Abstract
Knee pain is a leading cause of disability in the aging population and may indirectly accelerate biological aging processes. Chronological aging increases the risk of developing of knee pain and knee pain reduces physical function; however, limited data exist on how epigenetic aging, a known hallmark of biological aging shown to predict health span and mortality, may influence this relationship. The purpose of this study was to examine whether decreased physical performance associated with knee pain is mediated by markers of epigenetic aging. Participants (57.91 ± 8.04 years) with low impact knee pain (n=95), high impact knee pain (n=53) and pain-free controls (n=26) completed self-reported pain, a blood draw and a short physical performance battery (SPPB) that included balance, walking, and sit to stand tasks. We employed an epigenetic clock previously associated with knee pain and shown to predict overall mortality risk (DNAmGrimAge). Bootstrapped-mediation analyses were used to determine associations of DNAmGrimAge and SPPB between pain groups. Those with high impact and low impact pain had a biologically older epigenetic age (5.14y ± 5.66 and 1.32y ± 5.41, respectively). However, while there were direct effects of pain on overall physical performance, these were not explained by epigenetic aging. Epigenetic aging only mediated the effect of pain on balance performance. Future work is needed to examine pain’s impact on biological aging processes including epigenetic aging and its ultimate effect on physical function measures known to predict health span and mortality.
Keywords: Knee pain, epigenetic aging, biological clocks, aging, physical performance, balance
Introduction
Knee pain affects up to 70% of adults aged 65 and older (1, 2) and often impacts lower extremity physical function (3, 4). Knee pain can impair activities of daily living such as standing and walking, thus reducing quality of life (1, 5, 6). Physical function is usually characterized by self-reported experiences or observed physical performance (7). Performance-based tests of lower extremity function can be used to predict adverse events in older persons, such as disability, falls, hospitalization, and death (8–12).
Emerging evidence suggests that knee pain is complex and may be influenced by other factors that are not unique to the knee joint, including psychological factors (e.g., depression, and anxiety) (13, 14), environmental factors (socio-economic status, exercise and nutrition) (15, 16), and genetics (17). These factors may help begin to explain the observed discrepancy between symptom severity and level of disability experienced by those with knee complaints. Time spent on earth (chronological aging) is a key risk factor for knee pain (1, 2) and developing knee pain that severely limits daily activities (18–20), may indirectly accelerate biological aging processes, which further impacts physical function (21) leading to morbidity and disability. There is evidence that those individuals with chronic pain and/or physical function decrements demonstrate premature aging (22–24). While both chronic pain and physical function limitations are heterogeneous with advancing age, other biological and environmental “aging” processes may influence this variation.
Epigenetic changes are a hallmark of aging as certain epigenetic factors (within the genome and the external environment) can lead to biological changes within the human body, which may contribute to chronic pain. DNA methylation (DNAm) is an epigenetic mechanism that regulates gene expression (25) and changes in DNAm patterns have been linked to fundamental aging mechanisms (26). DNA methylation-based age prediction models (i.e., epigenetic clocks) are not only accurate in estimating chronological age, but can also estimate biological aging rates (27–29) and are among the most promising biomarkers of aging to date. Accelerated DNAm patterns using epigenetic clocks have been established in those with chronic pain (30) and these same clocks can predict all-cause mortality (31) and lifespan (32). One clock of interest; DNAmGrimAge uses a linear combination of chronological age, sex, and DNAm-based surrogate biomarkers for several plasma proteins and smoking pack-years, and has stronger relationships with a variety of health-related metrics compared to other epigenetic clocks (32). This epigenetic clock may be most associated with pain outcomes, since DNAmGrimAge outperforms other epigenetic clocks in their associations with age-related conditions and mortality and may be the most useful when establishing relationships between epigenetics, physical function and chronic pain in older adults (33).
While it is widely understood that chronological aging is a significant risk factor for developing knee pain (34, 35), and that knee pain can impact physical function (1, 5, 6), limited data exists on how epigenetic aging may influence this relationship. Individuals with high impact pain (i.e., pain that limits physical function) present with an older epigenome than those with low impact pain when using DNAmGrimAge to predict biological age. Furthermore, DNAmGrimAge is strongly associated with physical functioning (32, 33, 36) and is the only epigenetic clock associated with the complex, multidimensional pain experience (33). Therefore, the present study builds on this evidence and sought to identify associations between physical performance and DNAmGrimAge in individuals with knee pain and to examine whether DNAmGrimAge mediates the association between physical performance limits and self-reported knee pain. Based on previous work, we hypothesized that an older epigenome in persons with higher knee pain impact will significantly mediate the association between knee pain and physical performance measures.
