Abstract
Background
Because humans lack α-galactosidase, foods containing certain oligosaccharides from the raffinose family, such as soybeans and other legumes, may disrupt digestion and cause flatulence.
Results
Aspergillus niger NRC114 α-galactosidase was purified using protein precipitation, gel filtration, and ion exchange chromatography steps, which resulted in a 123-fold purification. The purified enzyme was found to be 64 kDa using the SDS-PAGE approach. The optimum pH and temperature of the purified α-galactosidase were detected at pH 3.5 and 60 ºC, respectively. The pure enzyme exhibited potent acidic pH stability at pH 3.0 and pH 4.0 for 2 h, and it retained its full activity at 50 ºC and 60 ºC for 120 min and 90 min, respectively. The enzyme was activated using 2.5 mM of K+, Mg2+, Co2+, or Zn2+ by 14%, 23%, 28%, and 11%, respectively. The Km and Vmax values of the purified enzyme were calculated to be 0.401 µM and 14.65 μmol min−1, respectively. The soymilk yogurt showed an increase in its total phenolic content and total flavonoids after enzyme treatment, as well as several volatile compounds that were detected and identified using GC–MS analysis. HPLC analysis clarified the enzymatic action in the hydrolysis of raffinose family oligosaccharides.
Conclusion
The findings of this study indicate the importance of A. niger NRC114 α-galactosidase enzyme for future studies, especially its applications in a variety of biological fields.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12896-023-00773-x.
Keywords: α-galactosidase, Purification, Soy bean, Nutritional properties, Treated-soymilk yogurt
Introduction
α-Galactosidase (α-D-galactoside galactohydrolase, EC 3.2.1.22) is a hydrolytic enzyme that catalyzes the degradation of terminal α-1,6-linked D-galactose residues in galactooligosaccharides and galactopolysaccharides [1]. It has miscellaneous usage in the nutraceutical, pharmaceutical industries and other industrial fields. α-Galactosidase has a great role in the sugar industry due to its catalytic degradation of raffinose sugar, which enhances the crystallization of sucrose and increases the product [2]. The enzyme is a good participant in soy food processing and animal feed processing [3]. At the level of medical applications, α-galactosidase was studied for the process of blood group transformation and the treatment of Fabry’s disease [4].
Although the α-galactosidase enzyme is excessively spread in most living systems, it is not formed in humans. In plants, α-Galactosidase has been studied in Vigna mungo [5] and Citrullus vulgaris [6]. It has been immobilized by the bacterium Thermus sp. T2 [7], is secreted by Lactobacilli during the fermentation of soy milk [8], and produced by lactic acid bacteria [9]. The purified enzyme from Bacillus stearothermophilus (NCIM-5146) could catalyze the hydrolysis of raffinose and stachyose [10].
Nowadays, many researchers and industrialists have given more attention to microbial enzymes in several industrial fields. These enzymes are characterized by low-cost production, plentiful yields, stability at a wide range of pH values and temperatures, and independence from seasonal production because the microbes have the ability to grow on inexpensive media and produce the enzymes under harsh circumstances [11]. Filamentous fungi and mushrooms are considered excellent sources for α-galactosidases production because they grow easily on different agriculture debris. α-galactosidases produced by fungi are characterized by their extracellular secretion and broad stability profiles [12].
It is well known that soybeans and other legumes provide humans with food rich in proteins and other valuable nutrients. Consequently, there is a growing demand for these meals of plant origin to minimize the bad effects of other foods from animal sources. Feeding on soybeans and other legumes as they contain some oligosaccharides of the raffinose family can disturb the digestion process and cause flatulence due to the absence of α-galactosidase in humans. Processing of legumes and their products by α-galactosidase can be used to degrade such oligosaccharides to monosaccharides which improve the quality and taste of these products. Substantial efforts and various studies have been made to eliminate or reduce the beany off-flavor, maximize the yield, and extend the soymilk shelf life [13].
Recently, there has been an increasing demand for functional foods due to their therapeutic and biological characteristics as well as their nutritional value. Among the preferred functional foods, yogurt is preferred because of the presence of lactic acid bacteria, which are responsible for the domestic therapeutic properties during the fermentation process of milk and provide significant digestible nutrients [14]. Soybean is one of the most important legumes that are considered functional foods, with significant consumption worldwide. It was characterized by a high content of protein, minerals, vitamins, and fiber. The healthy properties like antioxidant activity, lowering cholesterol, and reduction of heart diseases of soybeans and prepared products come from biological components such as isoflavones, saponins, phytosterols, and vitamin E. However, soybean and processed products' flavors are considered "off-flavor" with a negative effect on sensory properties. In the food industry, several attempts have been made to eliminate this undesirable off-flavor through enzyme treatments.
Purification procedures are created to obtain an enzyme in a timely, cost-effective, and pure manner. The present study aims to get the α-galactosidase enzyme in a pure form and study its properties to be a guide for its ability in the treatment of soymilk yogurt to improve its taste and increase its nutritive value. Accordingly, the changes in chemical composition, phytochemicals, antioxidant activity as well as volatile compounds of yogurt prepared from soybean after treatment with α-galactosidase and also the overall acceptability in comparison with plain yogurt was evaluated.
Materials and methods
Chemicals
Glucose, raffinose, sucrose, stachyose, galactose, p-nitrophenyl α-galactopyranoside (α-p-NPGal), and fructose were obtained from Sigma chemical company. Agar was provided by Fluka, Spain. All the other chemicals used were of analytical grade.
α-galactosidase activity assay
The activity of α-galactosidase was assessed in a reaction mixture contained: 0.2 ml of 4 mM p-nitrophenyl-α-D-galactopyranoside (pNP-α-D-Gal), 0.2 ml of 0.2 M citrate phosphate buffer (pH 3.5), and 0.1 ml of enzyme solution. The reaction was stopped after 10 min at 50 °C by adding 2 ml of a 0.2 M Na2CO3 solution. The released p-nitrophenol (pNP) was measured using a UV–Visible spectrophotometer (Cary 100 UV–Vis; Agilent Technologies, Germany) at 420 nm [12, 15]. One unit of activity was defined as the amount of enzyme capable of releasing 1 µmole of PNP per min. The protein content was measured at 595 nm using the Bradford method [16] and bovine serum albumin as a standard protein.
α-galactosidase purification
Aspergillus niger NRC114 was grown on media adjusted to the parameters referenced according to the application of central composite design (CCD) [12]. Cell-free filtrate (CFF) of the aforementioned culture was filtered by using Whatman filter paper No.1, which is considered as the crude α-galactosidase. The first step in the purification process was accomplished by adding ammonium sulfate (60–90% saturation) to precipitate the protein content in the filtrate. Using a HERMLE Z-323 K cooling centrifuge, the precipitation mixture was spun for 15 min at 12,000 rpm. The precipitate was re-dissolved in a 50 mM citrate phosphate buffer (pH 4.5) and dialyzed against distilled water overnight at 4 °C. After pre-equilibrating with the preceding buffer, the dialyzed enzyme was put onto a (2.5 × 50 cm) Sephadex G100 gel filtration chromatography column. Using a 50 mM citrate phosphate buffer (pH 4.5) at a flow rate of 1.5 ml/min, fractions containing α-galactosidase enzyme were collected using LKB Bromma 2070 Ultrorac® Fraction Collector. The α -galactosidase-active fractions were then combined, concentrated, and applied to a (1.5 × 35 cm) DEAE-Sephadex A-50 anion exchange chromatography column that had been pre-equilibrated with 50 mM citrate phosphate buffer (pH 4.5). The column was then eluted with the same buffer at a rate of 0.5 ml/min using a gradient of NaCl concentrations ranging from 0 to 1.0 M. The elution of resulted protein peaks were observed at A280 nm. The resulting fractions containing the active, purified α-galactosidase were collected and kept at − 20 °C for use in further studies.
Gel electrophoresis
To assess the effectiveness of the purification processes and determine the molecular weight of the enzyme, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used. According to Laemmli [17], SDS-PAGE was accomplished using 12% polyacrylamide. Using a 3 kDa MWCO Millipore Amicon® Ultra-15 centrifugal filter (Sigma-Aldrich, USA), protein sample concentration was achieved. It was carried out using the EZ-RunTM Pre-stained Rec Protein Ladder (Fisher, Bioreagents TM; BP3603). After 3 h of Coomassie Brilliant Blue-R250 staining, the gel was destained using a 40:10% solution of acetic acid and ethanol, respectively.
Temperature and pH dependency and stability of α-galactosidase
The purified enzyme (25.39 U/mL) was added to the substrate α-p-NPGal with the appropriate buffer and subjected to temperature values between 20 and 90 °C to detect the optimum temperature for the enzyme activity. The thermal stability of the enzyme was tested at 50–70 °C in the absence of the substrate over a time range of 10–120 min in a citrate phosphate buffer (50 mM, pH 3.5). The optimum pH value for the enzyme activity was determined by using Robinson buffer at pH values in the range of 2.0–7.0. The pH stability was revealed by incubating the enzyme with the same buffer at pH values of 3.0–7.0 at two different temperatures (30 °C and 60 °C) and different time intervals (15–120 min).
