osteoblast differentiation and bone mass. ZEB1 negatively regulates osteogenesis by modulating cell differentiation through a wnt/β-catenin signaling mechanism (Xu et al., 2021). Similarly, overexpression of POU2F2 is demonstrated to promote fracture healing through the induction of osteogenic regulators, including OSX, RUNX2, OSC, and COL10A. Like ZEB1, POU2F2 also promotes Wnt signaling through a POU2F2 binding site within the FZD (frizzled receptor) promoter (Katoh and Katoh, 2007b). The nuclear receptor NR4A1 is expressed in several tissues, including bone and has been identified as a common marker in osteoporosis. NR4A1 is demonstrated to play a significant role in osteogenic and adipogeneic differentiation, mesenchymal stromal cell migration and directly facilitates the expression of osteopontin (Lammi et al., 2004; Maijenburg et al., 2012). NR4A1 is also demonstrated to facilitate Wnt signaling in several cell types and plays a fundamental role in regulating an immunometabolic COX2-prosoglandin-NR4A-Wnt signaling axis (Wu et al., 2018).
Wnt and AhR pathways serve to modulate osteogenic and adipogenic differentiation in MSCs and function as master regulators of pluripotency and stem cell commitment through dysregulation of key pluripotency regulators OCT4, SALL4, NANOG, and SOX2 (Clevers and Nusse, 2012; Ko and Puga, 2017; Watson et al., 2019). Previously, we demonstrated that dysregulation of pluripotency markers by TCDD recapitulated expression levels observed in proliferating MSCs consistent with the theory that AhR activation serves as a transcriptional regulator of MSC pluripotency and fate commitment. Consistent with our observations, Gérard et al. (2019) identified both AhR and GLIS1 as primary drivers of MSC multipotency. AhR also plays a significant role in differentiating mouse embryonic stem cells into cardiomyocytes (Wang et al., 2010, 2013). These studies identified a selective feedback loop involving select pluripotency and polycomb factors that regulate AhR expression and subsequent stem cell lineage commitment (Ko et al., 2014). In a mouse model of cleft palate, TCDD is demonstrated to deregulate Oct4 expression in concert with disruptions in MSC proliferation and differentiation (Tao et al., 2020). Similarly, the Wnt/β-catenin signaling pathway is linked to cell commitment and pluripotency (Clevers and Nusse, 2012). This association may be due to the co-occupancy of T-cell factors (TCF) and core pluripotency regulators OCT4, NANOG, and SOX2 governing stem cell self-renewal and differentiation (Ho et al., 2013). Thus, in our system, there may be an interplay between Wnt, AhR, and pluripotency regulators that facilitate/modulate the osteogenic commitment of MSCs.
Lastly, we demonstrate that modulation of osteogenesis by TCDD in our MSC model results in gene enrichment for GO terms and disease pathways associated with alterations in bone mineral density, ECM organization, Ehlers-Danlos syndrome, osteogenesis imperfecta indicative of disruptions in skeletal system development. These outcomes are consistent with previous studies in MSCs and in vivo models demonstrating the effects of AhR activation by select ligands on osteogenic processes. Liu et al. (2020) demonstrated that TCDD inhibited ECM deposition and disruption of BMP/TGF/SMAD signaling pathways in palatal MSCs. Similarly, Tong et al. (2017) observed loss of mineralization capacity and attenuation in osteogenic markers and inhibition of β-catenin/Wnt expression following TCDD exposures.
There are, however, notable limitations to this study. Many recent studies have now demonstrated using scRNASeq that isolation protocols for hBMSC and adipose-derived MSC result in heterogeneous cell populations. Assessments of transcriptional profiles within distinct subpopulations demonstrate the presence of multipotent MSCs in addition to cells in various stages of osteogenic, chondrogenic, and adipogenic differentiation, as well as terminal-stage cells in the quiescent state (Wang et al., 2021). Although our results focus on cells that have undergone osteogenic differentiation, there may be some contributions from other cell lineages that influence the data outcomes of this study. Using a single-cell approach in future studies may help confirm the mechanistic inferences observed. Another limitation is the inherent individual variability of MSCs in responses to TCDD exposures. Although in previous studies we demonstrated a consistent response to TCDD across 3 individual MSC lines (Watson et al., 2019), in this study we utilized only a single MSC line for RNASeq analysis. Future studies with additional MSC lines are needed to confirm these findings. Lastly, external factors that may contribute to the variability of MSC response to TCDD were not documented from MSC doners. Factors other than gender, age, and osteoporotic status including race/ethnicity, weight/BMI, health status smoking, and other life style practices, may influence how MSCs respond to exogenous factors, including xenobiotic exposures.
