Abstract
The MYC oncogenic family is dysregulated in diverse tumors which is generally linked to the poor prognosis of tumors. The members in MYC family are transcription factors which are responsible for the regulation of various genes expression. Among them, c-MYC is closely related to the progression of tumors. Furthermore, c-MYC aberrations is tightly associated with the prevalence of breast cancer. Tumor microenvironment (TME) is composed of many different types of cellular and non-cellular factors, mainly including cancer-associated fibroblasts, tumor-associated macrophages, vascular endothelial cells, myeloid-derived suppressor cells and immune cells, all of which can affect the diagnosis, prognosis, and therapeutic efficacy of breast cancer. Importantly, the biological processes occurred in TME, such as angiogenesis, immune evasion, invasion, migration, and the recruition of stromal and tumor-infiltrating cells are under the modulation of c-MYC. These findings indicated that c-MYC serves as a critical regulator of TME. Here, we aimed to summarize and review the relevant research, thus to clarify c-MYC is a key mediator between breast cancer cells and TME.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01043-1.
Keywords: c-MYC, Breast cancer, Tumor microenvironment, Crosstalk
Introduction
c-MYC gene belongs to the MYC family, located on human chromosome 8, encodes the transcription factor c-MYC, which participates in cell cycle progression, proliferation, apoptosis, and cellular transformation [1, 2]. The expression levels of c-MYC are tightly modulated by several mechanisms involving transcriptional regulation of proximal promoter region [3]. As a transcription factor, c-MYC dimerizes with MAX (a helix-loop-helix leucine zipper protein), and then binds to DNA to regulate gene expression [4–6]. In breast cancer patients, c-MYC has been confirmed to be highly expressed and lead to the occurrence and development of breast cancer [7–10].
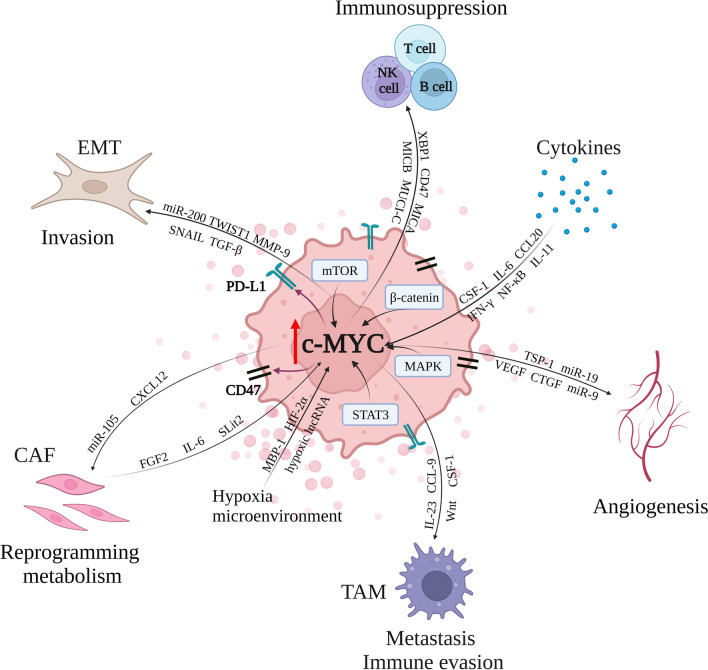
Accumulating evidence revealed that c-MYC is a critical regulator of the tumor microenvironment (TME), and involved in the stromal cell growth and angiogenesis (Fig. 1) [11–13]. TME contains many different types of cells, including cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), vascular endothelial cells (VECs), myeloid-derived suppressor cells (MDSCs), immune cells, inflammatory cells, adipocytes, and myoepithelial cells [14, 15]. Non-cellular components are also important parts of TME, such as extracellular matrix (ECM), extracellular vesicles (EVs), soluble cytokines and signaling molecules [16–18]. Moreover, the blood vessels and lymphatic system are also included in TME [19].
Fig. 1.
c-MYC is responsible for the crosstalk between breast cancer cells and tumor microenvironment. Within the tumor microenvironment, c-MYC can be regulated by several signaling proteins, such as STAT3, MAPK, β-catenin and mTOR. In addition, c-MYC contributes to the regulation of angiogenesis, the function of CAF and EMT. Moreover, c-MYC is able to modulate TAM, NK cell, T and B cell, as well as the expression of PD-L1 and CD47, thus leading to the immune evasion and immunosuppression. Besides, the tumor microenvironment can affect c-MYC expression in turn by various cytokines, the hypoxia microenvironment and the factors released from CAF
In breast cancer, increasing studies have revealed the close relationship between TME and tumor progression. This might be dependent on the c-MYC mediated crosstalk between TME and breast cancer cells [20–23]. Considering the vital role of c-MYC in both breast cancer cells and TME, this review aimed to summarize the functions and underlying mechanisms of c-MYC acts in the communication between breast cancer cells and TME.
