Abstract
STUDY QUESTION
What is the recommended management for medically assisted reproduction (MAR) in patients with a viral infection or disease, based on the best available evidence in the literature?
SUMMARY ANSWER
The ESHRE guideline on MAR in patients with a viral infection/disease makes 78 recommendations on prevention of horizontal and vertical transmission before, during and after MAR, and the impact on its outcomes, and these also include recommendations regarding laboratory safety on the processing and storage of gametes and embryos testing positive for viral infections.
WHAT IS KNOWN ALREADY
The development of new and improved anti-viral medications has resulted in improved life expectancy and quality of life for patients with viral infections/diseases. Patients of reproductive age are increasingly exploring their options for family creation.
STUDY DESIGN, SIZE, DURATION
The guideline was developed according to the structured methodology for the development of ESHRE guidelines. After the formulation of nine key questions for six viruses (hepatitis B virus, hepatitis C virus, human immunodeficiency virus, human papilloma virus, human T-lymphotropic virus I/II and Zika virus) by a group of experts, literature searches and assessments were performed. Papers published up to 2 November 2020 and written in English were included in the review. Evidence was analyzed by female, male or couple testing positive for the virus.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Based on the collected evidence, recommendations were formulated and discussed until consensus was reached within the guideline group. There were 61 key questions to be answered by the guideline development group (GDG), of which 12 were answered as narrative questions and 49 as PICO (Patient, Intervention, Comparison, Outcome) questions. A stakeholder review was organized after the finalization of the draft. The final version was approved by the GDG and the ESHRE Executive Committee.
MAIN RESULTS AND THE ROLE OF CHANCE
This guideline aims to help providers meet a growing demand for guidance on the management of patients with a viral infection/disease presenting in the fertility clinic.
The guideline makes 78 recommendations on prevention of viral transmission before and during MAR, and interventions to reduce/avoid vertical transmission to the newborn. Preferred MAR treatments and interventions are described together with the effect of viral infections on outcomes. The GDG formulated 44 evidence-based recommendations—of which 37 were formulated as strong recommendations and 7 as weak—33 good practice points (GPP) and one research only recommendation. Of the evidence-based recommendations, none were supported by high-quality evidence, two by moderate-quality evidence, 15 by low-quality evidence and 27 by very low-quality evidence. To support future research in the field of MAR in patients with a viral infection/disease, a list of research recommendations is provided.
LIMITATIONS, REASONS FOR CAUTION
Most interventions included are not well-studied in patients with a viral infection/disease. For a large proportion of interventions, evidence was very limited and of very low quality. More evidence is required for these interventions, especially in the field of human papilloma virus (HPV). Such future studies may require the current recommendations to be revised.
WIDER IMPLICATIONS OF THE FINDINGS
The guideline provides clinicians with clear advice on best practice in MAR for patients with a viral infection/disease, based on the best evidence currently available. In addition, a list of research recommendations is provided to stimulate further studies in the field.
STUDY FUNDING/COMPETING INTEREST(S)
The guideline was developed and funded by ESHRE, covering expenses associated with the guideline meetings, with the literature searches and with the dissemination of the guideline. The guideline group members did not receive any financial incentives, all work was provided voluntarily. A.D. reports research fees from Ferring and Merck, consulting fees from Ferring, outside the submitted work. C.P. reports speakers fees from Merck and MSD outside the submitted work. K.T. reports speakers fees from Cooper Surgical and Ferring and consultancy fees as member of the advisory board BioTeam of Ferring, outside the submitted work. The other authors have no conflicts of interest to declare.
DISCLAIMER
This guideline represents the views of ESHRE, which were achieved after careful consideration of the scientific evidence available at the time of preparation. In the absence of scientific evidence on certain aspects, a consensus between the relevant ESHRE stakeholders has been obtained.
Adherence to these clinical practice guidelines does not guarantee a successful or specific outcome, nor does it establish a standard of care. Clinical practice guidelines do not replace the need for application of clinical judgment to each individual presentation, nor variations based on locality and facility type.
ESHRE makes no warranty, express or implied, regarding the clinical practice guidelines and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. (Full disclaimer available at www.eshre.eu/guidelines.)
Keywords: viral disease, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, human papilloma virus, human T-lymphotropic virus I/II, Zika virus, cross-contamination, medically assisted reproduction, ESHRE guideline
WHAT DOES THIS MEAN FOR PATIENTS?
Patients living with a viral infection/disease now have a longer life expectancy and improved quality of life, which leads them to think about family creation. In some cases, the pregnancy can establish spontaneously and safely, while in certain circumstances medically assisted reproduction (MAR) treatments are required to help couples achieve a pregnancy. When embarking on MAR, patients and practitioners have to explore the best treatment options that reduce the risk of transmission of the virus and result in the highest chances of getting pregnant. This is where the present guideline aimed to bring clarity through evidence from the literature and expert opinion in order to increase the safety of therapy and offer reassurance to all involved, patients and practisers alike.
The current paper summarizes the ESHRE Guideline on MAR in patients with a viral infection/disease providing clinicians with evidence-based recommendations on how to manage these patients in the fertility clinic with the aim to prevent/reduce the risk of horizontal (person to person) and vertical viral transmission to the baby. In addition, the guideline also provides recommendations on providing information regarding the risks of vertical viral transmission to the newborn. The full guideline and a patient leaflet are available on https://www.eshre.eu/VirusGuideline.
Introduction
The development of new and improved anti-viral medications has resulted in improved life expectancy and quality of life for patients with viral infections/diseases. Patients of reproductive age are increasingly exploring their options for family creation. The management of medically assisted reproduction (MAR) in patients with a viral infection is regulated rather strictly in some European countries, however, not in all. While viral testing of all patients embarking on MAR is compulsory in Europe, detailed guidance on the correct conduit once a patient tests positive and wishes to proceed with MAR is lacking. This guideline aims to help providers meet a growing demand for guidance on the management of patients with a viral infection/disease presenting in the fertility clinic, and in particular the ones proceeding with MAR treatments.
Materials and methods
The guideline was developed according to a well-documented methodology that is universal to ESHRE guidelines (Vermeulen et al., 2020). The guideline development group (GDG) was composed of members of the ESHRE Special Interest Group (SIG) Safety and Quality in ART, Ethics and Law and members of the former task force on Viral Diseases, with the addition of experts in the field, including a virologist.
In short, 61 key questions were formulated by the GDG, of which 12 were answered as narrative questions and 49 as PICO (Patient, Intervention, Comparison, Outcome) questions. For each PICO question, databases (PUBMED/MEDLINE, Cochrane library, EMBASE and GIM) were searched from inception to 3 November 2020, limited to studies written in English. From the literature searches, studies were selected based on the PICO questions, assessed for quality and summarized in evidence tables. GDG meetings were organized where the evidence and draft recommendations were presented by the assigned GDG member and discussed until consensus was reached within the group. Each recommendation was labeled as strong or weak and a grade was assigned based on the strength of the supporting evidence (High ⊕⊕⊕⊕, Moderate ⊕⊕⊕◯, Low ⊕⊕◯◯, Very low ⊕◯◯◯). Good practice points (GPPs) based on clinical expertise were added, where relevant, to clarify the recommendations or to provide further practical advice. One ‘research only’ recommendation was also made, and this intervention should be applied only within the context of research, with appropriate precautions and ethical approval.
Strong recommendations should be used as a recommendation to be applied for most patients, while weak recommendations require discussion and shared decision-making.
For the narrative questions, a similar literature search was conducted. Collected data were summarized in a narrative summary and conclusions were formulated.
The guideline draft and an invitation to participate in the stakeholder review were published on the ESHRE website between 18 February and 1 April 2021. All comments were processed by the GDG, either by adapting the content of the guideline and/or by replying to the reviewer. The review process was summarized in the review report (Supplementary Data I), which is published on the ESHRE website (www.eshre.eu/Guidelines). Overall, 65% of the 109 comments resulted in an adaptation or correction in the guideline text. The full guideline document, the literature study and evidence tables can be found on the ESHRE website (www.eshre.eu/virusguideline).
This guideline will be considered for update 4 years after publication, with an intermediate assessment of the need for updating 2 years after publication.
