Abstract
Objective
To analyze the incidence and risk of hypertension associated with poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in cancer patients and provide reference for clinicians.
Methods
We used R software to conduct a meta-analysis of phase II/III randomized controlled trials (RCT) on PARP inhibitors for cancer treatment published in PubMed, Embase, Clinical Trials, Cochrane Library and Web of Science from inception to July 29th, 2022.
Results
We included 32 RCTs with 10,654 participants for this meta-analysis. For total PARP inhibitors, the incidence and risk ratio of all-grade hypertension were 12% and 1.22 (95% CI: 0.91–1.65, P = 0.19, I2 = 81%), and the incidence and risk ratio of grade 3–4 hypertension were 4% and 1.24 (95% CI: 0.74–2.08, P = 0.42, I2 = 68%). Compared with the control group, the niraparib group, olaparib 800 mg/day group, and olaparib plus cediranib group increased the risk of any grade and grade 3–4 hypertension, while the veliparib group and rucaparib group did not increase the risk of any grade and grade 3–4 hypertension, and olaparib 200 mg-600 mg/day group (exclude olaparib plus cediranib regime) reduced the risk of any grade and grade 3–4 hypertension.
Conclusion
Olaparib 200-600 mg/day (excluding olaparib plus cediranib regimen) may be the most suitable PARP inhibitor for cancer patients with high risk of hypertension, followed by veliparib and rucaparib. Niraparib, olaparib 800 mg/day and olaparib combined with cediranib may increase the risk of developing hypertension in cancer patients, clinicians should strengthen the monitoring of blood pressure in cancer patients and give medication in severe cases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10571-5.
Keywords: PARP inhibitors, Hypertension, Niraparib, Olaparib, Meta-analysis
Introduction
Cancer is a serious threat to human health, causing more than 8 million deaths each year [1]. Targeted therapy with high efficiency and low toxicity is the main strategy for the treatment of advanced cancer, which can specifically kill cancer cells with minimal harm to normal cells. For targeted therapy of cancer, it is of great significance to identify new drug targets and develop new targeted drugs [2]. DNA damage response (DDR) is a complex signal pathway network involving DNA damage repair, cell cycle checkpoint and apoptosis, which has become an important target in the development of new targeted therapeutic drugs [3]. In the past few years, DNA damage response and its related signal pathways have attracted considerable attention, and a large number of DDR inhibitors have emerged, such as PARP inhibitors, ataxia telangiectasia-mutated (ATM) inhibitors, ataxia telangiectasia and Rad3-related (ATR) kinase inhibitors and checkpoint kinase 1/2 (CHK1/2) inhibitors, etc. [4].
PARP inhibitors are currently the most widely studied DDR inhibitors, which can cause simultaneous impairment of two different DDR pathways (homologous recombination and base excision repair) by inhibiting the PARP protein, leading to apoptotic death of cancer cells through a mechanism known as "synthetic lethality" [5]. The PARP inhibitors developed so far include veliparib, rucaparib, olaparib, talazoparib, niraparib, pamiparib, iniparib, fuzuloparib etc. Surprisingly, It has been found that PARP inhibitors alone or in combination (e.g. platinum drugs) show promising clinical efficacy in various cancer patients, especially those with impaired homologous recombination [6, 7]. From 2014 to August 25, 2022, olaparib, rucaparib, talazoparib and niraparib have been clinically approved by FDA and/or the European Medicines Agency (EMA) for the treatment of various cancers (e.g. ovarian cancer, breast cancer, lung cancer) [8–11]. Niraparib has even been approved for the first-line maintenance treatment of platinum-responsive advanced ovarian cancer, and olaparib has been approved for the first-line maintenance treatment of advanced ovarian cancer with BRCA mutation and metastatic pancreatic cancer with gBRCA mutation [12–14]. In addition, fuzuloparib and pamiparib have recently been approved for ovarian, fallopian tube or primary peritoneal cancer in china [15, 16].
PARP inhibitors, like other targeted therapeutic, are associated with many adverse reactions, among which nausea, vomiting, fatigue, anemia, thrombocytopenia, neutropenia and hypertension are frequently reported. Interestingly, the reported incidence of PARP-related hypertension in clinical trials varies widely, ranging from approximately 1% to 76%, and the reported severity also varies greatly, ranging from grade 1 to grade 4, even serious hypertension. The reasons for the above differences are unclear, and it is also unclear whether there are differences among different PARP inhibitors, different cancer types and different treatment regimes. Hypertension is the leading cause of attributable deaths and burden of disease globally, which is also one of the important preventable risk factors for cardiovascular disease [17]. For clinicians, it is necessary to have a deep understanding of PARP inhibitor-related hypertension in cancer patients, so as to minimize the risk and harm of PARP inhibitor-related hypertension and ensure the maximum benefit of cancer patients. Given this background, we conducted a comprehensive meta-analysis of published Phase II and III RCTs of PARP inhibitors in the treatment of cancer to determine the incidence and risk of PARP inhibitors and to analyze the differences in the risk of hypertension among different PARP inhibitors, different cancer types and different treatment regimens. We hope to provide reference for clinicians to reasonably use PARP inhibitors and manage hypertension related to PARP inhibitors.
Methods
This study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.
Literature search
We searched PubMed, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov databases to identify relevant II/III randomised controlled trials published from inception to July 29, 2022, without language restrictions. We searched for the following keywords: veliparib, rucaparib, olaparib, talazoparib, niraparib, pamiparib, iniparib, fuzuloparib, PARP inhibitor, and used the RCTs filter or searched for randomly or randomized or randomization or random in the full text to identify possible RCTs. In addition, we have reviewed the references of the retrieved literature to identify any possible relevant studies.
Selection criteria
We searched for phase II or III RCTs of PARP inhibitors in the treatment of cancer patients. Inclusion criteria were based on the PICO-framework. Population (P): cancer patient. Intervention (I): Treatments containing PARP inhibitors. Comparison (C): Placebo or treatments without PARP inhibitors. Outcomes (O): any grade hypertension and grade 3–4 hypertension assessed according to the National Cancer Institute's Common Terminology Standard for Adverse Events (CTCAE) (version 3 or 4).
The exclusion criteria were as follows: (a) non-randomized controlled trials; (b) review and guideline; (c) trails with unavailable study data; (d) investigation; (e) conference articles; (f) both arms contain PARP inhibitors; (f) Phase I study. When there is a dispute between two reviewers, the decision is made by the third reviewer (YL).
Data extraction
We extracted data from articles, supplementary documents and ClinicalTrials.gov. Two reviewers (XC and XX) independently extracted the following information: author/year, national clinical trial (NCT) number, nation, study phase, interventions, sample size, median age, median treatment duration, median follow up duration and cancer type.
Quality assessment
According to the Cochrane Collaboration guidelines, we assessed the risk of bias for included RCTs from seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other types. The evaluation results are low, high and unclear, indicating low risk of bias, high risk of bias and unclear risk of bias, respectively.
Statistical analysis
All statistical analyses in our meta-analysis were performed by R software(version 4.0.2). To calculate the incidence of any grade hypertension and grade 3–4 hypertension, we determined the number of patients with any grade hypertension and grade 3–4 hypertension in patients receiving PARP inhibitors alone or in combination in each study and the total number of patients receiving PARP inhibitors alone or in combination. Freeman-Tukey double arcsine transformation was used to stabilize the variance when calculating the proportion of patients and 95% confidence intervals (CIs). Analyzing the risk of any grade hypertension and the risk of grade 3–4 hypertension associated with PARP inhibitors in cancer patients is our second objective. Risk ratio (RR) and 95% CI were used to determine the risk of hypertension with PARP inhibitors group compared to control group. Both random-effects(Mantel–Haenszel method) and fixed-effects models(Mantel–Haenszel method) were used to draw forest plots. We used Cochran 's Q test to assess heterogeneity among studies and the inconsistency index (I2 test) to assess the degree of heterogeneity. If there was no statistical heterogeneity among the studies (I2 < 50%), the fixed effects model was used for analysis; otherwise, the random effect model was used for analysis. Study exclusions and subgroup analyses were used to identify the main sources of heterogeneity. Publication bias was evaluated by visual inspection of funnel plots and Begg's tests. P < 0.05 was statistically significant.