c-MYC coordinates the crosstalk between breast cancer cells and angiogenesis
Like most solid tumors, new blood vessels are required if breast cancer cells are to grow beyond a few millimeters in diameter [24]. New blood vessels provide nutrients to tumor cells, allowing cells to proliferate and spread to other locations. Vascular endothelial growth factor (VEGF) is the major mediator of angiogenesis and functions by binding to its receptors on vascular endothelial cells. The oncogene expression, different growth factors, and hypoxia can induce the expression of VEGF [25–27]. Except for the stroma in the TME stimulates endothelial cell proliferation, the secret of VEGF from tumor cells can promote the formation of new blood vessels [28]. In breast cancer cells, targeting c-MYC has been demonstrated to inhibit tumor angiogenesis [29–31]. Mezquita et al. [32] found that VEGF expression was increased by c-MYC, which could stimulate the mRNA translation of VEGF. In addition, c-MYC can promote VEGF expression alone or in combination with Erα on the VEGF promoter [33]. By increasing the expression of miR-9, c-MYC caused the downregulation of E-cadherin and activation of β-catenin signaling, thus inducing the upregulation of VEGF and the tumor angiogenesis [34]. Pro-angiogenic miR-19 is also modulated by c-MYC, which directly targets anti-angiogenic proteins CTGF and TSP-1, thereby promoting angiogenesis [35]. Notably, c-MYC is able to affect angiogenesis by regulating VEGF, while VEGF can in turn affect c-MYC expression. As reported before, VEGF raised the expression level of c-MYC by activating STAT3 and enhances the self-renewal of breast cancer stem cells [36]. Except for inhibiting VEGF expression, c-MYC also decreased TSP-1 expression, facilitating tumor neovascularization [37, 38]. Combined with above, c-MYC can promote angiogenesis by affecting TME components such as VEGF and TSP-1 in breast cancer. Therefore, the expression of c-MYC can conversely modulated by VEGF in breast cancer.
c-MYC mediates the function of CAFs on breast cancer tumor growth
In TME, CAFs is the most common stromal cells, which deposits and modifies ECM. CAFs is generated due to the activation of normal fibroblasts under stimuli such as inflammatory signals or contact signals [39]. As previous studies reported, CAFs secrets soluble factors or modulates ECM to simulate tumor progression [18, 40–42]. Notably, c-MYC in breast cancer cells can promote tumor progression through CAFs. Yan et al. discovered that c-MYC increases the expression level of miR-105 in tumor cells vesicles. After receiving vesicles, c-MYC is upregulated in CAFs, inducing the reprogramming metabolism to promote tumor growth [43]. Besides, in mammary epithelial cells, c-MYC impact fibroblasts significantly. Mammary epithelial cells with c-MYC overexpression secrete IGF-I and IGF-II, which could subsequently induce fibroblasts via IGF-1R [44]. Valverius et al. [45] showed that the co-culture of human mammary epithelial cells overexpressing c-MYC with mammary fibroblasts induces both anchorage-independent and anchorage-dependent proliferation.
Moreover, the secretion of CAFs contributes to the expression of c-MYC in breast cancer cells, thereby promoting tumor growth. For instance, the medium of CAF induces cyclin D1, c-MYC, MMP-2 and MMP-9 expression in breast cancer cells, accelerating proliferation, migration and invasion [46, 47]. CAFs produce fibroblast growth factor 2 (FGF2), followed up with activation and recruition of ERα and PRBΔ4 to the MYC regulatory sequence leading to the breast cancer growth [48]. In breast cancer cells, TCF12 stimulates the activation of c-MYC/Cyclin D1 pathway to accelerate cancer growth via facilitate CXCL12 of CAFs [49]. Besides, c-MYC is required to maintain the cell growth of CAFs. Knockdown of c-MYC in CAFs reduced cyclin D1, cyclin E and E2F1 expression whereas increases p21clip1 expression, resulting in impaired proliferation of CAFs [50].
In addition to the promoting effect of CAFs on breast cancer, some studies have also shown that CAFs slow down breast cancer growth by downregulating c-MYC. Slit2, produced by stromal fibroblasts, can bind to the Robo1 receptor to inhibit carcinogenesis through blocking the nuclear translocation of β-catenin by modulating the PI3K/AKT axis and downregulating c-MYC [51]. In ERα-negative breast cancer cells, CAF-secreted IL-6 decreases c-MYC expression and suppresses tumor growth [52]. Starved fibroblast supernatants reduced MYC expression levels in breast cancer cells and induced cancer stem cells death [53]. Combined with above, CAFs have a dual function on breast cancer tumor growth and c-MYC is a critical mediator during the process.
c-MYC regulates the immune response in TME
Immune cells in TME mainly contains TAMs, dendritic cells, MDSCs, T cells, B cells, natural killer (NK) cells, neutrophils and others, which are tightly associated with the immune response [17, 54, 55]. Many researches have shown there is a close relationship between c-MYC and immune cells in TME.
c-MYC is elevated in TAMs
Macrophages are always divided into two groups according to their polarization and activation markers: M1 and M2. The M1 macrophage speeds up the inflammatory response, whereas the M2 type slows it down. Monocytes eventually develop into TAM after being enriched to tumor tissue via numerous signals in the tumor microenvironment [56, 57]. TAMs are considered M2-like macrophages because they suppress local immunity and support tumor growth. c-MYC is elevated in TAM and is involved in suppressing immunity, facilitating breast cancer tumor growth, and modulating the expression of proto-oncogenes (VEGF, MMP9, HIF-1α, TGF-β, MRC1, ALOX15) [58–60]. In TAM, Wnt ligands released from tumor cells can activate the Wnt/β-catenin axis, thereby activating c-MYC, leading to M2 polarization of TAM, which leads to tumor development, migration, and metastasis [61]. Liu and colleagues also found that c-MYC mRNA was significantly upregulated in macrophages after CSF-1 stimulation, driving macrophage proliferation. In addition, TAM infiltration is involved in the induction of c-MYC expression via IL-6/STAT3 pathway, indicating that there is a potential feedback loop mechanism to regulate the expression of c-MYC in TME [62]. Moreover, pro-inflammatory, stimulated by LPS and IFN-γ, inhibits the expression of c-MYC and the proliferation of macrophages [63]. Breast cancer cells with high c-MYC expression are more prevalent, promoting tumor progression through macrophages. By modulating SRC-1 expression, c-MYC activates colony-stimulating factor 1 (CSF-1) and enriches macrophages to enhance breast cancer metastasis [64]. The fusion of macrophages and breast cancer cells strengthened the activity TCF/LEF transcription factor and promoted the expression of downstream target genes (including cyclin D1 and c-MYC), leading to the promotion of tumor progression, metastasis, and EMT process [65]. Therefore, the intercommunication between breast cancer cells and TAM upregulates c-MYC expression, accelerating the breast cancer progression.