Effect of various metal ions on α-galactosidase activity
The effects of various metal ions, namely: MnCl2, HgCl2, CaCl2, KCl, MgSO4, CoSO4, ZnSO4, CuSO4, and FeSO4, on the activity of the pure enzyme (25.39 U/mL), were determined. EDTA was added to the reaction mixture to determine whether it required a metal ion to complete the catalytic process of the enzyme or not. The α-galactosidase activity in the absence of metal ions was recorded as 100%.
Kinetic studies
Using pNP-α-D-Gal as a substrate, the kinetic constants (Km and Vmax) for α-galactosidase were calculated at pH 3.5 and 60 °C. The Michaelis–Menten constant (Km) was identified to express the affinity of α-galactosidase for its substrate. The Vmax value was calculated by incubating the same amount of the enzyme (25.39 U/mL) and varying the substrate concentrations in the range of (0.04–0.8 µM) at the initial rate of hydrolysis of the substrate. The tests were performed in triplicate. The Lineweaver–Burk plots were created using the GraphPad Prism program to determine the Km and Vmax values.
Soymilk yogurt drink preparation
Soymilk was prepared as described by Elshafei et al. [12] and pasteurized at 72 °C for 15 s., then cooled down in an ice bath, and then inoculated with 10% of an activated probiotic mixture including Lactobacillus acidophilus CUL60, Bifidobacterium lactis HNO19, and Bifidobacterium bifidum CUL20. The inoculated soymilk was incubated at 42 °C for 24 h in sterilized bottles. After incubation for 24 h, the yogurt drink was stored in a refrigerator for 2 weeks.
Treatment of soymilk yogurt by the pure α-galactosidase
Ten milliliters of the pure α-galactosidase enzyme (25.39 U/mL) was added to 60 ml of soymilk yogurt in an Erlenmeyer flask 250 ml. This mixture was incubated for 8 h at 50 °C in a shaking state (200 rpm). At the end of the incubation period, the enzymatic activity was suppressed in a boiling water bath for 10 min.
Assessment of the proximate chemical composition of yogurt samples
Using the recommended techniques of the Association of Official Analytical Chemists [18], the proximate compositions of experimental soy yogurt and plain yogurt were measured in triplicate. The Bligh and Dyer [19] technique was used to calculate fat. Carbohydrate content was calculated according to the equation:
Determination of pH and titratable acidity
Yogurt samples, weighing 10 g each, were diluted in 10 mL of deionized water to detect the pH value using a pH meter (Hanna pH-meter HI 9021, Germany). These samples were subjected to a titration process using 0.01 N NaOH solution and 5 drops of 5 g/100 mL phenolphthalein solution. The total titratable acidity (TTA) was then calculated as lactic acid equivalents (g/L) as follows:
Phytochemicals and antioxidant activity
Preparation of yogurt water extract
Yogurt was combined with distilled water in a 1: 0.25 ratio to create yogurt water extract, which was then centrifuged (6708 xg, for 10 min) at 4 °C after being acidified to a pH of 4.0 with 1 M HCl and incubated at 45 °C for 10 min in a water bath. Thereafter, the supernatant was adjusted to pH 7.0 using 0.5 M of NaOH. Within 12 h of preparation, a further stage of centrifugation was performed to obtain yogurt water extract for use in pertinent experiments [20].
Total phenolic assay
Shetty et al. [21] methodology was used to calculate the total phenolic content (TPC). In which diluted Folin-Ciocalteu reagent was added to yogurt water extracts and a 95% ethanol mixture. A Shimadzu UV 1601 spectrophotometer (Japan) was used to determine the absorbance at 725 nm. Gallic acid was employed as a standard to represent the data as total phenolics, where gallic acid equivalents in micrograms per gram (µg GAE g−1) of sample were used.
Determination of total flavonoids
The total flavonoid content of yogurt samples was determined according to Barros et al. [22]. Total flavonoid content was calculated on the basis of the rutin calibration curve and represented as microgram equivalents of rutin per gram (µg RE g−1) of the sample [12].
Antioxidant activity using ABTS radical scavenging and ferric-reducing power
To evaluate the yogurt samples' ability to scavenge the ABTS radical in a reaction, Re et al. [23] technique was used. In which, 2, 2'-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt stock solution was oxidized with potassium persulfate (K2S2O8) to produce the ABTS radical solution. On the other hand, the protocol outlined by Benzie and Strain [24] was followed to conduct the ferric-reducing antioxidant power (FRAP) test. Ascorbic acid was used to create the calibration curve, and the findings were given as micrograms of ascorbic acid equivalents (AAE) per milliliter of extract.
Sugar analysis using HPLC
Absolute ethanol was added to large conical centrifuge tubes containing 10 g of yogurt samples to achieve a final ethanol concentration of 80% (v/v). To precipitate the proteins, slurries were combined and let stand for 20 min at room temperature. A total of 50 ml of ethanol (80% v/v) was then added. The supernatant was obtained after centrifuging the samples at 5000 xg for 5 min. The precipitate was washed with a further 25 mL of an 80% ethanol solution. Following that, the supernatants were all concentrated using a rotary vacuum evaporator (25 to 27 °C). In order to prepare the samples for eventual HPLC analysis, they were filtered using a 0.45 µm Metricel membrane, put in vials, sealed, and frozen at -10 °C. A Shimadzu HPLC Class-VPV 5.03 (Kyoto, Japan) was used to analyze the sugar in the filtrate. HPLC specifications and work conditions were as mentioned in Elshafei et al. [12].
Volatile compounds analysis by GC–MS
Yogurt samples (10 g) were mixed with NaCl (16% w/v) for mild shearing, then transferred to a volumetric flask, fixed to 100 mL with NaCl solution, and well shaken. The Solid Phase Microextraction (SPME) fiber was placed in a 20-mL headspace vial with processed samples (5 mL), which were then immediately sealed. The headspace vial was extracted for 30 min while being stirred at 200 rpm after being equilibrated in a water bath at a constant temperature for 15 min at 60 °C. An Agilent 7890 GC connected to a 5977 MS detector was used for the analyses. The GC–MS operating conditions and compounds recognition were achieved as described by Elshafei et al. [12].
Sensory assessment
As specified by Yang and Li [25], the plain and soy yogurt samples underwent sensory and an organoleptic evaluation test for consistency, odor or aroma, appearance or color, taste, and overall acceptability. A one-way analysis of variance (ANOVA) was used to assess the responses in a fully randomized setup [12].
Results and discussion
Purification and electrophoretic analysis of A. niger NRC114 α-galactosidase
The purification step depended on an optimized α-galactosidase enzyme produced by A. niger NRC114 through the central composite design (CCD) approach as described in our previous work [12]. Following the purification of various α-galactosidases, differential ammonium sulphate precipitations were used to isolate α-galactosidase [26]. As detailed in the Methods section, the crude enzyme was initially submitted to ammonium sulfate saturation, which revealed a total protein content of 124.40 mg and 3214.94 U of α-galactosidase activity. The activity of α-galactosidase was greater in the 60–90% saturation range among the various proteins precipitated at different ammonium sulfate saturations (Table 1). To further purify the α-galactosidase, ammounium sulfate (60–90%) fraction in a citrate phosphate buffer (pH 4.5, 0.05 M) was subjected to Sephadex G-100 column chromatography equilibrated with the same previous buffer (Fig. 1a). The step of Sephadex G-100 column chromatography resulted in a purification fold of 45 and a recovery percentage of 78.8% (Table 1). The active fractions were pooled, dialyzed against citrate phosphate buffer (pH 4.5, 0.02 M), concentrated, and subjected to ion exchange chromatography using a DEAE Sephadex A50 column. From this matrix, a stepwise gradient elution was carried out, and the fractions obtained were examined for protein and enzyme activity (Fig. 1b). The step of DEAE Sephadex A50 column chromatography resulted in a 123-fold purified enzyme. Table 1 summarizes the results of A. niger NRC114 α-galactosidase purification steps. When compared to the fold purification (12.7) achieved for soyabean enzyme [27] and 4.8 percent recovery for the Cicer enzyme [28], the fold purification (123-fold) and recovery (37.58%) from A. niger NRC114 α-galactosidase were greater.
Table 1.
Purification of A. niger NRC114 α-galactosidase
| Purification step | Total units (U) | Total protein (mg) | Specific activity (U/mg protein) | Recovery (%) | Purification fold |
|---|---|---|---|---|---|
| Crude enzyme | 3780.57 | 698.32 | 5.41 | 100.00 | 1.00 |
| Ammonium sulfate ppt. (60–90%) | 3214.94 | 124.40 | 25.84 | 85.04 | 4.77 |
| Sephadex G-100 column | 2981.21 | 12.20 | 244.42 | 78.86 | 45.15 |
| DEAE Sephadex A50 column | 1420.65 | 2.13 | 666.02 | 37.58 | 123.02 |
Fig. 1.
