Abstract
We conducted a systematic review on the state of the science related to sleep interventions for informal caregivers of persons with Alzheimer’s disease or related dementia (ADRD). This review included English-written, peer-reviewed articles that studied the effect of an intervention on sleep health outcomes for informal caregivers of persons with ADRD. Our search yielded 15 articles that met our a priori inclusion criteria. We categorized interventions into four categories: environmental, physical, cognitive, and collaborative. Intervention effects were heterogeneous, with most yielding nonsignificant sleep health effects. There is a need for theoretically sound and robust sleep health interventions for informal caregiver samples. Future research in this area could benefit from the use of more controlled, pragmatic, and adaptive research designs, and the use of objective measures that conceptually represent the multiple domains of sleep health to enhance intervention quality.
Keywords: Sleep, Caregiving, Dementia, Alzheimer’s disease
By 2050, more than 130 million individuals throughout the world will be afflicted with Alzheimer’s disease or related dementia (ADRD), exceeding more than US $1 trillion in associated costs (Gao et al., 2019; Prince et al., 2016; Wimo et al., 2017). ADRD is a progressive disease associated with decline in functional status, and informal caregivers are instrumental in providing care for those with ADRD, regardless of the disease’s stage or progression (Koca et al., 2017). In the United States alone, 16 million adults spend more than 20h per week serving as caregivers for persons with ADRD (Prudencio & Young, 2020). ADRD behavioral and psychological symptoms may compromise the sleep health of informal caregivers, which further impairs their ability to care for themselves and the person with ADRD (Baharudin et al., 2019; Mukherjee et al., 2017; Vaingankar et al., 2016; Worley, 2018).
Informal caregivers of persons with ADRD commonly report poor sleep health. However, there is a limited evidence base describing sleep-focused interventions for informal caregivers of those with ADRD. The majority of existing supportive interventions targeted psychological outcomes, such as psychological well-being, knowledge, and coping (Gilhooly et al., 2016). Few intervention studies evaluated any kind of subjective or objective physical health outcomes, with most reporting subjective levels of physical health (Cheng et al., 2019, 2020). A recent systematic review identified three sleep-focused behavioral interventions that improved the sleep of informal caregivers of those with ADRD (Gao et al., 2019). However, this work focused exclusively on sleep-related outcomes, specifically sleep quality and sleep duration, which fails to capture the complexity of sleep health, which can be measured by concepts other than quality and duration (e.g., hygiene and satisfaction) (Buysse, 2014; Knutson et al., 2017).
Given the detrimental health consequences associated with serving as an informal caregiver, the development of supportive interventions for informal caregivers of those with ADRD is a national research priority (Corriveau et al., 2017). There is ample evidence linking sleep health to physical and psychological health, yet little is known about the effects of sleep interventions for these caregivers. Such information may inform future development of supportive sleep interventions for these caregivers and other informal caregiving populations, as well as improve the health of care recipients (Gallagher-Thompson et al., 2020).
Therefore, the purpose of this study was to conduct a systematic literature review of sleep interventions for informal caregivers of persons with ADRD. Specifically, we sought to describe the types of sleep interventions and sleep-health outcomes of interest for targeted sleep interventions among these caregivers. Our research was guided by the following aims:
Describe the current state of the science as it relates to sleep interventions for informal caregivers of persons with ADRD.
Examine the effects of sleep interventions on the health of these caregivers.
Provide evidence-informed recommendations for future clinical research that address the limitations of the state of the science focused on sleep interventions for these caregivers.
Methods
Our study’s methods were guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA; Liberati et al., 2009). For this study, we specified inclusion criteria and analysis methods in advance; however, the protocol was not registered.
Eligibility Criteria, Information Sources, and Search
We included all English-written, peer-reviewed articles that examined the effect of a sleep intervention on any sleep-related outcome (Ibáñez et al., 2018), among informal caregivers of persons with ADRD, regardless of study design. We defined “informal caregiver” as an individual providing unpaid care to a person with whom they have a personal relationship (National Research Council, 2010). To improve breadth and comprehensiveness, we defined sleep interventions to include studies that examined the effect of any intervention type on a sleep-related outcome, even if it did not directly target sleep health (e.g., sleep quality, disturbance, daytime sleepiness, etc.; Knutson et al., 2017). Abstracts, conference proceedings, graduate theses/dissertations, and editorials were not included. We excluded review articles; however, we hand-searched review reference lists to identify additional relevant articles. We identified studies by searching electronic databases on March 14, 2020. We applied the same search strategy to PubMed, CINAHL, and Academic Search Complete. Specifically, our search terms contained the databases’ subject heading equivalent of “Dementia,” “Caregivers,” and “Sleep,” each connected with the “AND” Boolean operator. To enhance the relevance of our findings, we only included articles published from January 1, 2009 through December 31, 2019. Our study’s lead investigator exported the search results to EndNote and used one of the program’s functions to remove duplicate articles.
Study Selection, Data Extraction, and Bias Assessment
We used an unblinded systematic search strategy performed by two independent reviewers to screen studies for inclusion. We created a screening tool to maintain organization of the screening process (Polanin et al., 2019). Initially, our two study reviewers screened the title and abstract, and would search the full-text article to clarify the inclusion criteria, if necessary. Specifically, each reviewer verified the criteria of a single article in the following order: (a) written in English, (b) peer-reviewed, (c) included informal caregivers of a person with ADRD, (d) examined an informal caregiver sleep-related outcome (Ibáñez et al., 2018), (e) described the effect of an intervention on the sleep-related outcome, and (f) was not a review article. Upon completion of screening, the lead investigator examined the articles selected by the reviewers to verify congruence with the inclusion criteria and resolve screening discrepancies between the two reviewers.
We created a data extraction tool that was pilot-tested on the included articles by a single reviewer, and refined accordingly (Liberati et al., 2009; Randolph, 2009). Next, the two reviewers extracted data from the included studies and the lead investigator checked the extracted data for accuracy and completeness. They extracted the following methodological data: (a) study design (i.e., sampling method, group allocation methods, and comparison groups), (b) data collection time-points (i.e., frequency of data collection and distance between time points), (c) study setting, (d) sample descriptors (i.e., inclusion/exclusion criteria, age of caregivers and patients, gender distribution, and relationship), and (e) intervention characteristics (i.e., components, delivery method, dosing, and intervention fidelity). The extracted outcome data were the studies designated sleep outcomes (measurement and relationship to intervention exposure).