c-MYC inhibits tumor immune response
Tumor infiltrating lymphocytes (TILs) mainly clarified as T lymphocytes, B lymphocytes, and NK cells. In breast cancer, TIL acts a crucial role in mediating the response to chemotherapy [66]. Patients can benefit from treatment with increased TIL. TILs can be activated under the stimulation of various factors and participate in the immune response of tumors. Upon the activation of c-MYC, there is an immediate exclusion of T, B and NK cells within TME which are mainly caused by the up-regulation of chemokine CCL9 and IL-23 [67–69]. Interestingly, a study reported by Han and colleagues found that with c-MYC inhibitors, the percentage of CD3+ cells and natural killer cells was elevated addition whereas regulatory T cells were on the decline [70]. In triple-negative breast cancer (TNBC) cells, MUCI-C can recruit c-MYC to the promoter region of PD-L1, promoting PD-L1 expression. Inhibition of MUCI-C also suppressed c-MYC expression, resulting in an increase in IFN-γ in the CD8+ T cell population [71]. Upregulation of IFN-γ in CD8+ T cells has been demonstrated to aid tumor shrinking in studies [72]. CD47 is commonly upregulated in multiple tumors [73]. CD47 interacts with SIRPα, and initiates an inhibitory signaling pathway which causes tumor cells to evade phagocytosis by macrophages. PD-L1 is expressed on the surface of tumor cells to deliver inhibitory signals after interacting with ligands. It has been revealed that c-MYC could promote CD47 and PD-L1 expression by binding to the promoters of CD47 and PD-L1 genes to suppress antitumor immune responses [74]. As an immune checkpoint protein, PD-L1 on tumor cells can lead to the evasion of immune response, and then accelerate tumor progression. A variety of oncogenes, such as MAPK and AKT/mTOR, have been illustrated to regulate the expression of immune checkpoint [75, 76]. A possible mechanism is that these carcinogenic pathways are able to regulate c-MYC expression, which may be how they regulate the expression of immune checkpoints, such as PD-L1 [77]. Therefore, c-MYC may be a critical mediator between these carcinogenic signaling pathways and tumor immune response. In TNBC, c-MYC attenuates STING-dependent innate immunity by inhibiting the activation of dendritic cells and T cells. Breast cancers with c-MYC knockdown contain more CD3+ and CD8+ T cells, and c-MYC impairs T cell infiltration in vivo, leading to the formation of a “non-inflammatory tumor” microenvironment that induces immune evasion [78]. Furthermore, c-MYC inhibits antitumor immune responses by downregulating MICA and MICB via miR-17, resulting in reduced cell lysis in breast cancer cells [79]. In addition, XBP1 promotes NK cell proliferation in part by directly transactivating c-MYC expression [80].
In summary, c-MYC promotes the tumor progression of breast cancer by inhibiting the activation of immune cells in the TME.
Cytokines promotes the expression of c-MYC
Cytokines play an important role in creating an inflammatory TME and participate in the occurrence and development of tumors [81]. They are released by numerous cells, such as immunological cells, endothelial cells, and epidermal cells. Cytokines, most of which are small molecule polypeptides, exert their functions by interacting with receptors on the surface of cell membranes. Vast majority of cytokines contribute to tumor progression. In breast cancer cells, Sapi and colleagues demonstrated that cytokine CSF-1 induces expression of c-MYC, enhancing tumorigenicity as well as invasive potential [82]. Besides, c-MYC mRNA was significantly upregulated in macrophages after CSF-1 stimulation, boosting macrophage proliferation [63].Via JAK1/STAT3 signaling pathway, IL-11 induces c-MYC expression to promote breast cancer bone metastasis [83]. The chemokine CCL20 activates AKT pathways to enhance transcription of c-MYC, which is responsible for breast cell proliferation [84]. However, IFN-γ causes a decrease in c-MYC in normal human mammary epithelial cells and inhibits proliferation [85]. In addition, IL-6, VEGF and NF-κB promotes the expression of c-MYC via STAT3, leading to the development of breast cancer invasion and metastasis [86, 87]. Another study showed that breast cancer cells cultured with cytokines could activate Src, which promoted the upregulation of SOX2 and c-MYC. Furthermore, SOX2 stimulated c-MYC expression and enhanced cancer stem-like properties [88]. Taken together, multiple cytokines can promote breast cancer progression by upregulating c-MYC expression in cancer cells.
