Abstract
Itch sensation provokes the scratch reflex to protect us from harmful stimuli in the skin. Although scratching transiently relieves acute itch through activation of mechanoreceptors, it propagates the vicious itch-scratch cycle in chronic itch by further aggravating itch over time. Although well recognized clinically, the peripheral mechanisms underlying the itch-scratch cycle remain poorly understood. Here we show that mechanical stimulation of the skin results in activation of the Piezo2 channels on Merkel cells that pathologically promotes spontaneous itch in experimental dry skin. Three-dimensional reconstruction and immunoelectron microscopy revealed structural alteration of MRGPRA3+ pruriceptor nerve endings directed towards Merkel cells in the setting of dry skin. Our results uncover a functional miswiring mechanism under pathologic conditions, resulting in touch receptors triggering the firing of pruriceptors in the skin to drive the itch-scratch cycle.
One Sentence Summary:
A pathologic miswiring in the epithelial touch receptor results in adaptive activation of sensory pruriceptor to propagate the itch-scratch cycle.
INTRODUCTION
Itching is the most common symptom arising from the skin. However, due to poor understanding of its underlying mechanisms, effective treatments for chronic pruritus are lacking. Recent studies have begun to elucidate the functions of several classes of ion channels and G-protein coupled receptors (GPCRs) in mediating pruritogen-induced itch. For instance, various transient receptor potential (TRP) channels (1-5), Mas-related G protein-coupled receptors (MRGPRS) in primary sensory neurons (6-9), and receptors for gastrin-releasing peptide (GRP) and natriuretic polypeptide b (NPPB) in the spinal cord (10, 11), have emerged as critical components in promoting itch transduction, supporting the existence of itch-specific neuronal pathways.
Mechanical stimuli, such as touch and scratch, could transiently suppress itch in both healthy subjects and individuals with chronic itch (12, 13). Paradoxically, persistent scratching itself can trigger the release of a variety of proinflammatory mediators and aggravate itch sensation (known as the itch-scratch cycle) (14, 15). Whether skin cells participate in the switch from inhibition to activation of pruriceptive fibers under pathological conditions are not understood.
In the current study, we demonstrate that cutaneous Piezo2 activation in Merkel cells is critically involved in the generation of spontaneous itch in mice with experimental dry skin. Optogenetic activation of Merkel cells, rather than triggering the slowly-adapting type I (SAI) afferent firing that contributes to touch sensation, resulted in enhanced activation of pruriceptive C fibers and spontaneous scratching behavior in the setting of dry skin. Moreover, we detected structural alteration of MRGPRA3+ pruriceptor nerve endings directed towards Merkel cells, allowing for a pathologic touch receptor-pruriceptor circuit that promotes scratch-induced itch in mice. Collectively, our study unveils a miswiring mechanism between peripheral epithelia and sensory neurons, resulting in “short circuiting” of the mechanosensory Merkel cells with itch-producing MRGPRA3+ pruriceptors to promote the vicious itch-scratch cycle.
RESULTS
Merkel cell deficiency impairs C fiber firings and alleviates chronic itch
Cutaneous Piezo2 signaling in Merkel cells has been shown to critically contribute to the initiation of touch sensation (16, 17) and to the inhibition of mechanical itch under aged and dry skin conditions, by driving the activation of downstream slowly adapting type I (SAІ) Aβ fibers (18). To test if Merkel cells are also required to suppress other forms of itch, we employed a well-established dry skin associated chronic itch model by treating mice with acetone-ether-water (AEW) (19). The number of scratching bouts (20), as well as the total scratching time (21), were substantially reduced in Merkel cell-deficient (K14Cre; Atoh1f/f) mice when compared to littermate control mice subjected to AEW treatment starting after four days of AEW administration (Fig. 1A and fig. S1). Therefore, Merkel cells, rather than suppressing mechanical itch, were required for the induction of dry skin-induced spontaneous itch in this mouse model.