Results
Key questions and recommendations
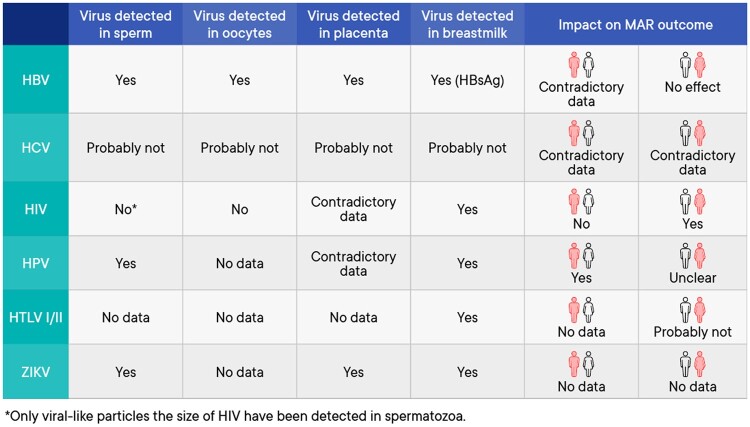
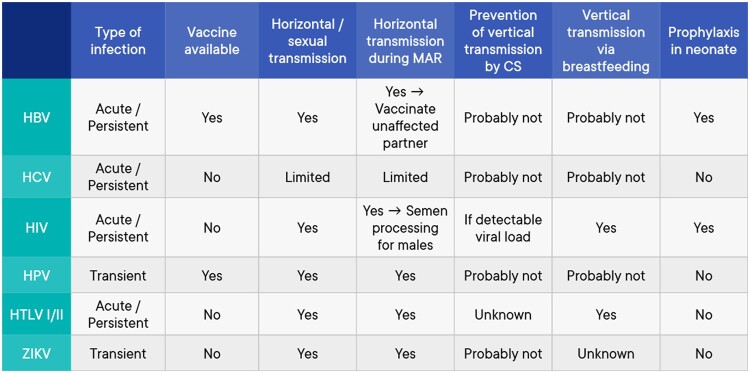
The current document summarizes all the key questions and the recommendations from the guideline ‘Medically assisted reproduction for patients with a viral infection/disease’. Figures 1 and 2 provide a summary of the available evidence from the literature on the topics included in the recommendations. Further background information and the supporting evidence for each recommendation can be found in the full version of the guideline available at https://www.eshre.eu/VirusGuideline.
Figure 1.
Summary of the available evidence on the topics included in the guideline. HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papilloma virus; HTLV I/II, human T-lymphotropic virus I/II; MAR, medically assisted reproduction; ZIKV, Zika virus.
Figure 2.
Summary of further evidence on the topics included in the guideline. CS, caesarean section; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papilloma virus; HTLV I/II, human T-lymphotropic virus I/II; MAR, medically assisted reproduction; ZIKV, Zika virus.
Hepatitis B virus
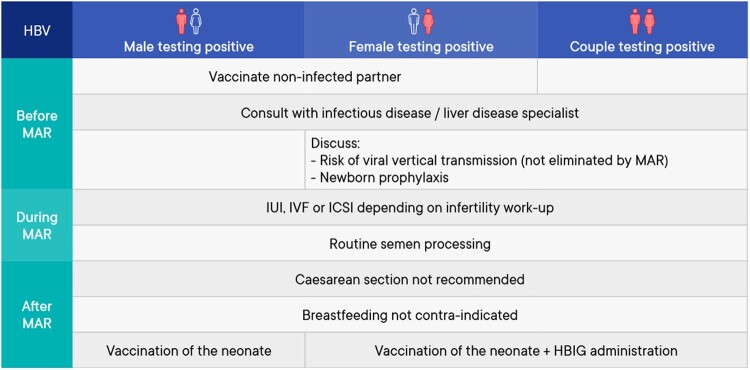
A summary of the management of medically assisted reproduction in patients testing positive for hepatitis B virus (HBV) can be found in Fig. 3.
Figure 3.
Summary of management of medically assisted reproduction in patients testing positive for hepatitis B virus. HBIG, hepatitis B immunoglobulin; HBV, hepatitis B virus; MAR: medically assisted reproduction.
Prevention of transmission before medically assisted reproduction
| Partners of hepatitis B virus (HBV)-positive individuals should be vaccinated. | Strong |
|---|---|
| ⊕○○○ | |
| Barrier contraception should be used until the completion of the HBV vaccination protocol (Inaba et al., 1979; Rosenblum et al., 1992; Hou et al., 1993; Huo et al., 1998; Katoonizadeh et al., 2018; Tufon et al., 2019). | Strong |
| ⊕⊕○○ | |
| Medically assisted reproduction (MAR) services staff should be vaccinated against HBV. | GPP |
| All patients with an active or chronic HBV-infection must be reviewed by an infectious disease/liver specialist before initiating any MAR treatment. | Strong |
| ⊕○○○ | |
| Commencing with MAR treatments in patients positive for HBV should be a joint decision between the patient, their partner, the fertility doctor and the infectious disease/liver specialist. | Strong |
| ⊕○○○ | |
| In the case of the female testing positive for HBV, the possibility of viral vertical transmission, the availability of vaccination during pregnancy and newborn prophylaxis should all be discussed. | GPP |
Assisted reproduction techniques and impact on outcomes
| The cause of infertility should dictate the specific technique (IUI/IVF/ICSI) used for MAR in couples where one or both partners test positive for HBV (Nie et al., 2019). | Strong |
|---|---|
| ⊕○○○ | |
| Women infected with HBV should be informed that MAR does not eliminate the risk of vertical transmission. | GPP |
| HBV can be detected in sperm cells, oocytes, granulosa cells and embryos. This equates with a theoretical risk of vertical HBV transmission that remains to be proven. | Conclusion |
| Existing evidence cannot clarify if the presence of HBV-infection in the male impacts the outcomes of MAR. Multiple studies showed no differences in reproductive outcomes following MAR when comparing seronegative with HBV-seropositive women. | Conclusion |
Prevention/reduction of transmission during assisted reproduction
| Men testing positive for HBV should be informed that no current semen preparation technique can select HBV DNA-free spermatozoa for use in MAR. | GPP |
|---|---|
| Routine semen processing according to the ESHRE ‘Guideline on good practice in the IVF laboratory’ should be used when performing MAR in men testing positive for HBV. | GPP |
| Based on the current evidence, HBV DNA testing on seminal fluid or sperm is not recommended (Ayoola et al., 1981; Hadchouel et al., 1985; Qian et al., 2005; Fei et al., 2015). | Strong |
| ⊕○○○ |
Reducing/avoiding vertical transmission
| Cesarean delivery is not recommended on the basis of maternal HBV positivity alone (Chen et al., 2019). | Strong |
|---|---|
| ⊕⊕○○ | |
| Breastfeeding is not contra-indicated in women testing positive for HBV (Zheng et al., 2011). | Conditional |
| ⊕⊕○○ | |
| All neonates born to HBV-positive couples should be vaccinated (Lee et al., 2006; Schillie and Murphy, 2013). | Strong |
| ⊕⊕⊕○ | |
| Administration of hepatitis B immunoglobulin (HBIG) in addition to vaccination is recommended for children born to mothers testing positive for HBV (Jin et al., 2014; Machaira et al., 2015). | Strong |
| ⊕⊕○○ | |
| HBIG administration should follow local or national guidelines. | GPP |
Hepatitis C virus
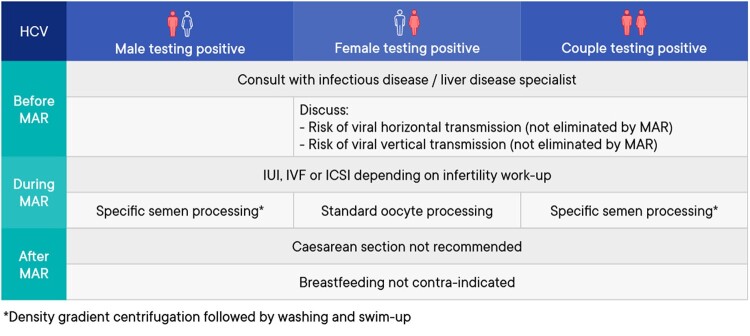
A summary of the management of medically assisted reproduction in patients testing positive for hepatitis C virus (HCV) can be found in Fig. 4.