Hypoxic TME induced c-MYC expression
Hypoxia is a vital feature of the TME, which is mainly due to the abnormal function of new blood vessels. Meanwhile, the rapid growth of tumor cells demands enough oxygen, exacerbating hypoxia. Many literatures report that hypoxia contribute to the rapid development of breast cancer [89]. Hypoxic TME has been reported to enhance HIF-2α expression, resulting the overexpression of c-MYC. This increases the stemness of breast cancer [90]. MBP-1 was reported to regulates the expression of c-MYC negatively. Hypoxia can block the interaction of MBP-1 with the c-MYC promoter, thus fades the negative regulation of MBP-1 on c-MYC. Furthermore, high expressed c-MYC stimulates aerobic glycolysis, reinforcing the adaptation of tumor cells to oxidative stress [91]. Hypoxia can also cause high levels of a novel hypoxic lncRNA KB-1980E6.3 expression. KB-1980E6.3 could elevate c-MYC protein level by maintaining the mRNA stability of c-MYC and is responsible for the breast cancer stem cells (BCSCs) self-renewal and stemness maintenance [92]. Under hypoxia-reoxygenation conditions, the increase of c-MYC decreases the tumor suppressor NDRG1 expression. Based on this, hypoxia-reoxygenation enhances the metastasis of breast cancer [93].
Conclusions and perspectives
As a crucial oncogenic transcription factor, c-MYC regulates numerous genes and factors in breast cancer cells and TME. Notably, the changes of TME are responsible for the progression of breast cancer. For breast cancer patients, TME is therefore a promising therapeutic target. In recent decades, some targeted therapies have been developed for breast cancer patients, however, the strategies are commonly focused on tumor cells themselves [94, 95]. The genome of breast cancer cells is yet unstable, and gene mutations very probably occur after targeted therapy. This may cause the occurrence and development of drug resistance. Compared to breast cancer cells, cells exist in TME are relatively stable on the genome. Therefore, the probability of cell genes mutation might be reduced significantly, as well as the drug resistance, if the therapy is targeted at the tumor microenvironment. The characterization of TME in breast cancer has uncovered the crosstalk between the diverse molecules involved, including c-MYC. c-MYC serves as a key mediator to closely connect the TME and breast cancer cells by regulating the diverse factors in TME. Interestingly, the expression of c-MYC can be influenced by the components of TME in turn, indicating c-MYC and TME are tightly related in breast cancer. Therefore, c-MYC possesses great potential to be an effective target for breast cancer by interfering the balance and blocking the crosstalk between TME and tumor cells. However, which factor is relatively more important during the regulation between c-MYC and TME in breast cancer, the angiogenesis? immune cells? or CAF? In addition, it has been reported in a previous study that nanomedicines containing c-MYC targeted inhibitors in M2 macrophages, but have less influence on M1 macrophages that kill tumor cells [60]. This suggests to us that with the advancement of technology, we can accurately target c-MYC in some specific cells, which contribute to the oncogenic process during the communication between breast cancer cells and TME.
Although c-MYC is a potential therapeutic target for cancers, specific molecules targeting c-MYC are hardly to construct, because of the largely intrinsically disordered structure, lacking catalytic activity, and its nuclear localization [96]. Nonetheless, a few strategies for targeting c-MYC have been developed [96, 97]. These strategies are mainly divided into direct and indirect treatment. Among them, the direct treatment is the therapy directly targeting c-MYC, such as OmoMYC [98]. OmoMYC is a 90-amino acid peptide that imitates the bHLHLZ domain of c-MYC which can antagonize c-MYC and attenuate cancer cell growth [99–101]. It was demonstrated that the intratumor injection of OmoMYC suppressed tumor growth via reducing PD-L1 in a TNBC model [102]. Dependent on its favorable efficacy and low toxicity, OmoMYC is being evaluated for clinical treatment. For small molecule inhibitors, KJ-Pyr-9 [103], Mycro3 [104], MYCMI-6 [105] and MYCi975 [70] are the promising low molecular weight c-MYC antagonists. Among them, KJ-Pyr-9 showed high binding affinity with c-MYC, and has been revealed to inhibit TNBC tumor growth without side toxicity [103]. Apart from above, some other potential strategies for targeting c-MYC have also been explored, such as inhibiting the translation of c-MYC [106], and breaking the interaction between c-MYC and binding proteins [107]. Currently, although none molecules targeting c-MYC has been approved in clinical application, increasing studies have focused on resolving the limitations mentioned above to develop feasible approaches for the treatment of tumors. Recently, as the first direct inhibitor of c-MYC to pass human first clinical test, OMO-103 has shown anti-tumor activity and safety in patients with advanced solid cancers according to first-in-human data presented at the EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, shedding light on the clinical treatment by targeting c-MYC [108]. In breast cancer patients, the alternative MYC and chromosome 8 copy number anomalies might characterize the responsive or nonresponsive subgroups of HER2+ tumors to trastuzumab [109]. Although some clinical trials involving c-MYC in breast cancer have been reported, the clinical trials of drugs direct targeting c-MYC in it are lacking [98]. Therefore, more studies focused on targeting c-MYC are needed for developing novel therapeutic strategies for breast cancer patients.
Collectively, c-MYC mediates the crosstalk between breast cancer cells and TME to regulate tumor progression. Nevertheless, more study is urgent for further characterizing more details about c-MYC in the mediation of TME and breast cancer cells, which will be beneficial for developing better and accurate therapeutic protocols for the management of breast cancer.
Acknowledgements
Not applicable.
