Abstract
Objective
Serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) and cystatin C (sCysC) are available clinically and beneficial in diagnosing acute kidney injury (AKI). Our purpose is to identify the performance of their combined diagnosis for AKI in critically ill patients.
Design
A prospectively recruited, observational study was performed.
Setting
Adults admitted to the intensive care unit of a tertiary hospital in China.
Participants
A total of 1222 critically ill patients were enrolled in the study.
Main outcome measures
To identify the performance of the combined diagnosis of serum NT-proBNP and sCysC for AKI in critically ill patients. The area under the receiver operating characteristic curve (AUC-ROC), category-free net reclassification index (NRI) and incremental discrimination improvement (IDI) were utilised for comparing the discriminative powers of a combined and single biomarker adjusted model of clinical variables enriched with NT-proBNP and sCysC for AKI.
Results
AKI was detected in 256 out of 1222 included patients (20.9%). AUC-ROC for NT-proBNP and sCysC to detect AKI had a significantly higher accuracy than any individual biomarker (p<0.05). After multivariate adjustment, a level of serum NT-proBNP ≥204 pg/mL was associated with 3.5-fold higher odds for AKI compared with those below the cut-off value. Similar results were obtained for sCysC levels (p<0.001). To detect AKI, adding NT-proBNP and sCysC to a clinical model further increased the AUC-ROC to 0.859 beyond that of the clinical model with or without sCysC (p<0.05). Moreover, the addition of these two to the clinical model significantly improved risk reclassification of AKI beyond that of the clinical model alone or with single biomarker (p<0.05), as measured by NRI and IDI.
Conclusions
In critically ill individuals, serum NT-proBNP, sCysC and clinical risk factors combination improve the discriminative power for diagnosing AKI.
Keywords: acute renal failure, adult intensive & critical care, nephrology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This prospective observational study established a cohort with an adequate sample size.
Rather than solely focusing on the effects of one biomarker, this study also assessed the combined efficacy of serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) and cystatin C (sCysC) in detection of acute kidney injury (AKI).
In this study, multiple logistic regression prediction models were established to detect AKI.
We evaluated the combined efficacy of serum NT-proBNP and sCysC in the diagnosis of AKI by calculating area under the curve and category-free net reclassification index and incremental discrimination improvement.
Dynamic assessment of NT-proBNP and sCysC changes was not available due to measured only once at admission.
Introduction
Acute kidney injury (AKI) is a predominant clinical syndrome affecting more than 50% of patients who underwent treatment at intensive care unit (ICU).1–3 Increased morbidity, mortality, hospitalisation length and cost are extremely related to AKI,4–6 so early recognition of AKI is critical to guiding management. Urine production and serum creatinine were employed as diagnostic criteria for AKI in accordance with the recommendations of Kidney Disease Improving Global Outcomes (KDIGO) guidelines.7 Changes in sCr or urine production cannot recognise early renal tubular injury prior to a reduction in glomerular filtration rate.8 9 Therefore, early and reliable AKI biomarkers are necessary to promote timely intervention and minimise complications. However, it is impossible for an individual biomarker to adequately evaluate the risk of AKI as a complex multifactorial syndrome.3 10 Combining diverse biomarkers in a clinical model evaluation could enhance early detection of AKI in critically ill patients.5 11 12
A haemodynamic marker stress, N-terminal pro-B-type natriuretic peptide (NT-proBNP), has recently received attention as a potential predictor of AKI in a wide diversity of clinical settings.13–17 High serum NT-proBNP level reflects haemodynamic instability, myocardial wall stress, myocardial ischaemia, volume overload, sympathetic nervous system and renin-angiotensin-aldosterone system activation, all of which may contribute to AKI incidence.18–20 However, there are finite data on clinical use of NT-proBNP for detecting AKI among critically ill patients.
Serum cystatin C (sCysC), a 122-amino acid low-molecular-weight protein (13 kDa), is a marker of glomerular filtration.9 CysC has a half-life of about one-third that of sCr, causing CysC to reach homeostasis three times faster. These properties promote sCysC as an alternative marker of renal function.8 To date, sCysC has been displayed to be conductive for the early identification of all-cause AKI.21
Although serum NT-proBNP and sCysC are mainly used for the prognosis assessment of heart disease and the prediction of acute cardiac events with AKI,22–26 the diagnostic accuracy of their combination for AKI in ICU remains unknown. We performed a prospective study in the present research to evaluate the performance of serum NT-proBNP and sCysC at ICU admission, both independently and in combination, for AKI determination among critically ill adults.
Methods
Study design and participants
At Guangdong Provincial People’s Hospital in China, a tertiary care hospital, a prospectively recruited observational research was performed in a mixed medical–surgical ICU. From December 2016 to December 2017, we consecutively enrolled patients aged 18 and up in a row. Pregnancy, renal replacement therapy prior to ICU admittance, nephrectomy, end-stage renal disease, renal transplant, rejection of consent or missing admission information were all exclusion criteria. The study protocol strengthened the reporting requirements of observational studies in epidemiology27 and standards for the reporting of diagnostic accuracy.28 This study protocol was authorised by ethics committee of Guangdong Provincial People’s Hospital. Additionally, all procedures were carried out consistent with applicable regulations and guidelines. All patients provided written informed consent.