Figure 4.
Summary of management of medically assisted reproduction in patients testing positive for hepatitis C virus. HCV, hepatitis C virus; MAR, medically assisted reproduction.
Prevention of transmission before medically assisted reproduction
| In a monogamous heterosexual relationship of more than 12 months, there is no indication for the use of barrier contraceptives to reduce the risk of hepatitis C virus (HCV) transmission in a serodiscordant-infected couple (Ackerman et al., 1998). | Conditional |
|---|---|
| ⊕⊕○○ | |
| All patients with an active or chronic HCV-infection must be reviewed by an infectious disease/liver specialist before initiating any medically assisted reproduction treatment (MAR). | GPP |
| Commencing with MAR treatments in patients positive for HCV should be a joint decision between the patient, their partner, the fertility doctor and the infectious disease/liver specialist. | Strong |
| ⊕○○○ | |
| In the case of the female testing positive for HCV, the possibility of viral vertical transmission should be discussed prior to MAR treatment. | GPP |
Assisted reproduction techniques and impact on outcomes
| The cause of infertility should dictate the specific technique (IUI/IVF/ICSI) used for MAR in couples where one or both partners test positive for HCV (Garrido et al., 2004; Nesrine and Saleh, 2012; Savasi et al., 2013). | Strong |
|---|---|
| ⊕○○○ | |
| Women testing positive for HCV should be informed that MAR does not eliminate the risk of vertical transmission. | GPP |
| The possibility of HCV RNA presence in oocytes cannot be excluded. However, the risk of Hepatitis C transmission through the use of reproductive material remains to be proven. | Conclusion |
| There are contradictory results evaluating effects of male HCV-infection on infertility treatments outcomes. Although the fertilization rate has been reported significantly lower in couples with HCV-RNA-positive men, other studies report that HCV-infection does not affect the IVF-ICSI cycle outcomes in these couples. | Conclusion |
| There are contradictory results evaluating effects of female HCV infection on infertility treatments outcomes. Although some studies report significantly reduced implantation rates, higher cycle cancelations, and higher FSH use in HCV positive women, other report no significant differences. | Conclusion |
Prevention/reduction of transmission during assisted reproduction
| There are no data regarding antiviral therapy in men or women with HCV without co-infections requiring MAR in order to reduce the risk of HCV transmission. None of the currently available HCV antiviral drugs are licensed for use in pregnancy. | Conclusion |
|---|---|
| A discontinuous gradient centrifugation, swim-up and washing is recommended for semen processing in patients testing positive for HCV (Bourlet et al., 2002, 2009; Cassuto et al., 2002; Meseguer et al., 2002; Canto et al., 2006; Garrido et al., 2006; Savasi et al., 2010; Leruez-Ville et al., 2013; Savasi et al., 2013; Molina et al., 2014). | Strong |
| ⊕○○○ | |
| After advanced semen processing, PCR testing for HCV is not necessary (Cassuto et al., 2002; Canto et al., 2006; Garrido et al., 2006; Bourlet et al., 2009; Savasi et al., 2010; Leruez-Ville et al., 2013; Molina et al., 2014). | Strong |
| ⊕○○○ | |
| Good laboratory practice regarding semen processing should be applied irrespective of whether only the male or both partners are testing positive for HCV. | GPP |
| High plasma HCV viral load is likely to be predictive of the presence of HCV RNA in semen. Strong evidence for the correlation of HCV viral load between serum and semen is currently lacking. | Conclusion |
Reducing/avoiding vertical transmission
| Cesarean delivery is not recommended on the basis of maternal HCV-positivity alone (Ghamar Chehreh et al., 2011) | Strong |
|---|---|
| ⊕○○○ | |
| Breastfeeding is not contra-indicated in HCV-positive women (Cottrell et al., 2013) | Strong |
| ⊕○○○ |
Human immunodeficiency virus
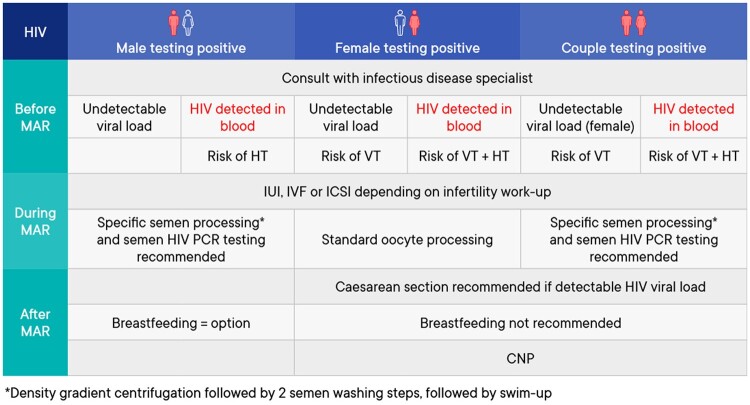
A summary of the management of medically assisted reproduction in patients testing positive for human immunodeficiency virus (HIV) can be found in Fig. 5.
Figure 5.
Summary of management of medically assisted reproduction in patients testing positive for human immunodeficiency virus. CNP, combined neonatal prophylaxis; HIV, human immunodeficiency virus; HT, horizontal transmission; MAR, medically assisted reproduction; VT, vertical transmission.
Prevention of transmission before medically assisted reproduction
| Human immunodeficiency virus (HIV)-1-serodiscordant couples should be informed that there is a risk of sexual transmission of the virus to the unaffected partner. To reduce this risk, couples must be advised to use barrier contraception and seek active therapy to reduce viral load (Baggaley et al., 2010; LeMessurier et al., 2018). | Strong |
|---|---|
| ⊕⊕○○ | |
| Individuals testing positive for HIV-1, antiretroviral therapy can suppress viral replication. These patients should remain on antiretroviral therapy and providing undetectable viral loads in serum can be achieved and sustained, the risk of horizontal transmission through unprotected intercourse is minimal in the absence of other sexually transmitted diseases (Attia et al., 2009). | Strong |
| ⊕⊕○○ | |
| Commencing with medically assisted reproduction (MAR) treatments in patients positive for HIV-1 or 2 should be a joint decision between the patient, their partner, the fertility doctor and the infectious disease specialist. | Strong |
| ⊕○○○ | |
| All patients testing positive for HIV, wishing to have a child should be counseled about the risk of horizontal and vertical transmission. In the case of the male testing positive for HIV, antiretroviral therapy can reduce the viral load in blood and semen to undetectable levels, allowing the possibility of natural conception. Reproductive counseling should include fertility and antiretroviral covariates. | GPP |
| In the case of the female testing positive for HIV-1 or 2, and even with undetectable viremia, the possibility of viral vertical transmission should be discussed prior to MAR treatment. | GPP |
Assisted reproduction techniques and impact on outcomes
| HIV infection status is not a reason to deny MAR treatment. | Strong |
|---|---|
| ⊕○○○ | |
| The cause of infertility should dictate the specific technique (IUI/IVF/ICSI) used for MAR in couples where one or both partners test positive for HIV (Vitorino et al., 2011; Barnes et al., 2014). | Strong |
| ⊕⊕○○ | |
| Advanced semen processing should be used for male patients testing positive for HIV-1 to reduce the likelihood of viral presence (Dussaix et al., 1993; Baccetti et al., 1994; Quayle et al., 1997; Deleage et al., 2011; Miller et al., 2019; Young et al., 2019). | Strong |
| ⊕○○○ | |
| No special laboratory techniques are needed for processing of oocytes from female patients testing positive for HIV. | Strong |
| ⊕○○○ | |