To evaluate the rigor of the included studies, our lead investigator examined each study for sources of bias using the revised Cochrane risk-of-bias tool for randomized trials (ROB 2; Sterne et al., 2019). The ROB 2 elicits evaluation of bias domains related to the study’s randomization process, intervention deviations, missing outcome data, outcome measurement, and reporting practices. Within each domain, a series of signaling questions are used to direct an algorithm that yields three possible bias outcomes from each domain (i.e., low risk, high risk, and some concern) and an overall bias evaluation. To clarify for this review, signaling questions involving specific outcomes were evaluated in terms of the specific sleep outcomes examined by each study.
Results
Our initial search yielded 643 articles to screen for inclusion; 43% were duplicates. Once our lead investigator removed the duplicates, the reviewers screened 365 full-text articles for eligibility. During the screening process, the reviewers excluded 350 articles for not meeting the inclusion criteria. The primary reasons for exclusion were not involving informal dementia caregivers (52%), not examining a sleep outcome (16%), and not describing the effect of an intervention on the sleep-related outcome (16%). Our hand search of the literature did not provide any additional articles that met our criteria. Overall, our search yielded 15 articles that met our criteria. The two screeners demonstrated acceptable interrater reliability (96.6% agreement; Cohen’s κ = .94). Figure 1 illustrates the processes for the search, extraction, and identification of articles that met the inclusion criteria.
Figure 1.
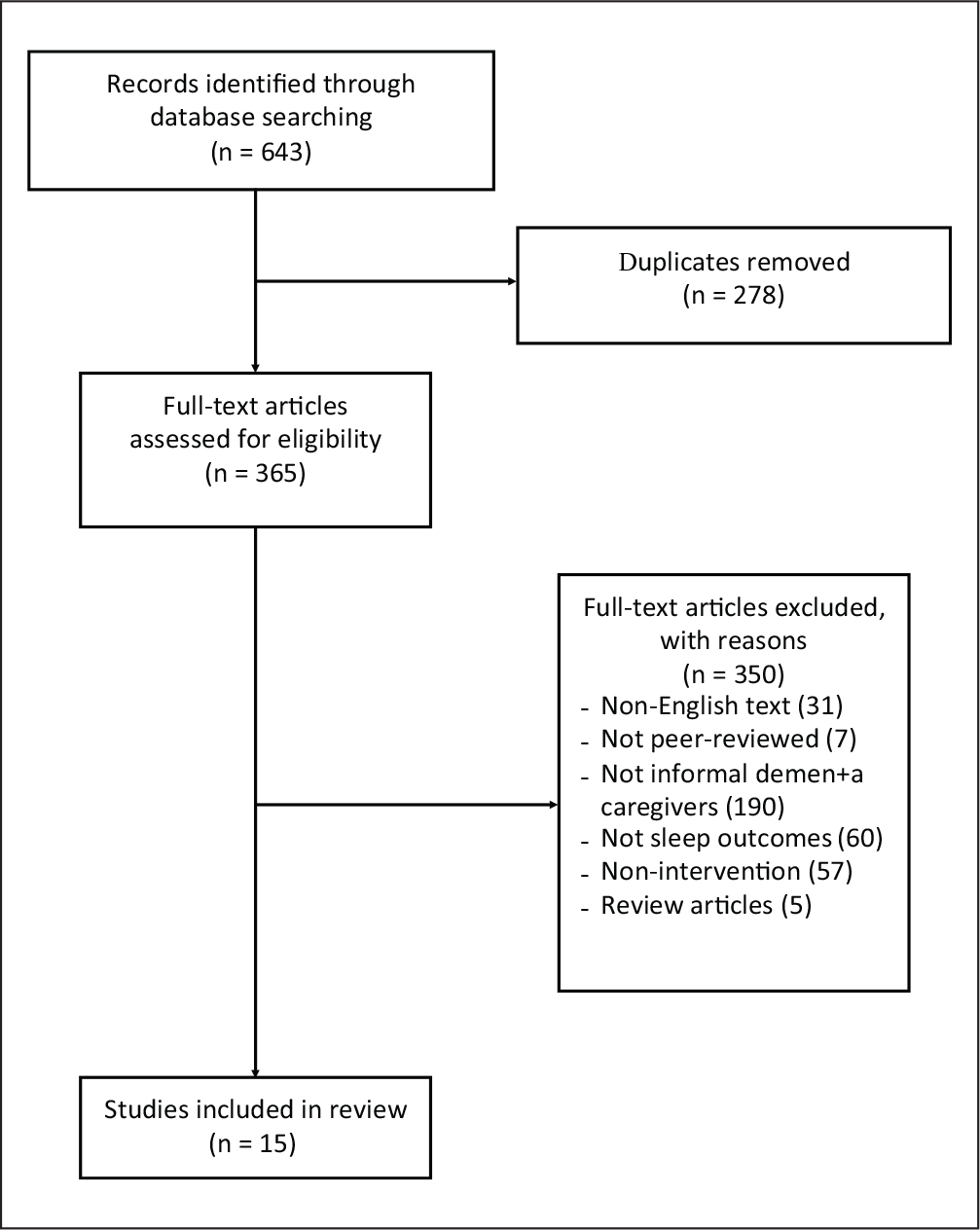
PRISMA Flow Diagram for Study Selection.
Bias Assessment
We used the ROB 2 tool to evaluate 14 of the 15 studies for bias; however, we did not use it for the Spring et al. (2009) study, because they reported only qualitative data. Of the 14 studies, 9 (64.3%) were appraised as having a “high risk” of bias, with the most common source stemming from the randomization process domain (Figueiro et al., 2015; Gibson et al., 2017; Jain et al., 2014; Paller et al., 2015; Simpson & Carter, 2010; Thomas et al., 2019). We deemed the remaining three studies as high risk due to significant concerns with measurement of the sleep outcomes (Elliott et al., 2010; Hirano et al., 2011) and intervention fidelity (Sloane et al., 2015). We categorized three studies as possessing “some concerns” due to concerns with measurement of the sleep outcome (Fowler et al., 2016; Rowe et al., 2010; Williams et al., 2019) missing sleep outcome data (Fowler et al., 2016) and the randomization process (Fowler et al., 2016). Thus, only two studies, both testing physical interventions, were deemed to possess a “low risk” of bias across all five bias domains (Table 1; Korn et al., 2009; Rose et al., 2009). This indicator of the quality of the included studies, when taken into consideration with other study characteristics such as the populations, settings, selected outcomes, and interventions, has adequate quality and an acceptable degree of bias for the critical appraisal and synthesis of the included studies.