Results
Selection of Eligible Studies
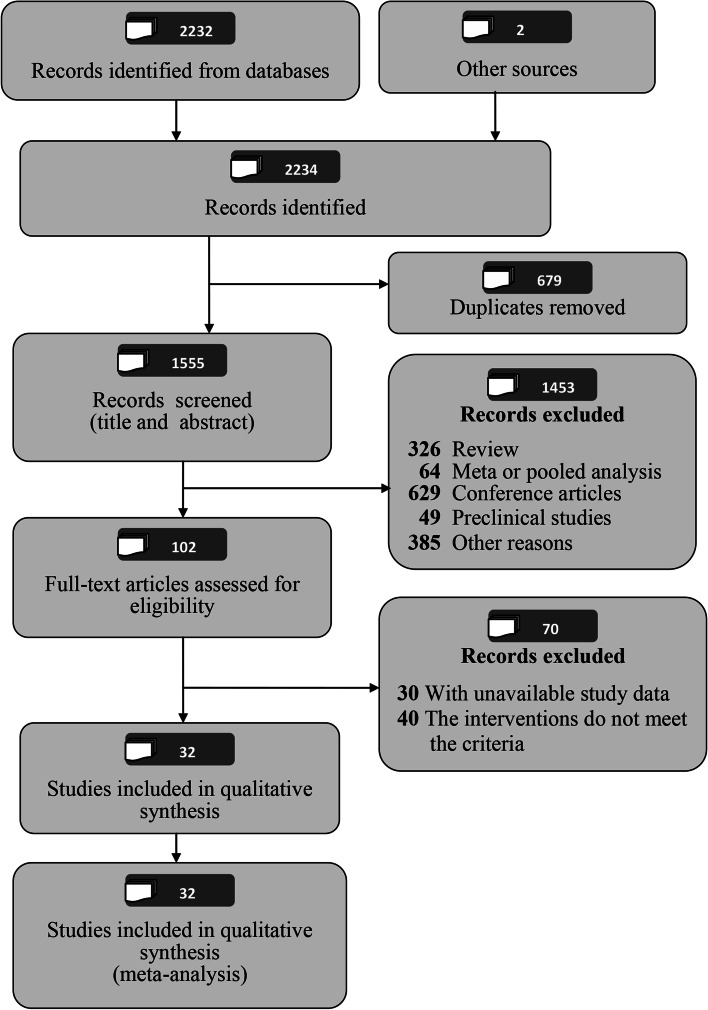
According to the search strategy, a total of 2234 articles were identified. First, we removed 679 duplicate articles with EndNote software. Then, we excluded 1453 articles after screening the title and abstract, and excluded 70 articles after reading the full text. Ultimately, 32 articles [18–49] were eligible for analysis. Figure 1 shows a flow chart depicting the articles selection process.
Fig. 1.
The PRISMA flowchart shows the selection process of the systematic review
Characteristics of Eligible Studies
This meta-analysis included 10,654 patients with ovarian, lung, breast and other cancers from 16 phase II [18–26, 31, 35, 41, 43, 47–49] studies and 16 phase III studies [27–30, 32–34, 36–40, 42, 44–46]. 6631 participants from the PARP inhibitor group received five PARP inhibitors niraparib (N = 4), olaparib (N = 14), veliparib (N = 10), rucaparib (N = 3),and iniparib (N = 1), alone or in combination with other anticancer drugs, and 4023 participants from the control group received placebo, paclitaxel, carboplatin, gemcitabine, and other anticancer drugs. The median duration of treatment with PARP inhibitors reported in the included studies ranged from 44 days to 14.7 months. The characteristics of the included studies are shown in Tables 1 and 2.
Table 1.
Characteristics of the RCTs and patients included in the meta-analysis
| Number | Author/year | NCT Number | Nation | Study phase | Interventions | Sample size | Median age (P/C) years | Median treatment duration (P/C) | Cancer type | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PARP group | Control group | PARP inhibitor group | Control group | ||||||||
| 1 |
Kaye [18] et.al 2011 |
NCT00628251 | United States, Australia, etc | II | Olaparib (400 mg/day) | Liposomal doxorubicin | 32 | 32 | 58.5/53 | NA | Ovarian cancer |
| Olaparib (800 mg/day) | 32 | 53.5/53 | NA | Ovarian cancer | |||||||
| 2 |
Ledermann [19] et.al 2012 |
NCT00753545 | United States, Austria, etc | II | Olaparib (800 mg/day) | Placebo | 136 | 128 | 58/59 | 206.5/141 days | Ovarian cancer |
| 3 |
Pusztai [20] et.al 2021 |
NCT01042379 | United States | II |
Olaparib (200 mg/day) + Durvalumab + Paclitaxel + Doxorubicin + Cyclophosphamide |
Paclitaxel + Doxorubicin + Cyclophosphamide | 73 | 299 | 46/48 | NA | Breast cancer |
| 4 |
Rugo [21] et.al 2016 |
II |
Veliparib (100 mg/day) + Carboplatin + Paclitaxel |
Carboplatin + Paclitaxel | 72 | 44 | 48.5/47.5 | 182/165 days | Breast Cancer | ||
| 5 |
Novello [22] et.al 2014 |
NCT01086254 | France, Germany, etc | II | Iniparib (11.2 mg/kg/week) + Gemcitabine + Cisplatin | Gemcitabine + Cisplatin | 78 | 39 | 59/58 | 15/13.9 weeks | Lung cancer |
| 6 |
Han [23] et.al 2018 |
NCT01506609 | United States, Australia, etc | II |
Veliparib (240 mg/day) + Carboplatin + Paclitaxel |
Placebo + Carboplatin + Paclitaxel |
93 | 96 | 44/46 | 36/30 weeks | Breast Cancer |
|
Veliparib (80 mg/day) + Temozolomide |
93 | 46/46 | 18/30 weeks | Breast Cancer | |||||||
| 7 |
Hussain [24] et.al 2017 |
NCT01576172 | United States | II | Veliparib (600 mg/day) + Abiraterone acetate + Prednisone | Abiraterone acetate + Prednisone | 79 | 74 | 68/69 | 36/36 weeks | Prostate Cancer |
| 8 |
Owonikoko [25] et.al 2020 |
NCT01642251 | United States | II |
Veliparib (200 mg/day) + Etoposide + Cisplatin |
Placebo + Etoposide + Cisplatin |
66 | 66 | 66/64 | NA | Lung Cancer |
| 9 |
Fennell [26] et.al 2022 |
NCT01788332 | United Kingdom | II | Olaparib (600 mg/day) | Placebo | 31 | 38 | 65/63 | 12/12 weeks | Lung cancer |
| 10 |
Banerjee [27] et.al 2021 |
NCT01844986 | United States, Canada, etc | III | Olaparib (600 mg/day) | Placebo | 260 | 130 | NA | 24.6/13.9 months | Ovarian cancer |
| 11 |
Mirza [28] et.al 2016 |
NCT01847274 | United States, Italy, etc | III | Niraparib (300 mg/day) | Placebo | 367 | 179 | NA | NA | Ovarian cancer |
| 12 |
Bang [29] et.al 2017 |
NCT01924533 | China, Japan, etc | III | Olaparib (200 mg/day) + Paclitaxel | Placebo + Paclitaxel | 262 | 259 | 58/59 | 73.5/59 days | Gastric cancer |
| 13 |
Ledermann [30] et.al 2021 |
NCT01968213 | United States, Australia, etc | III | Rucaparib (1200 mg/day) | Placebo | 372 | 189 | 61/62 | 8.3/5.5 months | Ovarian cancer |
| 14 |
Clarke [31] et.al 2018 |
NCT01972217 | United States, Canada, etc | II | Olaparib (600 mg/day) + Abiraterone | Pacebo + Abiraterone | 71 | 71 | 70/67 | 309/253 days | Prostate cancer |
| 15 |
Loibl [32] et.al 2018 |
NCT02032277 | United States, Australia, etc | III | Veliparib (100 mg/day) + Paclitaxe + Carboplatin |
Veliparib placebo + Paclitaxel + Carboplatin |
313 | 158 | 51/49 | 89/85.5 days | Breast cancer |
|
Veliparib placebo + Paclitaxel + Carboplatin placebo |
157 | 51/50 | 89/84 days | Breast cancer | |||||||
| 16 |
Diéras [33] et.al.2020 |
NCT02163694 | United States, Australia, etc | III | Veliparib (240 mg/day) + Carboplatin + Paclitaxel | Placebo + Carboplatin + Paclitaxel | 336 | 171 | 47/45 | NA | Breast cancer |
| 17 |
Golan [34] et.al 2020 |
NCT02184195 | United States, Canada, etc | III | Olaparib (600 mg/day) | Placebo | 90 | 61 | 57/57 | 6/3.7 months | Pancreatic Cancer |
| 18 |
Gorbunova [35] et.al 2019 |
NCT02305758 | North America, Australia etc | II |
Veliparib (400 mg/day) + Irinotecan + Leucovorin + Fluorouracil infusion ± Bevacizumab |
Irinotecan + Leucovorin + Fluorouracil bolus + Fluorouracil infusion ± Bevacizumab | 65 | 65 | 59/64 | NA | Colorectal Cancer |
| 19 |
Liu [36] et.al 2022 |