| Serodiscordant couples with a male partner testing positive for HIV-1 should be informed that the efficacy of MAR is not impacted compared to HIV seronegative couples (Bujan et al., 2007; Prisant et al., 2010; Sauer and Chang, 2002; Cito et al., 2019). | Strong |
| ⊕○○○ | |
| Serodiscordant couples with a female partner testing positive for HIV should be informed that the efficacy of IVF/ICSI could be reduced compared to HIV seronegative couples (Marques et al., 2015). | Conditional |
| ⊕○○○ |
Prevention/reduction of transmission during assisted reproduction
| The technique recommended for processing ejaculated semen for males testing positive for HIV is to perform a discontinuous density gradient centrifugation followed by 2 semen washing steps, followed by swim-up (Zafer et al., 2016). | Strong |
|---|---|
| ⊕⊕○○ | |
| Regardless of the semen processing technique used, the post-preparation sample that is going to be used in MAR from males tested positive for HIV should be HIV PCR tested (Zafer et al., 2016). | Strong |
| ⊕⊕○○ | |
| In serodiscordant couples with the male testing positive for HIV, only a HIV-negative tested sperm sample should be used for MAR (Zafer et al., 2016). | Strong |
| ⊕⊕○○ | |
| Good laboratory practice regarding semen processing should be applied irrespective of whether only the male or both partners are testing positive for HIV. | GPP |
| Advanced semen processing is recommended for male patients testing positive for HIV, regardless of the viral load in the serum and therapy status (Kalichman et al., 2008). | Strong |
| ⊕○○○ |
Reducing/avoiding vertical transmission
| Caesarean section is recommended in women with detectable HIV viral loads (Kennedy et al., 2017). | Strong |
|---|---|
| ⊕⊕○○ | |
| A female testing positive for HIV should refrain from breastfeeding when and where she has safe nutritional alternatives (De Martino et al., 1992; Tess et al., 1998; Coutsoudis, 2000; Olayinka et al., 2000; Mbori-Ngacha et al., 2002; Magoni et al., 2005; Kagaayi et al., 2008; Peltier et al., 2009; Imade et al., 2010; Assefa et al., 2017; Njom Nlend et al., 2018). | Strong |
| ⊕⊕○○ | |
| Combined neonatal prophylaxis (CNP) is recommended for neonates born to mothers testing positive for HIV. | Strong |
| ⊕⊕⊕○ |
Human papilloma virus
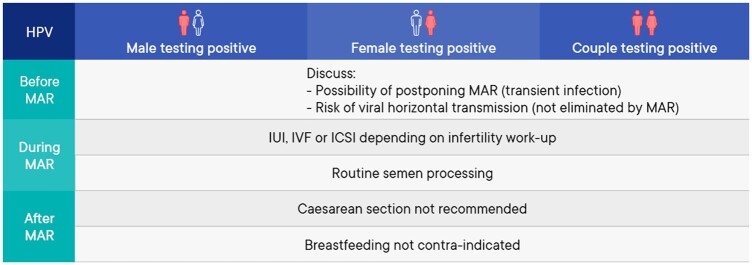
A summary of the management of medically assisted reproduction in patients testing positive for human papilloma virus (HPV) can be found in Fig. 6.
Figure 6.
Summary of management of medically assisted reproduction in patients testing positive for human papilloma virus. HPV, human papilloma virus; MAR, medically assisted reproduction.
Prevention of transmission before medically assisted reproduction
| The use of barrier contraception during sexual intercourse is advised to lower the risk of human papilloma virus (HPV) transmission (Dillner et al., 1996; Kjaer et al., 2001; Hernandez et al., 2008; Burchell et al., 2010; Widdice et al., 2013). | GPP |
| All women starting medically assisted reproduction (MAR) should undergo testing to detect HPV-related cervical lesions. | GPP |
Assisted reproduction techniques and impact on outcomes
| The cause of infertility should dictate the specific technique (IUI/IVF/ICSI) used for MAR in couples where one or both partners test positive for HPV. | Strong |
|---|---|
| ⊕○○○ | |
| Women infected with HPV should be informed that MAR does not eliminate the risk of vertical transmission. | GPP |
| The possibility of HPV testing could be discussed with couples undergoing IUI. | Research only |
| Couples with a known positive HPV test should be advised that HPV is a transient infection, and postponing MAR treatment is an option depending on the individual circumstances. | GPP |
Prevention/reduction of transmission during assisted reproduction
| There is weak evidence that therapeutic HPV vaccination in HPV-positive men may increase pregnancy rates in natural conception and reduce miscarriage rates. However, more studies are necessary. | Conclusion |
| HPV-positive males should be informed that no current semen preparation technique can eliminate the virus from the infected semen sample. | GPP |
Reducing/avoiding vertical transmission
| Caesarean delivery is not recommended on the basis of maternal HPV-positivity alone (Chatzistamatiou et al., 2016; Zouridis et al., 2018). | Strong |
|---|---|
| ⊕⊕○○ | |
| Breastfeeding is not contra-indicated in HPV-positive women (Yoshida et al., 2011; Glenn et al., 2012; Louvanto et al., 2017). | Conditional |
| ⊕○○○ |
Human T-lymphotropic virus I/II
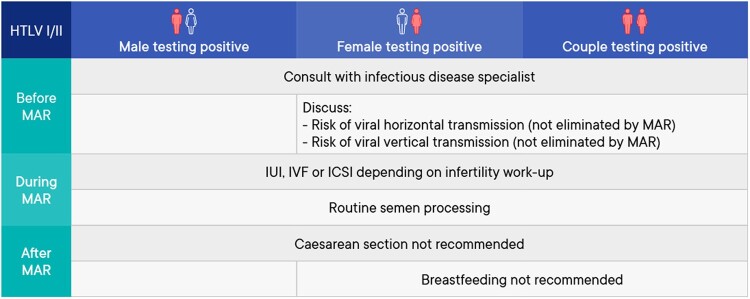
A summary of the management of medically assisted reproduction in patients testing positive for human T-lymphotropic virus (HTLV) I/II can be found in Fig. 7.
Figure 7.
Summary of management of medically assisted reproduction in patients testing positive for human T-lymphotropic virus I/II. HTLV, human T-lymphotropic virus; MAR, medically assisted reproduction.
Prevention of transmission before medically assisted reproduction
| It is suggested human T-cell lymphotropic virus (HTLV I/II)-serodiscordant couples should be informed that there is a risk of sexual transmission of the virus to the unaffected partner. To reduce this risk, couples could be advised to use barrier contraception and receive reproductive counseling if they want to conceive (Roucoux et al., 2005; Stuver et al., 1993). | Conditional ⊕○○○ |
Assisted reproduction techniques and impact on outcomes
| The cause of infertility should dictate the specific technique (IUI/IVF/ICSI) used for medically assisted reproduction (MAR) in couples where one or both partners test positive for HTLV I/II (Stuver et al., 1993; Kaplan et al., 1996; Paiva et al., 2017). | Strong |
|---|---|
| ⊕○○○ | |
| Women testing positive for HTLV I/II should be informed that MAR does not eliminate the risk of vertical transmission. | GPP |
| Studies on HTLV I/II viruses are dated and the technology to detect these viruses has changed a lot since. Therefore, the possibility of HTLV I/II presence in gametes or placenta cannot be confirmed or excluded. To date, the risk of HTLV I/II transmission through the use of infected semen or oocytes remains to be proven. | Conclusion |
| The impact of female HTLV I infection on MAR outcomes remains unknown. | Conclusion |
Prevention/reduction of transmission during assisted reproduction
No studies were identified comparing routine semen preparation with advanced semen processing in males testing positive for HTLV I/II. Similarly, no studies were identified investigating the correlation between viral load in semen and serum in HTLV I/II infected patients.
Reducing/avoiding vertical transmission
| Caesarean delivery is not recommended on the basis of maternal HTLV I/II positivity alone (Paiva et al., 2018). | Strong |
|---|---|
| ⊕○○○ | |
| A female testing positive for HTLV I/II should refrain from breastfeeding when and where she has safe nutritional alternatives (Boostani et al., 2018) | Strong |
| ⊕○○○ |
Zika virus
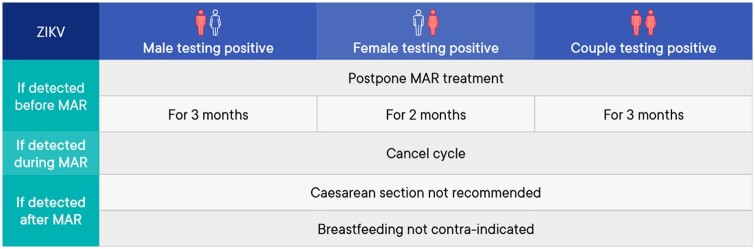
A summary of the management of medically assisted reproduction in patients testing positive for Zika virus (ZIKV) can be found in Fig. 8.
