Abstract
Background:
Racial disparities in childhood asthma outcomes result from a complex interplay of individual- and neighborhood-level factors.
Objectives:
To examine racial disparities in asthma-related emergency department (ED) visits between African American (AA) and European American (EA) children.
Methods:
This is a retrospective study of patients <18 years who visited the ED at Cincinnati Children’s for asthma, 2009–2018. The outcome was number of ED visits during a year. We assessed 11 social, economic, and environmental variables. Mediation and mixed-effects analyses were used to assess relationships between race, mediators, and number of ED visits.
Results:
A total of 31,114 children (46.1% AA, 53.9% EA) had 186,779 asthma-related ED visits. AA children had more visits per year than EA children (2.23 vs 2.15; p<0.001). Medicaid insurance was associated with a 7% increase in ED visits compared with commercial insurance (1.07; 95% Confidence interval [CI]=1.03–1.1). Neighborhood socioeconomic deprivation was associated with an increased rate of ED visits in AA, not EA children. Area-level PM2.5, pollen, and outdoor mold were associated with an increased rate of ED visits for both AA and EA children (all p<0.001). Associations between race and number of ED visits were mediated by insurance (standardized coefficient [sβ]=0.048; p<0.001) and area-level deprivation (sβ=0.084; p<0.001), PM2.5 (sβ=0.002; p<0.001), and outdoor mold (sβ=0.006; p<0.001), altogether accounting for 55% of race’s effect on ED visits. Race was not associated with number of ED visits (sβ=0.006; p=0.796) after accounting for mediators.
Conclusions:
Racial disparities in asthma-related ED visits are mediated by social, economic, and environmental factors which may be amenable to interventions aimed at improving outcomes and eliminating inequities.
Keywords: Asthma, racial disparities, mixed-effects analysis, mediation analysis, electronic health records, environmental exposure
Capsule summary
We determine the effect of a range of individual- and neighborhood-level factors on racial disparities for asthma-related ED visits using mediation and mixed-effects analyses.
Graphical Abstract
INTRODUCTION
Asthma is the most common non-communicable chronic disease of childhood.(1) It is the third ranking cause of hospitalization among children less than 18 years of age, and it is a leading cause of school absences.(2) The cost burden for the U.S. healthcare system alone surpassed $82 billion in 2019.(3) However, despite clinical advances, asthma prevalence, morbidity, and mortality all continue to be higher for children who identify as Black or African American (AA) compared to those who identify as white or European American (EA).(4) Hospitalization or death due to asthma are four and seven times higher in AAs than EAs, respectively.(5)
Previous studies have evaluated links between racial disparities in asthma outcomes and a range of risk factors. Hill et al reviewed the contribution of biological and environmental triggers to racial disparities among asthmatic children, suggesting these disparities are the result of gene-environment interactions, hardships, and ethnicity.(6) At a neighborhood-level, DePriest et al found that less greenspace and more violence were associated with increased asthma risk in AA children.(7) Keet et al found that living in poor, urban areas was a risk factor for child asthma morbidity.(8) More recently, Grunwell et al., found that neighborhood with high social vulnerability levels resulted in environmental triggers, affecting pediatric intensive care admissions in asthma.(9)
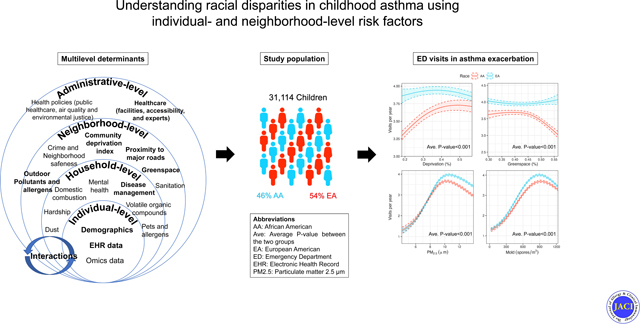
Despite this growing evidence, the study of multilevel asthma risk factors has been more limited, with scarce information available on the contributions and interplay between both individual and neighborhood-level exposures.(10) This limitation on understanding multilevel exposures may affect the unique position of physicians and policymakers to address modifiable individual- and neighborhood-level determinants of both asthma outcomes and disparities in those outcomes. Therefore, we sought to evaluate the role of asthma-associated risk factors, across multiple levels, in association with rate of asthma-related emergency department (ED) visits, using mediation and mixed effects negative binomial regression analyses. Specifically, we sought to examine differences in rate of ED visits among AA and EA children, considering a range of potentially underlying social, economic, and environmental exposures across individual and neighborhood levels.