Data collection
Clinical baseline data were collected prospectively. Within 1 hour following ICU admission, blood samples were collected contemporaneously to determine sCr, serum NT-proBNP and sCysC. Within 24 hours after collection, all samples were analysed in the Guangdong Provincial People’s Hospital central laboratory utilising standard protocol. When a patient is admitted to the ICU, sCr was measured and subsequently done at least once a day until discharge as part of routine clinical care. Throughout admission to ICU, urine production was also been recorded. We evaluated the following clinical variables: age, gender, body mass index, pre-existing clinical conditions, sepsis, admission type, baseline sCr, baseline estimated glomerular filtration rate (eGFR), Acute Physiology and Chronic Health Evaluation (APACHE) II score, ICU mortality, in-hospital mortality, duration of ICU admittance, length of hospitalisation, ICU costs and total costs. The outcome was the incidence of AKI following ICU enrolment within 1 week. To calculate eGFR, we employed the Chronic Kidney Disease (CKD) Epidemiology Collaboration creatinine equation.29
Definitions
The KDIGO classification criteria were utilised to define AKI: as a rise in sCr by ≥0.3 mg/dL (26.5 µmol/L) within 48 hours or a rise in sCr to ≥1.5 times the baseline within 1 week, or urine output <0.5 mL/kg/hour for 6 hour after ICU admission.7
Relying on following principles, ranked in descending order of preference, a baseline sCr was affirmed:30 (1) prior to ICU admittance, the most recent pre-ICU value between 30 and 365 days; (2) a stable pre-ICU value >365 days prior to ICU admittance for patients <40 years of age (stable definition is being within 15% of the lowest ICU measurement); (3) pre-ICU value >365 days prior to ICU admittance and lower than the initial sCr at ICU admission; (4) a pre-ICU value (within 3 and 39 days prior to ICU admittance) lower than or equal to the initial sCr on ICU admittance not obviously during AKI and (5) the least sCr value obtained at initial ICU admittance, the most recent ICU value or the lowest value achieved to a 365-day follow-up.
Biomarker measurement
The levels of sCysC and sCr were quantified through the UniCel D×C 800 Synchron system usage in compliance with the manufacturer’s instructions (Beckman Coulter, Brea, CA, USA). For sCysC, the intra-assay and interassay variation coefficients were 10% and 5%, respectively. Levels of serum NT-proBNP were quantified through an electrochemiluminescence immunoassay employing a Cobas e602 system usage (Roche Diagnostics, Germany). For NT-proBNP, the uppermost limit of normal for those who seem to be healthy (95th percentage) has been 125 pg/mL. The coefficient of interassay variation for NT-proBNP was <5%. Each patient’s clinical features were blinded to the personnel measuring the biomarkers.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Statistical analysis
For statistical analyses, we utilised SPSS V.21.0 (SPSS, Chicago, IL, USA), MedCalc V.18.2.1 (MedCalc Software, Ostend, Belgium) and R V.4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). The mean±SD or median (25th to 75th percentage, IQR) had been utilised to express continuous variables. Numbers (per cent) were utilised to represent categorical variables. For non-normally distributed continuous variables, the Wilcoxon rank-sum test was employed for intergroup difference measurement, and for categorical variables, the χ2 or Fisher’s exact test was deployed.
NT-proBNP concentrations were extremely skewed and therefore were log10 transformed before inclusion in the models. Areas under the receiver operating characteristic curves (AUC-ROCs) were computed. The method exploited by DeLong et al was used to compare AUC-ROCs between groups.31 The biomarkers’ sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios of the biomarkers were calculated. Youden’s index for AKI detection identified the optimal cut-off values for individual biomarkers and their combination.32
Logistic analysis was utilised to compute the ORs and 95% CIs for each factor: to identify the independent risk factors of AKI, with a forward stepwise method, in which the clinical variables with p<0.10 in univariate analysis were incorporated into the multivariate logistic model. We categorised NT-proBNP and sCysC levels according to their cut-off values and then performed logistic regression on the created variables. We conducted multiple logistic regression analyses to calculate the adjusted ORs of AKI, which was based on the clinical risk factors for AKI.
The performance of AKI detection after adding NT-proBNP and sCysC, or any of them, into the clinical model as categorical variables was assessed by AUC-ROC, category-free net reclassification improvement (NRI) index and integrated discrimination improvement (IDI) index, as described previously.33 34 To better quantify how accurately the reference and reclassification model would perform with independent data, we adopted a 10-fold cross-validation.35 All the tests were two-tailed, and p<0.05 was regarded statistically significant.
Results
Clinical data and outcomes
A total of 150 (10.9%) of the 1372 adult patients enrolled in the study were excluded (figure 1). Therefore, 1222 patients were enrolled and AKI occurred in 256 patients (20.9%). Patient baseline variables and outcomes are exhibited in table 1. In comparison to patients without AKI, patients with AKI were elderly and were observed more frequently in those with comorbidities, including CKD, hypertension, coronary artery disease (CAD), diabetes mellitus (DM), heart failure (HF), cerebrovascular disease and chronic obstructive pulmonary disease (COPD). Increased sCr, serum NT-proBNP and sCysC levels at admission, as well as increased APACHE II scores, were more prevalent in patients with AKI. The baseline sCr and eGFR did not show significant differences between the two groups.
Figure 1.
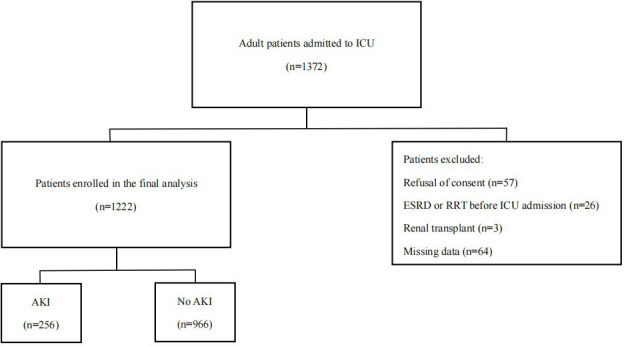
Flowchart for patient selection. AKI, acute kidney injury; ESRD, end-stage renal disease; ICU, intensive care unit; RRT, renal replacement therapy.