Methods
Participants
The present study is an ancillary investigation that aimed to determine brain and epigenetic aging patterns in knee pain, thus, only measures relevant to the study hypotheses are included and presented below. Participants between the ages of 45-85 (62.7% female) with self-reported knee pain were recruited from the University of Florida (UF; Gainesville, Florida, USA) and the University of Alabama at Birmingham (UAB; Birmingham, Alabama, USA). Individuals who self-identified as non-Hispanic and “African American/Black” or “White/Caucasian/European” and English speaking, were eligible for inclusion. Individuals were excluded if they reported 1) significant surgery to the index (i.e., most painful) knee (e.g., total knee replacement surgery); 2) cardiovascular disease or history of acute myocardial infarction; 3) uncontrolled hypertension (blood pressure > 150/95 mmHg); 4) systemic rheumatic diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia); 5) neuropathy; 6) chronic opioid use; 7) serious psychiatric illness; 8) neurological disease (e.g., Parkinson’s, multiple sclerosis, stroke with loss of sensory or motor function, or uncontrolled seizures); 9) pregnant; 10) significantly greater pain in a body site other than the knee. All participants provided written informed consent and the study was IRB approved and conducted with accordance with the Declaration of Helsinki.
Procedures
Sociodemographic (e.g., age, sex, race), and health information (e.g., knee OA symptoms, weight) were collected during initial phone screening. Once initial eligibility was determined, individuals were scheduled for a health assessment session, during which informed consent was obtained followed by health history, pain history, and physical exam to determine the most painful (i.e., index) knee. Objective measures of physical function were also assessed during the health assessment session. Self-reported pain measures were completed and blood collection occurred approximately one week following the health assessment session.
Performance-Based Physical Function
The Short Physical Performance Battery (SPPB) was used as an objective measure of physical function (9). The SPPB asks participants to complete three tasks to assess function of the lower extremities, including a balance task, a chair stand task, and a gait speed task. Tasks are scored from 0 (unable to complete) to 4 (highest level of performance), and summed for an overall score ranging from 0-12, with higher scores indicating greater functional ability.
Self-reported Pain
The Graded Chronic Pain Scale (GCPS) is a 7-item self-report measure used to assess characteristic pain intensity and pain interference over the past six months (20). Three items assess pain intensity (i.e., current, average, and worst), and three items assess pain interference, on a 0 (“no pain”/ “no interference”) to 10 (“pain as bad as it could be”/ “unable to carry out any activities”), respectively. An additional item asks participants to report “about how many days in the last six months have you been kept from your usual activities (work, school, or housework) because of knee pain”? Ratings are averaged and multiplied by 10 to produce a characteristic pain intensity (items 1-3), and pain disability (items 4-6) score ranging from 0-100, with higher scores indicating more severe symptoms.
Operational Definition of Pain Impact:
For the present study, we employed the GCPS pain severity grades where: grade 0 is no pain, grade I is low disability-low intensity, grade II is low disability-high intensity, grade III is high disability-moderately limiting, and grade IV is high disability-severely limiting (19, 20). Consistent with prior research (19) and following the recommendations from the Task Force for the Classification of Chronic Pain consensus for the 11th version of the International Classification of Diseases (ICD-11) of the World Health Organization (WHO) (37), participants with a Grade of 0 were categorized as “No Chronic Pain Controls”, Grades 1-2 were classified as “Low Impact Pain”, and Grade of 3-4 “High Impact Pain”.
Blood Collection and Processing
Blood samples were collected using a 10ml K2 EDTA tube that was subsequently used for DNA extraction and methylation analysis as previously reported by our group. The buffy coat was carefully extracted and stored at −80-degree until all samples were collected. The isolated DNA samples were qubit quantified, assessed for quality, and sent to the Molecular Genomics Core at the Moffitt Cancer Center (Tampa, FL 33612) for bisulfite conversion and EPIC methylation array analyses. Bisulfite conversion used qubit quantified 50 ul of 22ng/ul DNA in 96 well plate format. The Infinium Human Methylation EPIC Bead Chip kit was used covering over 850,000 CpG sites, with each CpG assay replicated. The internal controls assessed bisulfate conversion, staining, extension, hybridization, and assay performance. We used the BeadArray Controls reporter software for quality control assessment, with experimenters’ blinded using sample IDs to keep duplicate sample integrity.
DNA Methylation Age Calculation
Our prior work (30) showed that out of five epigenetic clocks, DNAmGrimAge was highly associated with the complex, multidimensional experience of pain in persons with knee pain. The DNAmGrimAge clock uses 1,030 CpG sites for its age calculation, and has shown predictability of mortality (i.e., Grim news) in previous work (32). As in our prior work, the raw data generated by illumina EPIC array (.idat files) were processed using R package minfi (38). Methylation beta values (percentage of methylation for each CpG site) were obtained and uploaded to the DNA Methylation Age online calculator (https://dnamage.genetics.ucla.edu/home (26) The normalized beta values were obtained using ChAMP (Chip Analysis Methylation Pipeline for Illumina HumanMethylation EPIC) protocol (38). These normalized beta values were extracted as .csv file to input dataset containing all the CpGs required for the online calculator as recommended by the calculator tutorial. The updated input dataset file and sample annotation file were uploaded in the online calculator as required to get the output file with processed DNA methylation age.