Figure 8.
Summary of management of medically assisted reproduction in patients testing positive for Zika virus. MAR, medically assisted reproduction; ZIKV, Zika virus.
Prevention of transmission before medically assisted reproduction
| A male diagnosed with ZIKV-infection or returning from a ZIKV endemic region should use barrier contraception with any partner, for 3 months. | GPP |
| A female diagnosed with ZIKV-infection or returning from a ZIKV endemic region should use barrier contraception and avoid pregnancy for 2 months. | GPP |
Assisted reproduction techniques and impact on outcomes
| If a patient or partner has been diagnosed with ZIKV-infection or returning from a ZIKV endemic region in the last 3 months, medically assisted reproduction (MAR) treatment should be postponed. | GPP |
| In case of fertility preservation, the approach should be tailored to the individual situation | GPP |
| In the case of fertility preservation, there is insufficient data on the risk of viral transmission using gametes potentially infected with Zika virus. An individual risk assessment is advised before using these gametes. | GPP |
| If ZIKV-infection is diagnosed in male or female during MAR treatment, the cycle should be stopped, and the couple should be advised to use barrier contraception for 3 months. | GPP |
Prevention/reduction of transmission during assisted reproduction
| There are currently no semen processing techniques available that can completely remove Zika virus from semen. | Conclusion |
|---|---|
| MAR is not advised even if serum is free of Zika virus because of poor correlation between serum and semen viral load (Joguet et al., 2017; Musso et al., 2017; Barzon et al., 2018; Mead et al., 2018; Paz-Bailey et al., 2018). | Strong |
| ⊕○○○ |
Reducing/avoiding vertical transmission
| The possibility of transmission of Zika virus through breastfeeding has only been assessed in 12 mother-child pairs. This provides insufficient evidence to establish a recommendation. | Conclusion |
Laboratory safety
Can separate cryo tank storage prevent cross contamination of stored material?
| Since viruses can survive and be transmitted via liquid nitrogen (LN2), separate storage of reproductive cells according to viral positive and viral negative status is recommended. | GPP |
|---|---|
| Emptied and dried cryo tanks and transport shippers should be disinfected according to local standard operating procedures to reduce the potential of cross-contamination. | GPP |
| Individual clinics must risk assess to decide the number of cryo tanks needed. | GPP |
| Separate cryopreservation dewars should be used to quarantine gametes and embryos from patients with unknown infectious status. | GPP |
Can the type of cryostorage environment (liquid versus vapor/open versus closed systems) prevent cross contamination of stored material?
| Vapor phase cryopreservation could be considered over liquid nitrogen in terms of safety to reduce the risk of cross-contamination (Bielanski, 2005; Grout and Morris, 2009; Mirabet et al., 2012; Molina et al., 2016) | Conditional |
|---|---|
| ⊕○○○ | |
| Provided the cryomaterial is not compromised, cryodevices, such as sealed semen straws/vials, should be cleaned with a disinfectant wipe after removal from LN2 storage to mitigate risk of transmission of pathogens from the cryodevice surface. | GPP |
Can the type of vials prevent cross-contamination of stored material?
| Hermetical sealing of cryovials with additional covers could reduce the risk of cross-contamination of stored material (Chen et al., 2006) | Conditional ⊕○○○ |
Can high security straws prevent cross-contamination of stored material?
| The use of high security straws in combination with thermal sealing is the preferred approach as it minimizes the risk of cross-contamination (Maertens et al., 2004). | Strong |
|---|---|
| ⊕○○○ | |
| At the time of thawing, decontamination of the exterior of the straw and the single use of sterile scissors will reduce the risk of contaminating the stored contents with potential pathogens. | GPP |
Can the use of separate labs prevent cross contamination?
| Given that personal protective equipment (PPE), laboratory equipment and exposed surfaces can be contaminated even after good laboratory practice, disinfection and changing PPE between cases can reduce the risk of cross-contamination. | GPP |
| The recommended procurement, processing, release and storage procedures should be used for all samples, not only virally positive samples. | GPP |
Discussion
The current paper summarizes the 78 recommendations on prevention of viral transmission before and during MAR, preferred MAR techniques, their effect on outcomes, and interventions to reduce/avoid vertical transmission to the newborn collated from the ESHRE guideline on ‘Medically assisted reproduction in patients with a viral infection/disease’. This guideline covers all aspects of the management of MAR in patients with a viral infection/disease, and was written by a multidisciplinary group of gynecologists and fertility specialists, embryologists and a virologist. It offers a broad scope for investigating, counseling and treating couples and individuals with a viral condition, be it transitory or chronic. As a basis for the current guideline, a broad and formal literature review was conducted. We identified very few randomized controlled trials with evidence for most interventions deriving from case series.
Considering the importance and clinical relevance of the topic, it was surprising to find that research data on many aspects, for example gamete–embyro–viral interaction in the MAR laboratory, are scarce. Even though the viruses discussed in the current guideline are of the most prevalent and/or ‘understood’ viruses at the present time, the current studies are limited in size and quality owing to the reduced number of patients with viral infection/disease presenting at fertility clinics or having access to MAR treatment. Furthermore, some viruses cause a transient event (i.e. ZIKA, HPV), many times asymptomatic, and the window of opportunity for long-term research is not present. A second deterrent for establishing robust research projects in the case of ZIKA, for example, is the current accepted guideline that pregnancy should be avoided, making any research proposal ethically questionable. The group reviewed the data on HPV infection and male and female infertility and even though this research is novel, this is a virus that deserves the attention of practitioners and patients alike.
In the case of HTLV I/II, its limited geographical prevalence means that even though the effects of the infection are significant for the affected individuals, the relevance to the global medical community remains low. In consequence, studies on the impact of HTLV I/II infection on MAR are lacking.
Research gaps were detected in several areas, and these are documented in a list of recommendations for further research (Supplementary Data II). As a matter of focus for the future, national data collection on outcomes of MAR treatment in patients with viral disease and the development of national centers of excellence where a larger cohort of affected patients can be offered therapy might help create the opportunity to find answers to questions that remain unanswered after this review, protected by an ethical and legal framework that could allow the opportunity to conduct high quality research on the topic.
Despite the limitations of guidelines in general, and the limitations in the evidence supporting the current guideline, the guideline group is confident that this document will help best practice in the management of MAR in patients with a viral infection/disease.
Data availability
The full guideline document, the literature study and evidence tables can be found on the ESHRE website (www.eshre.eu/virusguideline).
Supplementary Material
Acknowledgements
The Guideline Development Group (GDG) would like to express sincere thanks to Dr Mark Atkins for providing the virology expertise and oversight for this guideline development. In addition, the GDG would like to acknowledge the help of many clinicians and professional organizations who refereed the content of the guideline and submitted helpful comments to the draft version.
Authors’ roles
E.M. chaired the GDG and hence fulfilled a leading role in collecting the evidence, writing the manuscript and dealing with reviewer comments. N.L.C. as methodological expert, performed all literature searches for the guideline, provided methodological support and coordinated the guideline development. All other authors, listed in alphabetical order, as GDG members, contributed equally to the manuscript, by drafting key questions, synthesizing evidence, writing the different parts of the guideline and discussing recommendations until consensus within the group was reached.
Funding
The study has no external funding; all costs for meetings were covered by ESHRE.
Conflict of interest
A.D. reports research fees from Ferring and Merck, consulting fees from Ferring, outside the submitted work. C.P. reports speakers fees from Merck and MSD outside the submitted work. K.T. reports speakers fees from Cooper Surgical and Ferring and consultancy fees as member of the advisory board BioTeam of Ferring, outside the submitted work. The other authors have no conflicts of interest to declare.
Footnotes
†ESHRE Pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
Contributor Information
Edgar Mocanu, Department of Reproductive Medicine, Rotunda Hospital, Royal College of Surgeons in Ireland, Dublin, Ireland.
Andrew Drakeley, Department of Reproductive Medicine, Liverpool Women’s Hospital, Liverpool, UK.