Table 1.
Clinical data and outcomes
| Characteristics | Total (n=1222) | AKI (n=256) | No AKI (n=966) | P value |
| Age, years | 57 (45–67) | 63.5 (52–72) | 55 (44–65) | <0.001 |
| Male, n (%) | 666 (54.5) | 151 (59.0) | 515 (53.3) | 0.105 |
| BMI, kg/m2 | 22 (19.6–24.7) | 22.3 (19.9–25.0) | 21.9 (19.6–24.6) | 0.395 |
| Pre-existing clinical conditions | ||||
| Hypertension, n (%) | 252 (20.6) | 81 (31.6) | 171 (17.7) | <0.001 |
| Diabetes mellitus, n (%) | 121 (9.9) | 52 (20.3) | 69 (7.1) | <0.001 |
| Chronic kidney disease, n (%) | 26 (2.1) | 21 (8.2) | 5 (0.5) | <0.001 |
| Cerebrovascular disease, n (%) | 127 (10.4) | 50 (19.5) | 77 (8.0) | <0.001 |
| Chronic obstructive pulmonary disease, n (%) | 35 (2.9) | 18 (7.0) | 17 (1.8) | <0.001 |
| Coronary artery disease, n (%) | 53 (4.3) | 24 (9.4) | 29 (3.0) | <0.001 |
| Heart failure, n (%) | 31 (2.5) | 22 (8.6) | 9 (0.9) | <0.001 |
| Cancer, n (%) | 227 (18.6) | 50 (19.5) | 177 (18.3) | 0.659 |
| Sepsis, n (%) | 98 (8.0) | 70 (27.3) | 28 (2.9) | <0.001 |
| Admission type, n (%) | <0.001 | |||
| Elective surgical, n (%) | 863 (70.6) | 84 (32.8) | 779 (80.6) | |
| Emergency surgical, n (%) | 83 (6.8) | 33 (12.9) | 50 (5.2) | |
| Medical, n (%) | 276 (22.6) | 139 (54.3) | 137 (14.2) | |
| Baseline serum creatinine, mg/dL | 0.78 (0.64–0.96) | 0.76 (0.58–1.09) | 0.79 (0.65–0.94) | 0.949 |
| Baseline eGFR, mL/min/1.73 m2 | 119.4 (97.6–145.4) | 122.9 (83.3–158.7) | 118.7 (100.4–142.9) | 0.950 |
| Parameters at ICU admission | ||||
| sCr, mg/dL | 0.87 (0.72–1.07) | 0.97 (0.72–1.55) | 0.85 (0.72–1.02) | <0.001 |
| NT-proBNP, pg/mL | 97.1 (29.4–464.4) | 871.0 (225.8–2919.3) | 66.2 (23.7–206.2) | <0.001 |
| sCysC, mg/L | 0.78 (0.61–1.06) | 1.15 (0.83–1.85) | 0.75 (0.58–0.94) | <0.001 |
| APACHE Ⅱ score | 7 (4–10) | 11 (8–16) | 6 (4–9) | <0.001 |
| UP, mL/kg/hour | 1.67 (1.20–2.26) | 1.37 (0.89–2.20) | 1.73 (1.26–2.27) | <0.001 |
| Outcomes | ||||
| ICU mortality, n (%) | 40 (3.3) | 33 (12.9) | 7 (0.7) | <0.001 |
| In-hospital mortality, n (%) | 53 (4.3) | 40 (15.6) | 13 (1.3) | <0.001 |
| ICU stay, days | 2 (2–4) | 6 (3–13.3) | 2 (2–3) | <0.001 |
| Hospital stay, days | 16 (12–23) | 22 (13–33.3) | 15 (11–21) | <0.001 |
| ICU costs, CNY | 39 461.7 (28 966.2–59 437.2) | 66 140.1 (36 526.0–131 843.8) | 37 667.5 (27 881.5–48 238.4) | <0.001 |
| Total costs, CNY | 58 295.3 (44 201.6–98 602.8) | 128 945.6 (71 506.8–199 685.4) | 52 773.8 (40 856.2–78 718.9) | <0.001 |
Continuous variables are expressed as mean±SD or median (25th to 75th percentile, IQR). Categorical variables are expressed as a n (%).
AKI, acute kidney injury; BMI, body mass index; CNY, Chinese yuan; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; NT-proBNP, N-terminal pro-B-type natriuretic peptide; APACHE Ⅱ score, Acute Physiology and Chronic Health Evaluation Ⅱ score; sCr, serum creatinine; sCysC, serum cystatin C; UP, urine production first 24 hours after admission.
Indeed, patients with AKI had a higher risk of adverse outcomes, a higher percentage of ICU and in-hospital mortality, higher expenses, a longer hospitalisation duration and a longer stay in ICU (p<0.001) compared with those without AKI.
Detective abilities of the two biomarkers for AKI
To demonstrate the ability of these biomarkers for AKI detection, we used AUC-ROCs to calculate the two biomarkers, respectively, and in combination. AUC-ROCs for NT-proBNP and sCysC were computed for AKI detection (0.821 and 0.766, respectively). For AKI detection, NT-proBNP had a sensitivity of 78% and a specificity of 75%, while sCysC had high specificity but limited sensitivity. The cut-off values for NT-proBNP and sCysC were 204 pg/mL and 1.02 mg/L, respectively, yielding good sensitivity and specificity. We included NT-proBNP and sCysC in a multivariate logistic regression model to derive their combined AUC for comparison with the single biomarkers. The AUC-ROCs for AKI presented a better performance by NT-proBNP and sCysC (0.832) than any individual biomarker (p<0.05, table 2 and figure 2).