Statistical Analysis
Data analyses were performed using IBM SPSS v27.0 (Armonk, NY: IBM Corp) software and data were checked for normality, outliers, and conformance to assumptions prior to analysis of results. We used one-way analysis of variance (ANOVA) to compare groups with respect to continuous variables and χ2 analyses to assess associations with nominal variables. To compare chronological age to biological age using the aforementioned epigenetic clock, AgeAccelGrim was calculated using the difference between the two ages. SPPB scores and epigenetic differences in pain impact was assessed using a one-way analysis of covariance (ANCOVA), accounting for sex, race, chronological age, and study site, similar to our previous work (39) and adjusting for multiple comparisons using a Bonferroni correction. Pearson correlations were used to assess associations between SPPB domains, the epigenetic clocks, and self-reported pain severity and disability while controlling for sex, ethnicity/race, chronological age, weight and study site. Hierarchical linear regressions were performed to determine the association of pain impact with physical performance and epigenetic clocks after adjustment of covariates. Three models were tested; model 1 = pain impact, model 2 = model one plus sex, race, and study site, and model 3 = model 2 plus age and weight. Model assumptions were tested including tests of normality distribution and homogeneity of variances. Using the Hayes PROCESS macro (model 4) that was downloaded into IBM SPSS 27 (40), linear regression-based mediation analyses were used to assess pain grade (group, severity, and disability) and its association with SPPB domains with DNAmGrimAge as the mediator using direct and total indirect effects (Figure 1). We input pain variables (pain group, pain severity, and pain disability as independent predictors) as independent variables (X), DNAmGrimAge as the mediator (M), and SPPB domain (balance, sit to stand, walk, and total score) as the dependent variable (Y). Sex, race, age, weight, and study site were introduced as covariates. This model calculated direct effects of X on Y, the indirect effects of X on Y through M, and the total effects, which is the sum of the direct and indirect pathway. To overcome potential unmet assumptions commonly found in mediation analysis, bootstrapping procedures were employed for all analyses with 5,000 samples and reported as estimates and standard errors or as 95% bootstrapped confidence intervals. A probability less than 0.05 was considered statistically significant.
Figure 1:
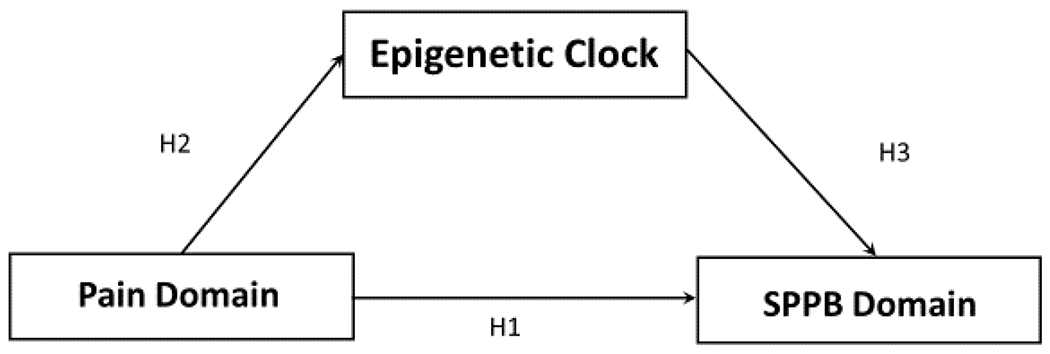
Hypothesized model of the associations between pain domain (pain impact, severity, and disability), physical performance (balance, sit to stand, walking and total SPPB) and epigenetic aging (DNAmGrimAge clock).
Results
Demographic and Subject Characteristics
Of the 213 participants who took part in the epigenetic sub-study that examined pain impact and epigenetic aging, 174 participants had complete pain, physical performance, and covariate data. The analytic sample age was 57.91 ± 8.04, was 62.7% female and 55.4% Non-Hispanic white. Self-reported pain and disability measures differed across pain impact groups as expected (p’s <0.001). Those with low impact pain had an older epigenome (p=0.015) relative to their chronological age (i.e., AgeAccelGrim). Similarly, those with high impact pain had an older AgeAccelGrim (p<0.001) relative to their chronological age. Pain characteristics by group are shown in Table 1.