NCT02446600 | United States, Canada, Japan | III | Olaparib (600 mg/day) | Platinum-based chemotherapy | 187 | 167 | NA | NA | Ovarian, Fallopian Tube, or Primary Peritoneal Cancer |
| Olaparib (400 mg/day) + Cediranib | 183 | NA | NA | Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | |||||||
| 20 |
Coleman [37] et.al 2019 |
NCT02470585 | United States, Australia, etc | III | Veliparib (300 mg/day) + Carboplatin + Paclitaxel + followed by veliparib (600 mg or 800 mg/day) maintenance |
Placebo + Carboplatin + Paclitaxel + followed by Placebo maintenance |
377 | 371 | 62/62 | NA | Ovarian, Fallopian Tube, or Primary Peritoneal Cancer |
| Veliparib (300 mg/day) + Carboplatin + Paclitaxel + followed by Placebo maintenance | 376 | 62/62 | NA | Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | |||||||
| 21 |
Ray-Coquard [38] et.al 2019 |
NCT02477644 | Austria, Belgium, etc | III |
Olaparib (600 mg/day) + Bevacizumab |
Placebo + Bevacizumab | 535 | 267 | 61/60 | 17.3/15.6 moths | Ovarian cancer |
| 22 |
González-Martín [39] et.al 2019 |
NCT02655016 | United States, France, etc | III | Niraparib (200 mg or 300 mg/day) | Placebo | 484 | 244 | 62/62 | NA | Ovarian cancer |
| 23 |
Kristeleit [40] et.al 2022 |
NCT02855944 | United States, Brazil, etc | III | Rucaparib (1200 mg/day) | Chemotherapy (administered per institutional guidelines) | 232 | 113 | 58/59 | 7.3/3.6 months | Ovarian cancer |
| 24 |
Chiorean [41] et.al 2021 |
NCT02890355 | United States | II |
Veliparib (400 mg/day) + Irinotecan + Folinic acid + 5-Fluorouracil infusion |
Irinotecan + Folinic acid + 5-Fluorouracil bolus + 5-Fluorouracil infusion |
56 | 50 | 67/67 | 8/10 weeks | Pancreatic Cancer |
| 25 |
Bono [42] et.al 2020 |
NCT02987543 | United States, Canada, etc | III | Olaparib (600 mg/day) | Physician’s choice of enzalutamide or abiraterone | 256 | 130 | NA | 7.4/3.9 months | Prostate Cancer |
| 26 |
Colombo [43] et.al 2022 |
NCT03314740 | Italy | II | Olaparib (600 mg/day) every day + Cediranib every day | Paclitaxel | 41 | 28 | 64.2/62.5 | NA | Ovarian cancer |
| Olaparib (600 mg/day) every day + Cediranib 5 days a week | 41 | 59.9/62.5 | NA | Ovarian cancer | |||||||
| 27 |
Ai [44] et.al 2021 |
NCT03516084 | China | III | Niraparib (300 or 200 mg/day) | Placebo | 125 | 60 | NA | 44/42.5 days | Lung Cancer |
| 28 |
Monk [45] et.al 2022 |
NCT03522246 | United States, Australia, etc | III | Rucaparib (1200 mg/day) | Placebo | 425 | 110 | 61/61 | 14.7/9.9 month | Ovarian cancer |
| 29 |
Wu [46] et.al 2020 |
NCT03705156 | China | III | Niraparib (300 mg or 200 mg/day) | Placebo | 177 | 88 | 53/55 | 369/171 days | Ovarian cancer |
| 30 |
Woll [47] et.al 2022 |
/ | United Kingdom | II | Olaparib (300 mg twice a day) | Placebo | 73 | 74 | 66/64 | 8/8 weeks | Lung cancer |
| Olaparib ( 200 mg three times a day) | Placebo | 73 | 63/64 | 19/8 weeks | Lung cancer | ||||||
| 31 |
Sun [48] et.al 2022 |
/ | China | II | Olaparib (400 mg/day) + Bevacizumab | Albumin-bound paclitaxel + Bevacizumab | 42 | 42 | NA | NA | Ovarian cancer |
| 32 |
O’Reilly [49] et.al 2020 |
/ | United States, Canada, Israel | II |
Veliparib (160 mg/day) + Cisplatin + Gemcitabine |
Cisplatin + Gemcitabine | 27 | 23 | 64/63 | NA | Pancreas Adenocarcinoma |
NCT number national clinical trial number, PARP poly(adenosine diphosphate-ribose) polymerase, NA not reported / Not registered on the ClinicalTrials.gov, P/C PARP inhibitor group/control group
Table 2.
Summary of included RCTs
| PARP Inhibitors | Number of Phase II Studies | Number of Phase III Studies | Sample Size (PARP group/control group) | Interventions | Median treatment duration (PARP group) | Cancer | |
|---|---|---|---|---|---|---|---|
| Treatment regime (PARP group versus control group) | Number of studies | ||||||
| Niraparib | 0 | 4 | 1153/571 | A | 4 | 44 days, 369 days | Ovarian cancer, Lung Cancer |
| Olaparib | 8 | 6 | 2418/1726 | A | 5 | 8 weeks ~ 24.6 months | Ovarian cancer, lung cancer, pancreatic cancer, prostate cancer, breast cancer, and gastric cancer |
| B | 3 | ||||||
| C | 6 | ||||||
| Veliparib | 7 | 3 | 1953/1275 | C | 10 | 8 weeks ~ 36 weeks | Ovarian cancer, breast cancer, prostate cancer, lung cancer, colorectal cancer, pancreatic cancer, pancreas adenocarcinoma |
| Rucaparib | 0 | 3 | 1029/412 | A | 2 | 7.3 months ~ 14.7 months | Ovarian cancer, peritoneal cancer |
| B | 1 | ||||||
| Iniparib | 1 | 0 | 78/39 | C | 1 | 15 weeks | Lung cancer |
| Totle | 16 | 16 | 6631/4023 | A,B,C | 32 | 44 days ~ 14.7 months | Ovarian cancer, lung cancer, pancreatic cancer, prostate cancer, breast cancer, peritoneal cancer, colorectal cancer, gastric cancer, pancreas adenocarcinoma |
PARP poly(adenosine diphosphate-ribose) polymerase, A: PARP inhibitors versus placebo, B: PARP inhibitors versus other anticancer drugs, C: PARP inhibitors + other anticancer drugs versus other anticancer drugs
Evaluation of the quality of RCTs
We assessed the quality of the 32 included double-blind randomized controlled trials [18–49] according to the Cochrane Collaboration guidelines. 11 [18, 20–22, 24, 36, 40–43, 49] of the 32 included studies were open-label studies and were not blinded. Of the remaining 21 studies, 2 studies [33, 37] mentioned that outcome assessors were not blinded, and one study [37] mentioned that drug allocation concealment was not performed. Most RCTs were conducted strictly according to the Cochrane Collaboration guidelines, and the overall quality was high. See Supplementary Table 1 for details.