METHODS
Study population
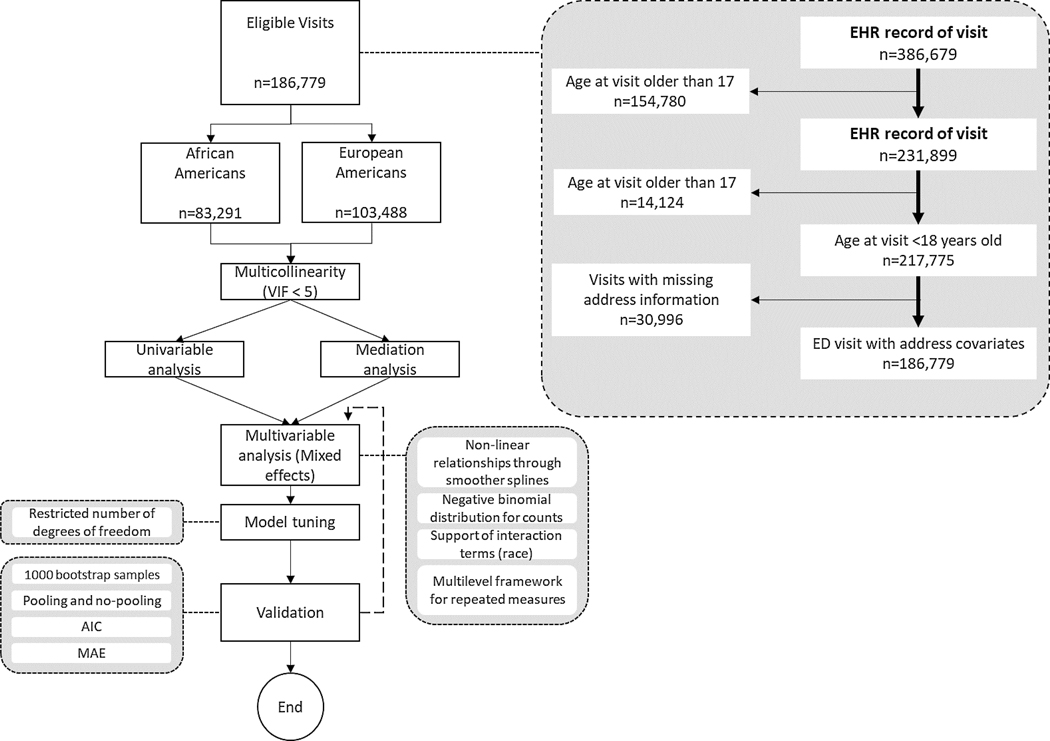
We conducted a retrospective population-based cohort study using data from and linked to Cincinnati Children’s Hospital Medical Center’s (CCHMC) electronic health record (EHR). CCHMC is an urban academic medical center in Southwestern Ohio serving residents of the Greater Cincinnati metropolitan area that includes counties in Indiana, Kentucky, and Ohio. EHR data for 31,114 patients that visited the ED for asthma between 2009 and 2018 were extracted based on ICD asthma codes (ICD-9: 493, ICD-10: J45). The exclusion criteria for ED visits were (1) those visits from children who self- or parent identified as a race other than AA or EA, (2) age at visit equal to or greater than 18 years, and (3) visits with incomplete information on covariates. Workflow of the study and the analytic framework is shown in Figure 1. ED encounters included key data for linked individuals, including sociodemographic characteristics, lab tests, and insurance provider. We also extracted the patient’s home address associated with the relevant encounter to enable geocoding and connection to potentially relevant neighborhood-level variables. Specifically, we geocoded the address to the census tract geography and linked the census tract to data from the 2015 American Community Survey 5-Year Estimates (ACS) for sociodemographic factors, satellite imagery for air pollutants, and ground-based stations for allergens.(11)
Figure 1.
Schematic diagram and exclusion criteria of this study. The study includes a total of 31,114 children with valid covariates.
Primary outcome
The outcome of interest was defined as the number of registered ED visits during a year. Repeated measures (patients with ED visits in more than one year) were included during the entire time course.
Individual-level factors
The primary independent variable was patient race, defined according to self or caregiver report (AA and EA). Additional individual-level independent variables were selected according to those known to be associated with asthma exacerbations and/or shown to affect the association between race and asthma-related healthcare reported on previous studies.(6, 10, 12–14) We obtained these variables from the EHR. Age at visit(s) within the same year and gender (male or female) were included due to their association with asthma exacerbations. Insurance type was categorized as commercial and Medicaid.
Neighborhood-level factors
Neighborhood-level factors linked to geocoded patients’ address at each visit included proximity, environmental and allergen measures. For proximity measures, we calculated distances to the CCHMC facility, to main roads (primary roadways, U.S. highway, state highway, or county highway system). Greenspace was calculated as the average normalized vegetation index (NDVI) at 0.5, 1.5, and 2.5 kms of patient’s location. Neighborhood-level socioeconomic deprivation index is an estimation of material deprivation using six different census-tract-level socioeconomic variables from the American Community Survey. (11) The index ranges from 0 to 1 where 0 indicates lowest and 1 indicates highest deprivation. Finally, we included weekly averages of daily particulate matter less than 2.5 μm [PM2.5], pollen, and outdoor mold. Risk factors were extracted longitudinally, then averaged for continuous variables and last registered for medical insurance over a study year of each patient’s follow-up period. The detailed description of deprivation index and outdoor environmental exposures can be found in the Methods section in the Online Repository.
Statistical analysis
Descriptive statistics were derived as means (with standard deviation [SD]) for continuous variables and frequencies (with percentages) for categorical variables. Associations between race and each individual- and neighborhood-level variable were tested using Chi-square (χ2) or t-tests, as appropriate. A Variance Inflation Factor (VIF) was examined to assure no multicollinearity (VIF<5) in the list of risk factors.(15) Mediation analysis was used to test the direct and indirect effect of race on number of ED visits through individual- and neighborhood-level risk factors.(16) We implemented a longitudinal modelling strategy using mixed effects negative binomial regression models to assess the association of risk factors and their interactions with rate of ED visits during the entire time course. Finally, the study cohort was split into three subsets (only AAs, only EAs, and both AAs and EAs together) where predictive models for rate of ED visits were built in the training data (75%) and tested in the testing data (25%). The Methods section in this article’s Online Repository at www.jacionline.org provides additional details about VIF, unadjusted visits rates, and 95% confidence intervals, (Table E1).
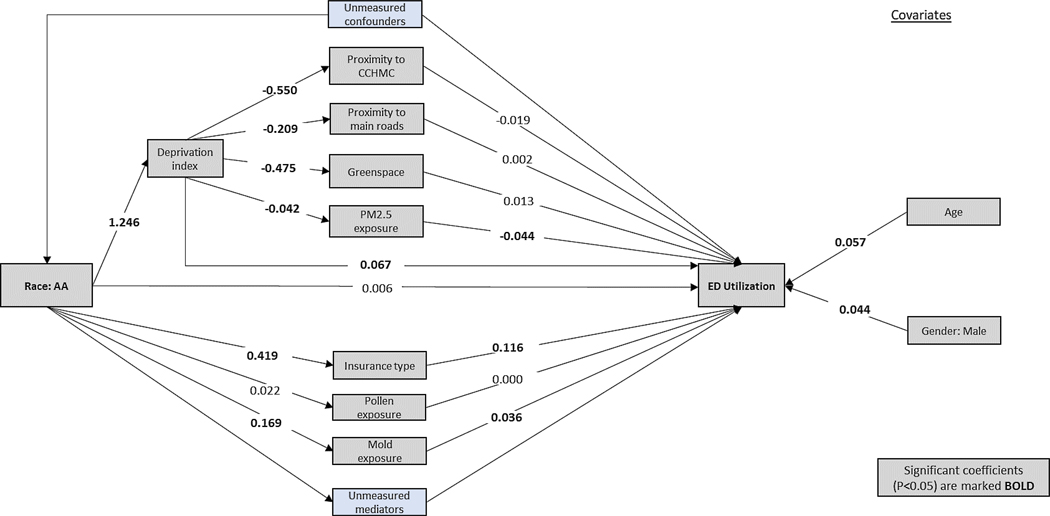
Mediation analysis
Following Mersha et. al., we determined the pathway between an independent variable X (race) and dependent variable Y (number of ED visits) using mediation analysis.(16) To implement the mediation analysis, number of ED visits were categorized into a binary outcome using the median of ED visits of all patients. In our mediation model (Figure 2), X → Y, X is thought to cause M (mediator), and M causes Y (X → M → Y representing the indirect effect of X on Y). We formulated hypotheses of the direct and indirect effect on the basis of previous reports.(17) Direct effects of race on number of ED visits were built following the illustration at Figure 2. estimated after adjusting for age and gender covariates, using Probit analysis. The total effect (φ) from race to number of ED visits was thought to be the sum of standardized direct effect (ϴ) and all indirect effects (δj), j=1,2, … J, where J is the total number of indirect paths from X to Y.