Table 1.
Bias Risk Assessment of Included Studies.
| Author | Randomization | Intervention Deviations | Missing Outcome Data | Outcome Measurement | Reporting Selection | Overall Risk of Bias |
|---|---|---|---|---|---|---|
|
| ||||||
| Elliott | Low | Low | Low | High | Low | High |
| Figueiro | High | Low | Low | Some concern | Low | High |
| Fowler | Some concern | Low | Some concern | Some concern | Low | Some concerns |
| Gibson | High | High | Some concern | Some concern | Low | High |
| Hirano | Some concern | Low | Some concern | High | Low | High |
| Jain | High | Low | Some concern | Low | Low | High |
| Korn | Low | Low | Low | Low | Low | Low |
| Paller | High | Low | Low | Low | Low | High |
| Rose | Low | Low | Low | Low | Low | Low |
| Rowe | Low | Low | Low | Some concern | Low | Some concerns |
| Simpson | High | Low | Low | Some concern | Low | High |
| Sloane | Low | High | Low | Some concern | Low | High |
| Thomas | High | Some concern | Some concern | Low | Low | High |
| Williams | Low | Low | Low | Some concern | Low | Some concern |
Design Characteristics of Studies
Seven (Elliott et al., 2010; Fowler et al., 2016; Hirano et al., 2011; Korn et al., 2009; Rose et al., 2009; Rowe et al., 2010; Williams et al., 2019) of the reviewed studies tested their intervention in a two-group, randomized controlled trial (RCT). The remaining studies used quasi or nonexperimental designs. The control groups in four (Elliott et al., 2010; Fowler et al., 2016; Hirano et al., 2011; Rowe et al., 2010) of the traditional RCTs were assigned a passive control (i.e., usual care condition), while the other three received an active control (Korn et al., 2009; Williams et al., 2019) or placebo (Rose et al., 2009). Figueiro et al. (2015) used the informal caregivers as a control group for the care recipients with ADRD.
Nine studies (Elliott et al., 2010; Figueiro et al., 2015; Fowler et al., 2016; Gibson et al., 2017; Hirano et al., 2011; Jain et al., 2014; Korn et al., 2009; Paller et al., 2015; Sloane et al., 2015) used a pre/posttest design, whereas the other six studies (Rose et al., 2009; Rowe et al., 2010; Simpson & Carter, 2010; Spring et al., 2009; Thomas et al., 2019; Williams et al., 2019) used repeated measures. The studies’ data collection schedules ranged from several weeks (Figueiro et al., 2015; Gibson et al., 2017; Hirano et al., 2011; Jain et al., 2014; Korn et al., 2009; Paller et al., 2015; Rose et al., 2009; Simpson & Carter, 2010; Sloane et al., 2015) to several months (Elliott et al., 2010; Fowler et al., 2016; Rowe et al., 2010; Spring et al., 2009; Thomas et al., 2019; Williams et al., 2019) Table 2 provides a summary of the included studies’ methodologies.
Table 2.
Summary of Study Methodologies.
| Authors | Design | Setting | Population |
|---|---|---|---|
|
| |||
| Elliott et al. (2010) | Randomized, multiple-site clinical trial | At home | Caregiver (n = 495) Age (M): 61.25 Gender (% female): 83.8 Race (% Caucasian): 32.2 |
| Person with ADRD (n = 495) Age (M): 78.8 Gender (% female): 55.7 Race (% Caucasian): not provided |
|||
| Figueiro et al. (2015) | Longitudinal, pre/post quasi-experiment | At home | Caregiver (n = 34) Age (M):71.8 Gender (% female): not provided Race (% Caucasian): not provided |
| Person with ADRD (n = 35) Age (M): 80.8 Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Fowler et al. (2016) | Randomized two-group pre/posttest design | At home | Caregiver (n = 28) Age (M): 63 Gender (% female): 46 Race (% Caucasian): 75 |
| Person with ADRD (n = 28) Age (M): 82 Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Gibson et al. (2017) | Mixed methods feasibility study | At home | Caregiver (n = 15) Age (M): not provided Gender (% female): not provided Race (% Caucasian): not provided |
| Person with ADRD (n = 15) Age (M): not provided Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Hirano et al. (2011) | Randomized two-group design | At home | Caregiver (n = 31) Age (M): 73.7 Gender (% female): 67.7 Race (% Caucasian): not provided |
| Person with ADRD (n = 31) Age (M): 76.9 Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Jain et al. (2014) | Open label feasibility study | In person sessions at in the community | Caregiver (n = 10) Age (M): 64 Gender (% female): 100 Race (% Caucasian): 80 |
| Person with ADRD (n = 10) Age (M): not provided Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Korn et al. (2009) | RCT | At home, specifically within the Native American community | Caregiver (n = 42) Age: 57% > 50 years Gender (% female): 90.5 Race (% Caucasian): 0 |
| Person with ADRD (n = 42) Age: 57.5% are >70 Gender (% female): not provided Race (% Caucasian): 0 |
|||
| Paller et al. (2015) | Pre/post quasi-experimental | Medical room conference center or local senior center | Caregiver (n = 20) Age (M): 62.5 Gender (% female): 80 Race (% Caucasian): not specified |
| Person with ADRD (n = 17) Age (M): 72.0 Gender (% female): 29 Race (% Caucasian): not provided |
|||
| Rose et al. (2009) | Randomized, double-blind, controlled pilot study | At home | Caregiver (n = 38) Age (M): 74.23 Gender (% female): 65.8 Race (% Caucasian): not provided |
| Person with ADRD (n = 38) Age (M): 76.075 Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Rowe et al. (2010) | Controlled clinical trial with pretest–posttest control group design and repeated measures, | At home | Caregiver (n = 45) Age (M): 62.165 Gender (% female): 81 Race (% Caucasian): 77.5 |
| Person with ADRD (n = 45) Age (M): 79.62 Gender (% female): 50 Race (% Caucasian): not provided |
|||
| Simpson and Carter (2010) | Quasi-experimental, repeated measures design | Location of caregiver’s choice, usually at home | Caregiver (n =10) Age (M): 63 Gender: predominantly women Race (% Caucasian): 60 |
| Person with ADRD (n = 10) Age (M): not provided Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Sloan et al. (2015) | Randomized control trial with crossover | At home | Caregiver (n =17) Age: 41% are >60 years Gender (% female): 77 Race (% Caucasian): 82 |
| Person with ADRD (n = 17) Age: 65% are >80 years Gender (% female): 65 Race (% Caucasian): 82 |
|||
| Spring et al. (2009) | Qualitative art of a mixed methods study | At home | Caregiver (n =14) Age (M): 63 Gender (% female): 93 Race (% Caucasian): 79 |
| Person with ADRD (n = 14) Age (M): not provided Gender (% female): not provided Race (% Caucasian): not provided |
|||
| Thomas et al. (2019) | A case study of one caregiver who participated in two separate studies | At home and through telemedicine | Caregiver (n = 1) Age: 71 Gender (% female): 100 Race: not provided |
| Person with ADRD (n = 1) Age: 74 Gender (% female): 0 Race: not provided |
|||
| Williams et al. (2019) | Randomized control trial | At home | Caregiver (n = 83) Age (M): 64 Gender (% female): 71 Race (% Caucasian): 86 |
| Person with ADRD (n = 71) Age (M): 75.7 Gender (% female): 41 Race (% Caucasian): 96 |
|||
Sample Characteristics
The majority (60%) of studies recruited participants from a single geographic area, most often a single community. However, Korn et al. (2009) recruited from four counties in the Pacific Northwest, Williams et al. (2019) recruited from two Midwest US research sites, and Elliott et al. (2010) recruited from five sites across the United States. Seven studies included caregiver/care recipient dyads (Elliott et al., 2010; Figueiro et al., 2015; Gibson et al., 2017; Paller et al., 2015; Rowe et al., 2010; Sloane et al., 2015; Williams et al., 2019), with the remaining studies studying only the informal dementia caregivers. Few studies had a sample size larger than 50, except for Williams et al. (2019) (N = 84) and Elliott et al. (2010) (N = 495). The informal caregivers were predominantly White female spouses between the approximate ages of 60 and 75 years living with and providing care for an older White male.