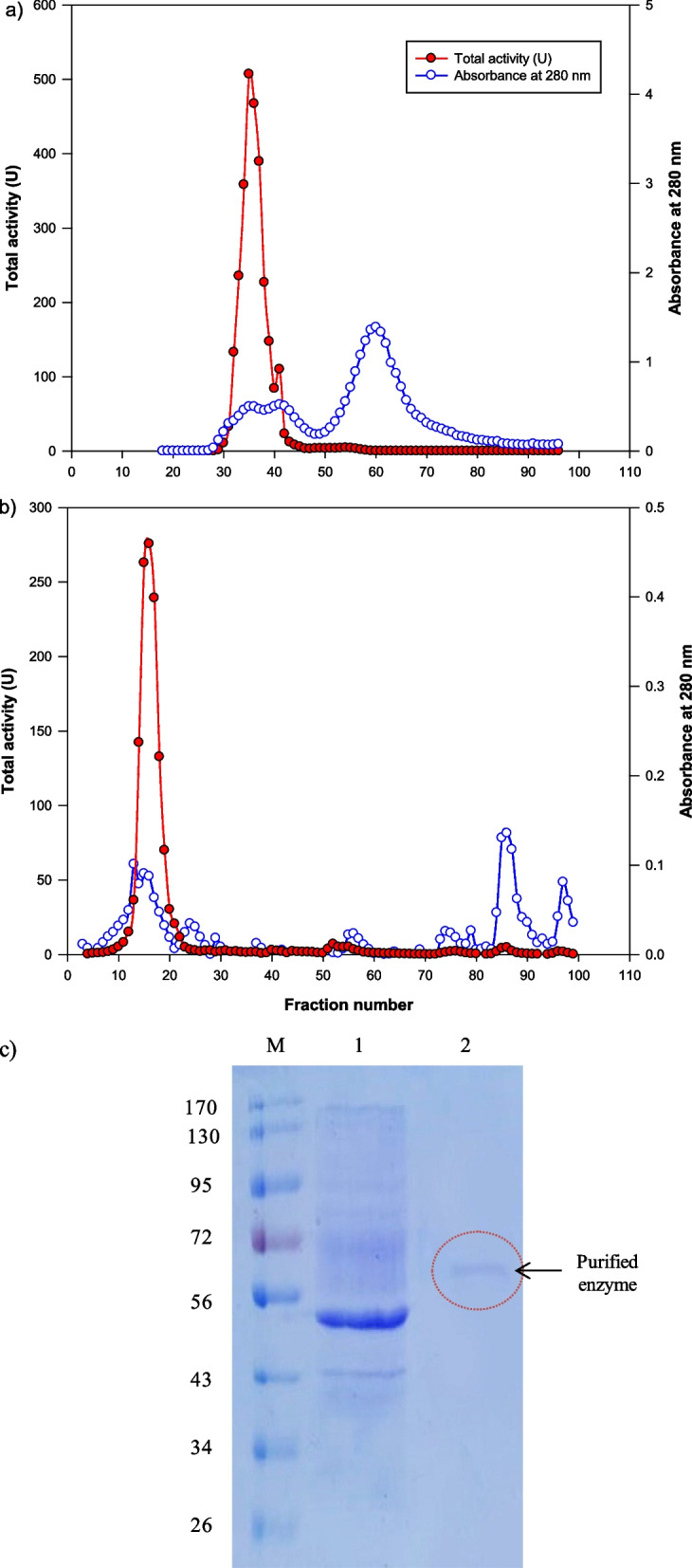
Purification of A. niger NRC114 α-galactosidase; (a) Sephadex G-100 column; (b) DEAE Sephadex A50 column; and (c) SDS-PAGE of A. niger NRC114 purified α-galactosidase enzyme; M: marker, lane 1: crude enzyme, and lane 2: purified α-galactosidase
The peak of activity was pooled and concentrated using Amicon centrifugal filter units (UFC9003) with a pore size of 3 kDa molecular weight cutoff (MWCO). Employing standard proteins, the molecular mass of the purified monomer enzyme that resulted from the last purification step was found to be 64 kDa using the SDS-PAGE approach (Fig. 1c). The protein that displays enzyme activity is eluted as a single peak, providing evidence that the enzyme is homogenous. According to the publications, α-galactosidases exist in several monomeric and multimeric variants with structural variety. The majority of α-galactosidases have molecular size extending between 30 and 100 kDa [29, 30]. Other α-galactosidases from Irpex lacteus and Aspergillus parasiticus also showed similar results, according to the observations [31, 32]. In this study, the purified enzyme from the DEAE Sephadex A50 column was used in all further studies.
Reaction time, temperature, and pH effects
The α-galactosidase activity was assessed at different time intervals between (0–60 min) at 60 °C. pNP-α-D-Gal was incubated with the pure enzyme and citrate phosphate buffer pH 4.5 (0.1 M), and then the released pNP was determined. Figure 2a showed that with the increase in time, the released amount of PNP increased until the incubation time was about 50 min. After that, the enzyme activity reached its maximum extent at 60 min. This result was taken into consideration when deciding to incubate the reaction mixture for 10 min, as it was found to be suitable for the enzyme assay in the next experiments.
Fig. 2.
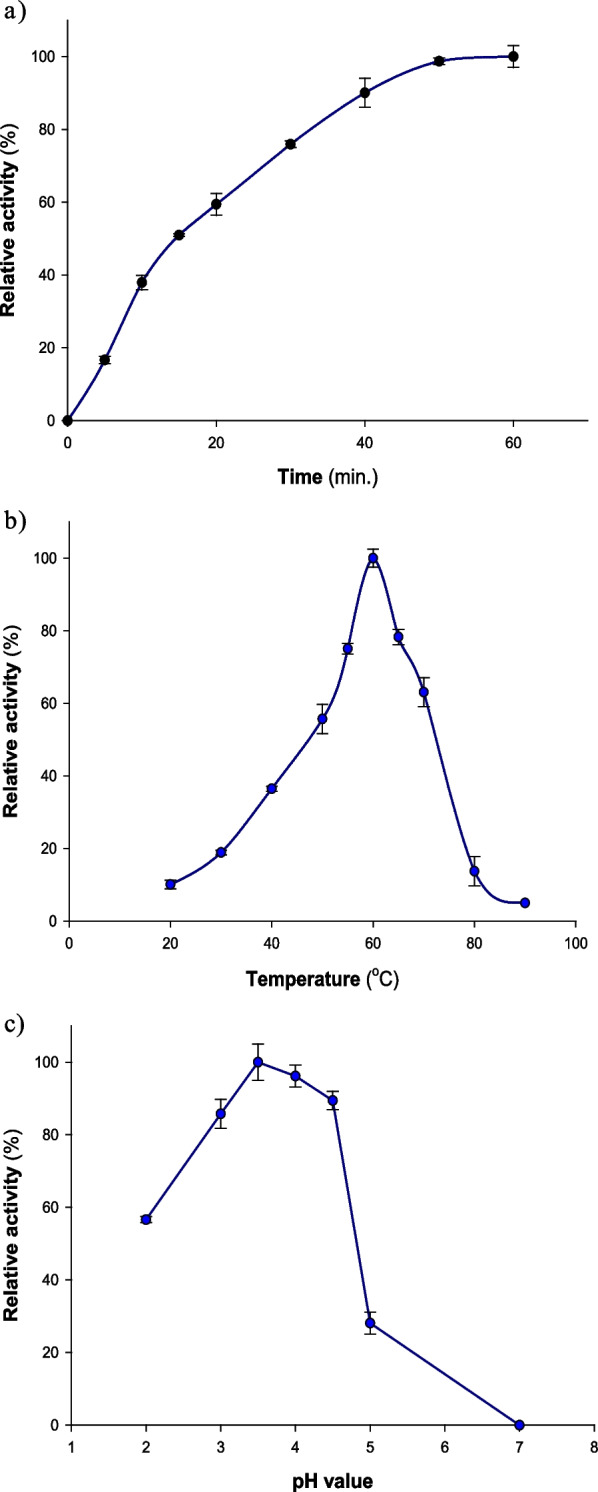
(a) Effect of incubation period on α-galactosidase activity. The α-galactosidase activity was assessed at different time intervals between (0–60 min) at 60 °C. pNP-α-D-Gal was incubated with the pure enzyme (25.39 U/mL) and citrate phosphate buffer pH 4.5 (0.1 M), and then the released pNP was determined. (b) Effect of temperature, and (c) effect of pH on α-galactosidase activity. The vertical bars show the standard deviation values, and each value reflects the mean of three replicate measurements
Figure 2b showed that the optimum temperature for α-galactosidase activity was detected at 60 °C. Before this degree, the enzyme activity exhibited a gradual increase in the range between 20 and 50 °C. On the other hand, the enzyme activity has gradually vanished at temperatures above the optimum degree, reaching 5% at 90 °C. α -galactosidases can be used directly in the processing of legume products. [33]. High-temperature processing has two major benefits: it reduces the risk of microbial contamination while also speeding up the processing time. Similar to other α-galactosidases from thermophilic fungi, including N. fischeri P1 [34] and R. miehei CAU432 [1], A. niger NRC114's was the most active at 60 °C, and much greater than those of the mesophilic species' α -galactosidases [35] and other α -galactosidases from seeds of Cassia, Pepino, Tachigali, Melon, and White Chickpea that have been previously reported (50 °C) [26, 29, 36]; and Cowpea α-galactosidase (35 °C) [37], and less than other α-galactosidases like Phaseolus coccineus α -galactosidase (70 °C) [38], Irpex lacteus α-galactosidase (70 °C) [31], Thermoanaerobacterium polysaccharolyticum α-galactosidase (77.5 °C) [39], and Sulfolobus solfataricus α-galactosidase (90 °C) [28].