The jth indirect effect δj is calculated as the product of all standardized coefficients (sβij), i = 1, …, Ij within the path j.(18)
To assess the magnitude of each mediator, a percentage over the total effect of race on number of ED visits is calculated for each significant mediator.
Figure 2.
Mediation model for the independent variable - race, mediators, covariates and the dependent variable – number of ED visits. Measured and unmeasured pathways. Measured variables are indicated by gray boxes, unmeasured variables are indicated by blue boxes. The values shown are standardized coefficients (sβ), with statistical significance (p<0.05) marked in bold.
Mixed-effects analysis
We implemented a mixed-effects negative binomial model with smooth splines. (19) The outcome variable Yij was the number of visits for patient i at year j. Independent individual-level variables were age, gender, race and insurance type. Independent neighborhood-level variables included proximity to CCHMC, proximity to a main road, greenspace, deprivation index, and outdoor exposures (PM2.5, pollen, and outdoor mold). Interactions between race and neighborhood-level variables were examined. The model also included the interaction effect between age and gender due to their association with asthma exacerbations:
In this equation, to refers to fixed effects including yearly average of proximity to CCHMC, proximity to main road, and greenspace, deprivation index, and outdoor exposures (PM2.5, pollen, and outdoor mold). The model also includes a subject-specific random intercept b0i for repeated measures (patients who experience ED visits in more than one year). Here, g(.) is the natural logarithm link function for negative binomial count data, and E(Yij) is the expected value. For interpretation purposes, partial effects were plotted using the exponential function, including the intercept. Complementary to expected rate of visits and 95% confidence intervals (CI), visit rate ratios (VRR) were constructed with specific contrasts values at Q1 and Q3 quartiles, for 25th percentile and 75th percentile, respectively.(20)
Prediction model development and validation
We then evaluated whether our model using both individual- and neighborhood-level factors would be predictive of rate of ED visits in three subsets of the cohort (only AAs, only EAs, and both AAs and EAs together). Our models were developed using the training data and overall performance of these models was evaluated using the mean absolute error (MAE) in both, training, and testing data. MAE quantifies how close predicted rates of visits are to the observed outcome. The MAE score can range from 0, for a perfect model, to ∞, for a model with very large errors. QQ plots are provided for all models. All analyses were conducted using the R environment, ggplot for graphics, lme4 for multivariable models, lavaan package for mediation analysis.(19, 21–23)
RESULTS
Visits characteristics and racial disparities
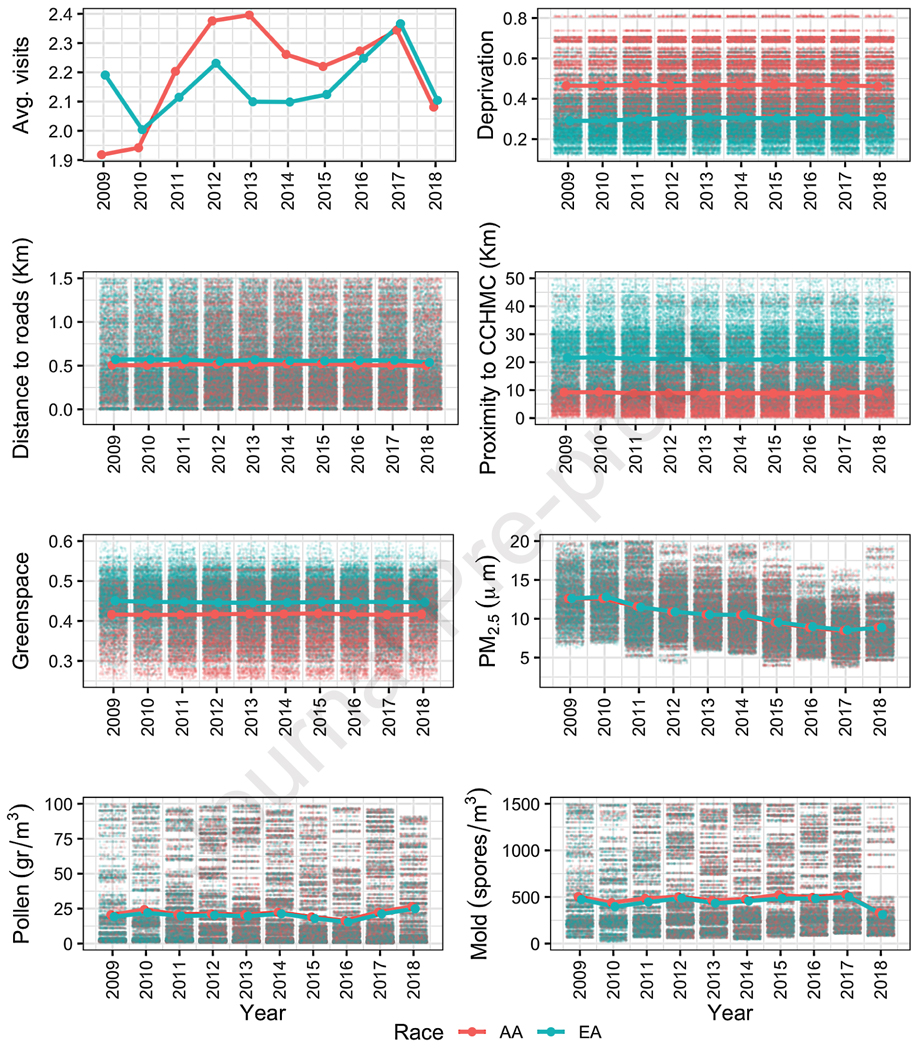
Of the 43,487 individuals who experienced an asthma-related ED visit, 31,114 were <18 years of age at index visit, AA or EA and had complete information on covariates. These included individuals contributed 186,779 total asthma-related ED visits where patients were <18 years of age at the visits. The cohort was comprised of 14,330 AA children, contributing 103,488 asthma-related ED visits, and 16,784 EA children, contributing 83,291 visits. Table 1 and Figure E2 present descriptive statistics of the sample included in the study. Among registered ED visits, there were more visits for AA children per year (2.23 vs 2.15; p<0.001), and there were more visits covered by Medicaid insurance for AA children (82.6% vs 43.3%; p<0.001).