Incidence of hypertension associated with PARP inhibitors
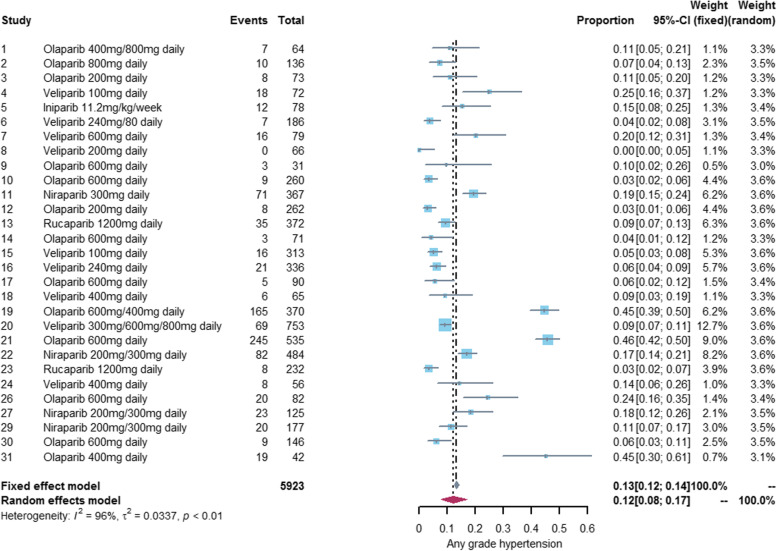
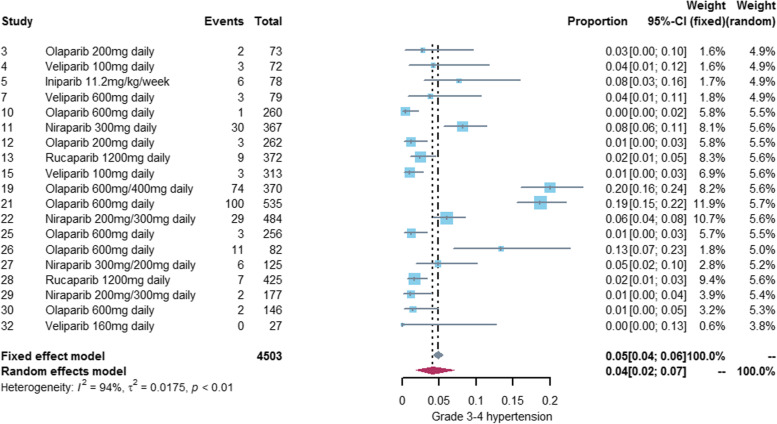
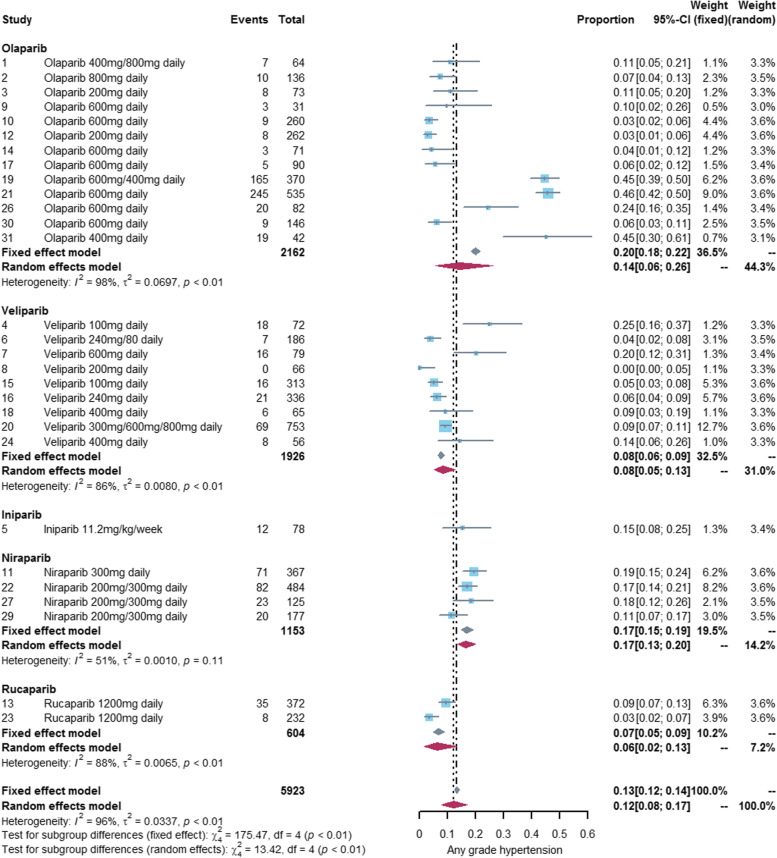
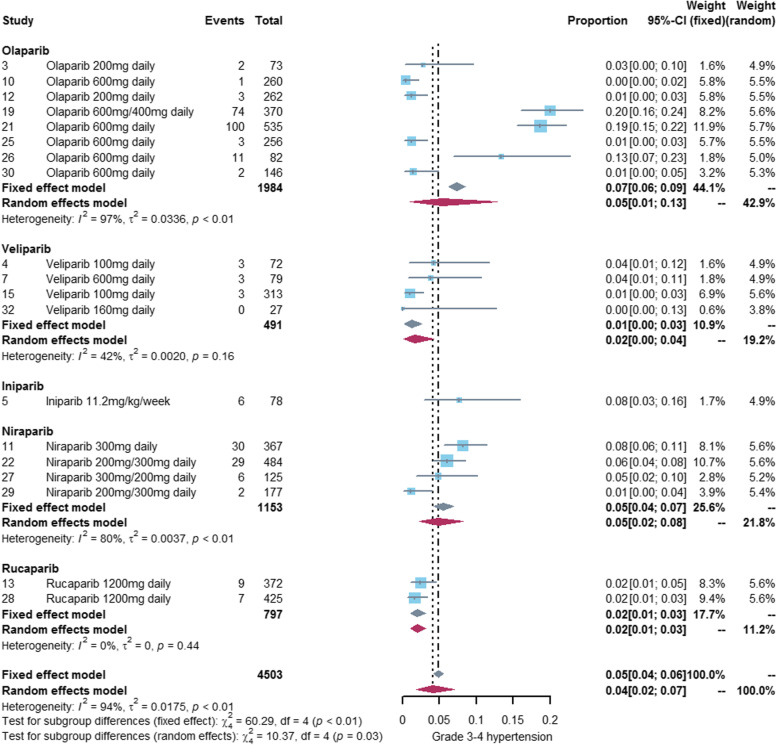
We performed a meta-analysis of 29 studies [18–41, 43, 44, 46–48] reporting any grade hypertension and 19 studies [20–22, 24, 27–30, 32, 36, 38, 39, 42–47, 49] reporting grade3-4 hypertension. The incidence of any grade hypertension was 12% (95%CI: 8%-17%) and the incidence of grade 3–4 hypertension was 4% (95%CI: 2%-7%). See Figs. 2 and 3 for details. The incidence of hypertension varies widely among different PARP inhibitors, with olaparib (any grade:14%, grade3-4: 5%) and niraparib (any grade:17%, grade3-4: 5%) exhibiting a higher incidence of hypertension than veliparib (any grade:8%, grade3-4: 1%) and rucaparib (any grade:6%, grade3-4: 2%). Only one study [22] reported hypertension associated with iniparib, so we did not conduct meta-analysis of iniparib alone. See Figs. 4 and 5 for details.