The most commonly evaluated sleep outcome was subjective sleep quality. The most common instrument used to measure subjective sleep quality was the Pittsburgh Sleep Quality Index (PSQI). However, Elliott et al. (2010) and Hirano et al. (2011) derived their own measure of sleep quality. Six studies also measured sleep quality objectively with actigraphy (Figueiro et al., 2015; Fowler et al., 2016; Gibson et al., 2017; Rowe et al., 2010; Simpson & Carter, 2010; Thomas et al., 2019). A small portion of the studies also measured sleep disturbance (Sateia, 2014) with investigator-derived instruments (Rowe et al., 2010), or validated subjective instruments such as the Insomnia Severity Index (Fowler et al., 2016), the General Sleep Disturbances Scale (GSDS) (Rose et al., 2009), the Medical Outcomes Study Sleep Scale (MOS-Sleep) (Sloane et al., 2015), and the Epworth Sleepiness Scale (Rowe et al., 2010). Two studies also used light meters to measure circadian stimulus, phasor angle, and phasor magnitude, which are indicative of physiologic responses to light (Figueiro et al., 2015; Sloane et al., 2015). Finally, two studies used qualitative responses to describe participant perceptions of how the intervention influenced their sleep (Gibson et al., 2017; Spring et al., 2009).
Intervention Design and Effects on Sleep Health
The described interventions varied in complexity and scope. Some interventions consisted of a single intervention component (Korn et al., 2009; Rose et al., 2009), whereas others contained multiple components (Elliott et al., 2010; Gibson et al., 2017; Simpson & Carter, 2010). Notably, only four (Korn et al., 2009; Rose et al., 2009; Spring et al., 2009; Williams et al., 2019) studies mentioned the use of a theory or framework to guide their intervention development or implementation. Korn et al. (2009) used polarity therapy theory, Rose et al. (2009) used psychoneuroimmunology theory, Spring et al. (2009) used grounded theory, and Williams et al. (2019) used a dementia behavior model. Given the intervention heterogeneity, we derived an interventional taxonomy to organize our synthesis of the interventions and their effect on sleep. This taxonomy classifies the study interventions by four intervention functionality themes: physical, environmental, cognitive, and collaborative. All of the environmental interventions (Figueiro et al., 2015; Rowe et al., 2010; Sloane et al., 2015; Spring et al., 2009; Thomas et al., 2019), three physical interventions (Gibson et al., 2017; Hirano et al., 2011; Rose et al., 2009), and two collaborative interventions (Fowler et al., 2016; Simpson & Carter, 2010) were designed to specifically target caregiver sleep, while others used to sleep as a secondary intervention outcome (Elliott et al., 2010; Spring et al., 2009; Williams et al., 2019) See Table 3 for a detailed summary of the interventions and their effects.
Table 3.
Summary of Study Interventions and Effects on Sleep Outcomes.