The optimum pH value of the enzyme activity was pH 3.5, as presented in Fig. 2c. Before and after this value, the enzyme activity starts to decrease to 28% at pH 5.0. The enzyme activity completely diminished at pH 7.0, which means that the enzyme is active at acidic pH values (Fig. 2c). Most other known fungal α-galactosidases have an ideal pH in the range of pH 4.0–5.5 [40–42], whereas A. niger NRC114's α-galactosidase demonstrated maximal activity at a pH value of 3.5, which is more acidic.
Thermal and pH stability
To determine the thermal stability of A. niger NRC114 α-galactosidase, the enzyme was incubated at 50, 60, 65, and 70 °C for 120 min and assayed periodically for its activity under the standard conditions. A. niger NRC114 α-galactosidase showed complete stability at 50 °C for 120 min, and 60 °C for 90 min. With the rise in temperature, the enzyme activity started to decrease gradually with the time factor (Fig. 3a). After 10 min of incubation, A. niger NRC114 α-galactosidase showed complete stability at 50 and 60 °C, whereas kept 97 and 19% of its initial activity at 65 and 70 °C, respectively. After 60 min, the enzyme still showed its full stability at 50 and 60 °C, but kept half its catalytic activity after exposure to 65 °C. After 120 min of incubation, A. niger NRC114 α-galactosidase still showed good stability behavior and kept 100, 95, and 41% of its initial activity at 50, 60 and 65 °C, respectively (Fig. 3a). Compared to several α-galactosidases from mesophilic microbes [35], A. niger NRC114 exhibits significantly superior thermo-stability. It demonstrated perfect stability at 50 °C for 120 min, a period of time that is significantly longer than that of other α-galactosidases from paddy soil metagenomic ((half-life of 42 min.) [35], Pontibacter sp. HJ8 h. (half-life of 5 min), and Streptomyces sp. HJG4 (half-life of 2 min) [43]. For industrial usage, an enzyme's strong thermostability, high optimum temperature, and reasonably wide pH stability range at room temperature (30 °C) are highly valued [40].
Fig. 3.
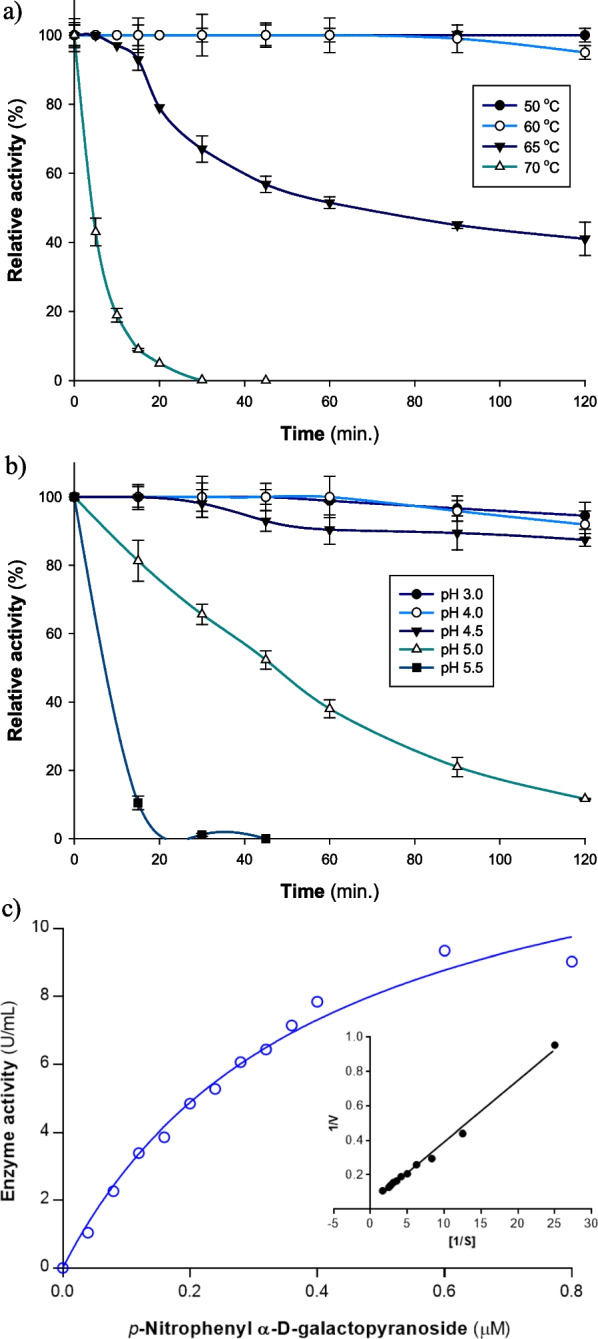
(a) thermal stability, and (b) pH stability (at 60 °C) of A. niger NRC114 purified α-galactosidase enzyme. Thermal stability was checked by incubating the enzyme (25.39 U/mL) at 50, 60, 65, and 70 °C for 120 min in a citrate phosphate buffer (50 mM, pH 3.5) and assayed periodically for its remaining activity under the standard conditions. For pH stability, the enzyme was incubated in a range of pH levels from pH 3.0 to 7.0 for 2 h at 60 °C and evaluated for its residual activity under typical circumstances. The vertical bars show the standard deviation values, and each value reflects the mean of three replicate measurements (c) Lineweaver–Burk plot of the reciprocal of initial velocities and pNP-α-D-Gal concentrations. Km and Vmax values were determined using the Lineweaver–Burk plot technique in GraphPad Prism version 6.01, and the experiments were performed in triplicate
When the purified A. niger NRC114 α-galactosidase was incubated in a range of pH levels from pH 3.0 to 7.0 for 2 h at 30 °C and evaluated for its residual activity under typical circumstances, the enzyme was discovered to be entirely stable in the tested pH range (data not shown). For that, the incubation temperature was increased to offer a bright stability behavior against the pH parameters. The purified A. niger NRC114 α-galactosidase was incubated in different pH values (pH 3.0–7.0) for 2 h at 60 °C and then assayed for its activity under standard conditions. The results obtained showed that the maximum stability was obtained at an acidic pH only, which is consistent with other previously described α-galactosidases [26, 44] proving that the α-galactosidase from A. niger NRC114 is acidic. After 15 min of incubation at 60 °C, A. niger NRC114 α-galactosidase showed varied stability and kept its complete activity at pH 3.0, 4.0, and 4.5, whereas kept 81, 10, and 3% of its initial activity at pH values of 5.0, 5.5, and 7.0, respectively. By increasing incubation time (120 min, 60 °C), the enzyme showed clear stability at acidic pH values, whereas its stability started to clearly diminish after pH 4.5. The relative percentages of remaining enzyme activity were 95, 92, 87, and 12% at pH values of 3.0, 4.0, 4.5, and 5.0, respectively, after 120 min. A. niger NRC114 α-galactosidase was completely inactivated at pH value of 5.5 after 30 min (Fig. 3b), which declare its acidic stability. Acidic α-galactosidases are crucial to raffinose family oligosaccharides (RFOs) metabolism [45]. Bacillus coagulans α-galactosidases showed stability at pH 5.0–10.0 [30] and RmgalB at pH 5.5–9.5 [1] as well as RmGal36 at pH 4.5–10.0 [46] originating from Rhizomucor miehei CAU432.
Kinetic parameters
Michaelis–Menten and Lineweaver–Burk plots were used to estimate the kinetic parameters of A. niger NRC114 α-galactosidase with pNP-α-D-Gal. The Km and Vmax of A. niger NRC114 α-galactosidase were calculated to be 0.401 µM and 14.65 µmol min-1, respectively (Fig. 3c). The recorded Km value on hydrolysis of pNP-α-D-Gal was lower than that reported with α-galactosidase from the seeds of Annona squamosal (0.67 mM) [29], Cicer α-galactosidase (0.67 mM) [26], Tachigali α-galactosidase (0.45 mM) [44], Pepino α-galactosidase (0.37 mM) [47], Soya bean α-galactosidase (0.33 mM) [27]. Higher affinity for the substrate is demonstrated by A. niger NRC114's lower Km value for the enzyme.
Metal ions effect on α-galactosidase activity
Of the metal ions assessed; K+, Mg2+, Co2+, and Zn2+ at a concentration of 2.5 mM revealed 14.39%, 23.46%, 28.99%, and 11.87% activation on the activity of A. niger NRC114 purified α-galactosidase enzyme respectively; whereas Mn2+, Hg2+, and Cu2+ at the same concentration inhibited the enzyme activity (Table 2). This inhibition effect of Cu2+ and Hg2+ has been reported by Çelem and Önal [48] and Geng et al., [49], respectively. The enzyme was activated by 16.57% when the concentration of Zn2+ was increased to 10 mM. Other metals, with the exception of Mg2+, induced enzymatic inhibition at the same dose. Similar to α-galactosidases from Mesorhizobium sp. JB07, Ca2+ had no discernible impact on the α-galactosidase activity of A. niger NRC114 [50], Streptomyces sp. S27 [51], and Hermetia illucens's gut metagenome [52]. Interestingly, EDTA had no effect on the pure α-galactosidase from A. niger NRC114 (Table 2), indicating that it is a non-metallo enzyme similar to the α-galactosidase found in Annona squamosa seeds [29].
Table 2.
