Abstract
The 2016 World Health Organization classification of prostate cancer with neuroendocrine (NE) differentiation includes NE cells in usual prostate cancer, adenocarcinoma with Paneth cell-like NE differentiation, well-differentiated NE tumor (carcinoid), small cell NE carcinoma, and large cell NE carcinoma. In this article, we report a rare case of primary prostatic carcinoma with de novo diffuse NE differentiation presenting with bilateral hydronephrosis in a 79-year-old man. This case did not fit into any of the existing classifications. The clinical, radiological, morphological, and immunohistochemical findings and response to androgen deprivation therapy (ADT) are presented. The proposed pathogenesis of NE differentiation via transdifferentiation from conventional prostatic adenocarcinoma whereby genomic alterations, coupled with ADT can induce lineage plasticity resulting in NE differentiation is described.
Keywords: prostate carcinoma, neuroendocrine differentiation, amphicrine carcinoma, androgen deprivation therapy, transdifferentiation
Introduction
The 2016 World Health Organization (WHO) classification of prostate cancer with neuroendocrine (NE) differentiation includes NE cells in usual prostate cancer, adenocarcinoma with Paneth cell-like NE differentiation, well-differentiated NE tumor (carcinoid), small cell NE carcinoma (SmCC) and large cell NE carcinoma (LCNEC).1 We describe a case of high-grade prostatic carcinoma that proved diagnostically challenging in terms of classification into one of these categories.
Case Report
A 79-year-old male was referred to a urologist with bilateral hydronephrosis. A computerized tomography (CT) scan revealed a large tumor involving the prostate and bladder trigone with obstruction of both ureteric orifices (Figure 1). The patient did not have any relevant past medical history and preoperative serum prostate-specific antigen (PSA) level was 21.3 μg/L. Cystoscopy revealed a large tumor involving the prostate and bladder trigone with obstruction of both ureteric orifices. Transurethral resection of the prostate and bladder was performed followed by bilateral ureteric stent insertion. The separately submitted prostate and bladder tissue showed similar morphological features, including a high-grade tumor with solid architecture (Figure 2A) and focal gland formation (Figure 2B), the latter involving ~10% of the tumor. Rosette-like areas were present (Figure 2C). The cells showed a range of morphology (Figure 2D and E) with moderate nuclear pleomorphism, one to several conspicuous nucleoli, vesicular to fine granular chromatin, and ample amphophilic to regions with minimal cytoplasm. Mitotic activity was brisk, up to 17 mitoses per 10 high-power fields (Figure 2F). No tumor necrosis was present. Immunohistochemically, the tumor cells were strongly and diffusely positive for prostatic markers including NK3 homeobox 1 (NKX3.1), prostate-specific membrane antigen (PSMA), androgen receptor (AR), and ets-related gene (ERG). PSA and prostatic-specific acid phosphatase (PSAP) displayed patchy positivity. The tumor co-expressed NE markers including synaptophysin, which was strongly positive in up to 80% of cells, and chromogranin A, which showed non-focal positivity in up to 40% of cells. The Ki-67 index was up to 80% (Figure 3).
Figure 1.