| Author | Intervention (Type) | Significant Sleep Outcomes | Nonsignificant Sleep Outcomes |
|---|---|---|---|
|
| |||
| Elliott et al. (2010) | REACH (collaborative), a multicomponent individualized intervention including educational materials for caregivers about self-care and health, and a telephone support group session. | Caregiver sleep quality (self-reported) improved in intervention group | None |
| Figueiro et al. (2015) | Lighting intervention (environmental) in which custom luminaires were installed into participants’ homes. | None | - Sleep duration (actigraphy): decreased insignificantly - Sleep minutes (actigraphy): - Sleep efficiency - PSQI global score (self-reported) decreased insignificantly |
| Fowler et al. (2016) | Virtual Healthcare Neighborhood (VHN; collaborative), a website that includes a blog for social support, educational material, and the opportunity to ask questions to an interdisciplinary healthcare team. | None | - Insomnia severity (self-reported) - Number of sleep interruptions (actigraphy) decreased insignificantly - Sleep score (actigraphy) improved insignificantly |
| Gibson et al. (2017) | Bright light therapy, exercise, and sleep hygiene education (physical). | Not provided | Not provided |
| Hirano et al. (2011) | Regular exercise of moderate intensity (physical). | - Quality of sleep scores decreased (self-reported) | None |
| Jain et al. (2014) | Central Meditation and Imagery Therapy for Caregivers (CMIT-C; cognitive), an in-person meditation and guided imagery group therapy program. | - Decreased insomnia symptoms | None |
| Korn et al. (2009) | Polarity therapy (physical), a type of biofield touch therapy where a practitioner strategically applies manual pressure to different anatomical points. | None | - Quality and patterns of sleep (self-reported) |
| Paller et al. (2015) | Mindfulness (cognitive) group sessions with elements drawn from dialectical behavioral therapy and from acceptance and commitment therapy. | - Among the participants who registered sleep problems initially, there was a significant improvement in sleep quality | None |
| Rose et al. (2009) | Cranial Electrical Stimulation, (CEM; physical) using a small device, which attaches using clips on the earlobes, that delivers low levels of alternating electrical current to the head. | - Decreased sleep onset latency (self-reported) | - Sleep disturbances (self-reported) - Sleep quality (self-reported) |
| Rowe et al. (2010) | Nighttime Monitoring System, (NMS; environmental), a device which alerts the caregiver when the care-recipient leaves their bed at night. | - Subjective improved sleep (self-reported) | - Objective sleep (actigraphy) |
| Simpson and Carter (2010) | Caregiver Sleep Intervention, (CASI; collaborative), a behavioral intervention incorporating stimulus control, relaxation therapy, cognitive therapy, sleep hygiene education, and goal setting and attainment. | None | - Sleep quality (self-reported) - Objective sleep measurement (actigraphy) |
| Sloan et al. (2015) | Blue–white light therapy (environmental) installed on during individuals’ normal waking hours. Control group was given yellow–white light. | Compared to control lighting: - Improved sleep efficiency (self-reported) - Improved sleep adequacy (self-reported) - Decreased sleep problems (self-reported) - Improved sleep index score (self-evaluated) Compared to usual light: - Decreased sleep problems (self-reported) - Decreased sleep disturbances (self-reported) - Improved sleep index score (self-evaluated) |
None |
| Spring et al. (2009) | NMS (environmental), which tracks the nighttime activities of the care recipient and both alerts the caregiver and allows the caregiver to track the recipient’s movements. | - Increased sleep disruptions affecting quality and quantity of sleep (self-reported) | None |
| Thomas et al. (2019) | Tele-STAR (cognitive, cognitive), telehealth consultations to discuss distressing care recipient behavior, and EVALUATE-AD (environmental), a motion sensor computer system that monitors caregiver behavior and burden. | - Decreased total sleep time (motion sensors) | None |
| Williams et al. (2019) | Supporting Family Caregivers with Technology for Dementia Home Care, FamTechCare (collaborative), a telehealth and technology intervention involving video cameras at home recording difficult situations and then tailored interventions based on those recordings made by an interdisciplinary team of experts. | None | - Sleep disturbance (self-reported) |
Environmental Interventions.
These interventions involved either modifying or monitoring the home environment of the participants. Caregiver recipients of the Rowe et al. (2010) nighttime monitoring intervention reported significantly lower sleep after wake onset levels than controls; however, no other significant objective or subjective differences in sleep quality were observed. The Spring et al. (2009) qualitative subanalysis of the Rowe et al. (2010) study found that the use of an in-home NMS increased self-reported sleep quality in some caregivers, while others reported more nighttime awakenings because of the system’s alarm. In addition, the second Thomas et al. (2019) case study explored the feasibility and preliminary efficacy of using home-based infrared motion sensors to identify digital biomarkers that may inform caregiver support. They found that the caregiver’s objective sleep time decreased slightly during the monitoring period (Thomas et al., 2019). Finally, Figueiro et al.’s (2015) and Sloane et al.’s (2015) studies tested the effects of prescribed in-home light therapy. Overall, receipt of both interventions corresponded to increases in the caregivers’ circadian stimulus and sleep efficiency. However, compared to controls, caregiver recipients of Sloane et al.’s (2015) light intervention reported significantly better sleep quality and fewer sleep problems, while Figueiro et al. (2015) reported no other significant sleep-related findings.
Physical Interventions.
Four studies tested physical interventions that involved some prescribed dose of physical contact or activity. For example, Korn et al.’s (2009) intervention was eight weekly polarity therapy sessions among a sample of Pacific Northwest American Indians for stress reduction. Similarly, participants in Rose et al.’s (2009) study received 60 min of daily cranial electric stimulation over the course of 4 weeks to improve sleep, depressive symptoms, and caregiver appraisal. Hirano et al. (2011) prescribed three weekly doses of moderate intensity exercise for 12 weeks to improve caregiver burden and bothersome physical symptoms. The prescribed exercise intervention was associated with a significant improvement in caregiver subjective sleep quality, whereas the polarity therapy and cranial electric stimulation interventions were not associated with significant follow-up changes in subjective sleep quality or sleep disturbance (Korn et al., 2009; Rose et al., 2009). Interestingly, Gibson et al.’s (2017) sleep-focused intervention trialed a combination of prescribed exercise and light therapy with sleep hygiene education for caregiver study participants. While they did not perform any formal hypothesis testing, they reported a quantitative trend toward improved sleep quality in the participants, who also provided qualitative support of the intervention’s beneficial impact on their sleep (Gibson et al., 2017).
Cognitive Interventions.
Three studies examined the effects of cognitive-focused interventions. These interventions were designed to reduce caregiver stress (Jain et al., 2014; Paller et al., 2015) and burden (Thomas et al., 2019). For example, Thomas et al. (2019) tested the effect of eight individual telehealth cognitive behavioral therapy sessions and three in-person caregiver group meetings within a caregiver/care recipient dyad. Alternatively, the Jain et al. (2014) and Paller et al. (2015) interventions involved attending eight weekly small group meetings that focused on the development of meditation (Jain et al., 2014) and mindfulness skills (Paller et al., 2015). The Jain et al. (2014) intervention was associated with fewer insomnia symptoms at study follow-up. Paller et al. (2015) also reported improved sleep quality among intervention recipients, yet the improvement was not significant. Thomas et al. (2019) did not perform formal statistical comparisons; however, they reported a progressive quantitative decline of the caregiver’s sleep quality resulting from the demands related to the clinical deterioration of the care recipient.
Collaborative Interventions.
The final four studies employed collaborative approaches to providing caregiver support. For instance, Williams et al. (2019) provided intervention recipients with a home video recording system that was reviewed by an interprofessional team to provide education, feedback, and develop caregiving strategies for problem behaviors. However, the intervention did not demonstrate any significant effects on measured sleep outcomes. Fowler et al. (2016) deployed a virtual healthcare neighborhood whose recipients did not report significant changes in sleep quality, sleep quantity, and insomnia symptoms, compared to the control group. Elliott et al. (2010) and Simpson and Carter (2010) tested face-to-face interventions that, like Williams et al. (2019), provided education and feedback, but also included intervention elements related to goal setting and provision of social support. The Simpson and Carter (2010) intervention was focused exclusively on improving caregiver sleep, while the Elliott et al. (2010) intervention was broader in scope. Nonetheless, Simpson and Carter (2010) reported no significant interventional sleep effects, and Elliott et al. (2010) reported a significant improvement in sleep for intervention recipients over time, but the improvement was not significantly better than the control group.