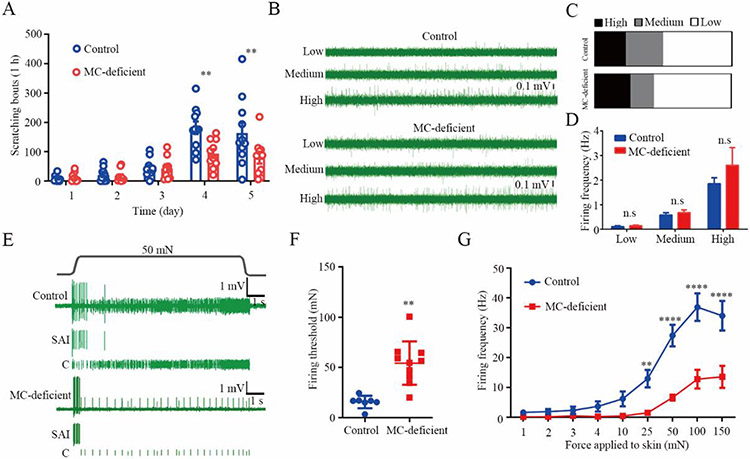
Fig 1. Correlation of Merkel cell-dependent mechanically activated C fiber firings and persistent scratching behavior in mice subjected to experimental dry skin.
(A) Time course of persistent scratching behavior in AEW-treated Merkel cell-deficient mice (n=10) and littermate controls (n=11). (B) Representative traces of spontaneous C fiber firings with different firing rates from littermate controls and Merkel cell-deficient mice. (C-D) Proportions (C) and firing frequencies (D) of three types of spontaneous C fiber firings. n=22 and 23 units from the littermate controls and Merkel cell-deficient group, respectively. The proportions of spontaneous C fibers with high, medium and low frequencies were 22.73%, 27.27%, 50.00% in the littermate controls and 26.09%, 17.39%, 56.52% in the Merkel cell-deficient group. (E) Representative traces showing both SAI and C fiber firings in response to 50 mN force applied to the touch dome area in a littermate control (top) and a Merkel cell-deficient mouse (bottom). (F-G) Summarized data of the Merkel cell-dependent mechanically activated C fiber firing thresholds (F) and firing frequencies (G) in both Merkel cell-deficient and littermate control groups. n=7 and 10 units from littermate controls and Merkel cell–deficient mice, respectively. **P<0.01, ****P<0.0001, two-way RM ANOVA with Bonferroni post hoc analysis in (A and G), Student t test in (D and F). n.s, not significant. MC-deficient, Merkel cell deficient mice.
Sensory nerves are generally classified by their diameter, conduction velocity, and extent of myelination. Whereas large-diameter myelinated Aβ fibers are known to mediate innocuous touch sensation, small-diameter unmyelinated C fibers primarily mediate nociceptive pain and itch sensations. We thus analyzed the firing properties of these nerve fibers in skin-nerve preparations (preps) (22) under normal and AEW conditions. Although the firing of Aβ fibers in Merkel cell-deficient mice was reduced under normal conditions as expected (16, 17), there was no difference in the firing frequency of Aβ fibers between Merkel cell-deficient mice and littermate controls subjected to experimental dry skin (fig. S2A). Thus, we hypothesized that, C fiber dysregulation might play a role in the setting of pathologic dry skin itch.