Abbreviations
- TME
Tumor microenvironment
- CAFs
Cancer-associated fibroblasts
- TAMs
Tumor-associated macrophages
- VECs
Vascular endothelial cells
- MDSCs
Myeloid-derived suppressor cells
- ECM
Extracellular matrix
- EMT
Epithelial–mesenchymal transition
- EVs
Extracellular vesicles
- VEGF
Vascular endothelial growth factor
- FGF2
Fibroblast growth factor 2
- NK
Natural killer
- CSF-1
Colony-stimulating factor 1
- TILs
Tumor infiltrating lymphocytes
- TNBC
Triple-negative breast cancer
- BCSCs
Breast cancer stem cells
Author contributions
XXG contributed to the conception and design of the study. FYG and XTL drafted the main text and graphical illustrations. KX and RTW participated in modifying the picture and editing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Key International Cooperation of the National Natural Science Foundation of China (No. 81920108029) and the Key Foundation for Social Development Project of the Jiangsu Province, China (No.BE2021741).
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks TA, Hurley LH. Targeting MYC expression through G-quadruplexes. Genes Cancer. 2010;1:641–649. doi: 10.1177/1947601910377493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kress TR, Sabo A, Amati B. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer. 2015;15:593–607. doi: 10.1038/nrc3984. [DOI] [PubMed] [Google Scholar]
- 5.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-MYC. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amati B, Dalton S, Brooks MW, Littlewood TD, Evan GI, Land H. Transcriptional activation by the human c-MYC oncoprotein in yeast requires interaction with max. Nature. 1992;359:423–426. doi: 10.1038/359423a0. [DOI] [PubMed] [Google Scholar]
- 7.Liao DJ, Dickson RB. c-MYC in breast cancer. Endocr Relat Cancer. 2000;7:143–164. doi: 10.1677/erc.0.0070143. [DOI] [PubMed] [Google Scholar]
- 8.Fallah Y, Brundage J, Allegakoen P, Shajahan-Haq AN. MYC-driven pathways in breast cancer subtypes. Biomolecules. 2017;7:53. doi: 10.3390/biom7030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neve RM, Sutterluty H, Pullen N, Lane HA, Daly JM, Krek W, Hynes NE. Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene. 2000;19:1647–1656. doi: 10.1038/sj.onc.1203470. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh AK, Grigorieva I, Steele R, Hoover RG, Ray RB. PTEN transcriptionally modulates c-MYC gene expression in human breast carcinoma cells and is involved in cell growth regulation. Gene. 1999;235:85–91. doi: 10.1016/s0378-1119(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 11.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a MYC-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitfield JR, Soucek L. Tumor microenvironment: becoming sick of MYC. Cell Mol Life Sci. 2012;69:931–934. doi: 10.1007/s00018-011-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meskyte EM, Keskas S, Ciribilli Y. MYC as a multifaceted regulator of tumor microenvironment leading to metastasis. Int J Mol Sci. 2020;21:7710. doi: 10.3390/ijms21207710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belli C, Trapani D, Viale G, D'Amico P, Duso BA, Della Vigna P, Orsi F, Curigliano G. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82:142–152. doi: 10.1159/000430499. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Dieterich LC, Bikfalvi A. The tumor organismal environment: role in tumor development and cancer immunotherapy. Semin Cancer Biol. 2020;65:197–206. doi: 10.1016/j.semcancer.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Tyan SW, Kuo WH, Huang CK, Pan CC, Shew JY, Chang KJ, Lee EY, Lee WH. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS ONE. 2011;6:e15313. doi: 10.1371/journal.pone.0015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao A, Gu F, Guo X, Zhang X, Fu L. Breast cancer-associated fibroblasts: their roles in tumor initiation, progression and clinical applications. Front Med. 2016;10:33–40. doi: 10.1007/s11684-016-0431-5. [DOI] [PubMed] [Google Scholar]
- 22.Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12:95. doi: 10.1186/s13045-019-0778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 27.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling: in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat Med. 2010;16:1107–1111. doi: 10.1038/nm1010-1107. [DOI] [PubMed] [Google Scholar]
- 29.Debnath S, Mukherjee A, Saha D, Dash J, Chatterjee TK. Poly-l-Lysine inhibits VEGF and c-MYC mediated tumor-angiogenesis and induces apoptosis in 2D and 3D tumor microenvironment of both MDA-MB-231 and B16F10 induced mice model. Int J Biol Macromol. 2021;183:528–548. doi: 10.1016/j.ijbiomac.2021.04.109. [DOI] [PubMed] [Google Scholar]
- 30.Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL. c-MYC is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Zhao WL, Wang JP, Xin BM, Shao RG. EBP50 suppresses the proliferation of MCF-7 human breast cancer cells via promoting Beclin-1/p62-mediated lysosomal degradation of c-MYC. Acta Pharmacol Sin. 2018;39:1347–1358. doi: 10.1038/aps.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mezquita P, Parghi SS, Brandvold KA, Ruddell A. MYC regulates VEGF production in B cells by stimulating initiation of VEGF mRNA translation. Oncogene. 2005;24:889–901. doi: 10.1038/sj.onc.1208251. [DOI] [PubMed] [Google Scholar]