Effect of metal ions on A. niger NRC114 α-galactosidase activity
| Metal salt | Relative activity (%) | |
|---|---|---|
| 2.5 mM | 10 mM | |
| Control | 100.00 ± 0.00 | 100.00 ± 0.00 |
| MnCl2 | 25.88 ± 0.003 | 43.29 ± 1.54 |
| HgCl2 | 12.78 ± 0.06 | 14.68 ± 0.49 |
| CaCl2 | 101.62 ± 1.98 | ND |
| KCl | 114.39 ± 0.00 | 86.68 ± 0.23 |
| MgSO4 | 123.46 ± 3.14 | 101.90 ± 1.56 |
| CoSO4 | 128.99 ± 0.67 | 95.07 ± 0.004 |
| ZnSO4 | 111.87 ± 2.55 | 116.57 ± 2.67 |
| CuSO4 | 93.73 ± 0.53 | 22.69 ± 0.37 |
| EDTA | 104.37 ± 0.88 | 101.55 ± 1.92 |
ND Not determined
Assessment of different changes of soymilk yogurt with α-galactosidase treatment
Proximate chemical composition
The treatment of soymilk yogurt by α-galactosidase showed a minor increase (0.86%) in the moisture content of soymilk yogurt at the binning of storage time (Table 3). A gradual increase in the moisture content was detected for all samples with storage at 4 °C. During storage, all samples exhibited a reduction in total solids gradually, and the maximum reductions were found at the end of storage. The obtained results are confirmed by Kumari et al. [53], who found that plain yogurt had a lower value of total solids compared to a symbiotic type. The results showed that the total solids of the enzyme treated-soymilk yogurts were less than those of the untreated ones by 1.12% at zero time and decreased through the storage time (Table 3). The data on protein and ash changes revealed the reduction in their values during storage. The lowest values of protein were observed in the enzyme-treated yogurt at the end of storage at 4 °C (Table 3). At the beginning of storage, ash values were recorded at 0.67% and 0.74% in untreated and enzyme-treated soymilk yogurts, respectively. The fat content had been determined and reduced as the storage time increased. The highest value of fat content (4.18%) was detected with the enzyme-treated soymilk yogurt compared to untreated soymilk yogurt, which had 3.76% at zero time (Table 3). The reduction of fat content during storage may be due to the consumption of strains used in the preparation of yogurt [54].
Table 3.
The proximate chemical composition of soymilk yogurt before and after enzyme treatment during the storage for two weeks at 4 °C
| Sample | Proximate composition (g/100 g)/Storage time (days) | ||
|---|---|---|---|
| Zero | 7 | 14 | |
| Moisture | |||
| Soymilk yogurt | 86.57 ± 0.15b | 89.13 ± 0.08b | 89.75 ± 0.07b |
| Treated soymilk yogurt | 87.43 ± 0.09a | 88.57 ± 0.04b | 89.62 ± 0.12b |
| Total solids | |||
| Soymilk yogurt | 14.28 ± 0.07b | 10.87 ± 0.08b | 10.28 ± 0.06b |
| Treated soymilk yogurt | 13.16 ± 0.03c | 10.23 ± 0.04b | 10.59 ± 0.3b |
| Protein | |||
| Soymilk yogurt | 4.79 ± 0.01b | 4.39 ± 0.02b | 3.85 ± 0.02b |
| Treated soymilk yogurt | 3.48 ± 0.01a | 3.26 ± 0.01a | 3.19 ± 0.01c |
| Fat | |||
| Soymilk yogurt | 3.76 ± 0.02b | 3.57 ± 0.03a | 3.15 ± 0.09b |
| Treated soymilk yogurt | 4.18 ± 0.03a | 3.72 ± 0.01b | 2.84 ± 0.05a |
| Ash | |||
| Soymilk yogurt | 0.67 ± 0.01b | 0.52 ± 0.01b | 0.48 ± 0.07b |
| Treated soymilk yogurt | 0.74 ± 0.02c | 0.68 ± 0.02c | 0.55 ± 0.09c |
| Carbohydrates | |||
| Soymilk yogurt | 4.21 ± 0.05b | 2.39 ± 0.02b | 2.82 ± 0.02b |
| Treated soymilk yogurt | 4.17 ± 0.04b | 3.77 ± 0.03c | 3.80 ± 0.03c |
| pH | |||
| Soymilk yogurt | 3.78 ± 0.01b | 3.65 ± 0.01b | 3.47 ± 0.02b |
| Treated soymilk yogurt | 4.39 ± 0.02a | 4.19 ± 0.03a | 4.08 ± 0.06a |
| TTA (g/L) | |||
| Soymilk yogurt | 2.16 ± 0.02b | 2.17 ± 0.04b | 2.69 ± 0.03b |
| Treated soymilk yogurt | 1.23 ± 0.02a | 1.24 ± 0.03a | 1.26 ± 0.05a |
| Phytochemicals (Total phenols, µg/g) | |||
| Soymilk yogurt | 52.68 ± 0.08b | 56.97 ± 0.03b | 60.34 ± 0.14b |
| Treated soymilk yogurt | 58.73 ± 0.11c | 61.24 ± 0.05c | 63.67 ± 0.09c |
| Total flavonoids (µg/g) | |||
| Soymilk yogurt | 39.43 ± 0.07b | 42.86 ± 0.07b | 45.62 ± 0.10b |
| Treated soymilk yogurt | 43.42 ± 0.09c | 47.52 ± 0.09c | 49.81 ± 0.12c |
| Antioxidant activity | |||
| Ferric-Reducing power (μg of ascorbic acid equivalents/mL) | |||
| Soymilk yogurt | 124.7 ± 0.40b | 138.4 ± 0.09b | 142.8 ± 0.32b |
| Treated soymilk yogurt | 125.2 ± 0.18b | 141.2 ± 0.05c | 143.9 ± 0.15b |
| ABTS assay (μg of ascorbic acid equivalents/mL) | |||
| Soymilk yogurt | 105.3 ± 0.21b | 109.5 ± 0.17b | 110.5 ± 0.16b |
| Treated soymilk yogurt | 107.6 ± 0.17c | 111.3 ± 0.15c | 112.4 ± 0.18c |
Values are expressed as Mean ± SD
Values in the same column with the same superscripts are not significantly different (P < 0.05).
The obtained pH values showed a gradual decrease during storage for two weeks at 4 °C in all yogurts under study. This reduction may be due to the alteration of lactose to lactic acid as well as sugar fermentation with prolonged storage time. The obtained results are confirmed by Lucey [55], who found a similar reduction in pH values in yogurt during storage. On the other hand, an increase in titratable acidity, especially in untreated enzyme yogurt, was observed compared with the treated samples. The acidity increase during storage is correlated with acid production and lactose metabolism subsequently by bacterial strains [56]. The quality of yogurt deteriorates with the increase of acidity and low pH with the increase of storage time due to bitterness and sourness.
The analysis of phytochemicals, including total phenolics and flavonoids, is given in Table 3. The increase of phytochemical content during storage was noticed, and the highest increase of phenolic content was recorded with the enzyme-treated soymilk yogurt, which was 63.67 µg/g at the end of storage in comparison with zero time (58.73 µg/g) (Table 3). A similar trend was found in total flavonoids, which increased with extended storage time. This rise may be explained by the breakdown of milk proteins caused by bacteria producing proteolytic enzymes and the release of certain phenolic acids and isoflavonoids found in soymilk [57].
The most widely used method for detecting antioxidant activity in food products is ABTS, which measures the scavenging activity of various natural products and can be used in media containing hydrophilic and lipophilic antioxidant components such as yogurt [23]. The second method used in the present study is FRAP, which is considered a powerful indicator of antioxidant status and determines the oxidative damage resulting from reactive oxygen [58]. The determination of antioxidant activity using Ferric-Reducing power and ABTS assays showed that the highest values were detected in the yogurt sample treated with α-galactosidase enzyme followed by the untreated one (Table 3). At the end of storage, a significant increase in antioxidant activity was observed compared to fresh samples in all studied samples (Table 3). In yogurt and other fermented milks, some of the antioxidant activity is related to bioactive peptides, which form during processing and fermentation, as well as during storage [59, 60]. The bioactive components in soymilk, like isoflavonoids (genistein and daidzein), vitamins, and proteins, have excellent antioxidant activity [61]. Also, the strong reducing power of prepared soymilk yogurts is attributed to the increased hydrogen ion availability as a result of fermentation by the strains used in the manufacture. The high antioxidant activity of the investigated yogurt samples correlated well with the high total phenolics and flavonoids.
Sugar content
The degradation of oligosaccharides present in soymilk yogurt by α-galactosidase was determined by HPLC analysis. The results cited in Additional file 1: Table S1 showed a dramatic decrease in the raffinose and stachyose content of enzyme-treated soymilk yogurt. About 65% and 98% of stachyose and raffinose were eliminated after storage at 4 °C for two weeks, respectively. A dramatic decrease in lactose and fructose was observed at the end of storage, and a reversible trend was found in glucose and galactose (Additional file 1: Table S1). The increase of glucose and galactose may be correlated with the decrease of raffinose and stachyose by the enzyme hydrolytic activity.