Table 1.
ED visits characteristics by self-reported race including individual-and neighborhood-level risk factors.
| Characteristic | Overall, No. (%) or Mean (SD) | AA, No. (%) orMean (SD) | EA, No. (%)or Mean (SD) | P-value* |
|---|---|---|---|---|
| Patients’ cohort | 31114 | 14330 (46.1%) | 16784 (53.9%) | |
| Total ED visits | 186779 | 103488 (55.4) | 83291 (44.6%) | |
| ED Visits per year | 2.20 (2.47) | 2.23 (2.54) | 2.15 (2.39) | <0.001 |
| Individual-level | ||||
| Age at visit (years) | 8.59 (4.69) | 8.78 (4.68) | 8.34 (4.69) | 0.015 |
| Gender | ||||
| Female | 75769 (40.6) | 6723 (42.8) | 6781 (40.4) | <0.001 |
| Male | 111010 (59.4) | 8983 (57.2) | 10006 (59.6) | |
| Insurance type | ||||
| Commercial | 65231 (34.9) | 17984 (17.4) | 47247 (56.7) | <0.001 |
| Medicaid | 121548 (65.1) | 85504 (82.6) | 36044 (43.3) | |
| Neighborhood-level | ||||
| Deprivation index | 0.39 (0.16) | 0.47 (0.15) | 0.30 (0.12) | <0.001 |
| Proximity to CCHMC’s facility (km) | 14.63 (11.03) | 9.02 (7.09) | 21.59 (11.08) | <0.001 |
| Proximity to major roads (km) | 0.71 (0.62) | 0.67 (0.59) | 0.76 (0.65) | <0.001 |
| Proportion of greenspace (%) | 0.43 (0.06) | 0.42 (0.06) | 0.45 (0.06) | <0.001 |
| PM2.5 (μm) | 10.48 (3.34) | 10.35 (3.28) | 10.63 (3.41) | <0.001 |
| Daily pollen exposure (gr/m3) | 108.54 (227.12) | 110.27 (227.18) | 106.39(227.03) | <0.001 |
| Daily outdoor mold exposure (spores/m3) | 1,102.89 (923.05) | 1,139.82(929.06) | 1,057.01(913.46) | <0.001 |
P-values are from either a X2 or t-test comparing the differences when grouped by self-reported race;
P-value<0.05.
Compared to EA children, AA children were more likely to live in areas with higher deprivation (0.47 vs 0.30; p<0.001) and were more likely to live closer to the CCHMC base facility (9.0 km vs 21.6 km; p<0.001) and main roads (0.67 km vs 0.76 km; p<0.001).
Conversely, the proportion of greenspace was on average 3 points higher in the areas in which EA children lived (0.45 vs 0.42; p<0.001). However, EA children were exposed to higher PM2.5 (10.63μm vs 10.35μm; p<0.001) compared to AA children. AA children had slightly higher exposure to pollen (110.27 gr/m3 vs 106.39 gr/m3, p=0.001) and outdoor mold (1,139.82 spores/m3 vs 1,057.01 spores/m3; p<0.001).
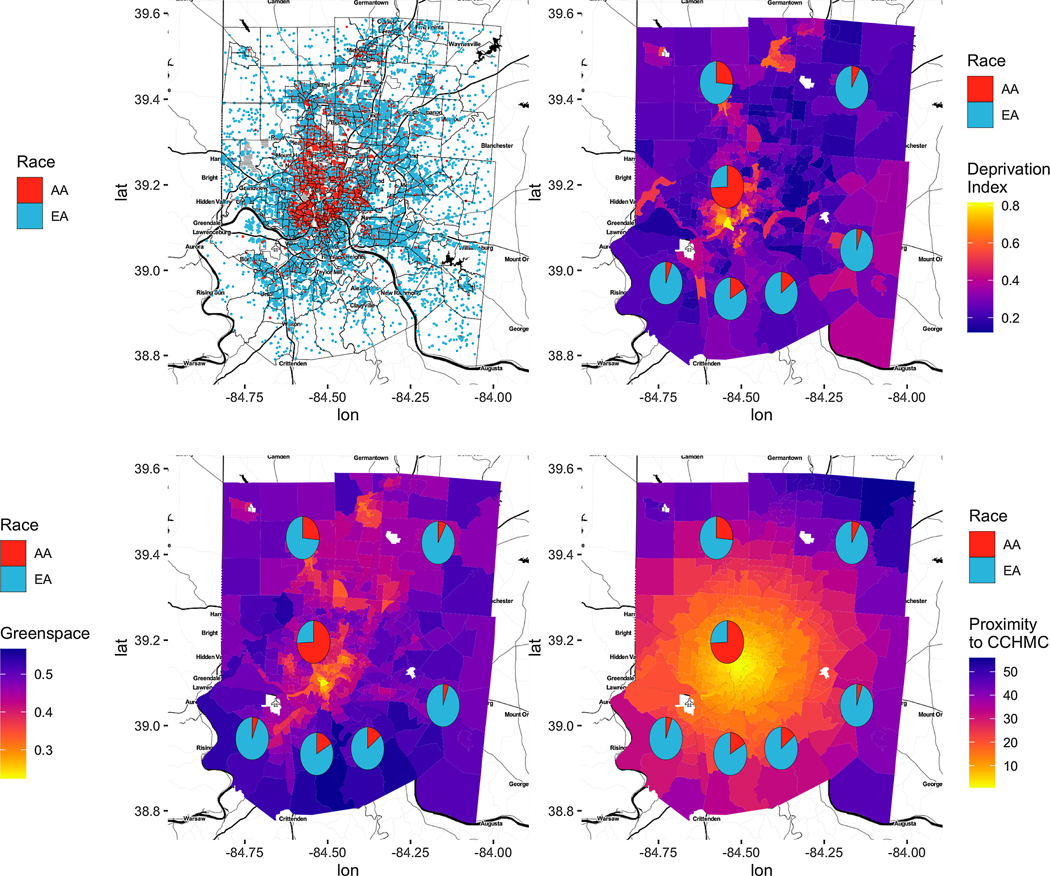
There was a spatial agreement between the number of visits registered and deprivation, greenspace, and proximity to CCHMC (figure 3). Visits from Hamilton County were more likely to be AA compared to other counties in Cincinnati metropolitan area where EA visits were more likely.