Table 1:
Demographic sample characteristics (n=174)
| No Pain (n=26) | Low impact Pain (n=95) | High Impact pain (n=53) | |
|---|---|---|---|
| Age | 59.58 ± 9.33 | 58.63 ± 7.96 | 56.63 ± 7.12 |
| Female | 18 (69) | 62 (65) | 30 (56) |
| Study location (UF) | 17 (65) | 62 (65) | 30 (56) |
| White * | 17 (65) | 59 (62) | 21 (39) |
| Height (cm) | 166.20 ± 7.65 | 168.04 ± 8.89 | 170.44 ± 9.37 |
| Weight (kgs) * | 83.68 ± 16.51 | 85.16 ± 18.41 | 92.54 ± 18.67 |
| BMI (kg/m2) | 30.30 ± 5.71 | 30.32 ± 6.68 | 31.87 ± 6.04 |
| Pain Score * | 0.00 ± 0.00 | 11.83 ± 6.90 | 20.30 ± 5.39 |
| Disability Score * | 9.38 ± 1.96 | 5.95 ± 5.88 | 50.96 ± 46.23 |
| AgeAccelGrim* | 0.09 ± 3.31 | 1.32 ± 5.41 | 5.14 ± 5.66 |
denotes significant difference between groups P<0.05
AgeAccelGrim = difference between age and DNAmGrimAge
Balance, walking, sit to stand, and total physical performance were compared between those with high pain impact pain, low impact pain, and no pain controls while controlling for sex, race, chronological age and study site (Figure 2). Individuals with high impact pain had lower balance scores than those with low impact pain (p=0.021). There were also differences in the sit to stand scores where individuals with high impact pain had lower scores compared to controls (p<0.001) and those with low impact pain (p=0.008). Individuals reporting high impact pain had lower walking scores than the control group (p=0.019). Individuals with high impact pain had lower total SPPB scores than the control group (p<0.001) and low impact pain group (p<0.001).
Figure 2:
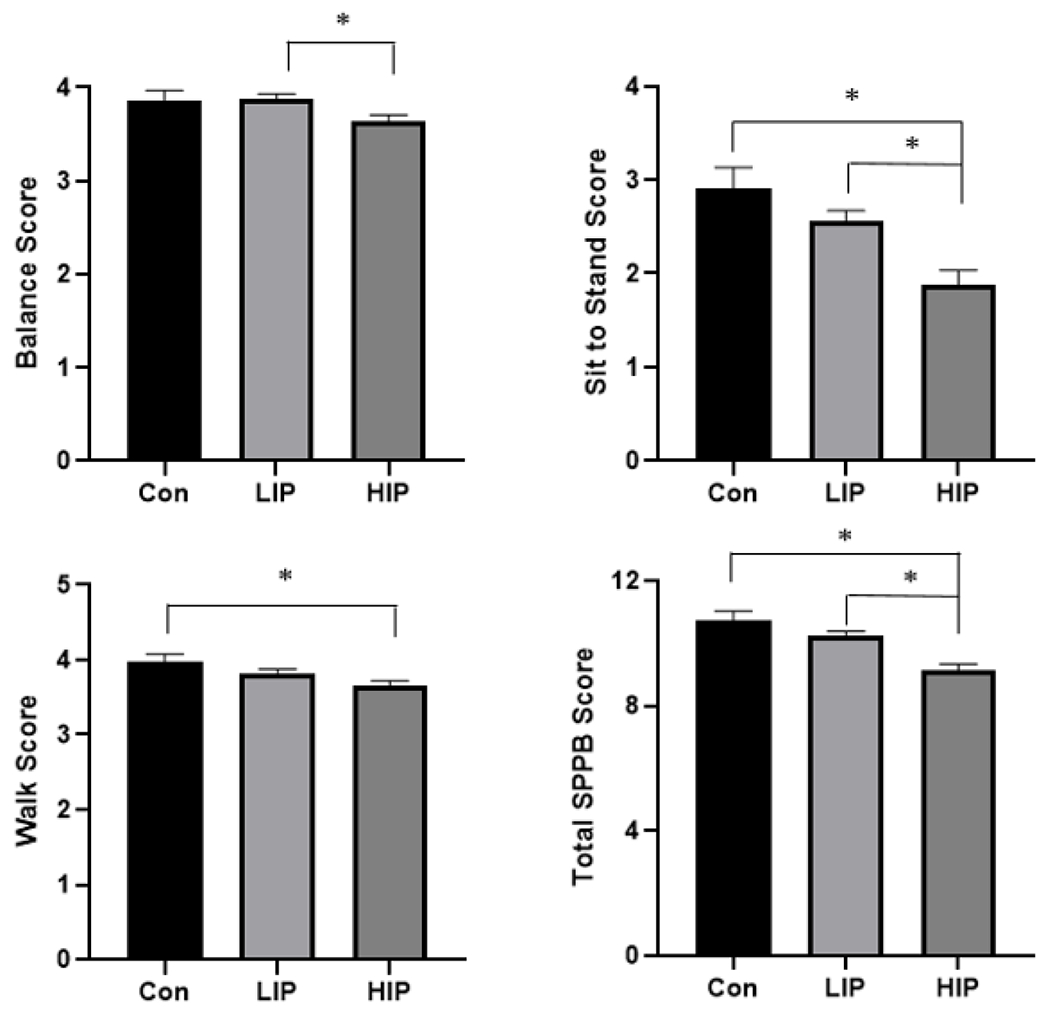
Adjusted means and SE differences when controlling for age, sex, race, and study site in physical performance domains between participants that did not have pain to those that had low impact pain (LIP) and high impact pain (HIP). *indicate significant difference between groups (p<0.001).