Markus S Kupka, Department Gynaecology and Obstetrics, Gynaekologicum Hamburg, Hamburg, Germany.
Evelin E Lara-Molina, Department of Egg Donation, IVI RMA Global Barcelona, Barcelona, Spain.
Nathalie Le Clef, European Society of Human Reproduction and Embryology, Grimbergen, Belgium.
Willem Ombelet, Genk Institute for Fertility Technology, ZOL Hospitals, Genk Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium.
Catherine Patrat, APHP Centre—University of Paris, Cochin, Service de Biologie de la Reproduction—CECOS, Paris, France.
Guido Pennings, Department of Philosophy and Moral Science, Bioethics Institute Ghent (BIG) Ghent University, Gent, Belgium.
Augusto Enrico Semprini, Department of Biomedical and Clinical Sciences, University of Milan, Milan, Italy.
Kelly Tilleman, Department for Reproductive Medicine, Ghent University Hospital, Gent, Belgium.
Mauro Tognon, Department of Medical Sciences, University of Ferrara School of Medicine, Ferrara, Italy.
Nino Tonch, Department of Reproductive Medicine, Amsterdam University Medical Centre, Location AMC, Amsterdam, The Netherlands.
Bryan Woodward, X&Y Fertility, Leicester, UK.
ESHRE Guideline Group on Viral infection/disease:
Harish M Bhandari, Thomas Mitchell, James Duffy, Anastasia Mania, Niki Konsta, Ippokratis Sarris, Pierre Boyer, Carlos Calhaz-Jorge, Stefan Matik, Qianhong Ma, Fang Ma, Charalampos Siristatidis, Liana Bosco, Kimball O Pomeroy, and Janek von Byern
References
- Ackerman Z, Paltiel O, Glikberg F, Ackerman E. Hepatitis C virus in various human body fluids: a systematic review. Hepatol Res 1998;11:26–40. [Google Scholar]
- Assefa M, Worku A, Yesuf A. HIV-free survival and morbidity among breast-fed and formula-fed infants and young children in a prevention of MTCT of HIV program in Addis Ababa, Ethiopia, 2014. Asian Pac J Trop Dis 2017;7:225–232. [Google Scholar]
- Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009;23:1397–1404. [DOI] [PubMed] [Google Scholar]
- Ayoola EA, Ladipo OA, Odelola HA. Antibody to hepatitis B core antigen, e-antigen and its antibody in menstrual blood and semen. Int J Gynaecol Obstet 1981;19:221–223. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Benedetto A, Burrini AG, Collodel G, Ceccarini EC, Crisa N, Di Caro A, Estenoz M, Garbuglia AR, Massacesi A. HIV-particles in spermatozoa of patients with AIDS and their transfer into the oocyte. J Cell Biol 1994;127:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010;39:1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A, Riche D, Mena L, Sison T, Barry L, Reddy R, Shwayder J, Parry JP. Efficacy and safety of intrauterine insemination and assisted reproductive technology in populations serodiscordant for human immunodeficiency virus: a systematic review and meta-analysis. Fertil Steril 2014;102:424–434. [DOI] [PubMed] [Google Scholar]
- Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, Cattelan AM, Palu G, Baldanti F. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin Infect Dis 2018;66:1173–1180. [DOI] [PubMed] [Google Scholar]
- Bielanski A. Non-transmission of bacterial and viral microbes to embryos and semen stored in the vapour phase of liquid nitrogen in dry shippers. Cryobiology 2005;50:206–210. [DOI] [PubMed] [Google Scholar]
- Boostani R, Sadeghi R, Sabouri A, Ghabeli-Juibary A. Human T-lymphotropic virus type I and breastfeeding; systematic review and meta-analysis of the literature. Iran J Neurol 2018;17:174–179. [PMC free article] [PubMed] [Google Scholar]
- Bourlet T, Levy R, Maertens A, Tardy J-C, Grattard F, Cordonier H, Laurent J-L, Guerin J-F, Pozzetto B. Detection and characterization of hepatitis C virus RNA in seminal plasma and spermatozoon fractions of semen from patients attempting medically assisted conception. J Clin Microbiol 2002;40:3252–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlet T, Lornage J, Maertens A, Garret AS, Saoudin H, Tardy JC, Jimenez C, Guerin JF, Pozzetto B, Levy R. Prospective evaluation of the threat related to the use of seminal fractions from hepatitis C virus-infected men in assisted reproductive techniques. Hum Reprod 2009;24:530–535. [DOI] [PubMed] [Google Scholar]
- Bujan L, Sergerie M, Kiffer N, Moinard N, Seguela G, Mercadier B, Rhone P, Pasquier C, Daudin M. Good efficiency of intrauterine insemination programme for serodiscordant couples with HIV-1 infected male partner: a retrospective comparative study. Eur J Obstet Gynecol Reprod Biol 2007;135:76–82. [DOI] [PubMed] [Google Scholar]
- Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sex Transm Dis 2010;37:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto CL, Segurado AC, Pannuti C, Cedenho A, Srougi M, Spaine D, Fernandes S, Carretiero N, Bernal MC, Levi JE. Detection of HIV and HCV RNA in semen from Brazilian coinfected men using multiplex PCR before and after semen washing. Rev Inst Med Trop Sao Paulo 2006;48:201–206. [DOI] [PubMed] [Google Scholar]
- Cassuto NG, Sifer C, Feldmann G, Bouret D, Moret F, Benifla JL, Porcher R, Naouri M, Neuraz A, Alvarez S et al. A modified RT-PCR technique to screen for viral RNA in the semen of hepatitis C virus-positive men. Hum Reprod 2002;17:3153–3156. [DOI] [PubMed] [Google Scholar]
- Chatzistamatiou K, Sotiriadis A, Agorastos T. Effect of mode of delivery on vertical human papillomavirus transmission—a meta-analysis. J Obstet Gynaecol 2016;36:10–14. [DOI] [PubMed] [Google Scholar]
- Chen HI, Tsai CD, Wang HT, Hwang SM. Cryovial with partial membrane sealing can prevent liquid nitrogen penetration in submerged storage. Cryobiology 2006;53:283–287. [DOI] [PubMed] [Google Scholar]
- Chen HL, Cai JY, Song YP, Zha ML, Qin G. Vaginal delivery and HBV mother to child transmission risk after immunoprophylaxis: a systematic review and a meta-analysis. Midwifery 2019;74:116–125. [DOI] [PubMed] [Google Scholar]
- Cito G, Coccia ME, Fucci R, Picone R, Cocci A, Russo GI, Rizzello F, Trotta M, Badolato L, Basile V et al. Influence of male human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection on the reproductive outcomes in serodiscordant couples: a case-control study. Andrology 2019;7:852–858. [DOI] [PubMed] [Google Scholar]
- Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158:109–113. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A. Influence of infant feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa. Ann N Y Acad Sci 2000;918:136–144. [DOI] [PubMed] [Google Scholar]
- De Martino M, Tovo PA, Tozzi AE, Pezzotti P, Galli L, Livadiotti S, Caselli D, Massironi E, Ruga E, Fioredda F et al. HIV-1 transmission through breast-milk: Appraisal of risk according to duration of feeding. AIDS 1992;6:991–997. [PubMed] [Google Scholar]
- Deleage C, Moreau M, Rioux-Leclercq N, Ruffault A, Jegou B, Dejucq-Rainsford N. Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo. Am J Pathol 2011;179:2397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J, Kallings I, Brihmer C, Sikstrom B, Koskela P, Lehtinen M, Schiller JT, Sapp M, Mardh PA. Seropositivities to human papillomavirus types 16, 18, or 33 capsids and to Chlamydia trachomatis are markers of sexual behavior. J Infect Dis 1996;173:1394–1398. [DOI] [PubMed] [Google Scholar]
- Dussaix E, Guetard D, Dauguet C, D'Almeida M, Auer J, Ellrodt A, Montagnier L, Auroux M. Spermatozoa as potential carriers of HIV. Res Virol 1993;144:487–495. [DOI] [PubMed] [Google Scholar]
- Fei QJ, Yang XD, Ni WH, Pan CS, Huang XF. Can hepatitis B virus DNA in semen be predicted by serum levels of hepatitis B virus DNA, HBeAg, and HBsAg in chronically infected men from infertile couples? Andrology 2015;3:506–511. [DOI] [PubMed] [Google Scholar]