We then examined the firing properties of C fibers in the dry skin preps and hypothesized that the spontaneous C fiber firing induced by AEW treatment would correlate with chronic itch behaviors, based on previous studies showing a similar correlation between spontaneous C-fiber firing and ongoing pain behaviors (23-26). We observed three distinct spontaneous firing patterns associated with low, medium, and high firing rates, respectively (Fig. 1B). However, the proportions of spontaneous C fibers with high, medium and low frequencies were 22.73%, 27.27%, 50.00% in the littermate controls, which were comparable with that of 26.09%, 17.39%, 56.52% in the Merkel cell-deficient group (Fig. 1C). Moreover, the firing frequencies of all three types of spontaneous C fiber firings were altered in the setting of Merkel cell deficiency compared with the presence of Merkel cells (Fig. 1D). We thus hypothesized that mechanical stimulation may unveil a role for Merkel cells on C fibers. To test this, we subjected skin-nerve preps from AEW-treated mice in both the presence and absence of Merkel cells to a range of mechanical forces (1 to 150 mN) applied to the Merkel cell-enriched touch dome area. Mechanical activation of Merkel cells promoted C fiber firing in the AEW-treated mice (Fig. 1E). In contrast, the same series of mechanical forces rarely evoked C fiber firing in vehicle-treated control mice independent of the presence of Merkel cells (figs. S2B, C). Although the C fiber conduction velocity was comparable between the Merkel cell-deficient mice and their littermate controls in the steady state (fig. S2D), in the dry skin state, there was an increase in firing frequency, and decrease in firing threshold, of the Merkel cell-dependent mechanically activated C fiber firings. This enhanced activity was markedly reduced in Merkel cell-deficient mice when compared with littermate controls (Figs. 1F, G). Collectively, these data suggest that where Merkel cells critically mediate homeostatic touch sensation via Aβ mechanoreceptors in the healthy state, they drive pathologic firing of pruriceptive C fibers under dry skin conditions. Based on these findings, we hypothesized that Merkel cells may be diverted to selectively interact with pruriceptors in the setting of dry skin.
Mechanosensitive Piezo2 channel is required for Merkel cell-dependent mechanically activated C fiber firing
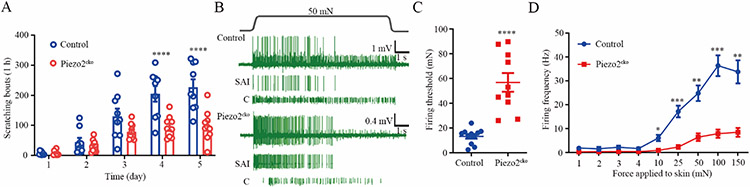
To determine the molecular basis underlying Merkel cell-driven C-fiber firing in the setting of experimental dry skin, we selectively knocked out the mechanosensitive Piezo2 channel from Merkel cells by crossing the Atoh1CreERT mice with Piezo2f/f mice. Consistent with the results from Merkel cell-deficient mice, the Merkel cell-specific Piezo2 knockout mice recapitulated the results on the reduction in persistent scratching bouts (Fig. 2A) and the total scratching time (fig. S3), decreased firing frequency and increased firing threshold of the Merkel cell-dependent C fiber firings (Figs. 2B-2D), without affecting the three types of spontaneous C fiber proportions, which were 8.10%, 27.00%, 64.90% in littermate control group and 11.90%, 26.20%, 61.90% in Merkel cell-specific Piezo2 deficient group (figs. S4A-C). Moreover, firing frequencies and conduction velocity of the spontaneous C fiber firings were comparable between the littermate controls and the Merkel cell-specific Piezo2 knockout mice subjected to AEW treatment (figs. S4D-E). Similar to Merkel cell-deficient mice, Piezo2 deficiency in Merkel cells did not cause further reduction in SAI Aβ firing when compared with their littermate controls after AEW treatment (fig. S4F). These studies confirm that the Piezo2-mediated signaling in Merkel cells promotes spontaneous itch in the pathologic dry skin state in addition to mediating homeostatic mechanosensation.
Fig 2. Merkel cell-expressed Piezo2 is required for the generation of AEW-induced persistent itch.
(A) Time course of persistent scratching behavior in both littermate controls (n=9) and Merkel cell-specific Piezo2 deficient (Piezo2cko) mice (n=10) treated with AEW. (B) Representative action potential firing traces for both SAI and C fiber in response to 50 mN force applied to the touch dome area in littermate controls and Merkel cell–specific Piezo2 deficient mice. (C-D) Summarized data of the Merkel cell-dependent mechanically activated C fiber firing threshold (C) and firing frequency (D). n=11 and 10 units from littermate controls and Merkel cell-specific Piezo2 deficient mice, respectively. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, two-way RM ANOVA with Bonferroni post hoc analysis in (A) and (D); Student t test in (C).
