Abstract
Background
Although a great deal of scientific evidence on the epidemiological risk factors for diabetes and prediabetes has been accumulated, there is still insufficient evidence to explore sex-related differences. The aim of this study was to examine sex-specific differences in the effect of the atherogenic index of plasma (AIP) on prediabetes and diabetes.
Methods
This cross-sectional study included data from 10099 American adults. The exposure variable was the AIP, which was defined as log10 (triglycerides/high-density lipoprotein cholesterol). The outcome variables included prediabetes and diabetes defined by the 2013 American Diabetes Association guidelines.
Results
The median age (mean ± SD) was 48.51 ± 18.42 years, and the average value (SD) of the AIP was − 0.09 (0.34). The prevalence of prediabetes was 40.24%, and that of diabetes was 21.32%. Overall, there was a significant positive association between the AIP and prediabetes and diabetes (per 1-unit increment in the AIP: OR, 2.49; 95% CI 1.75, 3.54). The multivariate logistic regression model demonstrated that for each unit increment in the AIP, the prediabetes and diabetes prevalence increased 4.96-fold among female participants (OR 4.96, 95% CI 2.68, 9.18) but not among male participants. We found that the AIP was not related to the prevalence of prediabetes or diabetes (OR 1.41; 95% CI 0.87, 2.29) among males. There was an interaction between sex and the AIP (P for interaction < 0.0001).
Conclusions
This study showed that a higher AIP was significantly associated with an increased prevalence of prediabetes and diabetes, and the above relationships occurred only among women and not men.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01740-8.
Keywords: Atherogenic index of plasma, Prediabetes, Diabetes, Sex differences, Females
Introduction
In recent decades, type 2 diabetes (T2D), the most common and clinically important metabolic disease, has become a global epidemic and a major medical burden worldwide. According to the diabetes map released by the International Diabetes Federation (IDF), the number of adult diabetic patients worldwide reached 537 million in 2021, approximately 1 in 10 adults worldwide. The number of patients with diabetes increased by 74 million, or 16 percent, compared to 2019. It is estimated that the prevalence of diabetes will further increase to 12.2%, and the number of patients will increase to 783 million by 2025. Approximately 6.7 million people died from diabetes or diabetes complications in 2021, accounting for 12.2% of all deaths [1]. Prediabetes is defined as a concentration of blood glucose below the diabetes threshold but above the normal level, but it is related to the high risk of diabetes and its complications [2]. We must also pay attention to this disease state. More importantly, a large number of epidemiological studies have shown that in patients with prediabetes, the kidneys, blood vessels and heart are damaged [3, 4]. The physiological basis of the pathogenesis of diabetes or prediabetes is closely related to insulin resistance (IR) [5, 6]. Therefore, we should prevent and treat diabetes and prediabetes according to their pathological mechanisms to reduce the clinical complications related to diabetes and the occurrence and development of cardiovascular diseases (CVDs).
The atherogenic index of plasma (AIP) was first proposed by Dobiásová as a biomarker for plasma atherosclerosis [7]. An increasing number of studies have shown that the AIP is a powerful marker for predicting CVD risks [8–10]. nationwide population-based cohort study showed that the AIP was significantly associated with cardiovascular risks after adjusting for traditional risk factors [11], and was an independent factor for the prediction of developing cardiovascular events and their related mortality [12].The results of a prospective cohort study conducted by Zhang et al. among 5538 nondiabetic patients with coronary heart disease after percutaneous coronary intervention showed that compared with patients in the lower AIP group, patients in the higher AIP group had a 37% (HR: 1.37, 95% CI 1.04–1.81; p = 0.025) risk of major adverse cardiac events (MACEs), and the relationship between the hazard ratio and the AIP appeared to be J-shaped [13]. Moreover, the AIP is an independent predictive marker for rapid plaque progression beyond traditional risk factors [14] and might be a strong biomarker that could be used to predict the risk of cardiovascular events among patients with T2DM [15]. Moreover, studies have shown that the AIP is a predictor of diabetes [16–21]. However, no study has been conducted to explore the relationship between the AIP and prediabetes. In fact, the number of patients with prediabetes is much larger than the number of patients with diabetes [22]. Early detection of patients with prediabetes is of great importance for preventing their progression to diabetes.