- 33.Dadiani M, Seger D, Kreizman T, Badikhi D, Margalit R, Eilam R, Degani H. Estrogen regulation of vascular endothelial growth factor in breast cancer in vitro and in vivo: the role of estrogen receptor alpha and c-MYC. Endocr Relat Cancer. 2009;16:819–834. doi: 10.1677/ERC-08-0249. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Psathas JN, Thomas-Tikhonenko A. MYC and the art of microRNA maintenance. Cold Spring Harb Perspect Med. 2014;4:a014175. doi: 10.1101/cshperspect.a014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao D, Pan C, Sun J, Gilbert C, Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D, et al. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate MYC and Sox2. Oncogene. 2015;34:3107–3119. doi: 10.1038/onc.2014.257. [DOI] [PubMed] [Google Scholar]
- 37.Cheng S, Guo M, Liu Z, Fu Y, Wu H, Wang C, Cao M. Morphine promotes the angiogenesis of postoperative recurrent tumors and metastasis of dormant breast cancer cells. Pharmacology. 2019;104:276–286. doi: 10.1159/000502107. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Cheng S, Fu G, Ji F, Wang C, Cao M. Postoperative administration of ketorolac averts morphine-induced angiogenesis and metastasis in triple-negative breast cancer. Life Sci. 2020;251:117604. doi: 10.1016/j.lfs.2020.117604. [DOI] [PubMed] [Google Scholar]
- 39.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 41.Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 43.Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, Liu X, Chen CH, Fadare O, Pizzo DP, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20:597–609. doi: 10.1038/s41556-018-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Vincenzo A, Belli S, Franco P, Telesca M, Iaccarino I, Botti G, Carriero MV, Ranson M, Stoppelli MP. Paracrine recruitment and activation of fibroblasts by c-MYC expressing breast epithelial cells through the IGFs/IGF-1R axis. Int J Cancer. 2019;145:2827–2839. doi: 10.1002/ijc.32613. [DOI] [PubMed] [Google Scholar]
- 45.Valverius EM, Ciardiello F, Heldin NE, Blondel B, Merlo G, Smith G, Stampfer MR, Lippman ME, Dickson RB, Salomon DS. Stromal influences on transformation of human mammary epithelial cells overexpressing c-MYC and SV40T. J Cell Physiol. 1990;145:207–216. doi: 10.1002/jcp.1041450204. [DOI] [PubMed] [Google Scholar]
- 46.Suh J, Kim DH, Surh YJ. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch Biochem Biophys. 2018;643:62–71. doi: 10.1016/j.abb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Suh J, Kim DH, Lee YH, Jang JH, Surh YJ. Fibroblast growth factor-2, derived from cancer-associated fibroblasts, stimulates growth and progression of human breast cancer cells via FGFR1 signaling. Mol Carcinog. 2020;59:1028–1040. doi: 10.1002/mc.23233. [DOI] [PubMed] [Google Scholar]
- 48.Giulianelli S, Riggio M, Guillardoy T, Perez Pinero C, Gorostiaga MA, Sequeira G, Pataccini G, Abascal MF, Toledo MF, Jacobsen BM, et al. FGF2 induces breast cancer growth through ligand-independent activation and recruitment of ERalpha and PRBDelta4 isoform to MYC regulatory sequences. Int J Cancer. 2019;145:1874–1888. doi: 10.1002/ijc.32252. [DOI] [PubMed] [Google Scholar]
- 49.Tang X, Tu G, Yang G, Wang X, Kang L, Yang L, Zeng H, Wan X, Qiao Y, Cui X, et al. Autocrine TGF-beta1/miR-200s/miR-221/DNMT3B regulatory loop maintains CAF status to fuel breast cancer cell proliferation. Cancer Lett. 2019;452:79–89. doi: 10.1016/j.canlet.2019.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang S, Hou Y, Zhang H, Tu G, Yang L, Sun Y, Lang L, Tang X, Du YE, Zhou M, et al. Oxidized ATM promotes abnormal proliferation of breast CAFs through maintaining intracellular redox homeostasis and activating the PI3K-AKT, MEK-ERK, and Wnt-beta-catenin signaling pathways. Cell Cycle. 2015;14:1908–1924. doi: 10.1080/15384101.2015.1041685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang PH, Hwang-Verslues WW, Chang YC, Chen CC, Hsiao M, Jeng YM, Chang KJ, Lee EY, Shew JY, Lee WH. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Res. 2012;72:4652–4661. doi: 10.1158/0008-5472.CAN-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dittmer A, Lange T, Leyh B, Dittmer J. Protein and growthmodulatory effects of carcinomaassociated fibroblasts on breast cancer cells: role of interleukin6. Int J Oncol. 2020;56:258–272. doi: 10.3892/ijo.2019.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pourbagher R, Akhavan-Niaki H, Jorsaraei SGA, Fattahi S, Sabour D, Zabihi E, Abedian Z, Ghasemi M, Golpour M, Mostafazadeh A. Targeting LA7 breast cancer stem cells of rat through repressing the genes of stemness-related transcription factors using three different biological fluids. Gene. 2020;734:144381. doi: 10.1016/j.gene.2020.144381. [DOI] [PubMed] [Google Scholar]
- 54.Dannenmann SR, Thielicke J, Stockli M, Matter C, von Boehmer L, Cecconi V, Hermanns T, Hefermehl L, Schraml P, Moch H, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. doi: 10.4161/onci.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. 2020;30:R246–R248. doi: 10.1016/j.cub.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 57.Munir MT, Kay MK, Kang MH, Rahman MM, Al-Harrasi A, Choudhury M, Moustaid-Moussa N, Hussain F, Rahman SM. Tumor-associated macrophages as multifaceted regulators of breast tumor growth. Int J Mol Sci. 2021;22:6526. doi: 10.3390/ijms22126526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A, Doni A, Nebuloni M, Swigart LB, Evan GI, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–421. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 59.Benner B, Scarberry L, Suarez-Kelly LP, Duggan MC, Campbell AR, Smith E, Lapurga G, Jiang K, Butchar JP, Tridandapani S, et al. Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro. J Immunother Cancer. 2019;7:140. doi: 10.1186/s40425-019-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esser AK, Ross MH, Fontana F, Su X, Gabay A, Fox GC, Xu Y, Xiang J, Schmieder AH, Yang X, et al. Nanotherapy delivery of c-MYC inhibitor targets protumor macrophages and preserves antitumor macrophages in breast cancer. Theranostics. 2020;10:7510–7526. doi: 10.7150/thno.44523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC, Han H, Liu WC, Qin HY. Crosstalk between hepatic tumor cells and macrophages via Wnt/beta-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. doi: 10.1038/s41419-018-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadjidaniel MD, Muthugounder S, Hung LT, Sheard MA, Shirinbak S, Chan RY, Nakata R, Borriello L, Malvar J, Kennedy RJ, et al. Tumor-associated macrophages promote neuroblastoma via STAT3 phosphorylation and up-regulation of c-MYC. Oncotarget. 2017;8:91516–91529. doi: 10.18632/oncotarget.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, Lu Y, Martinez J, Bi Y, Lian G, Wang T, Milasta S, Wang J, Yang M, Liu G, et al. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from MYC-dependent to HIF1alpha-dependent. Proc Natl Acad Sci U S A. 2016;113:1564–1569. doi: 10.1073/pnas.1518000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, Xu J. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci U S A. 2009;106:151–156. doi: 10.1073/pnas.0808703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang LN, Huang YH, Zhao L. Fusion of macrophages promotes breast cancer cell proliferation, migration and invasion through activating epithelial–mesenchymal transition and Wnt/beta-catenin signaling pathway. Arch Biochem Biophys. 2019;676:108137. doi: 10.1016/j.abb.2019.108137. [DOI] [PubMed] [Google Scholar]
- 66.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, Littlewood TD, Evan GI. MYC cooperates with ras by programming inflammation and immune suppression. Cell. 2017;171(1301–1315):e1314. doi: 10.1016/j.cell.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 69.Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28:207–212. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Han H, Jain AD, Truica MI, Izquierdo-Ferrer J, Anker JF, Lysy B, Sagar V, Luan Y, Chalmers ZR, Unno K, et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36(483–497):e415. doi: 10.1016/j.ccell.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maeda T, Hiraki M, Jin C, Rajabi H, Tagde A, Alam M, Bouillez A, Hu X, Suzuki Y, Miyo M, et al. MUC1-C induces PD-L1 and immune evasion in triple-negative breast cancer. Cancer Res. 2018;78:205–215. doi: 10.1158/0008-5472.CAN-17-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ligocki AJ, Brown JR, Niederkorn JY. Role of interferon-gamma and cytotoxic T lymphocytes in intraocular tumor rejection. J Leukoc Biol. 2016;99:735–747. doi: 10.1189/jlb.3A0315-093RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPalpha immune checkpoint. Immunity. 2020;52:742–752. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gutgemann I, Eilers M, Felsher DW. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lastwika KJ, Wilson W, 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 77.Casey SC, Baylot V, Felsher DW. The MYC oncogene is a global regulator of the immune response. Blood. 2018;131:2007–2015. doi: 10.1182/blood-2017-11-742577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu SY, Xiao Y, Wei JL, Xu XE, Jin X, Hu X, Li DQ, Jiang YZ, Shao ZM. MYC suppresses STING-dependent innate immunity by transcriptionally upregulating DNMT1 in triple-negative breast cancer. J Immunother Cancer. 2021;9:e002528. doi: 10.1136/jitc-2021-002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan J, Shen J, Si W, Du C, Chen D, Xu L, Yao M, Fu P, Fan W. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-MYC/miR-17 pathway. Oncotarget. 2017;8:65743–65758. doi: 10.18632/oncotarget.19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong H, Adams NM, Xu Y, Cao J, Allan DSJ, Carlyle JR, Chen X, Sun JC, Glimcher LH. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-MYC. Nat Immunol. 2019;20:865–878. doi: 10.1038/s41590-019-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kan CE, Cipriano R, Jackson MW. c-MYC functions as a molecular switch to alter the response of human mammary epithelial cells to oncostatin M. Cancer Res. 2011;71:6930–6939. doi: 10.1158/0008-5472.CAN-10-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sapi E, Flick MB, Rodov S, Kacinski BM. Ets-2 transdominant mutant abolishes anchorage-independent growth and macrophage colony-stimulating factor-stimulated invasion by BT20 breast carcinoma cells. Cancer Res. 1998;58:1027–1033. [PubMed] [Google Scholar]
- 83.Liang M, Ma Q, Ding N, Luo F, Bai Y, Kang F, Gong X, Dong R, Dai J, Dai Q, et al. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019;10:353. doi: 10.1038/s41419-019-1594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marsigliante S, Vetrugno C, Muscella A. CCL20 induces migration and proliferation on breast epithelial cells. J Cell Physiol. 2013;228:1873–1883. doi: 10.1002/jcp.24349. [DOI] [PubMed] [Google Scholar]
- 85.Harvat BL, Jetten AM. Gamma-interferon induces an irreversible growth arrest in mid-G1 in mammary epithelial cells which correlates with a block in hyperphosphorylation of retinoblastoma. Cell Growth Differ. 1996;7:289–300. [PubMed] [Google Scholar]