Chemogenetic activation of Merkel cells is sufficient to promote spontaneous itch behavior in dry skin mice
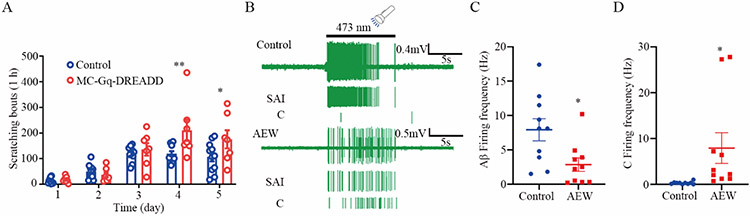
Upon mechanical stimulation, Merkel cells release numerous neurotransmitters and neurotrophic factors that activate and/or sensitize the postsynaptic primary afferents through either direct synaptic activation or paracrine signaling (27-29). To investigate whether activation of Merkel cells is sufficient to regulate the function of the touch dome-innervating pruriceptive fibers during chronic itch, we first generated the Merkel cell-Gq-DREADD (designer receptors exclusively activated by designer drugs)-expressing (Atoh1CreERT; Gq-DREADD) mice. After tamoxifen induction of Cre expression, chemogenetic activation of Merkel cells in these mice with the DREADD ligand clozapine-n-oxide (CNO) increased the number of the AEW-induced scratching bouts when compared with their littermate controls starting after 4 days of AEW administration (Fig. 3A). We also used an optogenetic model of Merkel cell activation. Activation of Merkel cells in the skin-nerve preps from AEW-treated mice expressing the excitatory channelrhodopsin-2 (ChR2) in Merkel cells (Atoh1CreERT; Ai32) using blue light illumination generated a canonical Aβ firing pattern, validating the expression of ChR2 in Merkel cells and confirming the action potential firing in Aβ fibers but not C fibers under normal conditions by Merkel cell stimulation (Fig. 3B). Consistent with a loss of Merkel cells in the AEW-treated skin preps (18, 30, 31), blue light illumination elicited reduced Aβ firings in the AEW-treated Merkel cell-ChR2 mice when compared with the vehicle-treated group in the skin-nerve preps (Fig. 3B and 3C). A robust C fiber firing was observed upon blue light stimulation directed to the touch tome area in the AEW-treated Merkel cell-ChR2 mice (Figs. 3B and 3D), suggesting that Merkel cells develop a functional connection with C fibers in the setting of chronic itch, that is not observed under normal conditions.
Fig 3. Stimulation of Merkel cells promotes persistent scratching and C fiber firing in chronic itch mice.
(A) Time course of persistent scratching behavior in response to 1 mM CNO in the AEW-treated control mice (n=11) and Merkel cell-Gq DREADD mice (n=7). CNO was applied through intraperitoneal injections. (B) Representative SAI Aβ fiber firing and C fiber firing in vehicle-treated (upper) and AEW-treated (lower) back skin-nerve preps from the Merkel cell-ChR2 mice in response to blue light illumination (3 mW, 10 s). (C-D) Statistical data of blue light-evoked Aβ fiber firing frequencies (C) and C fiber firing frequencies (D) in the water-treated (n=10 units) and AEW-treated (n=10 units) skin-nerve preps. *P<0.05, **P<0.01, ***P<0.001 two-way RM ANOVA with Bonferroni post hoc analysis in (A), Student t test in (C and D).
MRGPRA3+ C pruriceptors mediate Merkel cell-dependent spontaneous scratching
The transient receptor potential vanilloid 1-expressing (TRPV1+) sensory neurons contain the majority of the C pruriceptor populations (32-34). To test if the TRPV1+ C fibers are downstream mediators of the Merkel cell-dependent spontaneous itch in the experimental dry skin model, we chemically ablated TRPV1+ sensory nerves using the TRPV1-selective neurotoxin resiniferatoxin (RTX). After validation of the chemical ablation (fig. S5A), both RTX-treated and vehicle-treated mice were subjected to experimental dry skin before ex vivo skin-nerve recordings. RTX treatment nearly abolished not only the AEW-induced persistent itch (fig. S5B) but also the spontaneous C fiber firings of medium and high frequencies as the proportions of spontaneous C fiber firings with high, medium and low frequencies was 23.08%, 30.77%, 46.15% in the vehicle-treated group and 0%, 10.00%, 90.00% in the RTX-treated group (fig. S5C). Moreover, both the spontaneous C fiber firing frequencies (fig. S5D) and Merkel cell-dependent mechanically activated C fiber firing frequencies (fig. S5E) were diminished. Although the conduction velocity was not affected, the C fiber firing threshold was increased in the RTX-treated group when compared with that in the vehicle-treated group (fig. S5F-G). Taken together, these results suggest that TRPV1+ neurons are a broad population covering both types of C fibers.