Data from the National Health and Nutrition Examination Survey were used to conduct this cross-sectional study. The aim of this study was to examine sex-specific differences in the effect of the AIP on prediabetes and diabetes.
Methods
Study design and population
The data used in this study were all from the 2011–2018 National Health and Nutrition Examination Survey (NHANES) database. The NHANES is a continuous survey that selects a group of representative American people by means of complex and multistage probability sampling and aims to evaluate the health and nutrition status of American adults and children. The Ethics Review Committee of the National Center for Health Statistics (NCHS) approved the NHANES research plan. All the research participants provided written informed consent. More detailed information can be found at www.cdc.gov/nchs/nhanes/irba98.htm.
We conducted a cross-sectional study using data from the NHANES (2011–2018) study among patients ≥ 18 years of age (n = 10,978). The exclusion criterion was patients with missing AIP (n = 859), hemoglobin A1c or fasting plasma glucose (n = 20) data. Finally, 10099 subjects were analyzed.
Definitions of the exposure and outcome variables
The exposure variable was the AIP, which was defined as log10 (triglycerides/high-density lipoprotein cholesterol) with triglycerides and high-density lipoprotein cholesterol expressed in mmol/L [16]. The outcome variables included prediabetes and diabetes. Prediabetes was defined as any one of the following: 5.7% ≤ hemoglobin A1c (HbA1c) < 6.5%, fasting plasma glucose (FPG) between 5.6 mmol/L and 7.0 mmol/L, and a 2 h FPG value between 7.8 mmol/L and 11.1 mmol/L during an oral glucose tolerance test (OGTT) in accordance with the 2013 American Diabetes Association guidelines [23].Diabetes was defined as a self-reported physician diagnosis of diabetes or having an HbA1c level ≥ 6.5%, FPG level ≥ 7 mmol/L, or 2 h OGTT plasma glucose level ≥ 11.1 mmol/L. The combination of prediabetes and diabetes was regarded as an end event in this analysis.
Potential covariates
Covariables in this study included continuous variables (age, body mass index (BMI, kg/m2), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), triglycerides (TGs, mg/dL), total cholesterol (TC, mg/dL), estimated glomerular filtration rate (eGFR, mL/min/1.73 m2), and poverty income ratio) and categorical variables (sex, race, smoking status, alcohol intake, antihypertensive drugs, and lipoprotein-lowering drugs). The interviews collected demographic information on age, sex (male or female), poverty income ratio, race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other), smoking status (never, former, or current), alcohol intake (< 3, ≥ 3 drinks per day), antihypertensive drugs (no, yes), and lipoprotein-lowering drugs (no, yes). Anthropometric indicators included height, weight and blood pressure (BP). Body height and weight were collected without shoes and in light clothing and measured with a medical scale. Body mass index (BMI) in kg/m2 was calculated as weight divided by height squared. According to the standard blood pressure measurement protocol recommended by the American Heart Association at that time, a mercury sphygmomanometer was used to measure blood pressure. Three blood pressure readings were obtained continuously from the same arm. This study defined SBP and DBP as the average of three blood pressure measurements. Every subject was asked to provide an overnight rapid venous blood sample.
Fasting venous blood was drawn from each subject for TC and TG measurement. The formula used for the estimated glomerular filtration rate (eGFR) was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [24].
Statistical analysis
The statistical analyses of this study were performed via R, version 4.2.0 (R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). The level of statistical significance was set at p < 0.05.
Baseline characteristics are presented as the means and standard deviations (SDs) or medians (interquartile ranges) (IQRs) for continuous variables and proportions for categorical variables. The quartiles of AIP groups were compared with Student’s t test, the Mann–Whitney U test, and the chi-square test for categorical variables. Covariates that were known to be traditional or suspected risk factors for prediabetes and diabetes or the estimates of the AIP on prediabetes and diabetes changed by more than 10% in the multivariate logistic regression models [25]. The relationship between the AIP and prediabetes and diabetes in the overall population and among males and females was investigated with multivariate logistic regression analysis. Model 1 represented the unadjusted data. In Model 2, the data were adjusted for age, sex (only for the overall population), BMI, race, SBP, DBP, TGs, and TC. In Model 3, the results were adjusted for age, sex (only for the overall population), BMI, race, SBP, DBP, TGs, TC, eGFR, poverty income ratio, current smoking, alcohol intake, antihypertensive drugs, and lipoprotein-lowering drugs. The results from the logistic regression analysis are presented as odds ratios (ORs) and 95% confidence intervals (CIs). Moreover, the effect dose response between the AIP and prediabetes and diabetes was evaluated by a generalized additive model and fitting curve (penalized spline method). The interaction of the AIP with different sexes on prediabetes and diabetes was assessed by including stratification analysis and interaction tests in the regression model.