Figure 3.
Exploratory data analysis of ED visits and covariates. Only those covariates with spatial agreement are shown: (A) number of ED Visits by race, (B) deprivation index vs. race, (C) greenspace vs. race, and (D) and proximity to CCHMC vs race.
Mediation analysis
In our mediation analysis, we assessed associations among race, individual and neighborhood level variables, and number of ED visits (Figure 3). The direct effect of race on number of ED visits was not significant, after adjusting for all other variables (Table 2). However, the effect of race on ED visits was significantly mediated by Medicaid insurance (standardized coefficients [sβ]=0.048; p=<0.001), neighborhood socioeconomic deprivation (sβ=0.084; p<0.001), exposure to PM2.5 (sβ=0.002; p<0.001), and exposure to outdoor mold (sβ=0.006; p=<0.001). This effect was not mediated by proximity to CCHMC, proximity to main roads, greenspace, and exposure to pollen. Medical insurance, neighborhood socioeconomic deprivation, exposure to PM2.5, and exposure to outdoor mold accounted for 55% of race effect on number of ED visit
Table 2.
Summary of direct and indirect effects on number of ED visits. ED visits were categorized using the median ED visits in all patients (1.33). The sum of the standardized coefficients along each path is estimated for each indirect effect.
| Characteristic | Estimate | 95% CI | P-value* | Proportion | |
|---|---|---|---|---|---|
| Total effect | 0.252 | 0.209 | 0.295 | <0.001 | |
| Direct effects | |||||
| Race-AA | 0.006 | −0.039 | 0.051 | 0.796 | |
| Indirect effects, mediated by: | |||||
| Insurance-Medicaid | 0.048 | 0.034 | 0.062 | <0.001 | 19% |
| Deprivation index | 0.084 | 0.047 | 0.121 | <0.001 | 33% |
| Via Proximity to CCHMC | 0.013 | −0.003 | 0.029 | 0.078 | |
| Via Proximity to major roads | −0.001 | −0.005 | 0.003 | 0.765 | |
| Via Greenspace | −0.008 | −0.018 | 0.002 | 0.110 | |
| Via PM2.5 | 0.002 | 0.002 | 0.002 | <0.001 | 1% |
| Pollen exposure | 0.000 | 0.000 | 0.000 | 0.941 | |
| Outdoor mold exposure | 0.006 | 0.004 | 0.008 | <0.001 | 2% |
P-value<0.05; CI: Confidence interval; AA: African American.
Factors and interactions associated with rate of ED visits
Table 3 and Figure 4 summarize the results from the mixed-effects negative binomial models for the association between rate of ED visits and the assessed independent variables. For individual-level variables, we observed a non-linear interaction effect between age and gender. Age (Q3=12.2 years old vs Q1=4.4 years old) was associated with a 10% increase in rate of visits in both, females and males (p=0.001). Medicaid insurance was associated with a 7% increased rate of visits (p<0.001).
Table 3.
Expected rate of visits and visit rate ratios showing associations between risk factors and ED visits for asthma. Individual-level variables and interactions between race and neighborhood-level variables were examined.
| Factors | Expected visits (95% CI) | P-value* | Expected visits (95% CI) | P-value* |
|---|---|---|---|---|
| Gender | 0.709 | |||
| Female | Ref | |||
| Male | 1.02 (0.93 to 1.11) | |||
| Race | 0.545 | |||
| AA | Ref | |||
| EA | 1.13 (0.76 to 1.69) | |||
| Insurance type | <0.001 | |||
| Commercial | Ref | |||
| Medicaid | 1.07 (1.03 to 1.10) | |||
| Interactions | Group | P-value * | Group | P-value * |
| Deprivation (%) | AA | 0.033 | EA | 0.011 |
| Quartile 1: 0.27 | 3.51 (3.46 to 3.57) | 3.92 (3.86 to 3.97) | ||
| Quartile 3: 0.51 | 3.73 (3.65 to 3.8) | 3.9 (3.84 to 3.95) | ||
| Visits Ratio | 1.1 | 1.0 | ||
| Proximity to CCHMC by Race | AA | 0.025 | EA | 0.346 |
| Quartile 1: 6.6 | 3.69 (3.63 to 3.76) | 3.87 (3.81 to 3.92) | ||
| Quartile 3: 22.3 | 3.69 (3.64 to 3.75) | 3.89 (3.82 to 3.96) | ||
| Visits Ratio | 1.0 | 1.0 | ||
| Proximity to major roads (Km) | AA | 0.199 | EA | 0.009 |
| Quartile 1: 0.25 | 3.58 (3.52 to 3.64) | 3.84 (3.79 to 3.89) | ||
| Quartile 3: 1.04 | 3.61 (3.55 to 3.67) | 3.79 (3.74 to 3.85) | ||
| Visits Ratio | 1.0 | 1.0 | ||
| Greenspace (%) | AA | 0.007 | EA | 0.551 |
| Quartile 1: 0.40 | 3.69 (3.63 to 3.75) | 3.94 (3.89 to 4) | ||
| Quartile 3: 0.47 | 3.58 (3.52 to 3.65) | 3.96 (3.9 to 4.01) | ||
| Visits Ratio | 1.0 | 1.0 | ||
| PM2.5 (μm) | AA | <0.001 | EA | <0.001 |
| Quartile 1: 8.45 | 3.19 (3.13 to 3.25) | 3.3 (3.24 to 3.35) | ||
| Quartile 3: 12.08 | 3.41 (3.35 to 3.47) | 3.81 (3.76 to 3.87) | ||
| Visits Ratio | 1.1 | 1.2 | ||
| Pollen exposure (gr/m^3) | AA | <0.001 | EA | <0.001 |
| Quartile 1: 6.5 | 2.21 (2.15 to 2.27) | 2.27 (2.22 to 2.33) | ||
| Quartile 3: 118.65 | 3.93 (3.87 to 3.99) | 4.23 (4.17 to 4.28) | ||
| Visits Ratio | 1.8 | 1.9 | ||
| Outdoor mold exposure(Spores/m^3) | AA | <0.001 | EA | <0.001 |
| Quartile 1: 312.49 | 1.92 (1.86 to 1.98) | 2.06 (2 to 2.12) | ||
| Quartile 3: 1675.65 | 2.35 (2.29 to 2.41) | 2.39 (2.34 to 2.44) | ||
| Visits Ratio (VR) | 1.2 | 1.2 | ||
| Age at visit (years) | Female | <0.001 | Male | 0.001 |
| Quartile 1: 4.4 | 3.57 (3.51 to 3.63) | 3.65 (3.6 to 3.71) | ||
| Quartile 3: 12.2 | 3.81 (3.75 to 3.87) | 3.84 (3.78 to 3.9) | ||
| Visits Ratio | 1.1 | 1.1 |
P-value<0.05; CI: Confidence interval; Ref: Reference group
VR: Ratio of the rates between the two quartiles.