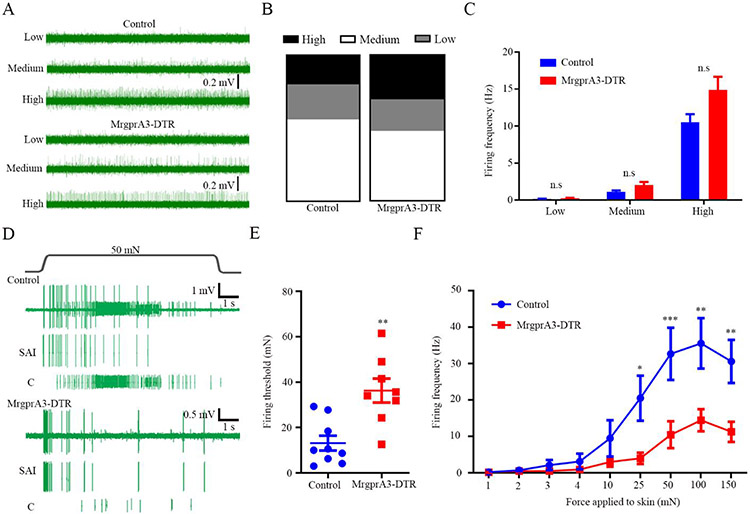
Skin-innervating MRGPRA3-expressing (MRGPRA3+) nerve fibers, which are not overlapped with Merkel cell-innervating NF200+ nerve fibers (fig. S6), comprise a small subset of the TRPV1+ population and mediate dry skin itch (7). To determine whether MRGPRA3+ C pruriceptors are downstream responders of the Merkel cell-dependent itch signaling in AEW-treated mice, we ablated MRGPRA3+ fibers using DTX in the MrgprA3-DTR (MrgprA3GFP-Cre; ROSA26DTR) mice. As expected, ablation of MRGPRA3+ fibers reduced AEW-induced spontaneous itch (fig. S7A). In ex vivo skin-nerve recordings, loss of MRGPRA3+ fibers didn’t affect the firing properties of the Aβ fibers (fig. S7B). Moreover, the proportions of spontaneous C fibers with high, medium and low frequencies were 20.00%, 24.00%, 56.00% in the control group, which are comparable with 30.43%, 21.74%, 47.83% in the MrgprA3-DTR group (Figs. 4A and 4B). Meanwhile, both the MRGPRA3+ neuron-ablated mice and their littermate controls displayed comparable firing frequencies of the spontaneous C fiber firings induced by AEW treatment (Figs. 4A and 4C), suggesting that MRGPRA3+ pruriceptors are not involved in the generation of the spontaneous C fiber firings. However, upon mechanical stimulation of the touch dome area in the AEW-treated skin, whereas the conduction velocity was unaffected (fig. S7C), the AEW-induced increase in firing frequency and reduction in firing threshold of the mechanically activated C fiber firings were substantially attenuated in the MRGPRA3-ablated mice compared to their littermate controls (Figs. 4D-4F). These findings recapitulate the firing properties of the spontaneous C fiber firings and Merkel cell-dependent mechanically activated C fiber firings observed in the Merkel cell-deficient mice, suggesting a Merkel cell-MRGPRA3+ pruriceptor axis resides at the center of this scratch-induced itch phenotype. RTX-treated mice as well as the MRGPRA3+ neuron-ablation mice displayed more Merkel cells per touch dome when compared with their littermate controls after AEW treatment (fig. S8), suggesting that ablation of MRGPRA3+/TRPV1+ fibers may protect the loss of Merkel cells and a bi-directional communication between Merkel cells and innervating pruriceptive C fibers in the setting of experimental dry skin-associated chronic itch.