Results
There were 10,099 participants, including 4913 males and 5186 females. The median age (mean ± SD) was 48.51 ± 18.42 years, and the average value (SD) of the AIP was − 0.09 (0.34). The prevalence of prediabetes was 40.24%, and that of diabetes was 21.32%. Table 1 shows participant demographic and clinical characteristics by quartiles of the baseline AIP. In the four AIP groups, all variables were statistically significant. Compared with the participants in the lower AIP group, the participants in the AIP Q4 group were often male, older, non-Hispanic white, current smokers and drinkers, had higher levels of SBP, DBP, TC, and TGs, had a lower poverty income ratio and eGFR, and had a higher proportion of antihypertensive drug and lipid-lowering drug use.
Table 1.
The demographic and clinical characteristics of the patients by quartiles of baseline AIP
| Variablea | AIP Quartiles | P value | |||
|---|---|---|---|---|---|
| Q1(< − 0.32) | Q2(− 0.32 to < − 0.10) | Q3(− 0.10 to < 0.12) | Q4(≥ 0.12) | ||
| Participants | 2522 | 2524 | 2528 | 2525 | |
| Males, N (%) | 916 (36.32%) | 1123 (44.49%) | 1292 (51.11%) | 1582 (62.65%) | < 0.001 |
| Age,year | 45.28 ± 19.34 | 47.96 ± 18.91 | 50.70 ± 18.13 | 50.11 ± 16.69 | < 0.001 |
| BMI, kg/m2 | 26.33 ± 6.57 | 28.31 ± 6.91 | 30.18 ± 7.05 | 31.61 ± 6.83 | < 0.001 |
| Race | < 0.001 | ||||
| Non-Hispanic White, N (%) | 858 (34.02%) | 900 (35.66%) | 948 (37.50%) | 1084 (42.93%) | |
| Non-Hispanic Black, N (%) | 821 (32.55%) | 640 (25.36%) | 437 (17.29%) | 276 (10.93%) | |
| Mexican American, N (%) | 216 (8.56%) | 329 (13.03%) | 405 (16.02%) | 447 (17.70%) | |
| Other Hispanic, N (%) | 195 (7.73%) | 244 (9.67%) | 314 (12.42%) | 319 (12.63%) | |
| Other races, N (%) | 432 (17.13%) | 411 (16.28%) | 424 (16.77%) | 399 (15.80%) | |
| Current smoking, N (%) | 508 (20.46%) | 528 (21.26%) | 602 (24.16%) | 678 (27.06%) | < 0.001 |
| alcohol intake,drinks per day | < 0.001 | ||||
| < 3 | 1124 (69.25%) | 1019 (65.74%) | 939 (61.41%) | 907 (59.17%) | |
| ≥ 3 | 499 (30.75%) | 531 (34.26%) | 590 (38.59%) | 626 (40.83%) | |
| SBP, mmHg | 120.27 ± 18.54 | 122.95 ± 19.13 | 125.28 ± 18.91 | 125.81 ± 17.55 | < 0.001 |
| DBP, mmHg | 68.21 ± 11.32 | 68.94 ± 12.06 | 70.29 ± 11.72 | 71.90 ± 12.01 | < 0.001 |
| Poverty income ratio | 2.58 ± 1.67 | 2.47 ± 1.64 | 2.40 ± 1.63 | 2.32 ± 1.57 | < 0.001 |
| TC, mg/dL | 179.59 ± 37.78 | 183.15 ± 37.80 | 188.26 ± 41.93 | 201.16 ± 45.18 | < 0.001 |
| TG, mg/dL | 51.00 (41.00–61.00) | 79.00 (68.00–92.00) | 112.00 (98.00–129.00) | 187.00 (154.00–244.00) | < 0.001 |
| eGFR, mL/min/1.73 m2 | 101.11 ± 24.31 | 96.83 ± 24.26 | 93.95 ± 24.08 | 92.71 ± 24.15 | < 0.001 |
| Glucose metabolism state | < 0.001 | ||||