Table 2.
Detective characteristics of the two biomarkers for AKI
| Logistic regression models | AUC-ROC* (95% CI) | Cut-off† | Sensitivity | Specificity | (+) LR | (−) LR | PPV | NPV |
| sCysC | 0.766 (0.741 to 0.789) | 1.02 mg/L | 0.66 | 0.80 | 3.28 | 0.43 | 0.47 | 0.90 |
| NT-proBNP | 0.821 (0.799 to 0.842) ‡ | 204.00 pg/mL | 0.78 | 0.75 | 3.12 | 0.29 | 0.45 | 0.93 |
| NT-proBNP+sCysC | 0.832 (0.809 to 0.852) § | 0.15¶ | 0.84 | 0.69 | 2.69 | 0.23 | 0.42 | 0.94 |
*Values are presented as AUC-ROC (95% CI).
†Ideal cut-off value according to Youden’s index.
‡P<0.05 vs sCysC (p=0.0011).
§P<0.05 vs NT-proBNP (p=0.0145), sCysC(p<0.0001).
¶Cut-off points of the biomarker panels were the predicted probabilities generated from the multiple logistic regression model.
AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curve; (+) LR, positive likelihood ratio; (-) LR, negative likelihood ratio; NPV, negative predictive value; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PPV, positive predictive value; sCysC, serum cystatin C.
Figure 2.
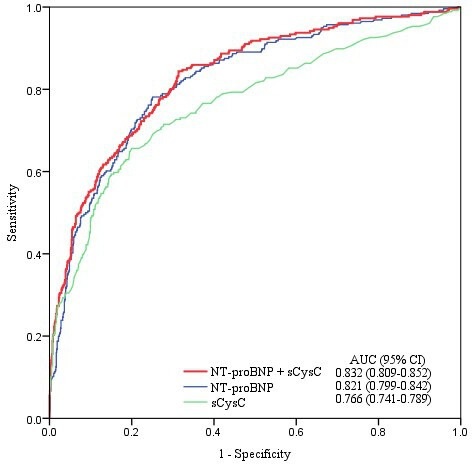
ROC analysis of the two biomarkers for AKI detection. Among 1222 patients, 256 were diagnosed as AKI. AKI, acute kidney injury; AUC, area under the receiver operating characteristic curve; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ROC, receiver operating characteristic; sCysC, serum cystatin C.
Multivariate logistic regression analyses of the two biomarkers for AKI detection
We stratified patients based on the two cut-off values of serum NT-proBNP and sCysC levels into two categories, respectively. Compared with those with serum NT-proBNP <204 pg/mL, patients with serum NT-proBNP ≥204 pg/mL on admission exhibited a higher incidence of AKI (7.2% vs 45.1%, p<0.001). AKI was found to have higher incidence (46.5%) in patients with sCysC ≥1.02 mg/L compared with those with sCysC <1.02 mg/L (10.2%) (p<0.001). Following clinical variables adjustment (including CKD, HF, sepsis, admission type, sCr at admission and APACHE II scores, table 3), a level of serum NT-proBNP ≥204 pg/mL was associated with 3.5-fold higher odds for AKI compared with NT-proBNP level below the cut-off value (p<0.001). Similarly, patients with sCysC ≥1.02 mg/L were linked to 2.6-fold greater odds for AKI compared with those with sCysC <1.02 mg/L (p<0.001) (table 4).
Table 3.
Logistic analyses of clinical risk factors for AKI detection
| Variables | Univariate analysis | Multivariate model | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.029 (1.019 to 1.038) | <0.001 | ||
| Hypertension | 2.152 (1.577 to 2.937) | <0.001 | ||
| Diabetes mellitus | 3.314 (2.242 to 4.898) | <0.001 | ||
| Chronic kidney disease | 17.175 (6.410 to 46.023) | <0.001 | 4.182 (1.250 to 13.998) | 0.020 |
| Cerebrovascular disease | 2.802 (1.903 to 4.126) | <0.001 | ||
| Chronic obstructive pulmonary disease | 4.222 (2.143 to 8.316) | <0.001 | ||
| Coronary artery disease | 3.342 (1.910 to 5.849) | <0.001 | ||
| Heart failure | 9.997 (4.544 to 21.997) | <0.001 | 3.487 (1.365 to 8.911) | 0.009 |
| Sepsis | 12.608 (7.914 to 20.084) | <0.001 | 5.033 (2.914 to 8.692) | <0.001 |
| Admission type | <0.001 | <0.001 | ||
| Elective surgical | 1.0 (referent) | 1.0 (referent) | ||
| Emergency surgical | 6.121 (3.735 to 10.030) | <0.001 | 3.493 (2.023 to 6.032) | <0.001 |
| Medical | 9.409 (6.791 to 13.037) | <0.001 | 3.237 (2.157 to 4.858) | <0.001 |
| sCr | 3.135 (2.385 to 4.120) | <0.001 | 1.538 (1.149 to 2.058) | 0.004 |
| APACHE Ⅱ score | 1.215 (1.182 to 1.249) | <0.001 | 1.101 (1.066 to 1.137) | <0.001 |
AKI, acute kidney injury; APACHEⅡ score, Acute Physiology and Chronic Health Evaluation Ⅱ score; sCr, serum creatinine.
Table 4.