Fig 4. MrgprA3+ fibers mediate Merkel cell-dependent mechanically activated C fiber firings induced by mechanical stimulation in the dry skin mice.
(A) Representative spontaneous C fiber firing traces with different firing rates from the control (upper) and MrgprA3-DTR mice (lower), respectively. Proportions (B) and firing frequencies (C) of three types of spontaneous C fiber firings in DTX treated MrgprA3-DTR mice and littermate controls. n=25 and 23 units for the control and MrgprA3-DTR group, respectively. The proportions of spontaneous C fibers with high, medium and low frequencies were 20.00%, 24.00%, 56.00% in the control group and 30.43%, 21.74%, 47.83% in the MrgprA3-DTR group. (D) Representative action potential firing traces from the DTX-treated control and MrgprA3-DTR mice subjected to AEW treatment in response to 50 mN force to the receptive fields. (E-F) Summarized data of the Merkel cell-dependent mechanically activated C fiber firing threshold (E) and firing frequency (F). n=9 and 8 units for the control and MrgprA3-DTR mice, respectively. *P<0.05, **P<0.01, ***P<0.001, n.s, not significant. Student t test in (C and E), two-way RM ANOVA with Bonferroni post hoc analysis in (F).
Structural basis of Merkel cell-MRGPRA3+ C pruriceptor interaction
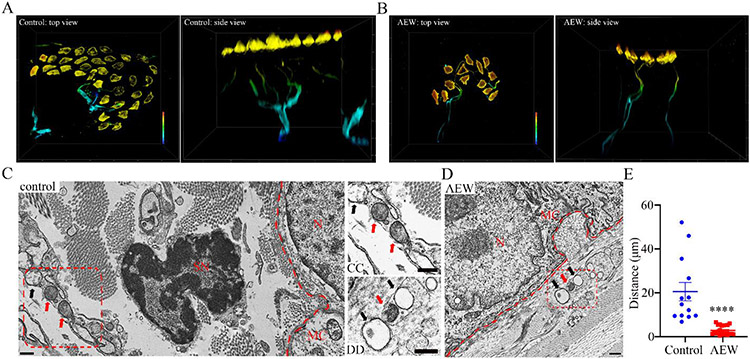
To visualize Merkel cell-MRGPRA3+ nerve ending interactions, we performed super-resolution microscopy with three-dimensional (3D) reconstruction and found that Merkel cells closely approximated NF200+ Aβ sensory nerve endings (figs. S9A and S9B and Movie. S1). On the other hand, we observed that MRGPRA3+ fibers densely innervating the inner dermal layer under all touch dome structures, although the nerve endings are not associated with Merkel cells under physiological (vehicle-treated) conditions (Fig. 5A and Movie. S2). AEW treatment induced an elongation of the MRGPRA3+ fibers towards the Merkel cell-enriched touch dome (Fig. 5B, fig. S9C and Movie. S3). Transmission electron microscopy (TEM) revealed that the postsynaptic structure remained intact as the distances between Merkel cells and Aβ sensory axons were comparable between the vehicle-treated and AEW-treated groups (figs. S9D-S9F). By using a validated polyclonal tdTomato antibody (fig. S9G) we showed that MRGPRA3+ axons emerged in the postsynaptic Aβ fiber bundles located ~2.8 micrometers from Merkel cells in the MrgprA3-tdTomato reporter mice subjected to experimental dry skin (Figs. 5D, E). In marked contrast, MRGPRA3+ axons were ~20.5 micrometers away from the border of epidermal layer where Merkel cells are located in the vehicle-treated MrgprA3-tdTomato reporter mice (Figs. 5C and 5E). These findings indicate that MRGPRA3+ pruriceptors migrate towards Merkel cell-enriched touch dome in the chronic itch skin, although they do not approximate Merkel cells in the steady state. This adaptive anatomic miswiring of Merkel cells with pruriceptors unveil how chronic itch can alter the normal function of skin cells to propagate the vicious itch-scratch cycle.