| none prediabetes | 1398 (55.43%) | 1100 (43.58%) | 811 (32.08%) | 573 (22.69%) | |
| prediabetes | 865 (34.30%) | 1021 (40.45%) | 1077 (42.60%) | 1101 (43.60%) | |
| diabetes | 259 (10.27%) | 403 (15.97%) | 640 (25.32%) | 851 (33.70%) | |
| Antihypertensive drugs | 104 (4.12%) | 144 (5.71%) | 171 (6.76%) | 166 (6.57%) | < 0.001 |
| Lipoprotein-lowering drugs | 352 (13.96%) | 497 (19.69%) | 624 (24.68%) | 660 (26.14%) | < 0.001 |
AIP atherogenic index of plasma, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, TG triglycerides, eGFR estimated glomerular filtration rate
aData are presented as number (%) or mean ± standard deviation
Regardless of whether the confounding factors were adjusted for, the AIP had a significant positive correlation with prediabetes and diabetes among all participants. The AIP was further divided into quartiles, and the Q1 group was used as the reference group to evaluate the relationship between the AIP and prediabetes and diabetes. After adjusting for age, sex, BMI, race, SBP, DBP, TGs, TC, eGFR, poverty income ratio, current smoking, alcohol intake, antihypertensive drugs, and lipoprotein-lowering drugs, compared with the Q1 reference group, the relative odds of prediabetes and diabetes of the participants in the Q2 (OR: 1.16; 95%: 0.97, 1.39), Q3 (OR: 1.46; 95%: 1.20, 1.78) and Q4 (OR: 1.95; 95%: 1.50, 2.55) groups increased linearly. With a P for trend < 0.0001, the AIP had a linear positive correlation with prediabetes and diabetes (Table 2). The results of multivariate regression analysis were consistent with those of the fitting curve (Fig. 1).
Table 2.
Relative odds of prediabetes and diabetes according to AIP in different models among all participantsa
| AIP | Events (%) | prediabetes and diabetes OR (95%CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Per 1 increment | 6217 (61.56%) | 5.46 (4.77, 6.25)*** | 2.85 (2.18, 3.74)*** | 2.49 (1.75, 3.54)*** |
| Quartile | ||||
| Q1(< − 0.32) | 1124 (44.57%) | 1 | 1 | 1 |
| Q2(-0.32 to < − 0.10) | 1424 (56.42%) | 1.61 (1.44, 1.80) *** | 1.25 (1.09, 1.43)** | 1.16 (0.97, 1.39) |
| Q3(− 0.10 to < 0.12) | 1717 (67.92%) | 2.63 (2.35, 2.95) *** | 1.57 (1.35, 1.82)*** | 1.46 (1.20, 1.78)*** |
| Q4(≥ 0.12) | 1952 (77.31%) | 4.24 (3.75, 4.79) *** | 2.10 (1.70, 2.59)*** | 1.95 (1.50, 2.55)*** |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | |
aValues are ORs (95% CIs) unless otherwise indicated
AIP Atherogenic index of plasma
*P < 0.05
**P < 0.01
***P < 0.001
Model 1 was adjusted for none
Model 2 was adjusted for age, sex, BMI, race, SBP, DBP, TG, TC
Model 3was adjusted for age, sex, BMI, race, SBP, DBP, TG, TC, eGFR, poverty income ratio, current smoking, alcohol intake, antihypertensive drugs, lipoprotein-lowering drugs
Fig. 1.