Multivariate logistic regression analyses of the two biomarkers for AKI detection
| Variables | AKI, % | Unadjusted OR (95% CI) | P value | Adjusted OR* (95% CI) | P value |
| NT-proBNP (pg/mL, n) | |||||
| <204, 779 | 7.2 | 1.0 (referent) | 1.0 (referent) | ||
| ≥204, 443 | 45.1 | 10.626 (7.639 to 14.781) | <0.001 | 3.460 (2.307 to 5.189) | <0.001 |
| sCysC layered by the cut-off value (mg/L, n) | |||||
| <1.02, 861 | 10.2 | 1.0 (referent) | 1.0 (referent) | ||
| ≥1.02, 361 | 46.5 | 7.646 (5.651 to 10.345) | <0.001 | 2.649 (1.842 to 3.810) | <0.001 |
*Adjusted for chronic kidney disease, heart failure, sepsis, admission type, serum creatinine at admission and Acute Physiology and Chronic Health Evaluation Ⅱ score.
AKI, acute kidney injury; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sCysC, serum cystatin C.
Discrimination and reclassification of the combination of biomarkers and clinical models for AKI
Considering the effect of adding NT-proBNP and sCysC, or any of them, to a clinical model as categorical variables for AKI detection, logistic regression analysis was employed. On ICU admittance for AKI diagnosis, potential available variables including age, sepsis, admission type, sCr and APACHE II scores as well as comorbidities, including hypertension, DM, CKD, cerebrovascular disease, COPD, CAD and HF were considered. The clinical model for detecting AKI involved CKD, HF, sepsis, admission type, sCr at admission and APACHE II scores (table 3). The cross-validated baseline performance characterised by accuracy and Kappa for the clinical model was 0.844 and 0.448.
To evaluate the enhancement of discriminative capacity, a panel of NT-proBNP and sCysC was introduced to the above-mentioned model. As shown in table 5, compared with the clinical model, the addition of NT-proBNP to the clinical model had a higher AUC-ROC (p<0.05), and no statistically significant variation existed when sCyC was added to the clinical model. However, the risk reclassification was markedly improved through the addition of NT-proBNP or sCyC to the clinical model, as measured by category-free NRI and IDI (p<0.05). Adding NT-proBNP and sCysC to a clinical model for AKI detection further increased the AUC-ROC to 0.859 beyond that of the clinical model with or without sCysC (p<0.05) (figure 3). The cross-validated baseline performance characterised by accuracy and Kappa for the clinical model enriched with NT-proBNP and sCysC was 0.848 and 0.475. Moreover, this panel addition to the clinical model significantly enhanced the risk reclassification of AKI beyond that of the clinical model with or without any individual biomarkers (p<0.05), with maximum NRI (0.531) and IDI (0.038).
Table 5.
Discrimination and reclassification of the combination of biomarkers and clinical model for AKI
| AUC-ROC (95% CI) | P value* | Category-free NRI (95% CI) | P value* | IDI (95% CI) | P value* | |
| Clinical model† | 0.840 (0.812 to 0.868) | |||||
| Clinical model+sCysC | 0.847 (0.819 to 0.874) | 0.163 | 0.193 (0.052 to 0.405) | 0.036 | 0.017 (0.009 to 0.026) | <0.001 |
| Clinical model+NT-proBNP | 0.855 (0.828 to 0.882) | 0.013 | 0.462 (0.196 to 0.747) | 0.001 | 0.028 (0.016 to 0.039) | <0.001 |
| Clinical model+NT-proBNP+sCysC | 0.859 (0.832 to 0.885) | 0.006 | 0.531 (0.238 to 0.741) | <0.001 | 0.038 (0.025 to 0.051) | <0.001 |
| Clinical model+NT-proBNP+sCysC vs clinical model+sCysC | – | 0.015 | 0.328 (0.139 to 0.553) | 0.002 | 0.021 (0.010 to 0.031) | <0.001 |
| Clinical model+NT-proBNP+sCysC vs clinical model+NT-proBNP | – | 0.223 | 0.167 (0.013 to 0.285) | 0.017 | 0.011 (0.004 to 0.018) | 0.003 |
Hosmer–Lemeshow goodness-of-fit test: for clinical model, χ2 value=14.249(p=0.075); for clinical model+sCysC, χ2 value=6.971 (p=0.54); for clinical model+NT-proBNP, χ2 value=9.362 (p=0.313) or clinical model+NT-proBNP+sCysC, χ2 value=4.245 (p=0.834).
*Biomarker+clinical model vs clinical model.
†The clinical model for detecting AKI is composed of chronic kidney disease, heart failure, sepsis, admission type, serum creatinine at admission and Acute Physiology and Chronic Health Evaluation Ⅱ score.
AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curve; IDI, incremental discrimination improvement; NRI, net reclassification index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sCysC, serum cystatin C.
Figure 3.
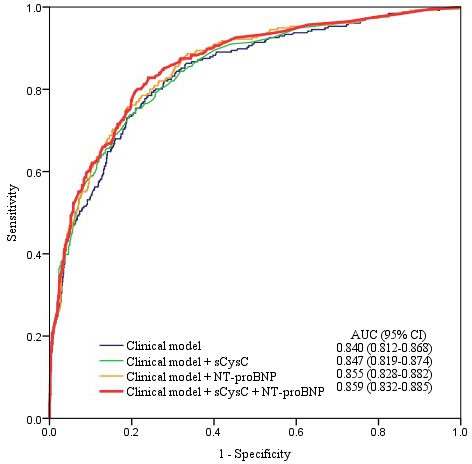
ROC analysis when two biomarkers were added to the clinical model for AKI detection. Among 1222 patients, 256 were diagnosed as AKI. AKI, acute kidney injury; AUC, area under the receiver operating characteristic curve; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ROC, receiver operating characteristic; sCysC, serum cystatin C.