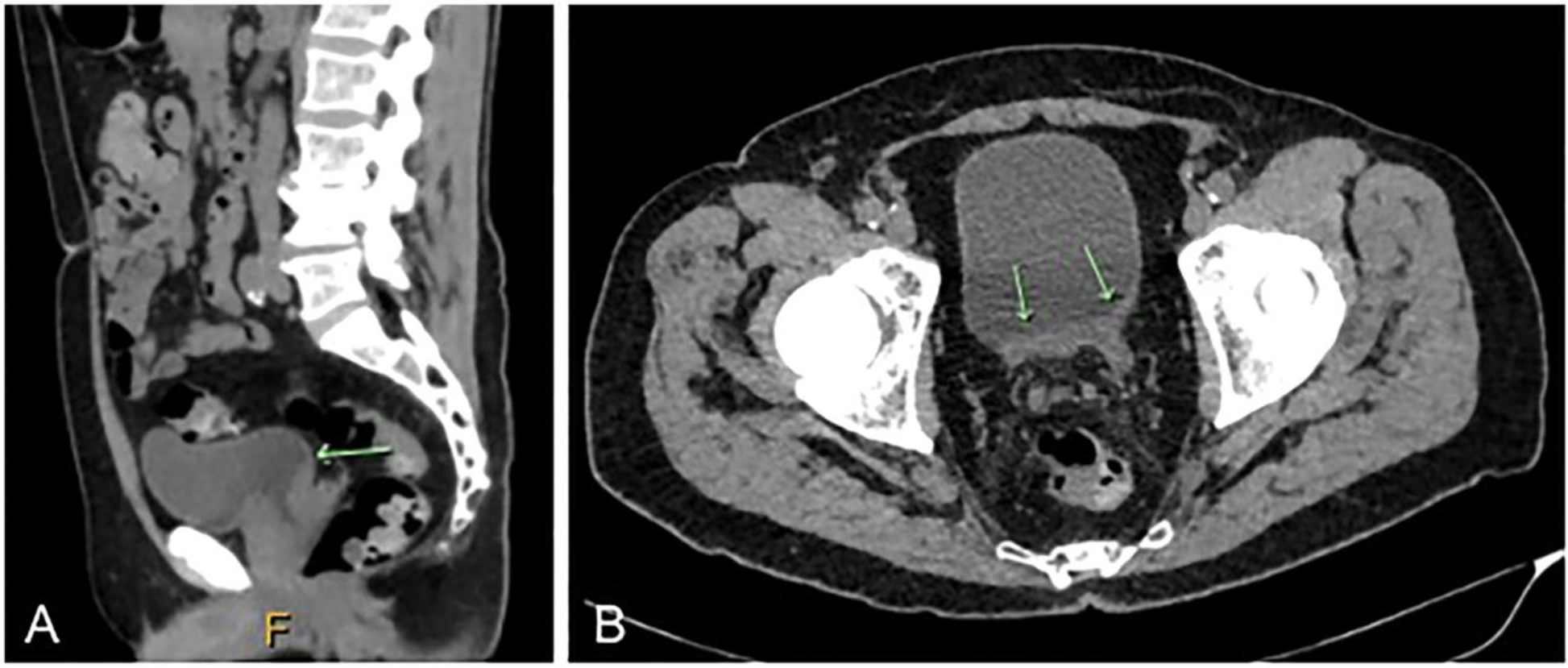
Computerized tomography (CT) scan showing tumor involving the prostate and bladder trigone. (A) Sagittal plane (arrow) and (B) axial plane (arrows).
Figure 2.
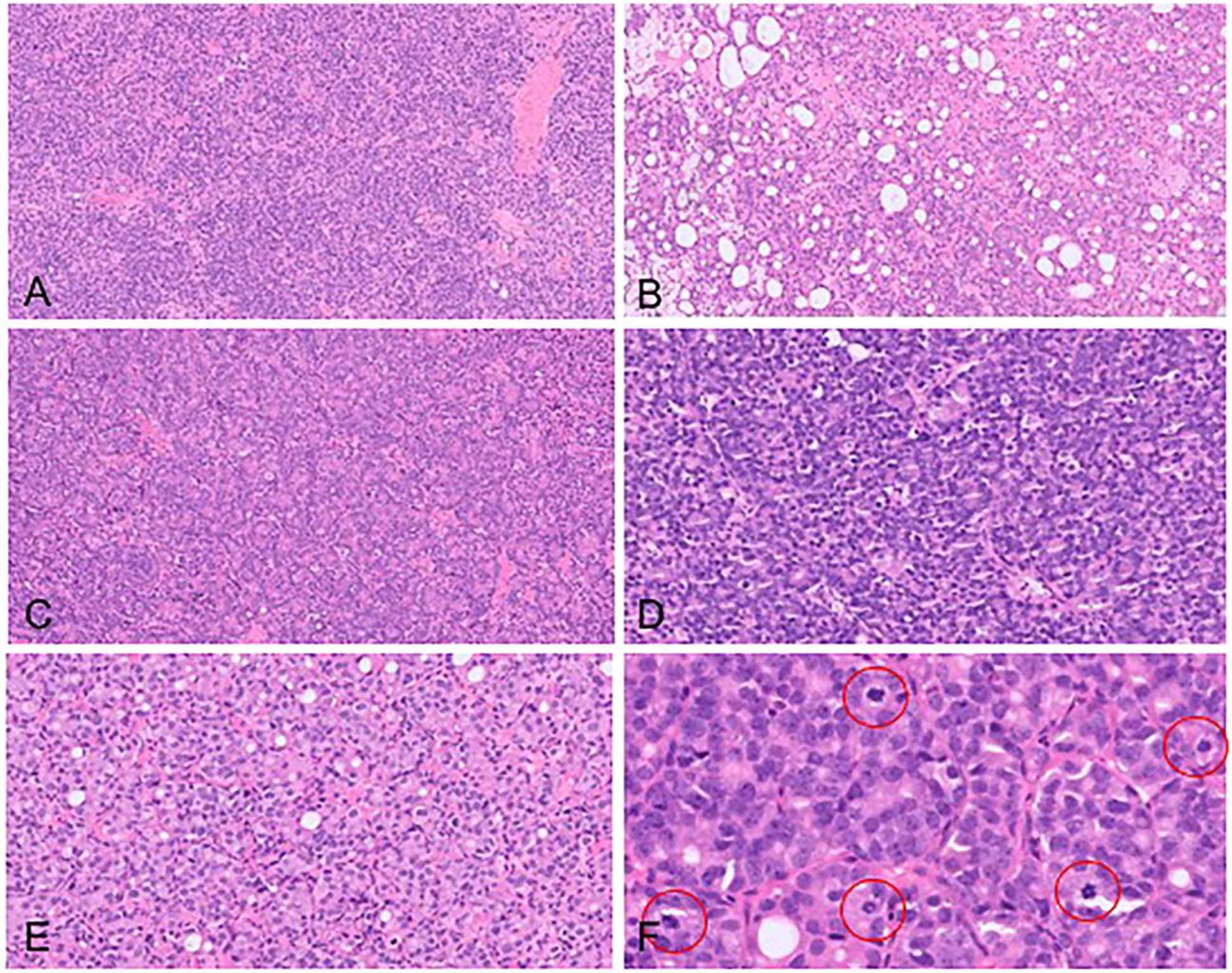
Morphological features. (A) Solid architecture, (B) focal gland formation, (C) rosette-like features, (D) cells with high nuclear to cytoplasmic ratios and limited cytoplasm, (E) cells with moderate amphophilic to eosinophilic cytoplasm, and (F) brisk mitotic activity (red circles showing mitoses).
Figure 3.
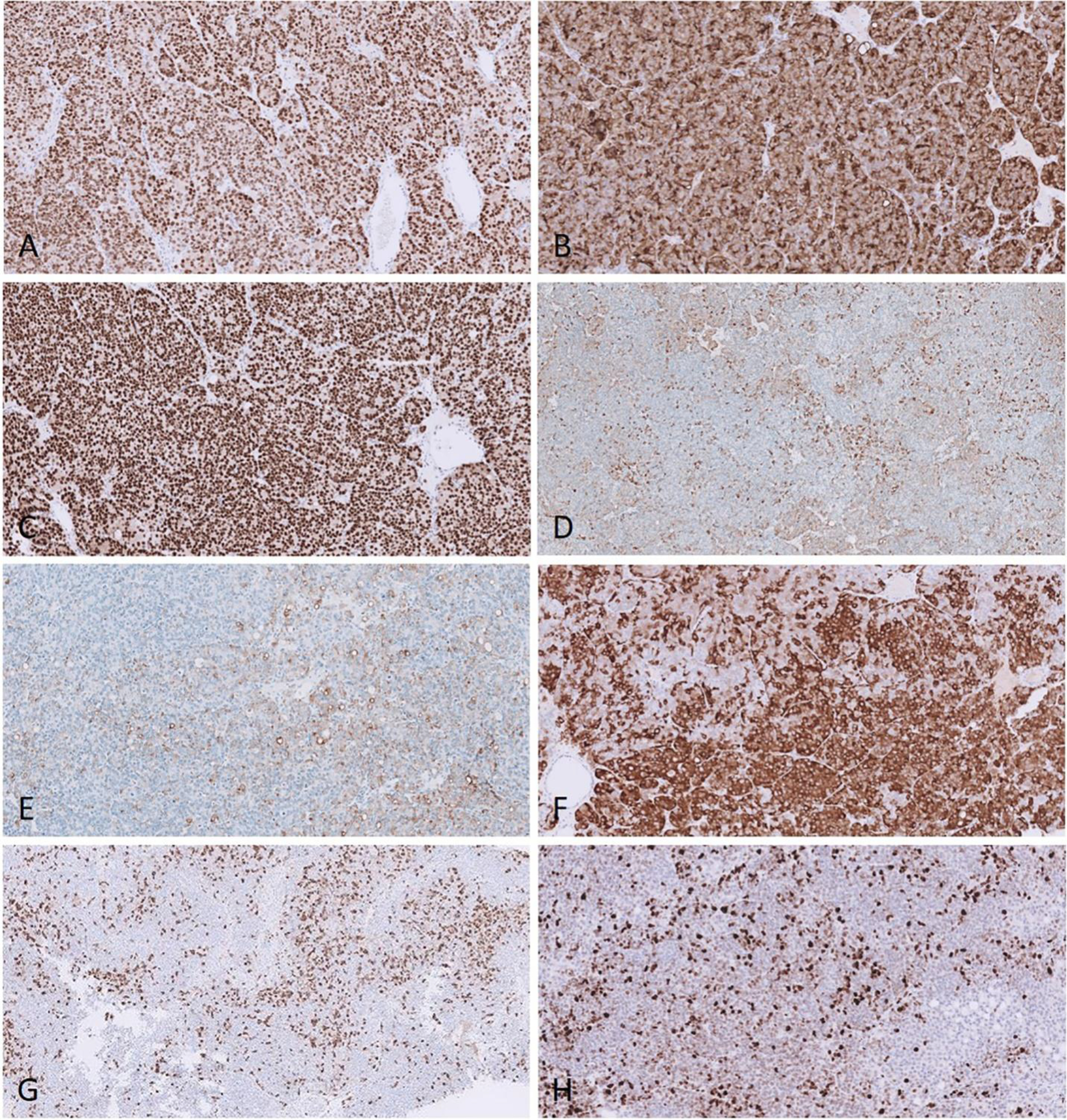