Figure 4.
Non-linear interactions associated with rate of ED visits: (A) Age vs. gender, (B) deprivation index vs. race, (C) proximity to CCHMC vs. race, (D) proximity to major roads vs. race, (E) greenspace vs. race, (F) PM2.5 vs. race, (G) pollen exposure vs. race, (H) and outdoor mold exposure vs. race.
For neighborhood-level variables, visits from AA children living in most deprived (Q1=0.51) vs those living in least deprived areas (Q3=0.27) were associated to 10% higher rate of visits (p=0.033). For outdoor environmental exposures, most polluted areas (Q3=12.08 μm) were associated with a 10% and 20% increased rate of visits in AA and EA children (both p<0.001), respectively. Also, pollen (Q3=118.65 gr/m3 vs Q1=6.5 gr/m3) was associated with higher rate of visits in both AA and EA (all p<0.001), 1.8 and 1.9 respectively. Similarly, an increase in outdoor mold exposure (Q3=1,675,65 spores/m3 vs Q1=312.49 spores/m3) was associated with 20% higher rate of visits in both AA and EA (all p<0.001). However, there were no significant interactions between EA race and deprivation, proximity to CCHMC, and greenspace, and between AA race and proximity to major roads, with respect to the relationship with rate of ED visits.
Prediction model and validation
Using MAE we evaluated errors of all models developed. The result showed that MAE metric for the testing data were similar to the training data estimates (Table 4). In all subsets in both datasets, training, and testing, mixed-effects negative binomials models had the lowest MAE scores, compared to classic negative binomial versions. QQ plots showed that mixed-effects models had the lowest difference between observed and predicted rate of ED visits (Figure E3).
Table 4.
Validation performance for ED visits models in three subsets of the cohort (only AAs, only EAs, and both AAs and EAs together) were examined. MAE including 95% CI were obtained for training and testing data, using 1000 bootstrap iterations and 3:1 random splits.
| Model | Dataset | MAE 95% CI |
|---|---|---|
| Full NB | Train | 1.403 (1.364 to 1.434) |
| Test | 1.396 (1.353 to 1.447) | |
| Full Mixed-effects NB | Train | 0.8 (0.778 to 0.805) |
| Test | 0.869 (0.864 to 0.934) | |
| AAs NB | Train | 1.428 (1.372 to 1.469) |
| Test | 1.444 (1.357 to 1.494) | |
| AAs Mixed-effects NB | Train | 0.797 (0.783 to 0.82) |
| Test | 0.957 (0.862 to 0.962) | |
| EAs NB | Train | 1.386 (1.323 to 1.419) |
| Test | 1.399 (1.312 to 1.436) | |
| EAs Mixed-effects NB | Train | 0.79 (0.761 to 0.803) |
| Test | 0.862 (0.838 to 0.932) |
NB: Negative binomial; AA: African American; EA: European American; MAE: Mean absolute error; CI: Confidence interval.
DISCUSSION
In this study, we used a large EHR dataset from 2009 to 2018 including more than 186,000 ED visits from about 31,000 children to systematically investigate racial disparities in asthma-related ED visits using association and mediation analyses. We were able to longitudinally adjust for individual factors and neighborhood-level information linked to the patient’s home address at the time of each ED event. ED visits were also more likely to occur among children covered by Medicaid insurance, particularly for AA children. AA children were also more likely to live in more deprived areas, live close to CCHMC, live close to greenspace, be more exposed to outdoor pollutants, and use the ED more frequently than EA children. Our findings support the notion that racial disparities in asthma-related ED visits are mediated by social, economic, and environmental factors at both individual and neighborhood levels.
There were differences in the exposures of children identifying as AA compared to those identifying as EA. Those differences influenced asthma-related ED visit frequency. For example, AA children were significantly more likely to live in more socioeconomically deprived neighborhoods. They were also more likely to be exposed to air pollutants and certain allergens. Our mixed-effects model showed that Medicaid-insured children (more common insurance type in AA children) were more likely to use ED frequently than commercially insured children. Several implications might be derived from these identified differences.(16, 24, 25) First, our exploratory data analysis revealed that visits from Hamilton County were more likely to be from AA children compared to other counties in Cincinnati metropolitan area where ED visits from EA children were more likely (Figure 3). As a consequence of living in urban areas such as Hamilton County, children might have complex family and social characteristics associated with their locations (hardship and accessibility) and be more exposed to environmental asthma “triggers” (e.g., closer to main roads, less greenspace availability, or higher PM2.5 exposure due to car pollution).(13, 26) Second, Medicaid-insured patients may use the ED as a more regular source of health care compared to children enrolled in commercial plans given differential access to primary or preventive care.(27) Also, minoritized groups may have more difficulty managing chronic disease like asthma due to limited access to additional health care supports (e.g., pharmacy), limited health literacy, reduced trust in health care providers, and additional factors rooted in structural racism.(17, 28, 29)
Higher exposure to PM2.5, pollen, and outdoor mold exposures were consistently associated with higher rates of ED visits in both AA and EA children. Comparing children living in more polluted to less polluted quartiles, AA and EA children had visits rate ratios of 1.1 and 1.2, respectively (p<0.001). Previous studies have shown that a causal relationship probably exists between early-life exposure to air pollutants, like PM2.5, and asthma burden.(30–32) Similarly, higher visits rate ratios were reported for high levels of pollen and outdoor mold. Although evidence of racial disparities in aeroallergen sensitization has been documented elsewhere, specific racial differences in exposure to pollens and outdoor molds are still not well understood.(33) Similarly, using genetic data from a prospective cohort study of 695 self-identified black and white children with an asthma-related admission, Mersha et al showed that although an increase in African ancestry is associated with increased odds of asthma readmission, genetic variations played far less of a role in explaining asthma readmission rates than social and environmental factors.(16) Although our mediation analysis varies some from Mersha’s ancestry analysis study in terms of independent variables and outcomes, deprivation was also the highest contributor as their estimate for hardship (33% vs. 40%), indicating the role of social, economic, and environmental risk factors in likelihood of asthma ED visits.