Fig. 2.
Forest plot of incidence of any grade hypertension related to PARP inhibitor
Fig. 3.
Forest plot of incidence of grade 3-4 hypertension related to PARP inhibitor
Fig. 4.
Forest plot of incidence of any grade hypertension related to different PARP inhibitors
Fig. 5.
Forest plot of incidence of grade 3-4 hypertension related to different PARP inhibitors
Risk of Hypertension Associated with PARP Inhibitors
We performed a meta-analysis of 29 studies [18–41, 43, 44, 46–48] reporting hypertension of any grade and 19 studies [20–22, 24, 27–30, 32, 36, 38, 39, 42–47, 49] reporting grade 3–4 hypertension, respectively. There was considerable heterogeneity among studies, so we used a random-effects model for analysis. There was no statistically significant difference in the risk of hypertension between the PARP inhibitor group and the control group(any grade: RR = 1.22, 95% CI: 0.91–1.65, P = 0.19, I2 = 81%; grade3-4: RR = 1.24, 95% CI: 0.74–2.08, P = 0.42, I2 = 68%). See Table 3 and Supplementary Fig. 1 for details.
Table 3.
Summary of the risk of hypertension associated with PARP inhibitors
| Subgroup | Any grade | Grade 3–4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR(95%CI) | P | I2 | Model | RR(95%CI) | P | I2 | model | |||
| PARP inhibitors | ||||||||||
| Olaparib | Olaparib plus cediranib regimen | 8.78(5.39,14.29) | < 0.01 | 0% | Fixed | 6.50(3.50,12.05) | < 0.01 | 0% | Fixed | |
| Olaparib (exclude olaparib plus cediranib regimen) | 200 mg-600 mg/day | 0.79(0.69,0.89) | < 0.01 | 22% | Fixed | 0.61(0.48,0.77) | < 0.01 | 0% | Fixed | |
| 800 mg/day | 2.71(1.10,6.69) | 0.03 | 23% | Fixed | / | |||||
| All dose | 0.82(0.72,0.92) | < 0.01 | 38% | Fixed | 0.61(0.48,0.77) | < 0.01 | 0% | Fixed | ||
| Olaparib (include olaparib plus cediranib regimen) | 1.12(0.67,1.87) | 0.67 | 86% | Random | 1.08(0.45,2.61) | 0.86 | 79% | Random | ||
| Veliparib | 1.01(0.80,1.28) | 0.94 | 3% | Fixed | 0.77(0.32,1.83) | 0.55 | 0% | Fixed | ||
| Niraparib | 3.47(2.36,5.09) | < 0.01 | 21% | Fixed | 4.20(2.04,8.68) | < 0.01 | 0% | Fixed | ||
| Rucaparib | 0.90(0.56,1.45) | 0.67 | 16% | Fixed | 0.77(0.34,1.74) | 0.53 | 15% | Fixed | ||
| Iniparib | / | / | ||||||||
| Cancer type | ||||||||||
| Ovarian cancer |
1.68(0.98,2.86) 1.54(0.93,2.55) |
0.10 | 91% | Random | 1.59(0.68,3.69) | 0.28 | 84% | Random | ||
| Lung cancer | 0.79(0.33,1.90) | 0.59 | 62% | Random | 1.11(0.45,2.75) | 0.83 | 37% | Fixed | ||
| Breast cancer | 1.21(0.85,1.73) | 0.41 | 23% | Fixed | 1.09(0.42,2.78) | 0.86 | 0% | Fixed | ||
| Prostate cancer | 0.78(0.45,1.35) | 0.38 | 0% | Fixed | 0.70(0.23,2.10) | 0.52 | 0% | Fixed | ||
| Pancreatic cancer | 1.17(0.52,2.62) | 0.71 | 0% | Fixed | / | |||||
| Treatment regime | ||||||||||
| PARP versus Placebo | 1.47(0.80,2.70) | 0.22 | 78% | Random | 1.37(0.57,3.29) | 0.48 | 60% | Random | ||
| PARP + Other anticancer drugs versus Other anticancer drugs | 1.19(0.78,1.80) | 0.42 | 87% | Random | 1.37(0.55,3.40) | 0.50 | 84% | Random | ||
| PARP versus Other anticancer drugs | 1.26(0.77,2.05) | 0.35 | 48% | Fixed | 0.40(0.16,1.00) | 0.05 | 0% | Fixed | ||
| Total | 1.22(0.91,1.65) | 0.19 | 81% | Random | 1.24(0.74,2.08) | 0.42 | 68% | Random | ||
PARP poly(adenosine diphosphate-ribose) polymerase, RR risk ratio, CI confidence interval, I2 The greater the value of I2, the greater the heterogeneity among studies. If I2 ≥ 50, the random effect model is used for analysis, otherwise the fixed effect model is used for analysis; P P < 0.05 indicates a statistical difference
Subgroup analysis of hypertension risk
We conducted subgroup analysis to explore the difference of hypertension risk among different PARP inhibitors, different cancer types and different treatment regimes.
Subgroup analysis of PARP inhibitors
Our subgroup analysis showed that the risk of hypertension varied widely among different PARP inhibitors. The risk of hypertension was significantly higher in the niraparib group compared with the control group (any grade: RR = 3.47, 95% CI: 2.36–5.09, P < 0.01, I2 = 21%; grade3-4: RR = 4.20, 95% CI: 2.04–8.68, P < 0.01, I2 = 0%). However, veliparib (any grade: RR = 1.01, 95% CI: 0.80–1.28, P = 0.94, I2 = 3%; grade 3–4: RR = 0.77, 95% CI: 0.32–1.83, P = 0.55, I2 = 0%) and rucaparib (any grade: RR = 0.90, 95% CI: 0.56–1.45, P = 0.67, I2 = 16%; grade 3–4: RR = 0.77, 95% CI: 0.34–1.74, P = 0.53, I2 = 15%) showed a comparable risk of hypertension as the control group. See Table 3 and Supplementary Fig. 2 for details.
There was great heterogeneity between olaparib group and the control group (any grade: I2 = 86%; grade 3–4: I2 = 79%), but the heterogeneity was significantly reduced when 2 studies [36, 43] of olaparib plus cediranib were excluded (any grade: I2 = 38%; grade3-4: I2 = 0%). The risk of hypertension with olaparib plus cediranib regime was significantly higher than that in the control group (any grade: RR = 8.78, 95% CI: 5.39–14.29, P < 0.01, I2 = 0%; grade 3–4: RR = 6.50, 95% CI: 3.50–12.05, P < 0.01, I2 = 0%), while the risk of hypertension with olaparib (exclude olaparib plus cediranib regime) alone or in combination with other anticancer drugs was lower than that in the control group (any grade: RR = 0.82, 95% CI: 0.72–0.92, P < 0.01, I2 = 38%; grade 3–4: RR = 0.61, 95% CI: 0.48–0.77, P < 0.01, I2 = 0%). Heterogeneity between olaparib group and control groups improved after further exclusion of 2 studies [18, 19] with olaparib 800 mg/day (any grade: 22%; grade 3–4: 0%). The results of our meta-analysis showed that olaparib 800 mg/day (without olaparib plus cediranib regime) may be associated with a higher risk of hypertension (any grade: RR = 2.71, 95% CI: 1.10–6.69, P = 0.03, I2 = 23%). However, olaparib 200 mg-600 mg/day(exclude olaparib plus cediranib regime) was associated with a lower risk of hypertension compared with the control group (any grade: RR = 0.79, 95% CI: 0.69–0.89, P < 0.01, I2 = 22%; grade 3–4: RR = 0.61, 95% CI: 0.48–0.77, P < 0.01, I2 = 0%). See Table 3 and Supplementary Fig. 3 for details.