Sensory evaluation
The sensory evaluation for soymilk yogurts either treated or untreated for taste, color, aroma, consistency, and overall acceptability is given in Table 4. The highest scores for all attributes were recorded for plain yogurt, followed by enzyme-treated soymilk yogurt, and the least values for yogurt manufactured without enzyme treatment. A significant negative effect for storage was found on sensory properties for both soymilk yogurts and the pronounced reduction of acceptability noticed in untreated enzyme soymilk yogurt (Table 4). The consumer preferred foods with healthy, nutritional, and therapeutic properties like yogurt [62]. However, the acceptability of these foods is critical. With respect to taste, aroma, color, and overall acceptability, the yogurt prepared from soymilk after enzyme treatment indicates the panelists liked this type, which recorded scores of more than five on a ten-point scale compared to untreated soymilk yogurt. An additional processing step that may improve the low scores of treated soymilk yogurt attributes, especially sour taste or beany-aroma, is adding flavoring agents like mango or chocolate during preparation. The enhancement of undesirable tastes may be obtained by adding sugar or other natural sweeteners like allulose. In the study carried out by Osundahunsi et al. [63], they tried to apply orange and strawberry flavorings to soymilk yogurt and found that strawberry yogurt was preferred over the orange type.
Table 4.
Sensory evaluation of plain and soymilk yogurts
| Attributes | Plain yogurt | Untreated soymilk yogurt | Treated-soymilk yogurt |
|---|---|---|---|
| Taste | 9.58 ± 0.23a | 4.56 ± 0.08b | 8.74 ± 0.15c |
| Colour | 9.92 ± 0.18a | 7.83 ± 0.15b | 9.23 ± 0.17a |
| Aroma | 8.95 ± 0.15a | 6.29 ± 0.16b | 7.82 ± 0.13a |
| Consistency | 8.16 ± 0.19a | 5.34 ± 0.12b | 7.95 ± 0.12a |
| OAA | 9.13 ± 0.24a | 5.26 ± 0.17b | 8.67 ± 0.09c |
Values in the same row with the same superscripts are not significantly different (P < 0.05)
OAA Overall acceptability
Volatile compounds
The extraction of volatile compounds using SPME and analysis by GC–MS showed that a total of 36 volatile compounds had been identified and are given in Additional file 1: Table S2. These identified volatile compounds belong to various chemical groups and can be classified as follows: 11 ketones as well as aldehydes, 7 esters, 6 acids, 5 alcohols, 3 terpenes, and 4 sulfur-containing compounds. Many of these volatile compounds are formed after fermentation and subsequent chemical changes during storage [64]. Acetaldehyde is the most important volatile compound that has a fresh, fruity, pungent taste, and it does originate from the metabolism of lactose, the decarboxylation of pyruvate, or the intermediate acetyl coenzyme A [65]. In all studied yogurt samples, acetaldehyde was found with significant variation either at zero time or after storage (Additional file 1: Table S2). The alcohol dehydrogenase enzyme had the ability to metabolize acetaldehyde into ethanol [66]. Also, the reduction of acetaldehyde during storage correlated well with the evaporation and/or hydrolysis by microbial enzymes.
It is well known that acetaldehyde can be oxidized easily, and the formation of acetate has occurred. The obtained results are confirmed by the study carried out by Hussein et al. [67] on plain and fortified yogurts with various additives. Among the identified aldehydes, hexanal, which gives a fruity note and originates from β-oxidation, was found with concentrations of 1.15% and 2.82% in untreated and treated soymilk-yogurt, respectively (Additional file 1: Table S2). The obtained data are in good agreement with previous studies carried out by Saint-Eve et al. [68] and Donkor et al. [69]. Another major volatile compound in yogurt is acetoin (diacetyl), which has a buttery, fatty, pungent odor and is formed by the acetolactate decarboxylase enzyme. The importance of acetoin in yogurt flavor appears when a low concentration of acetaldehyde has occurred [70]. Table S2 showed a reduction in acetoin in all yogurt samples at the end of storage. This phenomenon may be due to microbial hydrolysis by enzymes and the formation of new volatile compounds [71]. During storage in the refrigerator, acetoin was recorded in all studied yogurt samples, with the highest concentration in treated soymilk yogurt (1.70%) and the least concentration (1.01%) recorded in untreated soymilk yogurt (Additional file 1: Table S2). The previous studies by Chammas et al. [72] and Condurso et al. [64] confirmed our data.
Another volatile compound class that is formed via β-oxidation is ketones. The ketones such as 2-nonanone, 2-heptanone, and 2-propanone are responsible for the metallic flavor in ultrahigh-temperature (UHT) milk. The high concentrations of ketones such as 2,3-pentanedione and 2-heptanone, which are characterized by creamy and buttery and creamy and fresh flavors, respectively, impact the flavor in yogurt [73]. 2,3-Pentanedione is formed during the metabolism of isoleucine by α-aceto-α-hydroxybutyrate [74]. The most significant ketones were recorded as 2-heptanone, 2-nonanone, and 2-undecanone (Additional file 1: Table S2). Both soy yogurts prepared from either untreated or enzyme-treated soymilk exhibited higher concentrations of 2-undecanone, 2-nonanone (creamy and fresh notes), and 2-heptanone with a significant reduction at the end of storage (Additional file 1: Table S2). Herein, we could identify six ketones during the storage of yogurt samples. Among the identified ketones, 2,3-pentanedione and 2-nonanone were recorded in all samples, while 2,3-butanedione, 2-heptanone, and 2-butanone were identified in soymilk yogurts (Additional file 1: Table S2). This variation may be due to the chemical composition of milk and the effect of thermal treatment [70]. The identified ketones in the present study were found in small concentrations in all yogurt samples. The increase in ethanol in the current study is confirmed by several researchers [75]. On the other hand, our results contrast with those of Vah and Hruškar [76] and Ekinci and Gurel [71], who reported a significant reduction in ethanol during storage. With the increase of ethanol during storage, a reduction of acetaldehyde has been observed depending on the type of yogurt. Our results are in agreement with Demirci et al. [76].
In the current study, a total of seven esters were identified, and their concentrations are given in Additional file 1: Table S2. Ethyl acetate, which is responsible for the fruity and pineapple flavors, was recorded. The highest concentration of ethyl acetate was found in enzyme soymilk yogurt (2.85%), whereas the lowest concentration was recorded at 0.25% in an untreated soymilk yogurt sample at zero time (Additional file 1: Table S2). The presence of ethyl acetate has been recorded in yogurt samples [68]. Esters such as ethyl acetate and ethyl hexanoate play an important role in yogurt flavor and are responsible for the reduction of unpleasant odor and short-chain fatty acids [77]. Additional file 1: Table S2 showed a total of six volatile acids, including acetic, butanoic, pentanoic, hexanoic (capric acid), octanoic, and decanoic acids, which were identified with significant concentrations in soymilk yogurts. During storage, the soymilk yogurts had a higher concentration of the aforementioned acids, which may explain the sour taste and low scores of sensory attributes (Table 4). The concentrations of acetic acid were 11.91% and 7.33% in the untreated soymilk yogurt and the enzyme-treated one, respectively, at the end of storage. The study of Terpou et al. [78] found similar data for probiotics in yogurt after enrichment with bran from wheat. The analysis of the studied yogurt samples revealed four sulfur-containing compounds that had been identified, as shown in Table S2. Dimethyl sulfide was the major sulfur compound in enzyme soymilk yogurt with a concentration of 7.42%, followed by 3-(methylthio)-1-propanol, which recorded 4.80% at zero time, and these compounds were significantly reduced at the end of storage.
Conclusions
Purification of A. niger NRC114 α-galactosidase yielded a 123-fold purified enzyme. The characterization of the pure enzyme revealed that the pure enzyme has high levels of pH and thermal stability, especially at acidic conditions. The relative lower Km value of the enzyme under study indicates its higher affinity for substrate, which is highly recommended. The treated soymilk yogurt showed an increase in total phenolic and flavonoid contents, as well as several detected volatile compounds. In addition, the enzymatic action in the hydrolysis of raffinose family oligosaccharides was confirmed. These findings suggest that this enzyme has the potential to be used in a variety of important future studies, including the treatment of Fabry's disease, blood group conversion, and the eradication of immunogenic α-gal epitopes in xenotransplantation.
Supplementary Information
Additional file 1: Table S1: HPLC analysis of sugars (%) in soymilk yogurts. Table S2: Effect of storage on volatile compounds of soymilk yogurt samples
Acknowledgements
The National Research Centre (Egypt), which provided funding for this investigation under the research project number 12020114, is acknowledged by the authors.
Author contributions
The research was conceptualized and planned by AMO, AME, MAE, GEI, MMH, and NSM. Experiments were done by AMO, MAE, and GEI. The data was evaluated by all authors. The project was supervised by AME. The manuscript was written by AMO, MAE, and GEI. The article was reviewed and approved by all authors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This investigation was funded by the National Research Centre (Egypt) under the research project number 12020114.
Availability of data and materials
This article has all the data that was created or evaluated during this investigation.
Declarations
Ethics approval and consent to participate
There were no human or animal subjects used in any of the studies mentioned in this article.
Consent for publication
Not applicable.