Discussion
We identified four relatively distinct intervention taxonomies: physical, environmental, cognitive, and collaborative. Overall, light-based environmental interventions significantly improved circadian stimulus and sleep efficiency (Figueiro et al., 2015; Sloane et al., 2015). These findings are consistent with another systematic review of light therapy in neuropsychiatric illness (Faulkner et al., 2020), while a monitoring-based environmental intervention was associated with a decrease in wake after sleep onset (Rowe et al., 2010). Physical interventions consisting of prescribed exercise were significantly associated with improvements in sleep latency (Gibson et al., 2017) and sleep quality (Hirano et al., 2011). This finding is unsurprising, as an abundance of evidence suggests regular physical exercise promotes sleep health (Dolezal et al., 2017). Similarly, prescribed cognitive interventions were predictive of decreased insomnia symptomatology (Jain et al., 2014) and improved sleep quality which is also an extant finding in other primary care and community settings (Cheung et al., 2019; Paller et al., 2015).
All studies in this review were longitudinal, and 7 of the 15 reported using a diverse range of quasi or nonexperimental study designs. Furthermore, apart from Elliott et al. (2010) and Williams et al. (2019) the remaining studies all had sample sizes of less than 50 participants, of which less than half studied caregiver/care recipient dyads. The sampling was relatively homogeneous, with the included studies’ sample characteristics being consistent with one another and the extant literature (Brodaty & Donkin, 2009). Objective and subjective sleep quality was the most commonly evaluated outcome among the studies in this review, with a small number of studies measuring subjective symptoms of sleep disturbance (Fowler et al., 2016; Jain et al., 2014; Rowe et al., 2010; Sloan et al., 2015). However, several studies used only subjective (Elliott et al., 2010; Hirano et al., 2011; Korn et al., 2009; Paller et al., 2015; Rose et al., 2009; Williams et al., 2019) or objective (Fowler et al., 2016, Rowe et al., 2010; Sloane et al., 2015; Thomas et al., 2019) measures of sleep quality, or were scientifically limited by missing outcome data (Fowler et al., 2016; Gibson et al., 2017; Hirano et al., 2011; Jain et al., 2014). As such, the aforementioned limitations of the studies in this review curb our ability to draw confident conclusions regarding the effect of sleep interventions on the sleep health of informal caregivers of those with ADRD.
Considering the current state of the science regarding the effects of sleep interventions on the sleep health of informal caregivers of those with ADRD, we propose several recommendations for future clinical research. While heterogeneity in the study designs affirms the feasibility of conducting interventional work in this population, the future use of rigorously designed and adequately powered studies should be emphasized (e.g., RCT; Hariton & Locascio, 2018). Also, we encourage the inclusion of more diverse informal caregiver populations, as the present sampling homogeneity limits generalizability of the studies’ respective findings. In addition, we recommend that any future study in this population include dyadic interventions with caregiver/care recipient dyads from varied sociodemographic backgrounds, as they are feasible to conduct, and may be particularly effective when targeting caregiver outcomes related to burden, such as sleep (Brodaty et al., 2003; Poon, 2019).
Given the varied success of interventions across taxonomies, combination of various intervention taxonomies into multicomponent, group-focused intervention delivered over several weeks may yield superior effectiveness. This recommendation is consistent with prior work related to the use of multicomponent interventions for sleep health (Murawski et al., 2018; Schlarb et al., 2010) and other dementia caregiving populations (Gilhooly et al., 2016). This recommendation provides an opportunity for clinical researchers to develop effective interventions via scientifically rigorous means such as mixed methods designs (Gibson et al., 2017; Rowe et al., 2010; Spring et al., 2009), or advanced developmental designs like multiphase optimization strategy (MOST) or sequential multiple assignment randomized trial (SMART) designs (Collins et al., 2007). Furthermore, the limited effectiveness of the collaborative interventions further accentuates a broader sentiment among clinical scientists regarding the significance and need for the development of health promotion interventions that focus not only on education and feedback, but also incorporate biopsychosocial models of health maintenance (Collins et al., 2016; National Institute of Nursing Research, 2016; Ricon et al., 2019). Nonetheless, as the SARS-CoV-2 pandemic continues to disrupt global healthcare delivery systems and social functioning (Chakraborty & Maity, 2020; Ivanov, 2020), the feasibility of delivering remote interventions to this population is supported by the successful conduct of the collaborative studies in this review, and may be able to guide and expand upon future development of remote interventions.
Finally, the specific sleep outcome to target requires significant consideration. The National Sleep Foundation recommends joint examination of subjective and objective sleep quality measures, as well as additional indicators of sleep health (e.g., satisfaction, duration, and disturbances) and other related variables (e.g., sociodemographic factors, general health, sleep habits, sleep environment, and sleep beliefs) (Knutson et al., 2017; Ohayon et al., 2017). Therefore, we recommend clinicians and researchers design their interventions to not only target particular domains of sleep health, but also appreciate the conceptual complexity of sleep health. As such, interventions may be more effective if they possess multiple components targeting various objective and subjective domains of sleep health (Knutson et al., 2017).
Our study possessed notable limitations. To begin, we could have selected a more exhaustive search strategy, using additional terms such as “cognitive decline.” Therefore, it is possible our search did not yield every existing study that met our inclusion criteria. Furthermore, our bias assessment tool was designed for evaluation of randomized trials, limiting the conclusions to be drawn from the bias assessment of nonrandomized trials in this review. Also, heterogeneity in study designs and intervention types prevented us from providing definitive conclusions regarding intervention superiority. In addition, the results from the majority of the included studies should be cautiously interpreted, as their internal validities were most likely influenced by bias. Finally, the findings of our review may not be generalizable to underrepresented caregiver/care recipient populations, such as sociodemographic minorities.