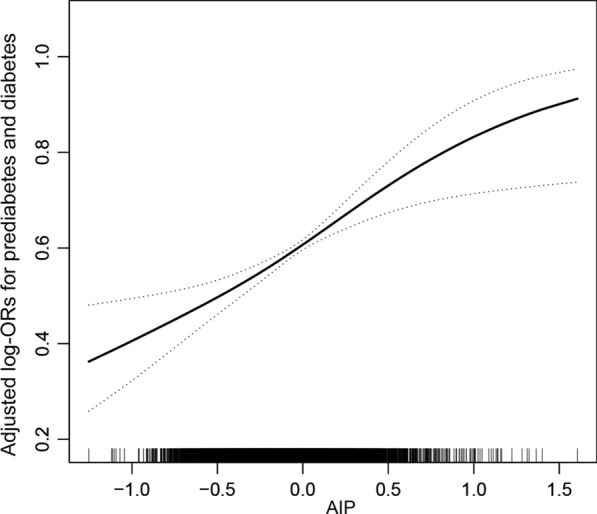
Association between AIP and the prevalence of prediabetes and diabetes. A linear association between AIP and the prevalence of prediabetes and diabetes was found (P < 0.05). The solid line and dashed line represent the estimated values and their corresponding 95% confidence interval. Adjustment factors included age, sex, BMI, race, SBP, DBP, TG, TC, eGFR, poverty income ratio, current smoking, alcohol intake, antihypertensive drugs, lipoprotein-lowering drugs
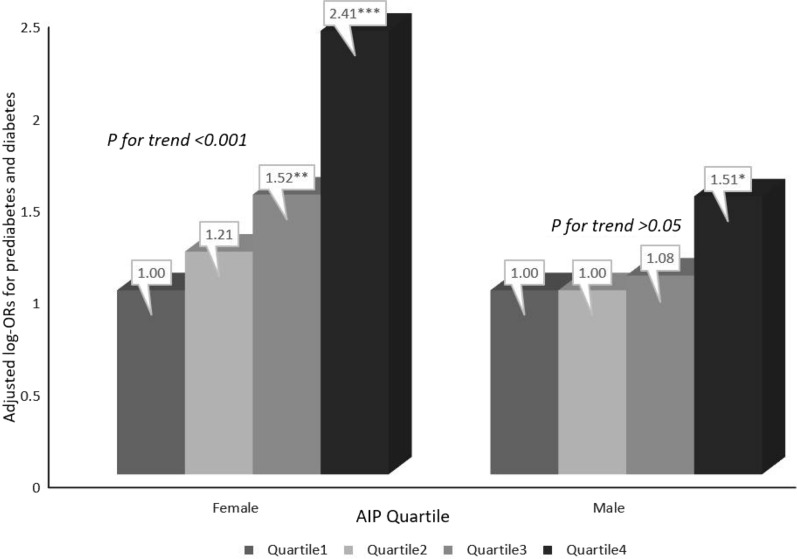
Table 3 shows the relationship between the AIP and prediabetes and diabetes among men and women; sex differences were found. With the increase in the AIP in three different models, the risk of prediabetes and diabetes among women was more significant than that among men (P for interaction < 0.0001). In addition, in fully adjusted Model 3, the AIP of male participants was not related to prediabetes or diabetes. Likewise, in addition to the AIP quartiles by sex, the influence of the AIP as a categorical variable on prediabetes and diabetes was analyzed. A multivariate regression analysis of the AIP, prediabetes and diabetes for the different sexes is shown in Fig. 2. Compared with the Q1 reference group, the relative odds of prediabetes and diabetes of the participants in the Q2 (OR: 1.21; 95%: 0.93, 1.58), Q3 (OR: 1.52; 95%: 1.11, 2.07) and Q4 (OR: 2.41; 95%: 1.53, 3.79) groups gradually increased among females (P for trend < 0.0001). However, we found that the fully adjusted Model 3 AIP for men was not related to prediabetes or diabetes, regardless of whether the AIP was a continuous variable or a categorical variable (P for trend = 0.055).
Table 3.