Discussion
The study’s key finding was that the combination of NT-proBNP and sCysC yields greater discriminative ability for AKI detection at ICU admission with or without a clinical model in critically ill adults. The finding indicates that assessing both serum NT-proBNP and sCysC levels on admission may assist with the early diagnosis and risk stratification of AKI in critically ill adults.
One of the most prevalent complications occurring in a variety of clinical settings is AKI, especially for critically ill patients.4 6 36 37 The development of AKI, as the same as its severity, is strongly associated with increased mortality.3 36 However, early identification is challenging when sCr or urine production changes are used to detect AKI,38 and precise clinical predictors are not widely known. Numerous studies have found and confirmed the accuracy and additional clinical benefits of these renal biomarkers for early AKI diagnosis, such as insulin-like growth factor-binding protein, matrix metalloproteinase-7, tissue inhibitor metalloproteinase-2, angiotensinogen, sCysC and neutrophil gelatinase-associated lipocalin.5 9 12 39 40 However, some novel biomarkers are not being used in clinical practice, due to insufficient evidence or previously unavailable commercially. Thus, the rational application of clinically available biomarkers is more practical and economical.
NT-proBNP, a widely used marker of haemodynamic stress, is a polypeptide secreted by the ventricles and its role is to facilitate natriuresis.17 41 In patients with elevated central venous pressure (CVP), the raised pressure may be transmitted to the renal veins, resulting in renal congestion and reduced glomerular filtration.14 42 43 It makes sense that NT-proBNP is associated with the development of AKI. The clinical application of serum NT-proBNP in cardiac disease has been extensively confirmed and finds its predictive value for AKI development in patients with HF, coronary angiography or percutaneous coronary intervention.19 23 44 45 Several studies have elucidated the link between NT-proBNP and AKI incidence after cardiac or non-cardiac surgery.13 46–48 However, its utility for AKI detection has not been fully evaluated in general ICU, and it is necessary to verify the reliability and universality of NT-proBNP in heterogeneous populations. The data demonstrated here both maintain NT-proBNP as a risk factor for AKI and also indicate that it can enhance the risk reclassification and discrimination for AKI in ICU.
A single biomarker is insufficient to express the multiple pathophysiological mechanisms of AKI, especially for critically ill patients, as AKI is a heterogeneous syndrome.9 10 There is no consensus on which specific markers should be combined to detect AKI. Numerous studies have revealed that combining different biomarkers utilised to detect AKI can improve predictive abilities.5 49 50 Naruse et al showed that combining urinary liver-type fatty-acid binding protein and serum NT-proBNP can enhance early prediction of AKI in patients in medical cardiac ICUs.15 Similar to such studies, we identified that the combination of two markers, serum NT-proBNP and sCysC, improved the diagnostic performance of AKI.
CysC is a glomerular filtration biomarker that can be utilised to anticipate the development of AKI and undesirable outcomes.8 9 The application and performance of CysC for AKI prediction have been demonstrated in various clinical settings.5 11 51–53 In individuals with ST-segment elevation myocardial infarction, a combination of B-type natriuretic peptide and CysC may contribute to risk stratification for AKI.25 In the present cohort, sCysC had a slightly higher specificity than NT-proBNP in the detection of AKI, but its sensitivity is limited. The ability of sCysC for detecting AKI was fine, but its AUC-ROC was not as great as NT-proBNP. Moreover, the combination of serum NT-proBNP and sCysC at ICU admission had the highest AUC-ROC. These data indicate that the simultaneous measurement of serum NT-proBNP and sCysC at ICU admission could improve the early identification of AKI.
Biomarkers for AKI, which indicates the various underlying pathophysiological mechanisms involved in AKI incidence, might be superior to individual biomarkers alone.3 6 It is also important that these biomarkers profit from being easily measurable, readily accessible, comparatively cheap and with an elevated level of sensitivity and specificity. In the present study, for AKI detection, NT-proBNP acted as a haemodynamic stress biomarker with high sensitivity and specificity, and sCysC had high specificity as a functional biomarker. Even after clinical risk variables adjustment, elevated NT-proBNP is an independent risk factor for AKI. The NT-proBNP addition to the clinical model significantly enhanced risk reclassification, as demonstrated by category-free NRI and IDI. Moreover, our results identified that the addition of NT-proBNP to sCysC markedly improved their detective abilities as biomarkers. Adding biomarkers to the clinical model further improved the diagnostic accuracy of AKI, as measured by AUC-ROCs. These data suggest that a single biomarker is insufficient for early diagnosis of AKI. Therefore, the method of combining different biomarkers may be of greater use.
There are some limitations for this study. First, it was a single-centre study, with an unproven external validity. Second, there were only 26 patients with CKD and 31 patients with HF enrolled, and hence we were unable to stratify our group relying on eGFR or cardiac function at baseline. Accordingly, future studies should be administrated in these subgroups. Last, we did not accomplish routine echocardiography in all patients to associate NT-proBNP levels with ventricular dilatation or other pathways that may promote NT-proBNP release. Despite these limitations, we believe that our findings have clinical implications and should facilitate further research to confirm our results.
Conclusion
In the present cohort, simultaneous measurement of NT-proBNP and sCysC at ICU admission increases the early identification of AKI beyond that of biomarker in isolation, and that the combination of the two biomarkers and clinical risk factors improves the discriminative ability for AKI detection in critically ill adults.
Supplementary Material
Acknowledgments
The authors thank all the doctors, nurses, technicians and patients at the Guangdong Provincial People’s Hospital for their dedication in the study.