In this study, we provide evidence to the growing body of literature that demonstrates MSC differentiation is sensitive to perturbation by TCDD, a prototypic AhR ligand. We demonstrate that the combination of expression data in conjunction with the iDREM regulatory model captures the transcriptional dynamics underlying the MSC differentiation under different conditions. Model predictions are consistent with known knowledge and provide an opportunity to identify novel pathways and TFs that may facilitate a better understanding of the MSC differentiation process, perturbation by exogenous agents, and potential intervention strategies by targeting novel pathways and TFs. Our findings further corroborate the established paradigm that skeletal development and bone formation, in which osteoblasts play an essential role, can be altered by numerous chemical and/or pharmaceutical agents known to act through distinct mechanisms (Alexander et al., 2016; Watson et al., 2019).
Supplementary Material
Contributor Information
AtLee T D Watson, Toxicology Program, North Carolina State University, Raleigh, North Carolina 27695, USA.
Aldo Carmona Baez, Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, USA; Bioinformatics Research Center, North Carolina State University, Raleigh, North Carolina 27695, USA.
Dereje Jima, Bioinformatics Research Center, North Carolina State University, Raleigh, North Carolina 27695, USA; Center for Human Health and the Environment, North Carolina State University, Raleigh, North Carolina 27695, USA.
David Reif, Bioinformatics Research Center, North Carolina State University, Raleigh, North Carolina 27695, USA; Center for Human Health and the Environment, North Carolina State University, Raleigh, North Carolina 27695, USA.
Jun Ding, Meakins-Christie Laboratories, Department of Medicine, McGill University Health Centre, Montreal, Quebec H4A 3J1, Canada.
Reade Roberts, Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, USA.
Seth W Kullman, Toxicology Program, North Carolina State University, Raleigh, North Carolina 27695, USA; Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, USA; Center for Human Health and the Environment, North Carolina State University, Raleigh, North Carolina 27695, USA.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Acknowledgment
The authors thank Andy Baltzegar for conducting their RNA-Seq experiment.
Funding
National Institute of Environmental Health Sciences (T32ES07046) Molecular Pathways to Pathogenesis in Toxicology; and National Institute of Environmental Health Sciences (P30ES025128) Center for Human Health and the Environment
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abbott B. D., Morgan K. S., Birnbaum L. S., Pratt R. M. (1987). TCDD alters the extracellular matrix and basal lamina of the fetal mouse kidney. Teratology 35, 335–344. [DOI] [PubMed] [Google Scholar]
- Abdallah B. M., Kassem M. (2008). Human mesenchymal stem cells: From basic biology to clinical applications. Gene Ther. 15, 109–116. [DOI] [PubMed] [Google Scholar]
- Abel J., Haarmann-Stemmann T. (2010). An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 391, 1235–1248. [DOI] [PubMed] [Google Scholar]
- Alexander P. G., Clark K. L., Tuan R. S. (2016). Prenatal exposure to environmental factors and congenital limb defects. Birth Defects Res. C Embryo Today 108, 243–273. [DOI] [PubMed] [Google Scholar]
- Aragon A. C., Kopf P. G., Campen M. J., Huwe J. K., Walker M. K. (2008). In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: Effects on fetal and adult cardiac gene expression and adult cardiac and renal morphology. Toxicol. Sci. 101, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge D., Shchelochkov O., Kelley B., Lee B. (2010). Signaling pathways in human skeletal dysplasias. Annu. Rev. Genomics Hum. Genet. 11, 189–217. [DOI] [PubMed] [Google Scholar]
- Baroncelli M., van der Eerden B. C., Kan Y. Y., Alves R. D., Demmers J. A., van de Peppel J., van Leeuwen J. P. (2018). Comparative proteomic profiling of human osteoblast-derived extracellular matrices identifies proteins involved in mesenchymal stromal cell osteogenic differentiation and mineralization. J. Cell. Physiol. 233, 387–395. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 57, 289–300. [Google Scholar]