Relative odds of prediabetes and diabetes according to AIP in different models among male and female
| AIP | Events (%) | prediabetes and diabetes OR (95%CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Male | ||||
| Per 1 increment | 3274 (66.64%) | 3.20 (2.66, 3.86)*** | 1.72 (1.17, 2.53)** | 1.41 (0.87, 2.29) |
| Q1(< − 0.25) | 695 (56.64%) | 1 | 1 | 1 |
| Q2(−0.25 to < -0.03) | 762 (62.00%) | 1.25 (1.06, 1.47)** | 0.97 (0.80, 1.17) | 1.00 (0.78, 1.27) |
| Q3(− 0.03 to < 0.20) | 856 (69.71%) | 1.76 (1.49, 2.08)*** | 1.19 (0.97, 1.47) | 1.08 (0.83, 1.40) |
| Q4(≥ 0.02) | 961 (78.19%) | 2.74 (2.30, 3.27)*** | 1.59 (1.20, 2.12)** | 1.51 (1.07, 2.14)* |
| P for trend | < 0.0001 | 0.0022 | 0.055 | |
| Female | ||||
| Per 1 increment | 2943 (56.75%) | 8.47 (6.90, 10.39) *** | 4.26 (2.68, 6.78)*** | 4.96 (2.68, 9.18)*** |
| Q1(< − 0.37) | 481 (37.09%) | 1 | 1 | 1 |
| Q2(− 0.37 to < -0.17) | 652 (50.31%) | 1.72 (1.47, 2.01)*** | 1.33 (1.10, 1.61)** | 1.21 (0.93, 1.58) |
| Q3(− 0.17 to < 0.05) | 825 (63.66%) | 2.97 (2.53, 3.49)*** | 1.64 (1.31, 2.05)*** | 1.52 (1.11, 2.07)** |
| Q4(≥ 0.05) | 985 (75.94%) | 5.36 (4.52, 6.35)*** | 2.23 (1.60, 3.10)*** | 2.41 (1.53, 3.79)*** |
| P for trend | < 0.0001 | < 0.0001 | 0.0003 | |
| P value for interaction* | < 0.0001 | < 0.0001 | < 0.0001 | |
*P < 0.05
**P < 0.01
***P < 0.001
Model 1 was adjusted for none
Model 2 was adjusted for age, BMI, race, SBP, DBP, TG, TC
Model 3 was adjusted for age, BMI, race, SBP, DBP, TG, TC, eGFR, poverty income ratio, current smoking, alcohol intake, antihypertensive drugs, lipoprotein-lowering drugs
Fig. 2.
Relative odds of prediabetes and diabetes according to AIP quartile among male and female#.#The adjustment factors included age, BMI, race, SBP, DBP, TG, TC, eGFR, poverty income ratio, current smoking, alcohol intake, antihypertensive drugs, lipoprotein-lowering drugs. *P<0.05 **P<0.01 ***P<0.001
Discussion
This was a large-sample cross-sectional study based on 2011–2018 National Health and Nutrition Survey data that found that an increased AIP increased the risk of prediabetes and diabetes. Further analysis found that an excessive AIP only increased the risk of prediabetes and diabetes among women. This is the first study to evaluate the relationship between sex differences in the AIP and prediabetes and diabetes.
Previous studies have evaluated the relationship between the AIP and diabetes, and all of them have shown a positive correlation between the AIP and diabetes [16–20]. A meta-analysis of 15 case–control studies showed that the ability of the AIP to predict the risk of diabetes was better than that of other lipid components [21]. Li and colleagues conducted a cohort study in the general population of Taiwan Province among 7670 individuals to assess the relationship between AIP levels and diabetes risk among participants of different ages and sexes. The results showed that only participants aged 40–64 years had a higher diabetes risk, and no sex differences were found [20]. However, we did not find any age differences in our research. We divided age by 20-year categories. The AIP was positively correlated with prediabetes and diabetes in each age group, but it did not reach statistical significance because there were too few people under 20 and over 80 years of age (see Additional file 1: Table S1 for specific results). A total of 2676 individuals aged 28–80 years in the general population were selected for the Turkish Adult Risk Factor Study, and in the observation of the relationship between the AIP and hypertension, diabetes and metabolic syndrome, the final result showed that the AIP had an independent positive correlation with diabetes among both men and women [18].The cross-sectional study found significant sex differences, which may be due to the different characteristics of the study participants.
In fact, there are more patients with prediabetes, and prediabetes easily develops into diabetes. Therefore, in this study, we used the combination of prediabetes and diabetes as an outcome variable for the first time to observe the relationship between the AIP and both prediabetes and diabetes. This cross-sectional analysis found that the AIP had a significant linear positive correlation with prediabetes and diabetes, regardless of whether confounding factors were adjusted for. Compared with the lowest AIP group, the risk of prediabetes and diabetes in the highest AIP group increased significantly by 95%. We further evaluated the relationship between the AIP and prediabetes and diabetes by sex and found that there was a linear positive correlation between the AIP and outcome events among females but not among males. One accepted hypothesis is that the higher risk of diabetes among women in late adulthood is due to hormonal changes in menopause, that is, estrogen consumption [26, 27]. Moreover, there are also epidemiological studies that show that the risk of diabetes and its related complications is higher among women than among men as they age [28, 29].