Footnotes
JD, LH, YL and LH contributed equally.
Contributors: JD, LLH, YFL and LHH equally contributed to the design of the research, analysis and interpretation of the data. CBC and JD contributed to the conception and design of the research as well as interpretation of the data, and critically revised the manuscript. JD, LLH, YFL, LHH, JX, HF and YL performed the research and collected data. All authors contributed to the acquisition and analysis of the data, drafted the manuscript and agreed to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript. CBC, as the guarantor, accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The study was supported by Chunbo Chen’s research grants from the National Natural Science Foundation of China (82172162), the Major Programme of Summit Project, Guangdong Province High-level Hospital Construction Project of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (DFJH2020028) and the Office of Talent Work Leading Group in Maoming (number of MaoRenCaiBan (2020)24).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets generated and/or analyzed during this study are not publicly available, owing to currently ongoing research studies, but the data are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. The ethics committee of the Guangdong Provincial People’s Hospital approved the study design, protocol, ethical issue and data and sample collection (No. GDREC2015396H(R1)). Participants gave informed consent to participate in the study before taking part.
References
- 1.Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. 10.1007/s00134-015-3934-7 [DOI] [PubMed] [Google Scholar]
- 2.Srisawat N, Kulvichit W, Mahamitra N, et al. The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: a prospective multicentre study. Nephrol Dial Transplant 2020;35:1729–38. 10.1093/ndt/gfz087 [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394:1949–64.:S0140-6736(19)32563-2. 10.1016/S0140-6736(19)32563-2 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, James MT. Acute kidney injury. Ann Intern Med 2017;167:ITC66–80. 10.7326/AITC201711070 [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Chi R, Chen S, et al. Evaluation of clinically available renal biomarkers in critically ill adults: a prospective multicenter observational study. Crit Care 2017;21:46. 10.1186/s13054-017-1626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu KD, Goldstein SL, Vijayan A, et al. Aki! now initiative: recommendations for awareness, recognition, and management of AKI. Clin J Am Soc Nephrol 2020;15:1838–47. 10.2215/CJN.15611219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury; 2012.
- 8.Teo SH, Endre ZH. Biomarkers in acute kidney injury (AKI). Best Pract Res Clin Anaesthesiol 2017;31:331–44.:S1521-6896(17)30076-9. 10.1016/j.bpa.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Albert C, Haase M, Albert A, et al. Biomarker-Guided risk assessment for acute kidney injury: time for clinical implementation? Ann Lab Med 2021;41:1–15. 10.3343/alm.2021.41.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: Workgroup statements from the tenth acute dialysis quality initiative consensus conference. Contrib Nephrol 2013;182:13–29. 10.1159/000349963 [DOI] [PubMed] [Google Scholar]
- 11.Deng Y, Ma J, Hou Y, et al. Combining serum cystatin C and urinary N-acetyl-beta-D-glucosaminidase improves the precision for acute kidney injury diagnosis after resection of intracranial space-occupying lesions. Kidney Blood Press Res 2020;45:142–56. 10.1159/000504599 [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Chen C, Tian J, et al. Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: a prospective, two-stage study. J Am Soc Nephrol 2015;26:2032–41. 10.1681/ASN.2014040408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardinale D, Cosentino N, Moltrasio M, et al. Acute kidney injury after lung cancer surgery: incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide. Lung Cancer 2018;123:155–9.:S0169-5002(18)30469-0. 10.1016/j.lungcan.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 14.Haines R, Crichton S, Wilson J, et al. Cardiac biomarkers are associated with maximum stage of acute kidney injury in critically ill patients: a prospective analysis. Crit Care 2017;21:88. 10.1186/s13054-017-1674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naruse H, Ishii J, Takahashi H, et al. Predicting acute kidney injury using urinary liver-type fatty-acid binding protein and serum N-terminal pro-B-type natriuretic peptide levels in patients treated at medical cardiac intensive care units. Crit Care 2018;22:197. 10.1186/s13054-018-2120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaub JA, Coca SG, Moledina DG, et al. Amino-Terminal pro-B-type natriuretic peptide for diagnosis and prognosis in patients with renal dysfunction: a systematic review and meta-analysis. JACC Heart Fail 2015;3:977–89.:S2213-1779(15)00619-8. 10.1016/j.jchf.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiese S, Breyer T, Dragu A, et al. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation 2000;102:3074–9. 10.1161/01.cir.102.25.3074 [DOI] [PubMed] [Google Scholar]
- 18.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015;36:1437–44. 10.1093/eurheartj/ehv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goussot S, Mousson C, Guenancia C, et al. N-Terminal fragment of pro B-type natriuretic peptide as a marker of contrast-induced nephropathy after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol 2015;116:865–71.:S0002-9149(15)01521-0. 10.1016/j.amjcard.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 20.Yamashita T, Seino Y, Ogawa A, et al. N-Terminal Pro-BNP is a novel biomarker for integrated cardio-renal burden and early risk stratification in patients admitted for cardiac emergency. J Cardiol 2010;55:377–83. 10.1016/j.jjcc.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 21.Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int 2004;66:1115–22. 10.1111/j.1523-1755.2004.00861.x [DOI] [PubMed] [Google Scholar]
- 22.Carrasco-Sánchez FJ, Pérez-Calvo JI, Morales-Rull JL, et al. Heart failure mortality according to acute variations in N-terminal pro B-type natriuretic peptide and cystatin C levels. J Cardiovasc Med (Hagerstown) 2014;15:115–21. 10.2459/JCM.0b013e3283654bab [DOI] [PubMed] [Google Scholar]