- Branam A. M., Davis N. M., Moore R. W., Schneider A. J., Vezina C. M., Peterson R. E. (2013). TCDD inhibition of canonical wnt signaling disrupts prostatic bud formation in mouse urogenital sinus. Toxicol. Sci. 133, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Pan P., Wei W., Zhang Y., Cui G., Yu Z., Guo X. (2022). Expression of Zeb1 in the differentiation of mouse embryonic stem cell. Open Life Sci. 17, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Nusse R. (2012). Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- D’Alimonte I., Lannutti A., Pipino C., Di Tomo P., Pierdomenico L., Cianci E., Antonucci I., Marchisio M., Romano M., Stuppia L., et al. (2013). Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. Rep. 9, 642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Hagood J. S., Ambalavanan N., Kaminski N., Bar-Joseph Z. (2018). iDREM: Interactive visualization of dynamic regulatory networks. PLoS Comput. Biol. 14, e1006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R. (2013). STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi G., Gourronc F. A., Ludewig G., Robertson L. W., Klingelhutz A. J. (2015). PCB126 inhibits adipogenesis of human preadipocytes. Toxicol. In Vitro 29, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard D., Schmidt F., Ginolhac A., Schmitz M., Halder R., Ebert P., Schulz M. H., Sauter T., Sinkkonen L. (2019). Temporal enhancer profiling of parallel lineages identifies AHR and GLIS1 as regulators of mesenchymal multipotency. Nucleic Acids Res. 47, 1141–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Eils R., Schlesner M. (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849. [DOI] [PubMed] [Google Scholar]
- Hamm J. T., Sparrow B. R., Wolf D., Birnbaum L. S. (2000). In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters postnatal development of seminal vesicle epithelium. Toxicol. Sci. 54, 424–430. [DOI] [PubMed] [Google Scholar]
- Ho R., Papp B., Hoffman J. A., Merrill B. J., Plath K. (2013). Stage-specific regulation of reprogramming to induced pluripotent stem cells by wnt signaling and T cell factor proteins. Cell Rep. 3, 2113–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Gao J. H., Liao Y. J., Tang S. J., Lu F. (2015). Tetrachlorodibenzo-p-dioxin delays palatal shelf elevation and suppresses Wnt5a and lymphoid enhancing-binding factor 1 signaling in developing palate. Cleft Palate. Craniofac. J. 52, 54–61. [DOI] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Hushka L. J., Williams J. S., Greenlee W. F. (1998). Characterization of 2,3,7,8-tetrachlorodibenzofuran-dependent suppression and AH receptor pathway gene expression in the developing mouse mammary gland. Toxicol. Appl. Pharmacol. 152, 200–210. [DOI] [PubMed] [Google Scholar]
- Katoh M., Katoh M. (2007a). WNT signaling pathway and stem cell signaling network. Clin. Cancer Res. 13, 4042–4045. [DOI] [PubMed] [Google Scholar]
- Katoh Y., Katoh M. (2007b). Conserved POU-binding site linked to SP1-binding site within FZD5 promoter: Transcriptional mechanisms of FZD5 in undifferentiated human ES cells, fetal liver/spleen, adult colon, pancreatic islet, and diffuse-type gastric cancer. Int. J. Oncol. 30, 751–755. [PubMed] [Google Scholar]
- Ko C. I., Fan Y., de Gannes M., Wang Q., Xia Y., Puga A. (2016). Repression of the aryl hydrocarbon receptor is required to maintain mitotic progression and prevent loss of pluripotency of embryonic stem cells. Stem Cells 34, 2825–2839. [DOI] [PubMed] [Google Scholar]
- Ko C. I., Puga A. (2017). Does the aryl hydrocarbon receptor regulate pluripotency? Curr. Opin. Toxicol. 2, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. I., Wang Q., Fan Y., Xia Y., Puga A. (2014). Pluripotency factors and polycomb group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells. Stem Cell Res. 12, 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi J., Huppunen J., Aarnisalo P. (2004). Regulation of the osteopontin gene by the orphan nuclear receptor NURR1 in osteoblasts. Mol. Endocrinol. 18, 1546–1557. [DOI] [PubMed] [Google Scholar]
- Liu X., Li X., Tao Y., Li N., Ji M., Zhang X., Chen Y., He Z., Yu K., Yu Z. (2020). TCDD inhibited the osteogenic differentiation of human fetal palatal mesenchymal cells through AhR and BMP-2/TGF-beta/Smad signaling. Toxicology 431, 152353. [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maijenburg M. W., Gilissen C., Melief S. M., Kleijer M., Weijer K., Ten Brinke A., Roelofs H., Van Tiel C. M., Veltman J. A., de Vries C. J. M., et al. (2012). Nuclear receptors Nur77 and Nurr1 modulate mesenchymal stromal cell migration. Stem Cells Dev. 21, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., de Crombrugghe B. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29. [DOI] [PubMed] [Google Scholar]
- Nottebrock C., Riecke K., Kruse M., Shakibaei M., Stahlmann R. (2006). Effects of 2,3,7,8-tetrachloro-dibenzo-p-dioxin on the extracellular matrix of the thymus in juvenile marmosets (Callithrix jacchus). Toxicology 226, 197–207. [DOI] [PubMed] [Google Scholar]
- Procházková J., Kabatkova M., Bryja V., Umannova L., Bernatik O., Kozubik A., Machala M., Vondracek J. (2011). The interplay of the aryl hydrocarbon receptor and beta-catenin alters both AhR-dependent transcription and wnt/beta-catenin signaling in liver progenitors. Toxicol. Sci. 122, 349–360. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2020). A Language and Environment for Statistical Computing. R Foundation for Statistical Computing [computer program; ], Vienna, Austria. [Google Scholar]