Subgroup analysis of cancer type
Based on the cancer type, we performed a subgroup analysis of five cancers including ovarian cancer, lung cancer, breast cancer, prostate cancer, and pancreatic cancer. All five subgroups showed no statistically significant difference in the risk of hypertension between the PARP inhibitor group and the control group. See Table 3 and Supplementary Fig. 4 for details. Other cancers were not analyzed separately because too few studies were included.
Subgroup analysis of treatment regime
Based on treatment regime, we divided the study into three subgroups: PARP inhibitors versus placebo, PARP inhibitors versus other anticancer drugs, PARP inhibitors + other anticancer drugs versus other anticancer drugs, and All three subgroups showed no statistically significant difference in the risk of hypertension between the PARP inhibitors group and the control group. See Table 3 and Supplementary Fig. 5 for details.
Publication Bias
For studies reporting hypertension of any grade and grades 3–4, neither the corresponding funnel plot nor Begg's test values indicated significant publication bias. See Supplementary Fig. 6 and Supplementary Table 2 for details.
Discussion
PARP inhibitors have shown good clinical efficacy in clinical trials, especially in BRCA-mutant ovarian cancer and breast cancer, but accompanied by some adverse events. At present, the systematic analysis of PARP inhibitor related adverse events mainly involves gastrointestinal adverse events [50], hematological adverse events [51], pneumonitis [52], myelodysplastic syndrome and acute myeloid leukaemia [53], peripheral neuropathy [54], etc. However, there is no comprehensive and systematic analysis of PARP inhibitor-related hypertension, although many clinical trials have reported different grades and proportions of PARP inhibitor-related hypertension. This is the first meta-analysis to systematically assess the incidence and risk of PARP inhibitor-related hypertension in cancer patients. We conducted a meta-analysis of 32 phase II or III RCTs involving 10,654 participants, and further analyzed the incidence of hypertension with different PARP inhibitors, as well as the risk of hypertension with different PARP inhibitors, different cancer types, and different treatment regimes. The results of our analysis involved olaparib, veliparib, niraparib, rucaparib and iniparib 5 PARP inhibitors.
Gastrointestinal and hematological adverse events are the most common adverse events of PARP inhibitors. The incidence of any grade hypertension associated with PARP inhibitors was 12%, which was lower than any grade gastrointestinal (nausea: 68.8%, vomiting: 47.8%, diarrhea: 25.3%, constipation: 25.3%) and hematological (anemia:47.8%, neutropenia: 39.6%, thrombocytopenia:23.0%) adverse events associated with PARP inhibitors [50, 51]. The incidence of grade 3–4 hypertension related to PARP inhibitors is 4%, which is higher than grade 3–4 gastrointestinal toxicity (nausea: 3.4%, vomiting: 2.0%, diarrhea: 1.7% and constipation: 1.4%) related to PARP inhibitors and lower than grade 3–4 hematological toxicity (anemia: 22.1%, neutropenia: 19.3%, thrombocytopenia: 15.4%) related to PARP inhibitors [50, 51]. There is a great difference in the incidence of hypertension among PARP inhibitors. Olaparib (any grade: 17%, grades 3–4: 7%) and niraparib (any grade: 16%, grades 3–4: 5%) all show high incidence of hypertension, and their incidence of grade 3–4 hypertension is similar to that of sorafenib [55] (5.7%, a tyrosine kinase inhibitors). However, the incidence of hypertension in veliparib (any grade: 8%, grade3-4: 1%) and rucaparib (any grade: 6%, grade3-4: 2%) is not high. PARP inhibitor-related hypertension may be due to an off target disruption of dopamine and nor epinephrine metabolism [56].
The results of our meta-analysis showed no statistically significant difference in the risk of hypertension between the PARP inhibitor group and the control group, but this result is not completely reliable because of large heterogeneity among studies (any grade: I2 = 80%, grade 3–4: I2 = 68%). Our subgroup analyses of cancer types and treatment regimes were consistent with the results of total PARP inhibitors, but there was also substantial heterogeneity across studies. Finally, we found that the varieties of PARP inhibitors maybe the main source of heterogeneity, and the risk of hypertension varied widely among different PARP inhibitors. Niraparib exhibited a significantly higher risk of hypertension than the control group. Niraparib-related hypertension may be attributable to off-target disruption of dopamine and norepinephrine metabolism and inhibition of DYRK1A (dual-specificity tyrosine phosphorylated and regulated kinase 1A) [57]. However, the risk of hypertension with veliparib and rucaparib was similar to the control group, and olaparib may even reduced the risk of hypertension in some cases.
Olaparib is currently the most widely investigated PARP inhibitor and has demonstrated promising efficacy in various cancers such as ovarian cancer and breast cancer [58, 59]. Our analysis of olaparib is very interesting. On the one hand, the risk of hypertension with olaparib was associated with combination therapy. The risk of hypertension was significantly higher in the olaparib plus cediranib regimen than in the control group, whereas olaparib alone or in combination with other anticancer drugs showed the opposite results. The results of the olaparib plus cediranib regimen was consistent with the meta-analysis of Guo et al. [60], which may be mainly attributed to the inhibition of vascular endothelial growth factor receptor by cediranib [61], or some mechanism of the combination of the two drugs. On the other hand, the risk of hypertension with olaparib is dose-related. Olaparib (without olaparib plus cediranib regimen) 800 mg/day having a significantly higher risk of hypertension than the control group, while olaparib200mg-600 mg/day(exclude olaparib plus cediranib regimen) had a lower risk of hypertension than the control group. One study [62] found that PARP inhibitors may have an inhibitory effect on angiotensin II (Ang II) in rats, so we speculate that olaparib may reduces the risk of hypertension by inhibiting renin angiotensin system (RAS), an important factor in the occurrence and maintenance of essential hypertension [63]. The mechanism of olaparib 800 mg/day increasing the risk of hypertension is unclear, and further research is needed.
Our previous results show that olaparib has a high incidence of hypertension, which seems to contradict the result that olaparib reduces the risk of hypertension. So, we excluded the study involving olaparib 800 mg/day and olaparib plus cediranib regimen and calculated the incidence of hypertension in the olaparib and control groups, respectively. The results showed that the incidence of hypertension in oalparib group (any grade: 11%, grade 3–4: 3%) was lower than that in the control group(any grade: 15%, grade 3–4: 5%), which was consistent with the result that olaparib reduced the risk of hypertension (Supplementary Fig. 3E and Supplementary Fig. 3F).
For patients receiving niraparib, olaparib 800 mg/day and the combination of olaparib and cediranib, some measures should be taken to prevent the development of hypertension, such as limiting salt intake (< 5 g/day), regular aerobic exercise supplemented by dynamic resistance exercise and flexible exercise, etc. [64, 65]. At the same time, clinicians should monitor and control patients' blood pressure and give medication in severe cases. According to relevant guidelines [66, 67], angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), beta receptor blockers, diuretics and calcium channel blockers (CCB) are currently the mainstream drugs for the treatment of hypertension. Clinicians can select appropriate drugs to treat cancer patients with PARP inhibitor-associated hypertension.