- Garrido N, Meseguer M, Bellver J, Remohi J, Simon C, Pellicer A. Report of the results of a 2 year programme of sperm wash and ICSI treatment for human immunodeficiency virus and hepatitis C virus serodiscordant couples. Hum Reprod 2004;19:2581–2586. [DOI] [PubMed] [Google Scholar]
- Garrido N, Remohi J, Pellicer A, Meseguer M. The effectiveness of modified sperm washes in severely oligoasthenozoospermic men infected with human immunodeficiency and hepatitis C viruses. Fertil Steril 2006;86:1544–1546. [DOI] [PubMed] [Google Scholar]
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, Alavian SM. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV− mothers: a meta-analysis. Arch Gynecol Obstet 2011;283:255–260. [DOI] [PubMed] [Google Scholar]
- Glenn WK, Whitaker NJ, Lawson JS. High risk human papillomavirus and Epstein Barr virus in human breast milk. BMC Res Notes 2012;5:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grout BW, Morris GJ. Contaminated liquid nitrogen vapour as a risk factor in pathogen transfer. Theriogenology 2009;71:1079–1082. [DOI] [PubMed] [Google Scholar]
- Hadchouel M, Scotto J, Huret JL, Molinie C, Villa E, Degos F, Brechot C. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line. J Med Virol 1985;16:61–66. [DOI] [PubMed] [Google Scholar]
- Hernandez BY, Wilkens LR, Zhu X, Thompson P, McDuffie K, Shvetsov YB, Kamemoto LE, Killeen J, Ning L, Goodman MT. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis 2008;14:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou MC, Wu JC, Kuo BI, Sheng WY, Chen TZ, Lee SD, Lo KJ. Heterosexual transmission as the most common route of acute hepatitis B virus infection among adults in Taiwan–the importance of extending vaccination to susceptible adults. J Infect Dis 1993;167:938–941. [DOI] [PubMed] [Google Scholar]
- Huo TI, Wu JC, Huang YH, Yang UC, Sheen IJ, Chang FY, Lee SD. Evidence of transmission of hepatitis B virus to spouses from sequence analysis of the viral genome. J Gastroenterol Hepatol 1998;13:1138–1142. [DOI] [PubMed] [Google Scholar]
- Imade PE, Uwakwe NO, Omoregie R, Eghafona NO. Post-natal maternal antiretroviral therapy and HIV prevalence among breast-fed infants in Benin, Nigeria. N Am J Med Sci 2010;2:427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba N, Ohkawa R, Matsuura A, Kudoh J, Takamizawa H. Sexual transmission of hepatitis B surface antigen. Infection of husbands by HBsAg carrier-state wives. Br J Vener Dis 1979;55:366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Zhao Y, Tan Z, Zhang X, Zhao Y, Wang B, Liu P. Immunization interventions to interrupt hepatitis B virus mother-to-child transmission: a meta-analysis of randomized controlled trials. BMC Pediatr 2014;14:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, Guyomard S, Prisant N, Lamarre P, Dejucq-Rainsford N et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017;17:1200–1208. [DOI] [PubMed] [Google Scholar]
- Kagaayi J, Gray RH, Brahmbhatt H, Kigozi G, Nalugoda F, Wabwire-Mangen F, Serwadda D, Sewankambo N, Ddungu V, Ssebagala D et al. Survival of infants born to HIV-positive mothers, by feeding modality, in Rakai, Uganda. PLoS One 2008;3:e3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis 2008;35:55–60. [DOI] [PubMed] [Google Scholar]
- Kaplan JE, Khabbaz RF, Murphy EL, Hermansen S, Roberts C, Lal R, Heneine W, Wright D, Matijas L, Thomson R et al. Male-to-female transmission of human T-cell lymphotropic virus types I and II: association with viral load. The Retrovirus Epidemiology Donor Study Group. J Acquir Immun Def Syndrom Human Retrovirol 1996;12:193–201. [DOI] [PubMed] [Google Scholar]
- Katoonizadeh A, Motamed-Gorji N, Sharafkhah M, Ostovaneh M, Esmaili S, Eslami L, Gharravi A, Khoshnia M, Shayanrad A, Katouli FS et al. Intra-familial transmission of chronic hepatitis B infection: a large population-based cohort study in northern Iran. Arch Iran Med 2018;21:436–442. [PubMed] [Google Scholar]
- Kennedy CE, Yeh PT, Pandey S, Betran AP, Narasimhan M. Elective cesarean section for women living with HIV: a systematic review of risks and benefits. AIDS 2017;31:1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer SK, Chackerian B, van den Brule AJ, Svare EI, Paull G, Walbomers JM, Schiller JT, Bock JE, Sherman ME, Lowy DR et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001;10:101–106. [PubMed] [Google Scholar]
- Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ 2006;332:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMessurier J, Traversy G, Varsaneux O, Weekes M, Avey MT, Niragira O, Gervais R, Guyatt G, Rodin R. Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: a systematic review. CMAJ 2018;190:E1350–E1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leruez-Ville M, Thiounn N, Poirot C, Launay O, Sogni P, Grabar S, Dulioust E. Intracytoplasmic sperm injection with microsurgically retrieved spermatozoa in azoospermic men infected with human immunodeficiency virus 1 or hepatitis C virus: the EP43 AZONECO ANRS study. Fertil Steril 2013;99:713–717. [DOI] [PubMed] [Google Scholar]
- Louvanto K, Sarkola M, Rintala M, Syrjanen K, Grenman S, Syrjanen S. Breast milk is a potential vehicle for human papillomavirus transmission to oral mucosa of the spouse. Pediatr Infect Dis J 2017;36:627–630. [DOI] [PubMed] [Google Scholar]
- Machaira M, Papaevangelou V, Vouloumanou EK, Tansarli GS, Falagas ME. Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg+/HBeAg– mothers: a systematic review and meta-analysis. J Antimicrob Chemother 2015;70:396–404. [DOI] [PubMed] [Google Scholar]
- Maertens A, Bourlet T, Plotton N, Pozzetto B, Levy R. Validation of safety procedures for the cryopreservation of semen contaminated with hepatitis C virus in assisted reproductive technology. Hum Reprod 2004;19:1554–1557. [DOI] [PubMed] [Google Scholar]
- Magoni M, Bassani L, Okong P, Kituuka P, Germinario EP, Giuliano M, Vella S. Mode of infant feeding and HIV infection in children in a program for prevention of mother-to-child transmission in Uganda. AIDS 2005;19:433–437. [DOI] [PubMed] [Google Scholar]
- Marques C, Guerreiro C, Soares SR. Lights and shadows about the effectiveness of IVF in HIV infected women: a systematic review. Infect Dis Obstet Gynecol 2015;2015:517208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: a randomized clinical trial. Obstet Gynecol Surv 2002;57:277–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Duggal NK, Hook SA, Delorey M, Fischer M, Olzenak McGuire D, Becksted H, Max RJ, Anishchenko M, Schwartz AM et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med 2018;378:1377–1385. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Garrido N, Gimeno C, Remohi J, Simon C, Pellicer A. Comparison of polymerase chain reaction-dependent methods for determining the presence of human immunodeficiency virus and hepatitis C virus in washed sperm. Fertil Steril 2002;78:1199–1202. [DOI] [PubMed] [Google Scholar]
- Miller RL, Ponte R, Jones BR, Kinloch NN, Omondi FH, Jenabian MA, Dupuy FP, Fromentin R, Brassard P, Mehraj V et al. HIV diversity and genetic compartmentalization in blood and testes during suppressive antiretroviral therapy. J Virol 2019;93:e00755-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabet V, Alvarez M, Solves P, Ocete D, Gimeno C. Use of liquid nitrogen during storage in a cell and tissue bank: contamination risk and effect on the detectability of potential viral contaminants. Cryobiology 2012;64:121–123. [DOI] [PubMed] [Google Scholar]