Associations between Self-Reported Pain, Physical Performance, and Epigenetic Aging
Pain impact, severity and disability were significant predictors of walking and total SPPB scores controlling for study site, sex, race, weight, and chronological age. Pain Impact predicted balance scores, and pain impact and pain severity predicted sit to stand scores. Pain impact, severity and disability were also predictors of DNAmGrimAge after controlling for study site, sex, race, weight, and chronological age. Results are presented in Table 2.
Table 2:
Hierarchical regression analysis of pain domains (pain impact, pain severity, and pain disability) predicting physical function and brain-PAD
|
|
||||||
|---|---|---|---|---|---|---|
| Pain Impact | Pain Severity | Pain Disability | ||||
| Dependent variables | Standardized b | p | Standardized b | p | Standardized b | p |
| Physical Function | ||||||
| Balance | −0.153 | 0.031 | −0.132 | 0.089 | −0.087 | 0.233 |
| Sit to Stand | −0.257 | <0.001 | −0.280 | <0.001 | −0.112 | 0.109 |
| Walk | −0.162 | 0.015 | −0.161 | 0.034 | −0.158 | 0.028 |
| Total SPPB score | −0.310 | <0.001 | −0.315 | <0.001 | −0.160 | 0.024 |
| Epigenetic Clock | ||||||
| DNAmGrimAge | 0.146 | <0.001 | 0.153 | <0.001 | 0.170 | <0.001 |
note: SPPB – Short physical performance battery
Mediation analysis
Pain impact, pain severity, and pain disability all predicted DNAmGrimAge (table 3) and DNAmGrimAge was associated with balance, but not sit to stand, walking or total SPPB scores (table 4). With SPPB domains as the dependent variables, DNAmGrimAge mediated the indirect effect of pain impact, pain severity and pain disability on balance scores and was statistically significant (i.e., did not include zero) as evaluated with the 95% bootstrapped confidence interval (table 5).
Table 3:
Path A - effect of pain impact (independent variable) on AgeAccelGrim (mediator)
| Coefficient | CI | P-value | |
|---|---|---|---|
| Pain Impact | 2.329 | 0.823; 3.835 | 0.003 |
| Pain Severity | 0.185 | 0.073; 0.298 | 0.001 |
| Pain Disability | 0.036 | 0.015; 0.058 | <0.001 |
Table 4:
Path B - effect of AgeAccelGrim (mediator) on dependent variable (SPPB scores)
| Coefficient | CI | P-value | |
|---|---|---|---|
| Balance | −0.025 | −0.046; −0.005 | 0.014 |
| Sit to Stand | −0.020 | −0.063; 0.024 | 0.370 |
| Walking | −0.009 | −0.029; 0.011 | 0.376 |
| Total SPPB Score | −0.047 | −0.100; 0.009 | 0.082 |
Note – SPPB = Short Physical Performance Battery
Table 5:
Mediated indirect effect estimates of Pain Impact Group and GCPS scores on SPPB outcomes, with bootstrapped confidence intervals.
| Indirect effect | ||
|---|---|---|
| effect | CI | |
| Pain Impact | ||
| Balance | −0.059 | −0.127; −0.013* |
| Sit to Stand | −0.045 | −0.163; 0.070 |
| Walking | −0.021 | −0.075; 0.059 |
| Total SPPB Score | −0.11 | −0.279; 0.026 |
| Pain Severity | ||
| Balance | −0.005 | −0.012; −0.001* |
| Sit to Stand | −0.004 | −0.014; 0.005 |
| Walking | −0.002 | −0.006; 0.002 |
| Total SPPB Score | −0.009 | −0.023; 0.001 |
| Pain Disability | ||
| Balance | −0.001 | −0.002; −0.001* |
| Sit to Stand | −0.001 | −0.003; 0.001 |
| Walking | 0 | −0.001; 0.000 |
| Total SPPB Score | −0.002 | −0.005; 0.000 |
Note – SPPB = Short Physical Performance Battery
Discussion
To our knowledge, this is the first study to examine the link between epigenetic aging and physical performance in adults with high and low impact knee pain. Several findings emerged. First, individuals with high impact pain had reduced balance, standing, walking, overall physical performance compared to persons with lower impact pain and pain-free controls. Second, while various pain measures were associated with multiple physical function measures, only balance was associated with epigenetic aging. Finally, epigenetic aging was only a significant mediator of the pain and balance association in our sample.