As the societal burden of ADRD exponentially increases, the need to develop effective interventions to support informal caregivers of those with ADRD is paramount (Brodaty & Donkin, 2009). Specifically, maintenance of sleep health is crucial to supporting the overall well-being of these caregivers, as well as their care recipients (Gao et al., 2019). We recommend future clinical researchers develop theoretically based and tailorable multicomponent interventions that target various domains of sleep health to inform future development and subsequent delivery of effective sleep interventions for this vulnerable population.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided in part by NIH grants KL2TR002547 and R01NR015750, and an American Nursing Foundation grant supported by the Sayre Memorial Fund and the Midwest Nursing Research Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring organizations.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Baharudin AD, Din NC, Subramaniam P, & Razali R (2019). The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health, 19(4), 447–447. 10.1186/s12889-019-6868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, & Donkin M (2009). Family caregivers of people with dementia. Dialogues in Clinical Neuroscience, 11(2), 217–228 10.31887/DCNS.2009.11.2/hbrodaty [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Green A, & Koschera A (2003). Meta-analysis of psychosocial interventions for caregivers of people with dementia. Journal of the American Geriatrics Society, 51(5), 657–664. 10.1034/j.1600-0579.2003.00210.x [DOI] [PubMed] [Google Scholar]
- Buysse DJ (2014). Sleep health: Can we define it? Does it matter? Sleep, 37(1), 9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty I, & Maity P (2020). COVID-19 outbreak: Migration, effects on society, global environment and prevention. Science of the Total Environment, 728, 138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ST, Au A, Losada A, Thompson LW, & Gallagher-Thompson D (2019). Psychological interventions for dementia caregivers: What we have achieved, what we have learned. Current Psychiatry Reports, 21(7), 59. 10.1007/s11920-019-1045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ST, Li KK, Losada A, Zhang F, Au A, Thompson LW, & Gallagher-Thompson D (2020). The effectiveness of nonpharmacological interventions for informal dementia caregivers: An updated systematic review and meta-analysis. Psychology and Aging, 35(1), 55–77. 10.1037/pag0000401 [DOI] [PubMed] [Google Scholar]
- Cheung JM, Jarrin DC, Ballot O, Bharwani AA, & Morin CM (2019). A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Medicine Reviews, 44, 23–36. 10.1016/j.smrv.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Collins LM, Kugler KC, & Gwadz MV (2016). Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS and Behavior, 20(1), 197–214. 10.1007/s10461-015-1145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, & Strecher V (2007). The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. American Journal of Preventive Medicine, 32(5), S112–S118. 10.1016/j.amepre.2007.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Koroshetz WJ, Gladman JT, Jeon S, Babcock D, Bennett DA, Carmichael ST, Dickinson SLJ, Dickson DW, & Emr M (2017). Alzheimer’s disease–related dementias summit 2016: National research priorities. Neurology, 89(23), 2381–2391. 10.1212/WNL.0000000000004717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal BA, Neufeld EV, Boland DM, Martin JL, & Cooper CB (2017). Interrelationship between sleep and exercise: A systematic review. Advances in Preventive Medicine, 2017, 1364387. 10.1155/2017/1364387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AF, Burgio LD, & DeCoster J (2010). Enhancing caregiver health: Findings from the resources for enhancing Alzheimer’s caregiver health II intervention. Journal of the American Geriatrics Society, 58(1), 30–37. 10.1111/j.1532-5415.2009.02631.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner SM, Dijk DJ, Drake RJ, & Bee PE (2020). Adherence and acceptability of light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuropsychiatric illness: A systematic review. Sleep Health, 6, 690–701. 10.1016/j.sleh.2020.01.014 [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Hunter CM, Higgins PA, Hornick TR, Jones GE, Plitnick B, Brons J, & Rea MS (2015). Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health, 1(4), 322–330. 10.1016/j.sleh.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CN, Kott K, Wicks MN, & Rutledge C (2016). Self-efficacy and sleep among caregivers of older adults with dementia: Effect of an interprofessional virtual healthcare neighborhood. Journal of Gerontological Nursing, 42(11), 39–47. 10.3928/00989134-20160901-02 [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Choryan Bilbrey A, Apesoa-Varano EC, Ghatak R, Kim KK, & Cothran F (2020). Conceptual framework to guide intervention research across the trajectory of dementia caregiving. The Gerontologist, 60(1), S29–S40. 10.1093/geront/gnz157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Chapagain NY, & Scullin MK (2019). Sleep duration and sleep quality in caregivers of patients with dementia: A systematic review and meta-analysis. JAMA Network Open, 2(8), e199891. 10.1001/jamanetworkopen.2019.9891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RH, Gander PH, Dowell AC, & Jones LM (2017). Non-pharmacological interventions for managing dementia-related sleep problems within community dwelling pairs: A mixed-method approach. Dementia, 16(8), 967–984. 10.1177/1471301215625821 [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Gilhooly MLM, Sullivan MP, McIntyre A, Wilson L, Harding E, Woodbridge R, & Crutch S (2016). A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatrics, 16(1), 106. 10.1186/s12877-016-0280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariton E, & Locascio JJ (2018). Randomised controlled trials - The gold standard for effectiveness research: Study design: Randomised controlled trials. BJOG, 125(13), 1716–1716. 10.1111/1471-0528.15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A, Suzuki Y, Kuzuya M, Onishi J, Ban N, & Umegaki H (2011). Influence of regular exercise on subjective sense of burden and physical symptoms in community-dwelling caregivers of dementia patients: A randomized controlled trial. Archives of Gerontology and Geriatrics, 53(2), e158–e163. 10.1016/j.archger.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Ibáñez V, Silva J, & Cauli O (2018). A survey on sleep assessment methods. PeerJ, 6, e4849. 10.7717/peerj.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov D (2020). Predicting the impacts of epidemic outbreaks on global supply chains: A simulation-based analysis on the coronavirus outbreak (COVID-19/SARS-CoV-2) case. Transportation Research Part E: Logistics and Transportation Review, 136, 101922. 10.1016/j.tre.2020.101922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain FA, Nazarian N, & Lavretsky H (2014). Feasibility of central meditation and imagery therapy for dementia caregivers. International Journal of Geriatric Psychiatry, 29(8), 870–876. 