This meta-analysis has five limitations. First of all, there is a lack of relevant single-arm studies when assessing the incidence of hypertension associated with PARP inhibitors. Second, more than one-third of RCTs are open-label studies that are not blinded. Thirdly, the duration of treatment, duration of follow-up, and median age varied widely among the included studies. Fourthly, we only retrieved one eligible study involving iniparib, and not any eligible studies involving pamiparib, fuzuloparib and talazoparib, because the relevant clinical studies were mainly concentrated in phase I. Finally, Because there are too few relevant studies, we did not compare the risk of hypertension between different doses of olaparib in cancer patients.
Conclusion
The incidence and risk of hypertension varied widely among different PARP inhibitors. Olaparib 200-600 mg/day (excluding olaparib plus cediranib regimen) may be the most suitable PARP inhibitor for cancer patients with high risk of hypertension, followed by veliparib and rucaparib. Niraparib, olaparib 800 mg/day, and the combination of olaparib and cediranib all have a high risk of hypertension. Therefore, cancer patients who use the above drugs should strengthen blood pressure monitoring and take some simple preventive measures, and receive appropriate medication in severe cases.
Supplementary Information
Additional file 1: Supplementary Table 1. Quality evaluation of RCTs according to Cochrane Collaboration Guidelines. Supplementary Figure 1. Risk of total PARP inhibitor-related hypertension. Supplementary Figure 2. Risk of hypertension with different PARP inhibitors. Supplementary Figure 3. Detailed analysis of olaparib-related hypertension. Supplementary Figure 4. Risk of hypertension in different types of cancer. Supplementary Figure 5. Risk of hypertension in different treatment regime. Supplementary Table 2. Begg's test results of any grade and grade 3-4 hypertension related to total PARP inhibitor. Supplementary Figure 6. Funnel plot of hypertension associated with total PARP inhibitors.
Acknowledgements
Not applicable.
Authors’ contributions
XC, YL, and JL conceived and wrote the article. XC and LK implement article retrieval and screening. XC, XX and YL collected the data. XX carried out the data sorting. XC and LK carried out the statistical processing. JL and QW carried out the analysis and interpretation of the results and the revision of the paper. JL and YL were responsible for the quality control and review of the article and analyzed the overall article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Sichuan Provincial Department of Education (SCYG2020-04, SCYG2019-04).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Li, Email: ljadoctor@swmu.edu.cn.
Yaling Li, Email: lylapothecary@swmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Zhang J, Gao Y, Li Y, Feng C, Song C, et al. LncSEA: a platform for long non-coding RNA related sets and enrichment analysis. Nucleic Acids Res. 2021;49(D1):D969–D980. doi: 10.1093/nar/gkaa806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- 4.Cheng B, Pan W, Xing Y, Xiao Y, Chen J, Xu Z. Recent advances in DDR (DNA damage response) inhibitors for cancer therapy. Eur J Med Chem. 2022;230:114109. doi: 10.1016/j.ejmech.2022.114109. [DOI] [PubMed] [Google Scholar]
- 5.Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 7.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschenbrenner DS. Olaparib Approved for Metastatic Pancreatic Cancer. Am J Nurs. 2020;120(4):22–23. doi: 10.1097/01.NAJ.0000660008.32418.6c. [DOI] [PubMed] [Google Scholar]
- 9.Ison G, Howie LJ, Amiri-Kordestani L, Zhang L, Tang S, Sridhara R, et al. FDA Approval Summary: Niraparib for the Maintenance Treatment of Patients with Recurrent Ovarian Cancer in Response to Platinum-Based Chemotherapy. Clin Cancer Res. 2018;24(17):4066–4071. doi: 10.1158/1078-0432.CCR-18-0042. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramaniam S, Beaver JA, Horton S, Fernandes LL, Tang S, Horne HN, et al. FDA Approval Summary: Rucaparib for the Treatment of Patients with Deleterious BRCA Mutation-Associated Advanced Ovarian Cancer. Clin Cancer Res. 2017;23(23):7165–7170. doi: 10.1158/1078-0432.CCR-17-1337. [DOI] [PubMed] [Google Scholar]
- 11.Hoy SM. Talazoparib: First Global Approval. Drugs. 2018;78(18):1939–1946. doi: 10.1007/s40265-018-1026-z. [DOI] [PubMed] [Google Scholar]
- 12.Arora S, Balasubramaniam S, Zhang H, Berman T, Narayan P, Suzman D, et al. FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist. 2021;26(1):e164–e172. doi: 10.1002/onco.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmer K, Kocher F, Puccini A, Seeber A. Targeting BRCA and DNA Damage Repair Genes in GI Cancers: Pathophysiology and Clinical Perspectives. Front Oncol. 2021;11:662055. doi: 10.3389/fonc.2021.662055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration . Zejula (niraparib) capsules, for oral use. Prescribing information. 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208447s015s017lbledt.pdf.
- 15.Lee A. Fuzuloparib: First Approval. Drugs. 2021;81(10):1221–1226. doi: 10.1007/s40265-021-01541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markham A. Pamiparib: First Approval. Drugs. 2021;81(11):1343–1348. doi: 10.1007/s40265-021-01552-8. [DOI] [PubMed] [Google Scholar]
- 17.Collaborators GBDRF Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 2020;396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 19.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 20.Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39(7):989–98 e5. doi: 10.1016/j.ccell.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rugo HS, Olopade OI, DeMichele A, Yau C, van’t Veer LJ, Buxton MB, et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novello S, Besse B, Felip E, Barlesi F, Mazieres J, Zalcman G, et al. A phase II randomized study evaluating the addition of iniparib to gemcitabine plus cisplatin as first-line therapy for metastatic non-small-cell lung cancer. Ann Oncol. 2014;25(11):2156–2162. doi: 10.1093/annonc/mdu384. [DOI] [PubMed] [Google Scholar]
- 23.Han HS, Dieras V, Robson M, Palacova M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29(1):154–161. doi: 10.1093/annonc/mdx505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, Kunju LP, et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012. J Clin Oncol. 2018;36(10):991–999. doi: 10.1200/JCO.2017.75.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL, 3rd, Srkalovic G, et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination With Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: ECOG-ACRIN 2511 Study. J Clin Oncol. 2019;37(3):222–229. doi: 10.1200/JCO.18.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fennell DA, Porter C, Lester J, Danson S, Blackhall F, Nicolson M, et al. Olaparib maintenance versus placebo monotherapy in patients with advanced non-small cell lung cancer (PIN): A multicentre, randomised, controlled, phase 2 trial. EClinicalMedicine. 2022;52:101595. doi: 10.1016/j.eclinm.2022.101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1721–1731. doi: 10.1016/S1470-2045(21)00531-3. [DOI] [PubMed] [Google Scholar]
- 28.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 29.Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1637–1651. doi: 10.1016/S1470-2045(17)30682-4. [DOI] [PubMed] [Google Scholar]
- 30.Ledermann JA, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(5):710–722. doi: 10.1016/S1470-2045(20)30061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975–986. doi: 10.1016/S1470-2045(18)30365-6. [DOI] [PubMed] [Google Scholar]
- 32.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. [DOI] [PubMed]
- 33.Dieras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(10):1269–1282. doi: 10.1016/S1470-2045(20)30447-2. [DOI] [PubMed] [Google Scholar]