Fig 5. Structural basis underlying the miswiring between Merkel cells and the MrgprA3+ fibers in chronic itch mice.
(A-B) Super-resolution 3D reconstruction of the Merkel cell-neurite structure. Vehicle-treated (A) and AEW-treated (B) MrgprA3 tdTomato reporter mice stained with K8 (Merkel cells) and dsRed (MrgprA3+ fibers). A warm-to-cool heat bar (80 μm depth) represents epidermal-to-dermal layer distance. Replications were performed in 4 mice per group, n=15 and 17 touch domes in the control and AEW treatment group. (C-D) Immunoelectron microscopy images of the Merkel-neurite complex from vehicle-treated (C) and AEW-treated (D) samples prepared from the MrgprA3 tdTomato reporter mice. CC and DD are higher magnification images of Merkel cell-innervating axons taken from the red boxes in C and D, respectively. Immunoelectron microscopy using an anti-dsRed antibody reveals specific expression of tdTomato-immunoreactivity (black dot) in the AEW-treated group (D and DD) and the vehicle-treated group (C and CC). The dermal-epidermal junction was marked with the red dash lines. Red arrows point to anti-dsRed antibody-labelled axons and black arrows point to the non-stained axons. MC, Merkel cell; N, Merkel cell nucleus; SN, Schwann cell nucleus. Scale bar: 500 nm. (E) Statistical data of the closest distance between Merkel cells and postsynaptic axons marked by anti-dsRed antibody. n=13 and 20 touch domes acquired from 3 mice per control and AEW group, respectively. ****P<0.0001, Student t test.
DISCUSSION
Chronic itch is a serious health problem for which there are no universally effective treatments.. One promising strategy for developing anti-pruritic therapies is to directly target the skin, the most superficial area involved in itch sensation. In our previous studies, we showed that aging may cause a partial loss of Merkel cell. Here, we used an experimental dry skin model in mice and showed a miswiring structure transiently formed by Merkel cell and MRGPRA3+ C fiber. Functionally, we uncovered Merkel cell as a key component in itch sensation, likely via two distinct signaling pathways (fig. S10): Under physiological conditions, Merkel cell drives SAI fiber firing to suppress the mechanical itch, whereas in chronic itch, Merkel cell drives C fiber firing to evoke scratch-induced itch.
Skin harbors a variety of different stromal, immune, and neural cell types. Although keratinocytes and skin immune cells are important sources of endogenous pruritogens (35-38), little is known about the functions of other cell types in the pathogenesis of itch. In mice, Piezo2 channels expressed by Merkel cell-neurite complexes contribute to light touch sensation through regulating the function of the SA1 Aβ low-threshold mechanoreceptors (LTMRs) (16, 17). Our recent studies also demonstrated that activation of Piezo2 channel in Merkel cells suppresses mechanical itch under physiological conditions (18). Here, we found that both Merkel cell-deficient mice and in animals with Piezo2 deletion in Merkel cells subjected to experiment dry skin displayed reduced pruriceptive C fiber firing and spontaneous itch. Thus, our data uncover a pathologic transition of Piezo2 signaling in Merkel cells from inhibiting mechanical itch at the steady state to promoting spontaneous itch in the setting of chronic itch.