Our findings support the existing body of research where knee pain is associated with decreased physical function and increased disability in aging individuals (41–44). Population-based studies have shown that high-impact chronic pain increases the odds of severe pain, physical disability, and cognitive impairment (19, 45), however, the contributors to lower physical function in persons with pain are likely complex and multifactorial, especially in aging individuals. While peripheral factors have been previously reported in persons with chronic pain (i.e., changes in structural joint, muscle mass, muscle biochemistry, peripheral afferents) (46–51), other more systemic contributors remain to be elucidated (i.e., brain, epigenetics) (39, 52, 53). Further, these associations are likely bidirectional in nature. For example, having both pain and physical disability could lead to physical inactivity, and in turn, accelerate epigenetic aging (54). On the other hand, regular physical activity protects against the development of chronic disease, improves health outcomes and slows down biological aging (55–57). While previous studies have reported multiple physical function domains to be associated with epigenetic aging (32, 33, 36), we found that only balance was associated with epigenetic aging in our chronic pain sample. This inconsistency may be explained by sample differences. Specifically, our participants were significantly younger (~ 58 years old) than those studied previously (~ 68-70 years old), with higher levels of physical and cognitive function, and lower levels of disability (33, 36). Another possibility may be the significantly smaller sample size of our study (n=174) compared to previous studies (n=413, n=920), where smaller effect sizes could be detected (33, 36). Future studies across the lifespan with a larger sample are needed to examine the consistency of these findings.
Finally, epigenetic aging only mediated pain’s impact on balance performance, and no other physical function measures. Although this was unexpected, our results may also be reflecting potential sample differences as explained above. Nonetheless, recent research has demonstrated that muscles have an epigenetic memory that influences muscle growth (58) and increased muscle mass can help with balance in older adults (59, 60), while the reverse are often present in persons with chronic pain (50, 61, 62). Additionally, fall risk, a consequence of poor balance, is a heritable, heterogeneous, and polygenic trait genetically correlated with fracture risk and grip strength (63). Fall risk has a large environmental influence, is multifactorial in origin and many different genetic pathways can contribute to the individual propensity to fall. While there is a small, but significant effect of gene expression on fall risk, it is more likely to be explained by environmental and lifestyle choices more aptly explained by epigenetics, however, this data does not currently exist. Therefore, the relationship between balance, fall risk, and epigenetic aging warrants further examination to explicate the association of balance with epigenetic aging.
The results of this study should be interpreted in light of a few considerations. First, data were collected as part of a larger study (n=216); however, only 174 participants completed the physical performance measures. Previous studies examining epigenetic aging and physical function have included much larger sample sizes (33, 36); it is therefore possible that our study was underpowered to detect additional associations. Another possibility is that the epigenetic aging measure used was not sensitive enough in our younger, high functioning sample of community-dwelling middle-to-older aged adults. Thus, future larger studies, with more comprehensive measures of biological aging that may be more sensitive in younger persons are needed to start measuring underlying contributors of pain’s impact on physical performance at earlier timepoints across the lifespan. Data were collected at a single time point; thus, the cross-sectional observations do not allow us to determine whether a specific epigenetic age preceded or was subsequent to pain and which was a contributor to physical performance. Future longitudinal studies should examine how chronic pain may accelerate the epigenome as well as other biological systems (i.e. telomere length, brain aging etc.) of an individual with chronic pain, especially high impact pain that limits physical functioning.
Despite these limitations, this study provides preliminary evidence that high impact knee pain may accelerate epigenetic aging processes that may ultimatelyinfluence balance performance in middle to older age community dwelling adults. Epigenetic aging may be a useful marker of general health across the lifespan, and may identify those at greatest risk of age-related functional deterioration and death. Future investigations examining biological aging processes and interactions with physical function over time in chronic pain populations are warranted to provide more actionable causative information.