The present study has notable strengths. We used longitudinally available individual-level information from an EHR which allowed more detailed specification of the outcome as well as adjustments for individual-level factors available across the entire time course. Presence of the patient’s home address tied to each included ED visit enabled addition of neighborhood-level information and exposures at the time of the visit. From the multivariable analysis, we accounted for non-linear interactions, retaining neighborhood-level exposures without arbitrary categorization and patients’ locations to avoid loss of information or bias. Third, our descriptive analysis showed differences, by race, in insurance type, deprivation, distance to CCHMC and main roads, greenspace availability, and outdoor exposures drive racial disparities in asthma outcomes. Similarly, our mixed effects model with multilevel factors showed significant effects and interactions of race with risk factors (deprivation, proximity to healthcare provider, proximity to major roads, greenspace, PM2.5 and allergens). Taking all these variables into a mediation analysis, we found no direct effect of race on number of ED visits. Instead, it was mediated through social, economic, and environmental variables.
Our study has several limitations worth noting. Although we found clear racial disparities in the rate of asthma-related ED visits, this study was conducted in a single geographic area and healthcare facility. However, as the only pediatric institution in the study area, we capture the majority of ED events.(34). In Cincinnati, and the areas in close proximity to the main CCHMC campus, there are higher concentrations of individuals who identify as Black or AA. Suburban communities that are further from the main CCHMC campus have higher concentrations of individuals who identify as white or EA. This is reflective of racial and economic segregation that persists in Cincinnati, like other parts of the country. It is possible that the further away a family lives from the ED, the more they are dissuaded from seeking care in that site. They may find alternative sites of care (e.g., adult facility, primary care office) or not seek care at all. That said, CCHMC dominates the regional market share for inpatient utilization and emergency pediatric care. Still, there may be ED visits missed, especially in those areas further from the main campus.
It is also possible that our findings will not generalize to other regions with differing demographic or health care access characteristics. Second, our race reporting systems in the EHR database make it difficult to assess those who see themselves as mixed race, multiracial, or of another race/ethnicity.(35) Third, the total effect obtained in the mediation analysis might include unmeasured mediators or confounders. Although both contextual (neighborhood) and individual level measures are critically important in defining disease risk, we had limited individual-level data available within the EHR. Future research might include additional potential individual-level variables (e.g., prescriptions, allergen sensitization, immunologic measures, and second-hand smoking), housing characteristics (e.g., in-home pests, indoor mold, and household income) that are not available in our study. Future studies may also wish to evaluate other biologically plausible interactions including race-sex and race-age interactions, each of which might influence the number of asthma-related ED visits.(36)
There are several implications of our findings. First, we could improve the deleterious environmental factors through healthy homes programs and clean air policies. This may reduce asthma exacerbations and the number of ED visits. Second, we can augment preventive care by enhancing access to primary care and pharmacies; this could include care extenders like community health workers (CHW) or care managers. CHW initiatives such as The Seattle-King County Healthy Homes Project have been particularly successful in support of behavior change in asthma modifiable risk-factors, reducing urgent health services and enhancing quality of life scores.(37) The fact that AA and EA children who have the same social, economic, and environmental circumstances visit the ED in a similar frequency suggests that we should recognize race for what it is, a sociopolitical construct emblematic of sociopolitical factors as opposed to genetic ones.
In conclusion, previous efforts to understand racial disparities in asthma-related ED visits have been limited by the variables used. Our analysis was designed to address multiple levels of exposures and mechanisms simultaneously. In addition, we implemented analyses that offer high-resolution insights into the underlying spatial risk factors in childhood asthma. The assessment of multi-level risk factors that drive racial disparities in asthma offers a good opportunity to inform the design of targeted intervention strategies to equitably reduce the asthma burden. Our approach is distinct from previous efforts in that we used individual and neighborhood-level exposures in mediation models to systematically assess racial disparities and identify areas at risk for childhood asthma which can be subsequently used to mitigate racial disparities in pediatric asthma. Our findings offer potential avenues for intervention for policymakers to address racial gaps in the experience of asthma and other complex diseases.
Supplementary Material
Key messages.
African American children were more likely to have Medicaid, live in socioeconomically deprived areas, be close to less green space availability, and visit the ED more frequently than European American children.
Medical insurance, neighborhood socioeconomic deprivation, exposure to PM2.5, and exposure to outdoor mold accounted for 55% of race’s effect on ED visit.
Race was not directly associated with a child’s number of ED visits. Racial disparities in number of ED visits were mediated by social, economic, and environmental factors at both individual and neighborhood levels.
Acknowledgement
This work was supported by the National Institutes of Health (NIH) [NHLBI (R01 HL132344) and NHGRI (R01 HG011411)] grants support.
Abbreviations
- AA
African American
- ACS
American Community Survey
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- EA
European American
- ED
Emergency Department
- EHR
Electronic health records
- ICD-9/10
International Classification of Disease 9/10.