Competing interests
No conflicting interests are disclosed by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katrolia P, Jia H, Yan Q, Song S, Jiang Z, Xu H. Characterization of a protease-resistant α-galactosidase from the thermophilic fungus Rhizomucor miehei and its application in removal of raffinose family oligosaccharides. Biores Technol. 2012;110:578–586. doi: 10.1016/j.biortech.2012.01.144. [DOI] [PubMed] [Google Scholar]
- 2.Ye F, Geng X, Xu L, Chang M, Feng C, Meng J. Purification and characterization of a novel protease-resistant GH27 α-galactosidase from Hericium erinaceus. Int J Biol Macromol. 2018;120:2165–2174. doi: 10.1016/j.ijbiomac.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Sirisha E, Potumarthi R, Naveen A, Mangamoori LN. Purification and characterisation of intracellular alpha-galactosidases from Acinetobacter sp. 3 Biotech. 2015;5:925–932. doi: 10.1007/s13205-015-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier EM, Osterrieder S, Whybra C, Ries M, Gal A, Beck M, et al. Disease manifestations and X inactivation in heterozygous females with Fabry disease. Acta Paediatr Suppl. 2006;95:30–38. doi: 10.1080/08035320600618809. [DOI] [PubMed] [Google Scholar]
- 5.Mutra R, Joseph JE, Panwar D, Kaira GS, Kapoor M. Low molecular weight α-galactosidase from black gram (Vigna mungo): Purification and insights towards biochemical and biophysical properties. Int J Biol Macromol. 2018;119:770–778. doi: 10.1016/j.ijbiomac.2018.06.093. [DOI] [PubMed] [Google Scholar]
- 6.Bayraktar H, Önal S. Concentration and purification of α-galactosidase from watermelon (Citrullus vulgaris) by three phase partitioning. Sep Purif Technol. 2013;118:835–841. [Google Scholar]
- 7.Filho M, Pessela BC, Mateo C, Carrascosa AV, Fernandez-Lafuente R, Guisán JM. Immobilization–stabilization of an α-galactosidase from Thermus sp. strain T2 by covalent immobilization on highly activated supports: selection of the optimal immobilization strategy. Enzyme Microbial Technol. 2008;42:265–271. [Google Scholar]
- 8.Singh BP, Vij S. α-Galactosidase activity and oligosaccharides reduction pattern of indigenous lactobacilli during fermentation of soy milk. Food Biosci. 2018;22:32–37. [Google Scholar]
- 9.Carević M, Banjanac K, Ćorović M, Jakovetić S, Milivojević A, Vukašinović-Sekulić M, et al. Selection of lactic acid bacteria strain for simultaneous production of α- and β-galactosidases. Zas Mat. 2016;57:265–273. [Google Scholar]
- 10.Gote MM, Khan MI, Gokhale DV, Bastawde KB, Khire JM. Purification, characterization and substrate specificity of thermostable α-galactosidase from Bacillus stearothermophilus (NCIM-5146) Process Biochem. 2006;41:1311–1317. [Google Scholar]
- 11.Gurung N, Ray S, Bose S, Rai V. A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int. 2013;2013:329121. doi: 10.1155/2013/329121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshafei AM, Othman AM, Elsayed MA, Ibrahim GE, Hassan MM, Mehanna NS. A statistical strategy for optimizing the production of α-galactosidase by a newly isolated Aspergillus niger NRC114 and assessing its efficacy in improving soymilk properties. J Genet Eng Biotechnol. 2022;20:36. doi: 10.1186/s43141-022-00315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju L-K, Loman AA, Mahfuzul Islam SM. α-galactosidase and its applications in food processing. In: encyclopedia of food chemistry. Elsevier; 2019. p. 124–8.
- 14.Buckley ND, Champagne CP, Masotti AI, Wagar LE, Tompkins TA, Green-Johnson JM. Harnessing functional food strategies for the health challenges of space travel—fermented soy for astronaut nutrition. Acta Astronaut. 2011;68:731–738. [Google Scholar]
- 15.Ohtakara A, Mitsutomi M, Uchida Y. Purification and enzymatic properties of α-galactosidase from Pycnoporus cinnabarinus. Agric Biol Chem. 1984;48:1319–1327. [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.AOAC. Official methods of analysistm, 21st Edition (2019). AOAC INTERNATIONAL. 2019. https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/. Accessed 27 Jun 2022.
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Behrad S, Yusof MY, Goh KL, Baba AS. Manipulation of probiotics fermentation of yogurt by Cinnamon and Licorice: effects on yogurt formation and inhibition of Helicobacter pylori growth in vitro. Int J Nutrition Food Eng. 2009;3:563–567. [Google Scholar]
- 21.Shetty K, Curtis O, Levin R, Witkowsky R, Ang W. Prevention of vitrification associated with in vitro shoot culture of oregano. (Origanum vulgare) by Pseudomonas spp. 1995. 10.1016/S0176-1617(11)82181-4.
- 22.Barros L, Cabrita L, Boas MV, Carvalho AM, Ferreira ICFR. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011;127:1600–1608. [Google Scholar]
- 23.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Li L. Physicochemical, textural and sensory characteristics of probiotic soy yogurt prepared from germinated soybean. Food Technol Biotechnol. 2010;48(4):490. [Google Scholar]
- 26.Singh N, Kayastha AM. Purification and characterization of α-galactosidase from white chickpea (Cicer arietinum) J Agric Food Chem. 2012;60:3253–3259. doi: 10.1021/jf204538m. [DOI] [PubMed] [Google Scholar]
- 27.de Fátima Viana S, Guimarães VM, José IC, e Oliveira MG, Costa NM, de Barros EG, Moreira MA, de Rezende ST. Hydrolysis of oligosaccharides in soybean flour by soybean α-galactosidase. Food Chem 2005;93(4):665-70.
- 28.Brouns SJJ, Smits N, Wu H, Snijders APL, Wright PC, de Vos WM, et al. Identification of a novel α-galactosidase from the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 2006;188:2392–2399. doi: 10.1128/JB.188.7.2392-2399.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakharayapatna Ranganatha K, Venugopal A, Chinthapalli DK, Subramanyam R, Nadimpalli SK. Purification, biochemical and biophysical characterization of an acidic α-galactosidase from the seeds of Annona squamosa (custard apple) Int J Biol Macromol. 2021;175:558–571. doi: 10.1016/j.ijbiomac.2021.01.179. [DOI] [PubMed] [Google Scholar]
- 30.Zhao R, Zhao R, Tu Y, Zhang X, Deng L, Chen X. A novel α-galactosidase from the thermophilic probiotic Bacillus coagulans with remarkable protease-resistance and high hydrolytic activity. PLoS ONE. 2018;13:e0197067. doi: 10.1371/journal.pone.0197067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang JM, Yang Y, Wang R, Bao H, Yuan H, Yang J. Characterization of a high performance α-galactosidase from Irpex lacteus and its usage in removal of raffinose family oligosaccharides from soymilk. Int J Biol Macromol. 2019;131:1138–1146. doi: 10.1016/j.ijbiomac.2019.04.060. [DOI] [PubMed] [Google Scholar]
- 32.Shivam K, Mishra SK. Purification and characterization of a thermostable α-galactosidase with transglycosylation activity from Aspergillus parasiticus MTCC-2796. Process Biochem. 2010;45:1088–1093. [Google Scholar]
- 33.Pugalenthi M, Siddhuraju P, Vadivel V. Effect of soaking followed by cooking and the addition of α-galactosidase on oligosaccharides levels in different Canavalia accessions. J Food Compos Anal. 2006;19:512–517. [Google Scholar]
- 34.Wang H, Shi P, Luo H, Huang H, Yang P, Yao B. A thermophilic α-galactosidase from Neosartorya fischeri P1 with high specific activity, broad substrate specificity and significant hydrolysis ability of soymilk. Biores Technol. 2014;153:361–364. doi: 10.1016/j.biortech.2013.11.078. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y-P, Liaw L-L, Kuo J-T, Wu H-T, Wang G-H, Chen X-Q, et al. Evaluation of synthetic gene encoding α-galactosidase through metagenomic sequencing of paddy soil. J Biosci Bioeng. 2019;128:274–282. doi: 10.1016/j.jbiosc.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z, Schaffer AA. A novel alkaline α-galactosidase from melon fruit with a substrate preference for raffinose1. Plant Physiol. 1999;119:979–988. doi: 10.1104/pp.119.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffigniez F, Briffaz A, Mestres C, Ricci J, Alter P, Durand N, et al. Kinetic study of enzymatic α-galactoside hydrolysis in cowpea seeds. Food Res Int. 2018;113:443–451. doi: 10.1016/j.foodres.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Du F, Zhu M, Wang H, Ng T. Purification and characterization of an α-galactosidase from Phaseolus coccineus seeds showing degrading capability on raffinose family oligosaccharides. Plant Physiol Biochem. 2013;69:49–53. doi: 10.1016/j.plaphy.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 39.King MR, White BA, Blaschek HP, Chassy BM, Mackie RI, Cann IKO. Purification and characterization of a thermostable α-galactosidase from Thermoanaerobacterium polysaccharolyticum. J Agric Food Chem. 2002;50:5676–5682. doi: 10.1021/jf0202281. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Yan Q, Guan L, Jiang Z, Yang S. Biochemical characterization of a novel protease-resistant α-galactosidase from Paecilomyces thermophila suitable for raffinose family oligosaccharides degradation. Process Biochem. 2020;94:370–379. [Google Scholar]