References:
- 1.Patel K, Dansie E, Guralnik J, Turk D. Prevalence and impact of pain among older adults in the United States: findings from the National Health and aging trends study. The Journal of Pain. 2013;14(4):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karttunen NM, Turunen JH, Ahonen RS, Hartikainen SA. Persistence of noncancer-related musculoskeletal chronic pain among community-dwelling older people: a population-based longitudinal study in Finland. The Clinical Journal of Pain. 2015;31(1):79–85. [DOI] [PubMed] [Google Scholar]
- 3.Dudgeon BJ, Gerrard BC, Jensen MP, Rhodes LA, Tyler EJ. Physical disability and the experience of chronic pain. Archives of physical medicine and rehabilitation. 2002;83(2):229–35. [DOI] [PubMed] [Google Scholar]
- 4.Hairi NN, Cumming RG, Blyth FM, Naganathan V. Chronic pain, impact of pain and pain severity with physical disability in older people—Is there a gender difference? Maturitas. 2013;74(1):68–73. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont LH, Bean JF, Guralnik JM, Leveille SG. Comparing pain severity versus pain location in the MOBILIZE Boston study: chronic pain and lower extremity function. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2009;64(7):763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. Jama. 2009;302(20):2214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckett LA, Brock DB, Lemke JH, De Leon CFM, Guralnik JM, Fillenbaum GG, et al. Analysis of change in self-reported physical function among older persons in four population studies. American journal of epidemiology. 1996;143(8):766–78. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(11):M691–M7. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(4):M221–M31. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New England Journal of Medicine. 1995;332(9):556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terroso M, Rosa N, Torres Marques A, Simoes R. Physical consequences of falls in the elderly: a literature review from 1995 to 2010. European Review of Aging and Physical Activity. 2014;11(1):51–9. [Google Scholar]
- 12.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. Journals of gerontology series a: biomedical sciences and medical sciences. 2016;71(9):1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scopaz KA, Piva SR, Wisniewski S, Fitzgerald GK. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Archives of physical medicine and rehabilitation. 2009;90(11):1866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. Journal of pain and symptom management. 2009;37(5):863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson KA, Terry EL, Sibille KT, Gossett EW, Ross EN, Bartley EJ, et al. At the intersection of ethnicity/race and poverty: knee pain and physical function. Journal of racial and ethnic health disparities. 2019;6(6):1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitaker KM, Gabriel KP, Laddu D, White DK, Sidney S, Sternfeld B, et al. Bidirectional associations of accelerometer measured sedentary behavior and physical activity with knee pain, stiffness, and physical function: The CARDIA study. Preventive medicine reports. 2021;22:101348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schutte DL, Mukhopadhyay N, Holwerda T, Sluka K, Rakel B, Govil M. Genetic predictors of knee pain in persons with mild to moderate osteoarthritis. Research in gerontological nursing. 2020; 13(4):191–202. [DOI] [PubMed] [Google Scholar]
- 18.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and Profile of High-Impact Chronic Pain in the United States. The Journal of Pain. 2019;20(2):146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–49. [DOI] [PubMed] [Google Scholar]
- 21.Badley EM, Rasooly I, Webster GK. Relative importance of musculoskeletal disorders as a cause of chronic health problems, disability, and health care utilization: findings from the 1990 Ontario Health Survey. The Journal of rheumatology. 1994;21(3):505–14. [PubMed] [Google Scholar]
- 22.Lahav Y, Levy D, Ohry A, Zeilig G, Lahav M, Golander H, et al. Chronic Pain and Premature Aging–The Moderating Role of Physical Exercise. The journal of pain. 2021;22(2):209–18. [DOI] [PubMed] [Google Scholar]
- 23.Sibille KT, Chen H, Bartley EJ, Riley J III, Glover TL, King CD, et al. Accelerated aging in adults with knee osteoarthritis pain: consideration for frequency, intensity, time, and total pain sites. Pain reports. 2017;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenholm S, Koster A, Valkeinen H, Patel KV, Bandinelli S, Guralnik JM, et al. Association of physical activity history with physical function and mortality in old age. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2016;71(4):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore LD, Le T, Fan G. DNA Methylation and Its Basic Function. Neuropsychopharmacology. 2013;38(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath S. DNA methylation age of human tissues and cell types. Genome Biology. 2013;14(10):3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Xia L, Tu K, Duan M, Kukurba K, Li-Pook-Than J, et al. Longitudinal personal DNA methylome dynamics in a human with a chronic condition. Nature medicine. 2018;24(12):1930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, McGue M, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging cell. 2016;15(1):149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salameh Y, Bejaoui Y, El Hajj N. DNA methylation biomarkers in aging and age-related diseases. Frontiers in Genetics. 