10.1002/gps.4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Phelan J, Paskow MJ, Roach A, Whiton K, Langer G, Hillygus DS, Mokrzycki M, Broughton WA, & Chokroverty S (2017). The National Sleep Foundation’s sleep health index. Sleep Health, 3(4), 234–240. 10.1016/j.sleh.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Koca E, Taşkapilioğlu Ö, & Bakar M (2017). Caregiver burden in different stages of Alzheimer’s disease. Archives of Neuropsychiatry 54(1), 82–86. 10.5152/npa.2017.11304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L, Logsdon RG, Polissar NL, Gomez-Beloz A, Waters T, & Rÿser R (2009). A randomized trial of a CAM therapy for stress reduction in American Indian and Alaskan Native family caregivers. The Gerontologist, 49(3), 368–377. 10.1093/geront/gnp032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, & Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62(10), e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Biswas A, Roy A, Biswas S, Gangopadhyay G, & Das SK (2017). Behavioural and psychological symptoms of dementia: Correlates and impact on caregiver cistress. Dementia and Geriatric Cognitive Disorders Extra, 7(3), 354–365. 10.1159/000481568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski B, Wade L, Plotnikoff RC, Lubans DR, & Duncan MJ (2018). A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Medicine Reviews, 40, 160–169. 10.1016/j.smrv.2017.12.003 [DOI] [PubMed] [Google Scholar]
- National Institute of Nursing Research. (2016). Advancing science, improving lives: A vision for nursing science [16-NR-7783]. National Institutes of Health. [Google Scholar]
- National Research Council. (2010). Informal caregivers in the United States: Prevalence, caregiver characteristics, and ability to provide care. In: Olson S (Ed.), The role of human factors in home health care: Workshop summary (pp. 322). National Academies Press. https://www.ncbi.nlm.nih.gov/books/NBK210048/ [Google Scholar]
- Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, Dauvilliers Y, Ferri R, Fung C, Gozal D, Hazen N, Krystal A, Lichstein K, Mallampalli M, Plazzi G, Rawding R, Scheer FA, Somers V, & Vitiello MV (2017). National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health, 3(1), 6–19. 10.1016/j.sleh.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Paller KA, Creery JD, Florczak SM, Weintraub S, Mesulam M-M, Reber PJ, Kiragu J, Rooks J, Safron A, & Morhardt D (2015). Benefits of mindfulness training for patients with progressive cognitive decline and their caregivers. American Journal of Alzheimer’s Disease & Other Dementias®, 30(3), 257–267. 10.1177/1533317514545377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanin JR, Pigott TD, Espelage DL, & Grotpeter JK (2019). Best practice guidelines for abstract screening largeevidence systematic reviews and meta-analyses. Research Synthesis Methods, 10(3), 330–342. 10.1002/jrsm.1354 [DOI] [Google Scholar]
- Poon E (2019). A systematic review and meta-analysis of dyadic psychological interventions for BPSD, quality of life and/or caregiver burden in dementia or MCI. Clinical Gerontologist, 1–21. 10.1080/07317115.2019.1694117 [DOI] [PubMed] [Google Scholar]
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, & Karagiannidou M (2016). World Alzheimer report 2016:Improving healthcare for people living with dementia: Coverage, quality and costs now and in the future. Alzheimer’s Disease International. [Google Scholar]
- Prudencio G, & Young H (2020). Caregiving in the US 2020: What does the latest edition of this survey tell us about their contributions and needs? Innovation in Aging, 4(1), 681–681. 10.1093/geroni/igaa057.2372 [DOI] [Google Scholar]
- Randolph J (2009). A guide to writing the dissertation literature review. Practical Assessment, Research, and Evaluation, 14(1), 13. 10.7275/b0az-8t74 [DOI] [Google Scholar]
- Ricon I, Hanalis-Miller T, Haldar R, Jacoby R, & Ben-Eliyahu S (2019). Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of β-adrenergic and cyclooxygenase 2 signaling. Cancer, 125(1), 45–56. 10.1002/cncr.31594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KM, Taylor AG, & Bourguignon C (2009). Effects of cranial electrical stimulation on sleep disturbances, depressive symptoms, and caregiving appraisal in spousal caregivers of persons with Alzheimer’s disease. Applied Nursing Research, 22(2), 119–125. 10.1016/j.apnr.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MA, Kairalla JA, & McCrae CS (2010). Sleep in dementia caregivers and the effect of a nighttime monitoring system. Journal of Nursing Scholarship, 42(3), 338–347. 10.1111/j.1547-5069.2010.01337.x [DOI] [PubMed] [Google Scholar]
- Sateia MJ (2014). International classification of sleep disorders-Third edition. Chest, 146(5), 1387–1394. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- Schlarb AA, Velten-Schurian K, Poets CF, & Hautzinger M (2010). First effects of a multicomponent treatment for sleep disorders in children. Nature and Science of Sleep, 3, 1–11. 10.2147/NSS.S15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C, & Carter PA (2010). Pilot study of a brief behavioral sleep intervention for caregivers of individuals with dementia. Research in Gerontological Nursing, 3(1), 19–29. 10.3928/19404921-20090731-02 [DOI] [PubMed] [Google Scholar]
- Sloane P, Figueiro M, Garg S, Cohen L, Reed D, Williams C, Preisser J, & Zimmerman S (2015). Effect of home-based light treatment on persons with dementia and their caregivers. Lighting Research & Technology, 47(2), 161–176. 10.1177/1477153513517255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring HJ, Rowe MA, & Kelly A (2009). Improving caregivers’ well-being by using technology to manage nighttime activity in persons with dementia. Research in Gerontological Nursing, 2(1), 39–48. 10.3928/19404921-20090101-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, & Eldridge SM (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ, 366, 14898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Thomas NW, Lindauer A, & Kaye J (2019). EVALUATE-AD and Tele-STAR: Novel methodologies for assessment of caregiver burden in a telehealth caregiver intervention–A case study. Dementia and geriatric cognitive disorders, 47(3), 176–184. 10.1159/000497805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaingankar JA, Chong SA, Abdin E, Picco L, Shafie S, Seow E, Pang S, Sagayadevan V, Chua BY, Chua HC, & Subramaniam M (2016). Psychiatric morbidity and its correlates among informal caregivers of older adults. Comprehensive Psychiatry, 68, 178–185. 10.1016/j.comppsych.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Williams KN, Perkhounkova Y, Shaw CA, Hein M, Vidoni ED, & Coleman CK (2019). Supporting family caregivers with technology for dementia home care: A randomized controlled trial. Innovation in Aging, 3(3), igz037. 10.1093/geroni/igz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina AM, Winblad B, Jönsson L, Liu Z, & Prince M (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s & Dementia, 13(1), 1–7. 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley SL (2018). The extraordinary importance of sleep: The detrimental effects of inadequate sleep on health and public safety drive an explosion of sleep research. P & T, 43(12), 758–763. https://pubmed.ncbi.nlm.nih.gov/30559589 [PMC free article] [PubMed] [Google Scholar]