- MAE
Mean absolute error
- NB
Negative binomial
- PM2.5
Particulate matter 2.5 μm
quantile-quantile
- sβ
Standardized coefficients
- SD
Standard deviation
- VIF
Variance Inflation Factor
- VRR
Visits rate ratio
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
none
REFERENCES
- 1.WHO. Asthma Fact sheet No 307. 2013. Geneva:WorldHealthOrganization2013. [Google Scholar]
- 2.Centers for Disease Control (CDC). Asthma Facts- National Asthma Control Program. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2013. [Google Scholar]
- 3.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15(3):348–56. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Most Recent National Asthma Data: Centers for Disease Control and Prevention; 2019
- 5.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J Allergy Clin Immunol. 2014;134(3):547–53 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill TD, Graham LM, Divgi V. Racial Disparities in Pediatric Asthma: A Review of the Literature. Current Allergy and Asthma Reports. 2011;11(1):85–90. [DOI] [PubMed] [Google Scholar]
- 7.DePriest K, Butz A, Gross D. Investigating the relationships among neighborhood factors and asthma control in African American children: A study protocol. Research in Nursing & Health. 2018;41(5):428–39. [DOI] [PubMed] [Google Scholar]
- 8.Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. Journal of Allergy and Clinical Immunology. 2017;140(3):822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunwell JR, Opolka C, Mason C, Fitzpatrick AM. Geospatial Analysis of Social Determinants of Health Identifies Neighborhood Hot Spots Associated With Pediatric Intensive Care Use for Life-Threatening Asthma. The Journal of Allergy and Clinical Immunology: In Practice. 2022;10(4):981–91.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePriest K, Butz A. Neighborhood-Level Factors Related to Asthma in Children Living in Urban Areas: An Integrative Literature Review. The Journal of School Nursing. 2016;33(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bureau C. American Community Survey 2014–2018 5-Year Estimates: U.S. Census Bureau; [Retrieved from https://data.census.gov/cedsci/table?g=0100000US.050000&tid=ACSST5Y2018.S0101&hidePreview=false&vintage=2018&layer=VT_2018_050_00_PY_D1&cid=DP05_0001E]. [Google Scholar]
- 12.Achakulwisut P, Brauer M, Hystad P, Anenberg SC. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO2 pollution: estimates from global datasets. The Lancet Planetary Health. 2019;3(4):e166–e78. [DOI] [PubMed] [Google Scholar]
- 13.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. Journal of Allergy and Clinical Immunology. 2015;135(3):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dick S, Friend A, Dynes K, AlKandari F, Doust E, Cowie H, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4(11):e006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gareth James DWTHRT. An introduction to statistical learning : with applications in R: New York: : Springer, [2013] ©2013; 2013. [Google Scholar]
- 16.Mersha TB, Qin K, Beck AF, Ding L, Huang B, Kahn RS. Genetic ancestry differences in pediatric asthma readmission mediated by socio-environmental factors. Journal of Allergy and Clinical Immunology. [DOI] [PMC free article] [PubMed]
- 17.Beck AF, Huang B, Auger KA, Ryan PH, Chen C, Kahn RS. Explaining Racial Disparities in Child Asthma Readmission Using a Causal Inference Approach. JAMA Pediatrics. 2016;170(7):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kline RB. Principles and practice of structural equation modeling, 3rd ed. New York, NY, US: Guilford Press; 2011. xvi, 427–xvi, p. [Google Scholar]
- 19.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 20.Shepherd BE, Rebeiro PF, the Caribbean C, epidemiology SAnfH. Brief Report: Assessing and Interpreting the Association Between Continuous Covariates and Outcomes in Observational Studies of HIV Using Splines. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2017;74(3):e60–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. 3.5.2 ed. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 22.Wickham H. ggplot2: Elegant Graphics for Data Analysis: Springer-Verlag New York; 2016.
- 23.Rosseel Y. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software. 2012;48(2):1–36. [Google Scholar]
- 24.Beck AF, Huang B, Simmons JM, Moncrief T, Sauers HS, Chen C, et al. Role of Financial and Social Hardships in Asthma Racial Disparities. Pediatrics. 2014;133(3):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population–based cohort study. Annals of Epidemiology. 2019;30:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz J. Air Pollution and Children’s Health. Pediatrics. 2004;113(Supplement 3):1037. [PubMed] [Google Scholar]
- 27.Chang J, Freed GL, Prosser LA, Patel I, Erickson SR, Bagozzi RP, et al. Comparisons of Health Care Utilization Outcomes in Children With Asthma Enrolled in Private Insurance Plans Versus Medicaid. Journal of Pediatric Health Care. 2014;28(1):71–9. [DOI] [PubMed] [Google Scholar]
- 28.Shum M, Poureslami I, Liu J, FitzGerald J. Perceived Barriers to Asthma Therapy in Ethno-Cultural Communities: The Role of Culture, Beliefs and Social Support. Health.9(7):1029–46. [Google Scholar]
- 29.Young HN, Len-Rios ME, Brown R, Moreno MM, Cox E. How does patient-provider communication influence adherence to asthma medications? Patient Education and Counseling. 2017;100(4):696–702. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, et al. Early-Life Air Pollution and Asthma Risk in Minority Children. The GALA II and SAGE II Studies. American Journal of Respiratory and Critical Care Medicine. 2013;188(3):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achakulwisut P, Brauer M, Hystad P, Anenberg SC. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO pollution: estimates from global datasets. The Lancet Planetary Health. 2019;3(4):e166–e78. [DOI] [PubMed] [Google Scholar]
- 32.Anenberg Susan C, Henze Daven K, Tinney V, Kinney Patrick L, Raich W, Fann N, et al. Estimates of the Global Burden of Ambient PM2.5, Ozone, and NO2 on Asthma Incidence and Emergency Room Visits. Environmental Health Perspectives.126(10):107004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wegienka G, Johnson CC, Zoratti E, Havstad S. Racial Differences in Allergic Sensitization: Recent Findings and Future Directions. Current Allergy and Asthma Reports. 2013;13(3):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck AF, Moncrief T, Huang B, Simmons JM, Sauers H, Chen C, et al. Inequalities in Neighborhood Child Asthma Admission Rates and Underlying Community Characteristics in One US County. The Journal of Pediatrics. 2013;163(2):574–80.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Human Genomics. 2015;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor Air Pollution and Asthma in Children. Proceedings of the American Thoracic Society. 2010;7(2):102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: A Randomized, Controlled Trial of a Community Health Worker Intervention to Decrease Exposure to Indoor Asthma Triggers. American Journal of Public Health. 2005;95(4):652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.