2020;11:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz-Almeida Y, Sinha P, Rani A, Huo Z, Fillingim RB, Foster T. Epigenetic aging is associated with clinical and experimental pain in community-dwelling older adults. Molecular pain. 2019; 15:1744806919871819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Föhr T, Törmäkangas T, Lankila H, Viljanen A, Rantanen T, Ollikainen M, et al. The Association Between Epigenetic Clocks and Physical Functioning in Older Women: A 3-Year Follow-up. The Journals of Gerontology: Series A. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis and cartilage. 2015;23(11):1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeser RF. Aging processes and the development of osteoarthritis. Current opinion in rheumatology. 2013;25(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. International journal of epidemiology. 2015;44(4):1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nugraha B, Gutenbrunner C, Barke A, Karst M, Schiller J, Schäfer P, et al. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain. 2019;160(1):88–94. [DOI] [PubMed] [Google Scholar]
- 38.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz-Almeida Y, Fillingim RB, Riley JL 3rd, Woods AJ, Porges E, Cohen R, et al. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain. 2019;160(5):1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach: Guilford publications; 2017. [Google Scholar]
- 41.Ham SA. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis & Rheumatism (Arthritis Care & Research). 2003;49(1):129–35. [DOI] [PubMed] [Google Scholar]
- 42.Campbell R, Evans M, Tucker M, Quilty B, Dieppe P, Donovan J. Why don’t patients do their exercises? Understanding non-compliance with physiotherapy in patients with osteoarthritis of the knee. Journal of Epidemiology & Community Health. 2001;55(2):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Focht BC, Rejeski WJ, Ambrosius WT, Katula JA, Messier SP. Exercise, self-efficacy, and mobility performance in overweight and obese older adults with knee osteoarthritis. Arthritis Care & Research. 2005;53(5):659–65. [DOI] [PubMed] [Google Scholar]
- 44.Ling SM, Fried LP, Garrett ES, Fan M-Y, Rantanen T, Bathon JM. Knee osteoarthritis compromises early mobility function: The Women’s Health and Aging Study II. The Journal of rheumatology. 2003;30(1):114–20. [PubMed] [Google Scholar]
- 45.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report. 2018;67(36):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Annals of the rheumatic diseases. 1997;56(11):641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauchard GC, Vançon G, Meyer P, Mainard D, Perrin PP. On the role of knee joint in balance control and postural strategies: Effects of total knee replacement in elderly subjects with knee osteoarthritis. Gait & Posture. 2010;32(2):155–60. [DOI] [PubMed] [Google Scholar]
- 48.Hafez AR, Alenazi AM, Kachanathu SJ, Alroumi A, Mohamed E. Knee osteoarthritis: a review of literature. Phys Med Rehabil Int. 2014;1(5):8. [Google Scholar]
- 49.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. Bmj. 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo JJ, Cho NH, Lim SH, Kim HA. Relationships between body mass index, fat mass, muscle mass, and musculoskeletal pain in community residents. Arthritis & Rheumatology. 2014;66(12):3511–20. [DOI] [PubMed] [Google Scholar]
- 51.Ayoub S, Berbéri A, Fayyad-Kazan M. Cytokines, Masticatory Muscle Inflammation, and Pain: an Update. Journal of Molecular Neuroscience. 2020;70(5):790–5. [DOI] [PubMed] [Google Scholar]
- 52.Cruz-Almeida Y, Rosso A, Marcum Z, Harris T, Newman AB, Nevitt M, et al. Associations of musculoskeletal pain with mobility in older adults: potential cerebral mechanisms. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2017;72(9):1270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, et al. Aging, the Central Nervous System, and Mobility. The Journals of Gerontology: Series A. 2013;68(11):1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart KJ. Physical activity and aging. Annals of the New York Academy of Sciences. 2005; 1055(1):193–206. [DOI] [PubMed] [Google Scholar]
- 55.O’Donovan G, Lee I-M, Hamer M, Stamatakis E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA internal medicine. 2017;177(3):335–42. [DOI] [PubMed] [Google Scholar]
- 56.Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity–a systematic review of longitudinal studies. BMC public health. 2013;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Löllgen H, Böckenhoff A, Knapp G. Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. International journal of sports medicine. 2009;30(03):213–24. [DOI] [PubMed] [Google Scholar]
- 58.Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, Van Someren KA, et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Scientific reports. 2018;8(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piastra G, Perasso L, Lucarini S, Monacelli F, Bisio A, Ferrando V, et al. Effects of two types of 9-month adapted physical activity program on muscle mass, muscle strength, and balance in moderate sarcopenic older women. BioMed research international. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—the MINOS study. Journal of Bone and Mineral Research. 2005;20(5):721–9. [DOI] [PubMed] [Google Scholar]
- 61.Sakai Y, Matsui H, Ito S, Hida T, Ito K, Koshimizu H, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporosis and sarcopenia. 2017;3(4):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbunt JA, Seelen HA, Vlaeyen JW, van de Heijden GJ, Heuts PH, Pons K, et al. Disuse and deconditioning in chronic low back pain: concepts and hypotheses on contributing mechanisms. European journal of pain. 2003;7(1):9–21. [DOI] [PubMed] [Google Scholar]
- 63.Trajanoska K, Seppala LJ, Medina-Gomez C, Hsu Y-H, Zhou S, van Schoor NM, et al. Genetic basis of falling risk susceptibility in the UK Biobank Study. Communications biology. 2020;3(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