- Molina I, Carmen Del Gonzalvo M, Clavero A, Angel Lopez-Ruz M, Mozas J, Pasquau J, Sampedro A, Martinez L, Castilla JA. Assisted reproductive technology and obstetric outcome in couples when the male partner has a chronic viral disease. Int J Fertil Steril 2014;7:291–300. [PMC free article] [PubMed] [Google Scholar]
- Molina I, Mari M, Martínez JV, Novella-Maestre E, Pellicer N, Pemán J. Bacterial and fungal contamination risks in human oocyte and embryo cryopreservation: open versus closed vitrification systems. Fertil Steril 2016;106:127–132. [DOI] [PubMed] [Google Scholar]
- Musso D, Richard V, Teissier A, Stone M, Lanteri MC, Latoni G, Alsina J, Reik R, Busch MP, Detection of Zika virus RNA in semen of asymptomatic blood donors. Clin Microbiol Infect 2017;23:1001.e1–1001.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesrine F, Saleh H. Hepatitis C virus (HCV) status in newborns born to HCV positive women performing intracytoplasmic sperm injection. Afr Health Sci 2012;12:58–62. [PMC free article] [PubMed] [Google Scholar]
- Nie R, Wang M, Liao T, Qian K, Zhu G, Jin L. Assisted conception does not increase the risk for mother-to-child transmission of hepatitis B virus, compared with natural conception: a prospective cohort study. Fertil Steril 2019;111:348–356. [DOI] [PubMed] [Google Scholar]
- Njom Nlend AE, Motaze ACN, Sandie A, Fokam J. HIV-1 transmission and survival according to feeding options in infants born to HIV-infected women in Yaounde. BMC Pediatr 2018;18:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayinka B, Oni AO, Mbajiorgu FE. Impact of infant feeding practices on the risk of mother to child transmission of HIV-1 in Zimbabwe. J Paediatr Child Health 2000;36:313–317. [DOI] [PubMed] [Google Scholar]
- Paiva A, Smid J, Haziot MEJ, Assone T, Pinheiro S, Fonseca LAM, de Oliveira ACP, Casseb J. High risk of heterosexual transmission of human T-cell lymphotropic virus type 1 infection in Brazil. J Med Virol 2017;89:1287–1294. [DOI] [PubMed] [Google Scholar]
- Paiva AM, Assone T, Haziot MEJ, Smid J, Fonseca LAM, Luiz ODC, de Oliveira ACP, Casseb J. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci Rep 2018;8:7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, Perez-Padilla J, Medina FA, Waterman SH, Gubern CG et al. Persistence of Zika virus in body fluids—final report. N Engl J Med 2018;379:1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, Pharm CO, Ndimubanzi PC, Courteille O, Arendt V. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS 2009;23:2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisant N, Tubiana R, Lefebvre G, Lebray P, Marcelin AG, Thibault V, Rosenblum O, Bonmarchand M, Vauthier-Brouzes D, Golmard JL et al. HIV-1 or hepatitis C chronic infection in serodiscordant infertile couples has no impact on infertility treatment outcome. Fertil Steril 2010;93:1020–1023. [DOI] [PubMed] [Google Scholar]
- Qian WP, Tan YQ, Chen Y, Peng Y, Li Z, Lu GX, Lin MC, Kung HF, He ML, Shing LK. Rapid quantification of semen hepatitis B virus DNA by real-time polymerase chain reaction. World J Gastroenterol 2005;11:5385–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis 1997;176:960–968. [DOI] [PubMed] [Google Scholar]
- Rosenblum L, Darrow W, Witte J, Cohen J, French J, Gill PS, Potterat J, Sikes K, Reich R, Hadler S. Sexual practices in the transmission of hepatitis B virus and prevalence of hepatitis delta virus infection in female prostitutes in the United States. JAMA 1992;267:2477–2481. [PubMed] [Google Scholar]
- Roucoux DF, Wang B, Smith D, Nass CC, Smith J, Hutching ST, Newman B, Lee TH, Chafets DM, Murphy EL; HTLV Outcomes Study Investigators. A prospective study of sexual transmission of human T lymphotropic virus HTLV-I and HTLV-II. J Infect Dis 2005;191:1490–1497. [DOI] [PubMed] [Google Scholar]
- Sauer MV, Chang PL. Establishing a clinical program for human immunodeficiency virus 1-seropositive men to father seronegative children by means of in vitro fertilization with intracytoplasmic sperm injection. Am J Obstet Gynecol 2002;186:627–633. [DOI] [PubMed] [Google Scholar]
- Savasi V, Oneta M, Parrilla B, Cetin I. Should HCV discordant couples with a seropositive male partner be treated with assisted reproduction techniques (ART)? Eur J Obstet Gynecol Reprod Biol 2013;167:181–184. [DOI] [PubMed] [Google Scholar]
- Savasi V, Parrilla B, Ratti M, Oneta M, Clerici M, Ferrazzi E. Hepatitis C virus RNA detection in different semen fractions of HCV/HIV-1 co-infected men by nested PCR. Eur J Obstet Gynecol Reprod Biol 2010;151:52–55. [DOI] [PubMed] [Google Scholar]
- Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine 2013;31:2506–2516. [DOI] [PubMed] [Google Scholar]
- Stuver SO, Tachibana N, Okayama A, Shioiri S, Tsunetoshi Y, Tsuda K, Mueller NE. Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in southwestern Japan: an initial report from the Miyazaki Cohort Study. J Infect Dis 1993;167:57–65. [DOI] [PubMed] [Google Scholar]
- Tess BH, Rodrigues LC, Newell ML, Dunn DT, Lago TD. Infant feeding and risk of mother-to-child transmission of HIV-1 in Sao Paulo State, Brazil. Sao Paulo Collaborative Study for Vertical Transmission of HIV-1. J Acquir Immun Def Syndrom Human Retrovirol 1998;19:189–194. [DOI] [PubMed] [Google Scholar]
- Tufon KA, Meriki HD, Kwenti TE, Tony NJ, Malika E, Bolimo AF, Kouanou YS, Nkuo-Akenji T, Anong DN. HBV transmission risk assessment in healthcare workers, household and sexual contacts of HBV infected patients in the Southwest Region of Cameroon. Oman Med J 2019;34:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen N, Le Clef N, D'Angelo A, Tilleman K, Veleva Z, Nelen W. Manual for ESHRE guideline development. ESHRE 2020. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Guideline-development-process (16 August 2021, date last accessed).
- Vitorino RL, Grinsztejn BG, de Andrade CA, Hokerberg YH, de Souza CT, Friedman RK, Passos SR. Systematic review of the effectiveness and safety of assisted reproduction techniques in couples serodiscordant for human immunodeficiency virus where the man is positive. Fertil Steril 2011;95:1684–1690. [DOI] [PubMed] [Google Scholar]
- Widdice L, Ma Y, Jonte J, Farhat S, Breland D, Shiboski S, Moscicki AB. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 2013;207:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Furumoto H, Abe A, Kato T, Nishimura M, Kuwahara A, Maeda K, Matsuzaki T, Irahara M. The possibility of vertical transmission of human papillomavirus through maternal milk. J Obstet Gynaecol 2011;31:503–506. [DOI] [PubMed] [Google Scholar]
- Young CD, Tatieng S, Kongmanas K, Fongmoon D, Lomenick B, Yoon AJ, Kiattiburut W, Compostella F, Faull KF, Suree N et al. Sperm can act as vectors for HIV-1 transmission into vaginal and cervical epithelial cells. Am J Reprod Immunol 2019;82:e13129. [DOI] [PubMed] [Google Scholar]
- Zafer M, Horvath H, Mmeje O, van der Poel S, Semprini AE, Rutherford G, Brown J. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples: a systematic review and meta-analysis. Fertil Steril 2016;105:645–655.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Lu Y, Ye Q, Xia Y, Zhou Y, Yao Q, Wei S. Should chronic hepatitis B mothers breastfeed? A meta analysis. BMC Public Health 2011;11:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouridis A, Kalampokas T, Panoulis K, Salakos N, Deligeoroglou E. Intrauterine HPV transmission: a systematic review of the literature. Arch Gynecol Obstet 2018;298:35–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full guideline document, the literature study and evidence tables can be found on the ESHRE website (www.eshre.eu/virusguideline).