- 86.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci U S A. 2012;109:9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: a review. Int J Cancer. 2016;138:2570–2578. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, Morata-Tarifa C, Kim M, Ince TA, Azzam DJ, et al. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Zhang B, Bao L, Jin L, Yang M, Peng Y, Kumar A, Wang JE, Wang C, Zou X, et al. ZMYND8 acetylation mediates HIF-dependent breast cancer progression and metastasis. J Clin Invest. 2018;128:1937–1955. doi: 10.1172/JCI95089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan Y, Liu F, Han L, Zhao L, Chen J, Olopade OI, He M, Wei M. HIF-2alpha promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res. 2018;37:256. doi: 10.1186/s13046-018-0925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sedoris KC, Thomas SD, Miller DM. Hypoxia induces differential translation of enolase/MBP-1. BMC Cancer. 2010;10:157. doi: 10.1186/1471-2407-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu P, He F, Hou Y, Tu G, Li Q, Jin T, Zeng H, Qin Y, Wan X, Qiao Y, et al. A novel hypoxic long noncoding RNA KB-1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-MYC mRNA stability. Oncogene. 2021;40:1609–1627. doi: 10.1038/s41388-020-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lai LC, Su YY, Chen KC, Tsai MH, Sher YP, Lu TP, Lee CY, Chuang EY. Down-regulation of NDRG1 promotes migration of cancer cells during reoxygenation. PLoS ONE. 2011;6:e24375. doi: 10.1371/journal.pone.0024375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 95.Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O'Shaughnessy J, Gradishar W, Schmid P, Winer E, Kelly C, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36:884–890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duffy MJ, O'Grady S, Tang M, Crown J. MYC as a target for cancer treatment. Cancer Treat Rev. 2021;94:102154. doi: 10.1016/j.ctrv.2021.102154. [DOI] [PubMed] [Google Scholar]
- 97.Whitfield JR, Beaulieu ME, Soucek L. Strategies to Inhibit MYC and their clinical applicability. Front Cell Dev Biol. 2017;5:10. doi: 10.3389/fcell.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y, Zhu C, Tang L, Chen Q, Guan N, Xu K, Guan X. MYC dysfunction modulates stemness and tumorigenesis in breast cancer. Int J Biol Sci. 2021;17:178–187. doi: 10.7150/ijbs.51458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Masso-Valles D, Soucek L. Blocking MYC to treat cancer: reflecting on 2 decades of omomyc. Cells. 2020;9:883. doi: 10.3390/cells9040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung LA, Gebhardt A, Koelmel W, Ade CP, Walz S, Kuper J, von Eyss B, Letschert S, Redel C, d'Artista L, et al. OmoMYC blunts promoter invasion by oncogenic MYC to inhibit gene expression characteristic of MYC-dependent tumors. Oncogene. 2017;36:1911–1924. doi: 10.1038/onc.2016.354. [DOI] [PubMed] [Google Scholar]
- 101.Demma MJ, Mapelli C, Sun A, Bodea S, Ruprecht B, Javaid S, Wiswell D, Muise E, Chen S, Zelina J, et al. Omomyc reveals new mechanisms to inhibit the MYC oncogene. Mol Cell Biol. 2019;39:e00248-19. doi: 10.1128/MCB.00248-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang E, Sorolla A, Cunningham PT, Bogdawa HM, Beck S, Golden E, Dewhurst RE, Florez L, Cruickshank MN, Hoffmann K, et al. Tumor penetrating peptides inhibiting MYC as a potent targeted therapeutic strategy for triple-negative breast cancers. Oncogene. 2019;38:140–150. doi: 10.1038/s41388-018-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hart JR, Garner AL, Yu J, Ito Y, Sun M, Ueno L, Rhee JK, Baksh MM, Stefan E, Hartl M, et al. Inhibitor of MYC identified in a Krohnke pyridine library. Proc Natl Acad Sci U S A. 2014;111:12556–12561. doi: 10.1073/pnas.1319488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiessling A, Sperl B, Hollis A, Eick D, Berg T. Selective inhibition of c-MYC/Max dimerization and DNA binding by small molecules. Chem Biol. 2006;13:745–751. doi: 10.1016/j.chembiol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 105.Castell A, Yan Q, Fawkner K, Hydbring P, Zhang F, Verschut V, Franco M, Zakaria SM, Bazzar W, Goodwin J, et al. A selective high affinity MYC-binding compound inhibits MYC:MAX interaction and MYC-dependent tumor cell proliferation. Sci Rep. 2018;8:10064. doi: 10.1038/s41598-018-28107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wiegering A, Uthe FW, Jamieson T, Ruoss Y, Huttenrauch M, Kuspert M, Pfann C, Nixon C, Herold S, Walz S, et al. Targeting translation initiation bypasses signaling crosstalk mechanisms that maintain high MYC levels in colorectal cancer. Cancer Discov. 2015;5:768–781. doi: 10.1158/2159-8290.CD-14-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Struntz NB, Chen A, Deutzmann A, Wilson RM, Stefan E, Evans HL, Ramirez MA, Liang T, Caballero F, Wildschut MHE, et al. Stabilization of the Max homodimer with a small molecule attenuates MYC-driven transcription. Cell Chem Biol. 2019;26(711–723):e714. doi: 10.1016/j.chembiol.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Direct MYC Inhibitor Passes First Clinical Test. Cancer Discov 2022:OF1.
- 109.Perez EA, Jenkins RB, Dueck AC, Wiktor AE, Bedroske PP, Anderson SK, Ketterling RP, Sukov WR, Kanehira K, Chen B, et al. C-MYC alterations and association with patient outcome in early-stage HER2-positive breast cancer from the north central cancer treatment group N9831 adjuvant trastuzumab trial. J Clin Oncol. 2011;29:651–659. doi: 10.1200/JCO.2010.30.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.