- Rojewski M. T., Weber B. M., Schrezenmeier H. (2008). Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus. Med. Hemother. 35, 168–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. P., Holz J. D., Mulcahey M., Sheu T. J., Gasiewicz T. A., Puzas J. E. (2007). Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. J. Bone Miner. Res. 22, 1571–1580. [DOI] [PubMed] [Google Scholar]
- Samee N., Geoffroy V., Marty C., Schiltz C., Vieux-Rochas M., Levi G., de Vernejoul M. C. (2008). Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am. J. Pathol. 173, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A. J., Branam A. M., Peterson R. E. (2014). Intersection of AHR and wnt signaling in development, health, and disease. Int. J. Mol. Sci. 15, 17852–17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. M., Jensen E. D., Westendorf J. J. (2005). Runx2: A master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. C Embryo Today 75, 213–225. [DOI] [PubMed] [Google Scholar]
- Simpson G. (1943). Mammals and the nature of continents. Am. J. Sci. 241, 1–31. [Google Scholar]
- Tao Y., Liu X., Cui L., Liu X., Chen Y., He Z., Ji M., Gao Z., Li N., Wan Z., et al. (2020). Oct4 plays a role in 2,3,7,8-tetrachlorobenzo-p-dioxin (TCDD) inducing cleft palate and inhibiting mesenchymal proliferation. Toxicology 438, 152444. [DOI] [PubMed] [Google Scholar]
- Tong Y., Niu M., Du Y., Mei W., Cao W., Dou Y., Yu H., Du X., Yuan H., Zhao W. (2017). Aryl hydrocarbon receptor suppresses the osteogenesis of mesenchymal stem cells in collagen-induced arthritic mice through the inhibition of beta-catenin. Exp. Cell Res. 350, 349–357. [DOI] [PubMed] [Google Scholar]
- Tsang H., Cheung T. Y., Kodithuwakku S. P., Chai J., Yeung W. S., Wong C. K., Lee K. F. (2012). 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses spheroids attachment on endometrial epithelial cells through the down-regulation of the wnt-signaling pathway. Reprod. Toxicol. 33, 60–66. [DOI] [PubMed] [Google Scholar]
- Vezina C. M., Hardin H. A., Moore R. W., Allgeier S. H., Peterson R. E. (2010). 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits fibroblast growth factor 10-induced prostatic bud formation in mouse urogenital sinus. Toxicol. Sci. 113, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Chen J., Ko C. I., Fan Y., Carreira V., Chen Y., Xia Y., Medvedovic M., Puga A. (2013). Disruption of aryl hydrocarbon receptor homeostatic levels during embryonic stem cell differentiation alters expression of homeobox transcription factors that control cardiomyogenesis. Environ. Health Perspect. 121, 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan Y., Puga A. (2010). Dioxin exposure disrupts the differentiation of mouse embryonic stem cells into cardiomyocytes. Toxicol. Sci. 115, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Kurita H., Carreira V., Ko C. I., Fan Y., Zhang X., Biesiada J., Medvedovic M., Puga A. (2016). Ah receptor activation by dioxin disrupts activin, BMP, and WNT signals during the early differentiation of mouse embryonic stem cells and inhibits cardiomyocyte functions. Toxicol. Sci. 149, 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li X., Yang J., Gong Y., Zhang H., Qiu X., Liu Y., Zhou C., Chen Y., Greenbaum J., et al. (2021). Single-cell RNA sequencing deconvolutes the in vivo heterogeneity of human bone marrow-derived mesenchymal stem cells. Int. J. Biol. Sci. 17, 4192–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. T. D., Nordberg R. C., Loboa E. G., Kullman S. W. (2019). Evidence for aryl hydrocarbon receptor-mediated inhibition of osteoblast differentiation in human mesenchymal stem cells. Toxicol. Sci. 167, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Amarachintha S., Xu J., Oley F., Du W. (2018). Mesenchymal COX2-PG secretome engages NR4A-WNT signalling axis in haematopoietic progenitors to suppress anti-leukaemia immunity. Br. J. Haematol. 183, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Shi H., Jiang X., Fan Y., Huang D., Qi X., Cheng Q. (2021). ZEB1 mediates bone marrow mesenchymal stem cell osteogenic differentiation partly via wnt/beta-catenin signaling. Front. Mol. Biosci. 8, 682728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C., Weiner J. A., Chun D. S., Yun J., Cook R. W., Schallmo M. S., Kannan A. S., Mitchell S. M., Freshman R. D., Park C., et al. (2017). Mechanistic insight into the effects of aryl hydrocarbon receptor activation on osteogenic differentiation. Bone Rep. 6, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Kanno Y., Nakayama M., Makimura M., Ohara S., Inouye Y. (2012). Activation of the aryl hydrocarbon receptor represses mammosphere formation in MCF-7 cells. Cancer Lett. 317, 192–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.