Immunohistochemical features. (A) NKX3.1, (B) PSMA, (C) AR, (D) PSA, (E) PSAP, (F) synaptophysin, (G) chromogranin A, and (H) Ki-67. Abbreviations: NKX3.1, NK3 homeobox 1; AR, androgen receptor; PSMA, prostate-specific membrane antigen; PSA, prostate-specific antigen; PSAP, prostatic-specific acid phosphatase.
Though the overall features were of a high-grade prostatic carcinoma with NE differentiation, features typical of SmCC, including lack of prominent nucleoli, nuclear molding, crush artifact, and necrosis were not present. Peripheral palisading and geographic necrosis described in the exceptionally rare LCNEC were similarly lacking. In addition, typical reduction or lack of expression of prostatic exocrine markers such as PSA, PSMA, and AR2,3 seen in SmCC and LCNEC, contrasted with the strong, diffuse positivity for similar markers in our case. Given that the tumor did not fit into the WHO-endorsed categories, a diagnosis of prostatic carcinoma Grade Group 5 (Gleason score 5+4=9) with NE differentiation was rendered, with an indication to the urologist that the tumor did not show features of SmCC/LCNEC. A subsequent bone scan showed sclerotic metastases to the left fifth rib and L4 vertebra. A CT scan of the chest, abdomen, and pelvis did not show any appreciable visceral or lymph node metastases. The patient was commenced on androgen deprivation therapy (ADT). At 7 months follow-up, the PSA dropped to 0.127 μg/L and he remains alive with stable disease.