The mechanism of the AIP in prediabetes and diabetes is not clear, but the following biological mechanisms can be explained. The AIP is calculated by combining TGs and HDL, so the levels of TGs and HDL in the human body are closely related to the pathogenesis of diabetes and prediabetes. High levels of TGs in plasma reduce the number and activity of insulin receptors on adipocytes and prevent insulin from binding to receptors by competing with glucose to enter cells, leading to diabetes, while lower HDL levels also lead to decreased insulin secretion and sensitivity [30].Abnormal blood lipid levels may cause insulin resistance (IR) by causing inflammation, endoplasmic reticulum stress and lipotoxicity [31]. Moreover, IR increases TG and plasma-free fatty acid levels, while HDL-C levels are decreased. Both are causal, which verifies the "vicious circle" hypothesis of diabetes development. As an early state of diabetes, prediabetes has the same risk factors as diabetes [32]. Some studies have shown that there are metabolic abnormalities in prediabetic patients, such as abnormal blood glucose levels, dyslipidemia, IR, a procoagulant state, endothelial dysfunction, oxidative stress and inflammation [33]. Therefore, the causes of prediabetes and diabetes by the AIP are still based on IR and dyslipidemia.
Limitations
There are some shortcomings in this study. First, this was a cross-sectional study, a design that is not as comprehensive as a cohort study. In addition, this study’s ability to explore the etiology hypothesis was limited, and its ability to test the etiology hypothesis and extrapolation was not sufficient. Other cohort studies are needed to verify the correlation between the AIP and prediabetes and diabetes. Second, confounding by unknown or unmeasurable factors could not be completely ruled out. Finally, due to the differences between countries, the results may not be extrapolated to other countries.
Perspectives and significance section
Sex differences exist throughout the life cycle, but their specific mechanisms and consequences are still unclear. Knowing the sex differences in risk factors for diabetes and prediabetes can help clinicians implement more personalized prevention strategies correctly.
Conclusions
This cross-sectional study was conducted among 10,099 American adults and showed a positive correlation between the AIP and prediabetes and diabetes, and the risk of prediabetes and diabetes increased gradually with the increase in the AIP. Moreover, we found an interaction between sex and the AIP and found that the AIP was positively correlated with prediabetes and diabetes only among women and not among men.
Supplementary Information
Additional file1: Table S1. Relative odds of prediabetes and diabetes according to AIP in different ages.
Acknowledgements
A special thanks to all of the NHANES participants who freely gave their time to make this and other studies possible.
Author contributions
YMS participated in literature search, study design, data collection, data analysis, data interpretation, and wrote the manuscript. YMS and MHW conceived of the study, and participated in its design, coordination, data collection and analysis. MHW participated in study design and provided the critical revision. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China, Regional Science Foundation (82060063), Jiangxi Youth Science Foundation (20202BABL216004).
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethical approval and consent to participate
All procedures performed in studies involving human participants were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yumeng Shi, Email: shiyumeng666@126.com.
Minghua Wen, Email: wmh618@126.com.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9 (th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Edwards CM, Cusi K. Prediabetes: a worldwide epidemic. Endocrinol Metab Clin North Am. 2016;45(4):751–764. doi: 10.1016/j.ecl.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Brannick B, Dagogo-Jack S. Prediabetes and cardiovascular disease: pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am. 2018;47(1):33–50. doi: 10.1016/j.ecl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald IA. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur J Nutr. 2016;55(Suppl 2):17–23. doi: 10.1007/s00394-016-1340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Franco OH, Lamballais S, Ikram MA, Schoufour JD, Muka T, Voortman T. Associations of specific dietary protein with longitudinal insulin resistance, prediabetes and type 2 diabetes: the rotterdam study. Clin Nutr. 2020;39(1):242–249. doi: 10.1016/j.clnu.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Dobiásová M. AIP–atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek. 2006;52(1):64–71. [PubMed] [Google Scholar]