- 41.Wang C, Wang H, Ma R, Shi P, Niu C, Luo H, et al. Biochemical characterization of a novel thermophilic α-galactosidase from Talaromyces leycettanus JCM12802 with significant transglycosylation activity. J Biosci Bioeng. 2016;121:7–12. doi: 10.1016/j.jbiosc.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Ma R, Shi P, Huang H, Yang P, Wang Y, et al. Insights into the substrate specificity and synergy with mannanase of family 27 α-galactosidases from Neosartorya fischeri P1. Appl Microbiol Biotechnol. 2015;99:1261–1272. doi: 10.1007/s00253-014-6269-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Liu Y, Lu Q, Zhang R, Wu Q, Li C, et al. Characterization of a glycoside hydrolase family 27 α-galactosidase from Pontibacter reveals its novel salt-protease tolerance and transglycosylation activity. J Agric Food Chem. 2016;64:2315–2324. doi: 10.1021/acs.jafc.6b00255. [DOI] [PubMed] [Google Scholar]
- 44.Fialho L da S, Guimarães VM, Callegari CM, Reis AP, Barbosa DS, Borges EE de L, et al. Characterization and biotechnological application of an acid α-galactosidase from Tachigali multijuga Benth. seeds. Phytochemistry. 2008;69: 2579–85. [DOI] [PubMed]
- 45.Blöchl A, Peterbauer T, Hofmann J, Richter A. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta. 2008;228:99–110. doi: 10.1007/s00425-008-0722-4. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Yan Q, Jiang Z, Liu Y, Li Y. High-level expression of a novel α-galactosidase gene from Rhizomucor miehei in Pichia pastoris and characterization of the recombinant enyzme. Protein Expr Purif. 2015;110:107–114. doi: 10.1016/j.pep.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Şen A, Eryılmaz M, Bayraktar H, Önal S. Purification of α-galactosidase from pepino (Solanum muricatum) by three-phase partitioning. Sep Purif Technol. 2011;83:130–136. [Google Scholar]
- 48.Çelem EB, Önal S. Sepabeads EC-EP immobilized α-galactosidase: immobilization, characterization and application in the degradation of raffinose-type oligosaccharides. Process Biochem. 2022;116:136–147. [Google Scholar]
- 49.Geng X, Yang D, Zhang Q, Chang M, Xu L, Cheng Y, et al. Good hydrolysis activity on raffinose family oligosaccharides by a novel α-galactosidase from Tremella aurantialba. Int J Biol Macromol. 2020;150:1249–1257. doi: 10.1016/j.ijbiomac.2019.10.136. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Lu Q, Zhang R, Wang Y, Wu Q, Li J, et al. Characterization of two glycoside hydrolase family 36 α-galactosidases: novel transglycosylation activity, lead–zinc tolerance, alkaline and multiple pH optima, and low-temperature activity. Food Chem. 2016;194:156–166. doi: 10.1016/j.foodchem.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Cao Y, Yuan T, Shi P, Luo H, Li N, Meng K, et al. Properties of a novel α-galactosidase from Streptomyces sp. S27 and its potential for soybean processing. Enzyme Microbial Technol. 2010;47:305–312. [Google Scholar]
- 52.Lee C-M, Kim S-Y, Song J, Lee Y-S, Sim J-S, Hahn B-S. Isolation and characterization of a halotolerant and protease-resistant α-galactosidase from the gut metagenome of Hermetia illucens. J Biotechnol. 2018;279:47–54. doi: 10.1016/j.jbiotec.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Kumari AGIP, Ranadheera S, Prasanna PHP, Senevirathne N, Vidanarachchi J. Development of a rice incorporated synbiotic yogurt with low retrogradation properties. Int Food Res J. 2015;22:2032–2040. [Google Scholar]
- 54.Ye M, Ren L, Wu Y, Wang Y, Liu Y. Quality characteristics and antioxidant activity of hickory-black soybean yogurt. LWT Food Sci Technol. 2013;51:314–318. [Google Scholar]
- 55.Lucey JA. Cultured dairy products: an overview of their gelation and texture properties. Int J Dairy Tech. 2004;57:77–84. [Google Scholar]
- 56.Bensmira M, Jiang B. Organic acids formation during the production of a novel peanut-milk kefir beverage. British J Dairy Sci. 2011;2(1):18–22. [Google Scholar]
- 57.McCue PP, Shetty K. Phenolic antioxidant mobilization during yogurt production from soymilk using Kefir cultures. Process Biochem. 2005;40:1791–1797. [Google Scholar]
- 58.Küçük M, Kolaylı S, Karaoğlu Ş, Ulusoy E, Baltacı C, Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007;100:526–534. [Google Scholar]
- 59.Awad S, El-Sayed MI, Wahba A, El Attar A, Yousef MI, Zedan M. Antioxidant activity of milk protein hydrolysate in alloxan-induced diabetic rats. J Dairy Sci. 2016;99:8499–8510. doi: 10.3168/jds.2015-10626. [DOI] [PubMed] [Google Scholar]
- 60.Şanlıdere Aloğlu H, Öner Z. Determination of antioxidant activity of bioactive peptide fractions obtained from yogurt. J Dairy Sci. 2011;94:5305–5314. doi: 10.3168/jds.2011-4285. [DOI] [PubMed] [Google Scholar]
- 61.Mahmoudi I, Moussa OB, Hassouna M. Symbiotic, hypocholesterolemic and antioxidant effects of potential probiotic Lactobacilli strains isolated from Tunisian camel milk. Adv Microbiol. 2017;7:328–342. [Google Scholar]
- 62.Hekmat S, Soltani H, Reid G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov Food Sci Emerg Technol. 2009;10:293–296. [Google Scholar]
- 63.Osundahunsi OF, Amosu D, Ifesan BOT. Quality evaluation and acceptability of soy-yoghurt with different colours and fruit flavours. Am J Food Technol. 2007;2:273–280. [Google Scholar]
- 64.Condurso C, Verzera A, Romeo V, Ziino M, Conte F. Solid-phase microextraction and gas chromatography mass spectrometry analysis of dairy product volatiles for the determination of shelf-life. Int Dairy J. 2008;18:819–825. [Google Scholar]
- 65.Chaves ACSD, Fernandez M, Lerayer ALS, Mierau I, Kleerebezem M, Hugenholtz J. Metabolic engineering of acetaldehyde production by Streptococcus thermophilus. Appl Environ Microbiol. 2002;68:5656–5662. doi: 10.1128/AEM.68.11.5656-5662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamime AY, Deeth HC. Yogurt: technology and biochemistry 1. J Food Prot. 1980;43:939–977. doi: 10.4315/0362-028X-43.12.939. [DOI] [PubMed] [Google Scholar]
- 67.Hussein MM, Hassan FAM, Abdel Daym HH, Salama A, Enab AK, Abd El-Galil AA. Utilization of some plant polysaccharides for improving yoghurt consistency. Annals Agricul Sci. 2011;56:97–103. [Google Scholar]
- 68.Saint-Eve A, Lévy C, Le Moigne M, Ducruet V, Souchon I. Quality changes in yogurt during storage in different packaging materials. Food Chem. 2008;110:285–293. doi: 10.1016/j.foodchem.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 69.Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Rheological properties and sensory characteristics of set-type soy yogurt. J Agric Food Chem. 2007;55:9868–9876. doi: 10.1021/jf071050r. [DOI] [PubMed] [Google Scholar]
- 70.Kaminarides S, Stamou P, Massouras T. Comparison of the characteristics of set type yoghurt made from ovine milk of different fat content. Int J Food Sci Tech. 2007;42:1019–1028. [Google Scholar]
- 71.Ekinci FY, Gurel M. Effect of using propionic acid bacteria as an adjunct culture in yogurt production. J Dairy Sci. 2008;91:892–899. doi: 10.3168/jds.2007-0244. [DOI] [PubMed] [Google Scholar]
- 72.Chammas GI, Saliba R, Corrieu G, Béal C. Characterisation of lactic acid bacteria isolated from fermented milk “laban”. Int J Food Microbiol. 2006;110:52–61. doi: 10.1016/j.ijfoodmicro.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 73.Ott A, Fay LB, Chaintreau A. Determination and origin of the aroma impact compounds of yogurt flavor. J Agricul Food Chem. 1997;45(3):850–858. [Google Scholar]
- 74.Imhof R, Glättli H, Bosset JO. Volatile organic compounds produced by thermophilic and mesophilic single strain dairy starter cultures. LWT Food Sci Technol. 1995;28:78–86. [Google Scholar]
- 75.Pinto SM, Clemente MDG, De Abreu LR. Behaviour of volatile compounds during the shelf life of yoghurt. Int J Dairy Technol. 2009;62:215–223. [Google Scholar]
- 76.Demirci T, Öztürk Negiş Hİ, Oraç A, Konak Göktepe Ç, Sözeri Atik D, Aktaş K, et al. Immature wheat grain as a potential prebiotic ingredient in set-type yoghurts: impact on antioxidative, textural properties and survival of different probiotics. J Food Sci Technol. 2019;56:5474–5483. doi: 10.1007/s13197-019-04019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curioni PMG, Bosset JO. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int Dairy J. 2002;12:959–984. [Google Scholar]
- 78.Terpou A, Gialleli A-I, Bekatorou A, Dimitrellou D, Ganatsios V, Barouni E, et al. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod Process. 2017;101:184–192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: HPLC analysis of sugars (%) in soymilk yogurts. Table S2: Effect of storage on volatile compounds of soymilk yogurt samples
Data Availability Statement
This article has all the data that was created or evaluated during this investigation.