Discussion
This case shows similar features to a series of 5 cases published by Prendeville et al,4 which they termed prostate carcinoma with amphicrine features. Amphicrine carcinoma refers to a rare distinct type of carcinoma characterized by synchronous exocrine and endocrine differentiation within the same tumor cells.5 These tumors have been described in several anatomical locations, particularly within the gastrointestinal tract.6 Such tumors are not included in the current classification of the NE tumors of the prostate. As in our case, the 5 cases described showed similar high-grade morphology with immunohistochemical evidence of dual exocrine (AR, PSA, and PSAP positive) and NE endocrine differentiation (synaptophysin and chromogranin A positive), though no gland formation was identified. Two of the patients were diagnosed with de novo amphicrine carcinoma on their primary diagnostic biopsies and were hormone naive. Each had metastatic disease to bone and lymph nodes, respectively, at presentation.4 Wu et al7 also reported a case of an aggressive prostatic “hybrid carcinoma” composed entirely of dual exocrine and NE differentiation. The term “prostate cancer with overlapping features of small cell and acinar adenocarcinoma” has also been proposed in tumors where there is a morphologic concern for NE carcinoma with sheet-like architecture and scant cytoplasm, but in which the morphological features, particularly the cytology, are not typical of either small cell carcinoma or conventional carcinoma.8 In a report reflecting the proceedings of the United States & Canadian Academy of Pathology Prostate Long Course, Fine proposed reporting these tumors as “prostatic carcinoma with diffuse NE differentiation” with a descriptive note to separate them from well-established categories of high-grade NE carcinoma.9
In the advanced prostate cancer setting, a NE phenotype is thought to arise via trans-differentiation whereby genomic alterations coupled with ADT-induced and environmental factors can induce lineage plasticity resulting in NE differentiation. The extent of NE differentiation and the degree of loss of exocrine differentiation determine the spectrum of NE manifestations ranging from prostatic adenocarcinoma with focal divergent NE differentiation to high-grade NE carcinoma.10,11
Prostate carcinomas with amphicrine features are thought to remain AR-driven and to lie somewhere along the axis of this lineage plasticity pathway. This is supported by the fact that our case, as well as the cases mentioned above, showed a good clinical response to ADT. Additionally, the distribution of metastases (bone and lymph nodes) and type of metastases (sclerotic) suggest that amphicrine carcinomas are still androgen driven, rather than androgen independent as occurs in high-grade NE carcinoma (SmCC/LCNEC) in which visceral and/or lytic bone metastases are more typical.
Routine application of NE marker immunohistochemistry is not recommended in otherwise typical acinar adenocarcinoma.1 Our case, and those in prior publications4,7, showed nuclear pleomorphism and markedly increased mitotic figures, features not typically observed in conventional prostatic carcinoma. Such cases may have been classified as high-grade conventional prostatic adenocarcinoma or as other established prostatic NE categories in the past.9,10 Judicious use of immunohistochemistry has been suggested in such aggressive, highly proliferative prostatic carcinomas.4,10
Our case presented de novo with no prior history of ADT for prostate cancer. However, given that NE differentiation is in part ADT-induced, it is possible that in already diagnosed prostate cancer patients who live longer with more potent ADT and/or increased biopsy rate in the castration-resistant prostate cancer setting, pathologists will encounter prostate carcinoma with NE differentiation more often.8 Better categorization of such challenging lesions may assist in better characterization in terms of prognosis, appropriateness of grading, molecular correlation, and optimal management.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
Ethics approval was not required as this is a case report.
Informed Consent
Written informed was obtained from the patient.
Trial Registration
Not applicable, because this article does not contain any clinical trials.
References
- 1.Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. IARC Press; 2016. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65–71. [DOI] [PubMed] [Google Scholar]
- 3.Evans AJ, Humphrey PA, Belani J, et al. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol. 2006;30:684–693. [DOI] [PubMed] [Google Scholar]
- 4.Prendeville S, Al-Bozom I, Compérat E, et al. Prostate carcinoma with amphicrine features: further refining the spectrum of neuroendocrine differentiation in tumours of primary prostatic origin? Histopathology. 2017;71:926–933. [DOI] [PubMed] [Google Scholar]
- 5.Lewin K Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11:71–86. [DOI] [PubMed] [Google Scholar]
- 6.Huang D, Ren F, Ni S, et al. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int. 2019;19:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Wyatt AW, Lapuk AV, et al. Integrated genome and transcriptome sequencing identifies a novel form of hybrid and aggressive prostate cancer. J Pathol. 2012;227:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine SW. Neuroendocrine tumors of the prostate. Mod Pathol. 2018;31:S122–S132. [DOI] [PubMed] [Google Scholar]
- 10.Bellur S, Van der Kwast T, Mete O. Evolving concepts in prostatic neuroendocrine manifestations: from focal divergent differentiation to amphicrine carcinoma. Hum Pathol. 2019;85:313–327. [DOI] [PubMed] [Google Scholar]
- 11.Beltran H, Hruszkewycz A, Scher HI, et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin Cancer Res. 2019;25:6916–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]