- 8.Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17(1):197. doi: 10.1186/s12944-018-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu TT, Zheng YY, Yang YN, Li XM, Ma YT, Xie X. Age, sex, and cardiovascular risk attributable to lipoprotein cholesterol among Chinese individuals with coronary artery disease: a case-control study. Metab Syndr Relat Disord. 2019;17(4):223–231. doi: 10.1089/met.2018.0067. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019;50(5):285–294. doi: 10.1016/j.arcmed.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Cho YK, Kim YJ, Jung CH, Lee WJ, Park JY, Huh JH, Kang JG, Lee SJ, Ihm SH. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol. 2022;21(1):81. doi: 10.1186/s12933-022-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi M, Heshmat-Ghahdarijani K, Talaei M, Safaei A, Sarrafzadegan N, Roohafza H. The predictive value of atherogenic index of plasma in the prediction of cardiovascular events; a fifteen-year cohort study. Adv Med Sci. 2021;66(2):418–423. doi: 10.1016/j.advms.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Li C, Yang J, Seery S, Qi Y, Wang W, Zhang K, Shao C, Tang YD. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: a prospective study of the long-term outcomes in China. Cardiovasc Diabetol. 2022;21(1):29. doi: 10.1186/s12933-022-01459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won KB, Heo R, Park HB, Lee BK, Lin FY, Hadamitzky M, Kim YJ, Sung JM, Conte E, Andreini D, et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021;324:46–51. doi: 10.1016/j.atherosclerosis.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Fu L, Zhou Y, Sun J, Zhu Z, Xing Z, Zhou S, Wang Y, Tai S. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):201. doi: 10.1186/s12933-021-01393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onat A, Can G, Kaya H, Hergenç G. "Atherogenic index of plasma" (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4(2):89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Yi Q, Ren Z, Bai G, Zhu S, Li S, Li C, Wu H, Zhu Y, Song P. The longitudinal effect of the atherogenic index of plasma on type 2 diabetes in middle-aged and older Chinese. Acta Diabetol. 2022;59(2):269–279. doi: 10.1007/s00592-021-01801-y. [DOI] [PubMed] [Google Scholar]
- 18.Hu YM, Tian HM, Liu R, Chen X. Atherogenic index of plasma is associated with carotid intima-media thickness in patients with type 2 diabetes mellitus. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35(5):696–698. [PubMed] [Google Scholar]
- 19.Manohar SM, Vaikasuvu SR, Deepthi K, Sachan A, Narasimha SR. An association of hyperglycemia with plasma malondialdehyde and atherogenic lipid risk factors in newly diagnosed Type 2 diabetic patients. J Res Med Sci. 2013;18(2):89–93. [PMC free article] [PubMed] [Google Scholar]
- 20.Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11(1):9900. doi: 10.1038/s41598-021-89307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu XW, Deng FY, Lei SF. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. 2015;9(1):60–67. doi: 10.1016/j.pcd.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Vatcheva KP, Fisher-Hoch SP, Reininger BM, McCormick JB. Sex and age differences in prevalence and risk factors for prediabetes in mexican-Americans. Diabetes Res Clin Pract. 2020;159:107950. doi: 10.1016/j.diabres.2019.107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349. doi: 10.2105/AJPH.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. 2011;203(1):259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurka MJ, Vishnu A, Santen RJ, DeBoer MD. Progression of metabolic syndrome severity during the menopausal transition. J Am Heart Assoc. 2016;5(8):3609. doi: 10.1161/JAHA.116.003609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Ritter R, Sep S, van der Kallen C, van Greevenbroek M, de Jong M, Vos RC, Bots ML, Reulen J, Houben A, Webers C, et al. Sex differences in the association of prediabetes and type 2 diabetes with microvascular complications and function: the maastricht study. Cardiovasc Diabetol. 2021;20(1):102. doi: 10.1186/s12933-021-01290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, Beiser A, Borenstein AR, Crane PK, Haan M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2 3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Kelley DE. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus. Curr Diab Rep. 2002;2(3):216–222. doi: 10.1007/s11892-002-0086-2. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Fu J, Koonen DP, Kuivenhoven JA, Snieder H, Hofker MH. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance. Atherosclerosis. 2014;233(1):130–138. doi: 10.1016/j.atherosclerosis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui S, Zainal H, Harun SN, Sheikh Ghadzi SM, Ghafoor S. Gender differences in the modifiable risk factors associated with the presence of prediabetes: a systematic review. Diabetes Metab Syndr. 2020;14(5):1243–1252. doi: 10.1016/j.dsx.2020.06.069. [DOI] [PubMed] [Google Scholar]
- 33.Mahat RK, Singh N, Arora M, Rathore V. Health risks and interventions in prediabetes: a review. Diabetes Metab Syndr. 2019;13(4):2803–2811. doi: 10.1016/j.dsx.2019.07.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file1: Table S1. Relative odds of prediabetes and diabetes according to AIP in different ages.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.