- 23.Linzbach S, Samigullin A, Yilmaz S, et al. Role of N-terminal pro-brain natriuretic peptide and cystatin C to estimate renal function in patients with and without heart failure. Am J Cardiol 2009;103:1128–33. 10.1016/j.amjcard.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 24.Ruan ZB, Zhu L, Yin YG, et al. Cystatin C, N-terminal probrain natriuretic peptides and outcomes in acute heart failure with acute kidney injury in a 12-month follow-up: insights into the cardiorenal syndrome. J Res Med Sci 2014;19:404–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Tung Y-C, Chang C-H, Chen Y-C, et al. Combined biomarker analysis for risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. PLoS One 2015;10:e0125282. 10.1371/journal.pone.0125282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Han S, Tong F, et al. Predictive value of the serum cystatin C/prealbumin ratio in combination with NT-proBNP levels for long-term prognosis in chronic heart failure patients: a retrospective cohort study. Front Cardiovasc Med 2021;8:684919. 10.3389/fcvm.2021.684919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 28.Bossuyt PM, Reitsma JB, Bruns DE, et al. Toward complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Acad Radiol 2003;10:664–9. 10.1016/s1076-6332(03)80086-7 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endre ZH, Walker RJ, Pickering JW, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int 2010;77:1020–30. 10.1038/ki.2010.25 [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 32.YOUDEN WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 33.Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 2008;54:17–23. 10.1373/clinchem.2007.096529 [DOI] [PubMed] [Google Scholar]
- 34.Pencina MJ, D’Agostino RB, D’Agostino RB, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 35.Albert C, Haase M, Albert A, et al. Urinary biomarkers may complement the Cleveland score for prediction of adverse kidney events after cardiac surgery: a pilot study. Ann Lab Med 2020;40:131–41. 10.3343/alm.2020.40.2.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015;10:1510–8. 10.2215/CJN.02140215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Nie S, Zhang A, et al. Acute kidney injury among hospitalized children in China. Clin J Am Soc Nephrol 2018;13:1791–800. 10.2215/CJN.00800118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins CB, De Bels D, Honore PM, et al. Early prediction of acute kidney injury by machine learning: should we add the urine output criterion to improve this new tool? J Transl Int Med 2020;8:201–2. 10.2478/jtim-2020-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C, Yang X, Lei Y, et al. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol 2016;11:1536–44. 10.2215/CJN.00910116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Chen C, Teng S, et al. Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3373–82. 10.1681/ASN.2017020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–68. 10.1016/j.jacc.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 42.Gambardella I, Gaudino M, Ronco C, et al. Congestive kidney failure in cardiac surgery: the relationship between central venous pressure and acute kidney injury. Interact Cardiovasc Thorac Surg 2016;23:800–5. 10.1093/icvts/ivw229 [DOI] [PubMed] [Google Scholar]
- 43.Redant S, Honoré PM, De Bels D. Fifty shades of central venous pressure in the cardiorenal syndrome. J Transl Int Med 2020;8:1–2. 10.2478/jtim-2020-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Liu C, Mao Z, et al. Brain natriuretic peptide for predicting contrast-induced acute kidney injury in patients with acute coronary syndrome undergoing coronary angiography: a systematic review and meta-analysis. J Interv Cardiol 2020;2020:1035089. 10.1155/2020/1035089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin KY, Wu ZY, You ZB, et al. Pre-procedural N-terminal pro-B type natriuretic peptide predicts contrast-induced acute kidney injury and long-term outcome in elderly patients after elective percutaneous coronary intervention. Int Heart J 2018;59:926–34. 10.1536/ihj.17-573 [DOI] [PubMed] [Google Scholar]
- 46.Greenberg JH, Parsons M, Zappitelli M, et al. Cardiac biomarkers for risk stratification of acute kidney injury after pediatric cardiac surgery. Ann Thorac Surg 2021;111:191–8.:S0003-4975(20)30506-3. 10.1016/j.athoracsur.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel UD, Garg AX, Krumholz HM, et al. Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgery. Circulation 2012;125:1347–55. 10.1161/CIRCULATIONAHA.111.029686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao BC, Zhuang PP, Lei SH, et al. Pre-Operative N-terminal pro-B-type natriuretic peptide for prediction of acute kidney injury after noncardiac surgery: a retrospective cohort study. Eur J Anaesthesiol 2021;38:591–9. 10.1097/EJA.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 49.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014;64:2753–62.:S0735-1097(14)06685-6. 10.1016/j.jacc.2014.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang C-H, Chang C-H, Chen T-H, et al. Combination of urinary biomarkers improves early detection of acute kidney injury in patients with heart failure. Circ J 2016;80:1017–23. 10.1253/circj.CJ-15-0886 [DOI] [PubMed] [Google Scholar]
- 51.Deng Y, Wang L, Hou Y, et al. The influence of glycemic status on the performance of cystatin C for acute kidney injury detection in the critically ill. Ren Fail 2019;41:139–49. 10.1080/0886022X.2019.1586722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang S, Shi M, Bai Y, et al. The effect of glucocorticoids on serum cystatin C in identifying acute kidney injury: a propensity-matched cohort study. BMC Nephrol 2020;21:519. 10.1186/s12882-020-02165-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang D, Gao L, Ye H, et al. Impact of thyroid function on cystatin C in detecting acute kidney injury: a prospective, observational study. BMC Nephrol 2019;20:41. 10.1186/s12882-019-1201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The datasets generated and/or analyzed during this study are not publicly available, owing to currently ongoing research studies, but the data are available from the corresponding author on reasonable request.