- 34.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381(4):317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorbunova V, Beck JT, Hofheinz RD, Garcia-Alfonso P, Nechaeva M, Cubillo Gracian A, et al. A phase 2 randomised study of veliparib plus FOLFIRI+/-bevacizumab versus placebo plus FOLFIRI+/-bevacizumab in metastatic colorectal cancer. Br J Cancer. 2019;120(2):183–189. doi: 10.1038/s41416-018-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. Olaparib With or Without Cediranib Versus Platinum-Based Chemotherapy in Recurrent Platinum-Sensitive Ovarian Cancer (NRG-GY004): A Randomized, Open-Label, Phase III Trial. J Clin Oncol. 2022;40(19):2138–2147. doi: 10.1200/JCO.21.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med. 2019;381(25):2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Martin A, Pothuri B, Vergote I, DePont CR, Graybill W, Mirza MR, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 40.Kristeleit R, Lisyanskaya A, Fedenko A, Dvorkin M, de Melo AC, Shparyk Y, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(4):465–478. doi: 10.1016/S1470-2045(22)00122-X. [DOI] [PubMed] [Google Scholar]
- 41.Chiorean EG, Guthrie KA, Philip PA, Swisher EM, Jalikis F, Pishvaian MJ, et al. Randomized Phase II Study of PARP Inhibitor ABT-888 (Veliparib) with Modified FOLFIRI versus FOLFIRI as Second-line Treatment of Metastatic Pancreatic Cancer: SWOG S1513. Clin Cancer Res. 2021;27(23):6314–6322. doi: 10.1158/1078-0432.CCR-21-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 43.Colombo N, Tomao F, Benedetti Panici P, Nicoletto MO, Tognon G, Bologna A, et al. Randomized phase II trial of weekly paclitaxel vs. cediranib-olaparib (continuous or intermittent schedule) in platinum-resistant high-grade epithelial ovarian cancer. Gynecol Oncol. 2022;164(3):505–13. doi: 10.1016/j.ygyno.2022.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Ai X, Pan Y, Shi J, Yang N, Liu C, Zhou J, et al. Efficacy and Safety of Niraparib as Maintenance Treatment in Patients With Extensive-Stage SCLC After First-Line Chemotherapy: A Randomized, Double-Blind, Phase 3 Study. J Thorac Oncol. 2021;16(8):1403–1414. doi: 10.1016/j.jtho.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Monk BJ, Parkinson C, Lim MC, O'Malley DM, Oaknin A, Wilson MK, et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022.10.1200/JCO2201003. [DOI] [PMC free article] [PubMed]
- 46.Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial() Ann Oncol. 2021;32(4):512–521. doi: 10.1016/j.annonc.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Woll P, Gaunt P, Danson S, Steele N, Ahmed S, Mulatero C, et al. Olaparib as maintenance treatment in patients with chemosensitive small cell lung cancer (STOMP): A randomised, double-blind, placebo-controlled phase II trial. Lung Cancer. 2022;171:26–33. doi: 10.1016/j.lungcan.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Liu C, Li Y. Effect of PARP Inhibitor Combined with Bevacizumab on Platinum-Resistant Recurrent Ovarian Epithelial Carcinoma. Comput Math Methods Med. 2022;2022:4600145. doi: 10.1155/2022/4600145. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.O’Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J Clin Oncol. 2020;38(13):1378–88. [DOI] [PMC free article] [PubMed]
- 50.Liu Y, Meng J, Wang G. Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: a meta-analysis of published trials. Drug Des Devel Ther. 2018;12:3013–3019. doi: 10.2147/DDDT.S164553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Li J. Haematologic toxicities with PARP inhibitors in cancer patients: an up-to-date meta-analysis of 29 randomized controlled trials. J Clin Pharm Ther. 2021;46(3):571–584. doi: 10.1111/jcpt.13349. [DOI] [PubMed] [Google Scholar]
- 52.Ma Z, Sun X, Zhao Z, Lu W, Guo Q, Wang S, et al. Risk of pneumonitis in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials and a pharmacovigilance study of the FAERS database. Gynecol Oncol. 2021;162(2):496–505. doi: 10.1016/j.ygyno.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Morice PM, Leary A, Dolladille C, Chretien B, Poulain L, Gonzalez-Martin A, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021;8(2):e122–e134. doi: 10.1016/S2352-3026(20)30360-4. [DOI] [PubMed] [Google Scholar]
- 54.Balko R, Hurley R, Jatoi A. Poly (ADP-Ribose) Polymerase Inhibition for Chemotherapy-Induced Peripheral Neuropathy: A Meta-Analysis of Placebo-Controlled Trials. J Palliat Med. 2019;22(8):977–980. doi: 10.1089/jpm.2018.0572. [DOI] [PubMed] [Google Scholar]
- 55.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9(2):117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 56.LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–e28. doi: 10.1016/S1470-2045(18)30786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandhu D, Antolin AA, Cox AR, Jones AM. Identification of different side effects between PARP inhibitors and their polypharmacological multi-target rationale. Br J Clin Pharmacol. 2022;88(2):742–752. doi: 10.1111/bcp.15015. [DOI] [PubMed] [Google Scholar]
- 58.Marchetti C, Imperiale L, Gasparri ML, Palaia I, Pignata S, Boni T, et al. Olaparib, PARP1 inhibitor in ovarian cancer. Expert Opin Investig Drugs. 2012;21(10):1575–1584. doi: 10.1517/13543784.2012.707189. [DOI] [PubMed] [Google Scholar]
- 59.Robert M, Frenel JS, Gourmelon C, Patsouris A, Augereau P, Campone M. Olaparib for the treatment of breast cancer. Expert Opin Investig Drugs. 2017;26(6):751–759. doi: 10.1080/13543784.2017.1318847. [DOI] [PubMed] [Google Scholar]
- 60.Guo X, Qian X, Jin Y, Kong X, Qi Z, Cai T, et al. Hypertension Induced by Combination Therapy of Cancer: A Systematic Review and Meta-Analysis of Global Clinical Trials. Front Pharmacol. 2021;12:712995. doi: 10.3389/fphar.2021.712995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maki-Petaja KM, McGeoch A, Yang LL, Hubsch A, McEniery CM, Meyer PAR, et al. Mechanisms Underlying Vascular Endothelial Growth Factor Receptor Inhibition-Induced Hypertension: The HYPAZ Trial. Hypertension. 2021;77(5):1591–1599. doi: 10.1161/HYPERTENSIONAHA.120.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Wang L, Zhang F, Zhang C, Deng S, Wang R, et al. Inhibition of PARP prevents angiotensin II-induced aortic fibrosis in rats. Int J Cardiol. 2013;167(5):2285–2293. doi: 10.1016/j.ijcard.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 63.Li FJ, Zhang CL, Luo XJ, Peng J, Yang TL. Involvement of the MiR-181b-5p/HMGB1 Pathway in Ang II-induced Phenotypic Transformation of Smooth Muscle Cells in Hypertension. Aging Dis. 2019;10(2):231–248. doi: 10.14336/AD.2018.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaleski A. Exercise for the prevention and treatment of hypertension – implications and application. In: American College of Sports Medicine. 2019; Available from: https://www.acsm.org/blog-detail/acsm-certified-blog/2019/02/27/exercise-hypertensionprevention-treatment. Accessed 29 Aug 2022.
- 65.WHO Guideline: Sodium Intake for Adults and Children, In: World Health Organization (WHO): Geneva, Switzerland, 2012. Available from: https://www.who.int/publications/i/item/9789241504836. [PubMed]
- 66.Jones NR, McCormack T, Constanti M, McManus RJ. Diagnosis and management of hypertension in adults: NICE guideline update 2019. Br J Gen Pract. 2020;70(691):90–91. doi: 10.3399/bjgp20X708053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Quality evaluation of RCTs according to Cochrane Collaboration Guidelines. Supplementary Figure 1. Risk of total PARP inhibitor-related hypertension. Supplementary Figure 2. Risk of hypertension with different PARP inhibitors. Supplementary Figure 3. Detailed analysis of olaparib-related hypertension. Supplementary Figure 4. Risk of hypertension in different types of cancer. Supplementary Figure 5. Risk of hypertension in different treatment regime. Supplementary Table 2. Begg's test results of any grade and grade 3-4 hypertension related to total PARP inhibitor. Supplementary Figure 6. Funnel plot of hypertension associated with total PARP inhibitors.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.