A ~80 μm sectional projection of multiple stacks taken from the epidermis layer to the dermis layer showed that touch domes in human skin associate with peptidergic and nonpeptidergic C fibers (39), suggesting C fibers may participate in innocuous mechanoreception and/or nociception. Our studies showed that chemical ablation of the TRPV1+ fibers reduced experimental dry skin itch, further supporting the essential role of the TRPV1+ C fibers in the generation of chronic itch (40). Previous data have shown that the increased excitability of MRGPRA3+ and MRGPRD+ neurons may mediate the spontaneous itch- and pain behavior in a mouse model of contact dermatitis (41), strongly supporting our findings that MrpgrA3+ neurons displayed a functional plasticity under pathological condition. Moreover, a subset of peptidergic C fiber nociceptors expressing the nicotinic acetylcholine receptor subunit alpha-3 (Chrna3) receptor served as “silent” mechanoreceptors and displayed mechanosensitivity upon NGF-mediated sensitization of Piezo2 channels (42). Although we found that direct optogenetic activation of Merkel cells drove C fiber firings in mice subjected to experimental dry skin, our results could not exclude the possibility that these Chrna3+/Piezo2+ neurons might also display mechanosensitivity and mediate the scratch-induced itch in the dry skin condition. Future studies will be required to reveal the multiple cell types involved in the generation of scratch-induced itch.
A recent report showed that experimental dry skin induced hyperinnervation of MRGPRA3+ itch-sensing neurons in the skin (43). However, how the MRGPRA3+ fibers are activated remains unclear and warrants further investigation. We showed that the MRGPRA3+ fibers were required for the Merkel cell-induced action potential firings in pruriceptive C fibers and these MRGPRA3+ fibers were reoriented to Merkel cells under experimental dry skin condition. Thus, there appears to be a miswiring between Merkel cell and pruriceptive MRGPRA3+ fiber in chronic itch.
There are a few limitations in this study, first, the neurotransmitters in Merkel cell-mediated activation of pruriceptive C fibers under pathological conditions have not been identified, although it was demonstrated that serotonin and/or norepinephrine released from Merkel cells activated downstream Aβ fibers under physiological conditions (28, 29). Second, due to a lack of specific Piezo2 inhibitors, the role of Piezo2 in chronic itch has been investigated mainly through genetic approaches. Third, our data uncovered the dynamic crosstalk between touch receptor and itch receptor in a mouse model of dry skin associated chronic itch, future studies should be performed to determine whether the miswiring mechanism also contributes to the development of itch-scratch cycle in other types of chronic itch. Finally, although the human MRGPRX1 shares similar expression pattern and chemical activation property with mouse MRGPRA3, the translational potential for targeting MRGPRX1 to treat chronic itch in human patients requires further clinical studies. Nevertheless, our results indicate that the Piezo2 signaling in Merkel cells, whereas important for touch sensation in the healthy state, emerges as a critical mediator of pathologic spontaneous itch. At mechanistic level, there might be a reduction in canonical activation of Aβ mechanoreceptors and formation of maladaptive functional interactions between Merkel cells and C pruriceptors in the skin (fig. S11). These findings reveal how miswiring of Merkel cell-C fiber in the skin can propagate the itch-scratch cycle in a mouse model and highlight the dynamic plasticity of cutaneous epithelia-sensory nerve interactions under pathological conditions.
MATERIALS AND METHODS
Study design
A multidisciplinary approach using electrophysiological and pharmacological approaches in combination to electron microscopy, genetics and behavioral methods to understand the cellular and molecular mechanism of the vicious itch-scratch cycle in the chronic dry skin mouse model. Randomization: In behavioral tests, body weight- and gender-matched mice were genotyped and allocated to experimental groups or control groups at 8 to 12-weeks of age. Blinding: All behavioral tests were done by experimenters blind to the treatments or genotypes of animals. Replication: Experiments were designed to minimize the number of animals used. To minimize the variances in inter- and intra-groups, we included enough sample size (indicated in each figure legend) for reliable statistical analysis.
Animals
All experiments were performed in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain, and were approved by the Animal Studies Committee at Washington University School of Medicine. All mice were housed under a 12 h light/dark cycle with food and water provided ad libitum. C57BL/6J mice were obtained from the Jackson Laboratories. To specifically ablate Merkel cell and associated Piezo2 channels, Atoh1flox/flox mice and Piezo2flox/flox mice were mated with K14cre/+ (TgKRT14-cre1Amc/J) mice and Atoh1CreERT mice, respectively. For chemogenetic and optogenetic stimulations of Merkel cells, mice were engineered by crossing the Atoh1CreERT mice with Gq-DREADD (B6N.129-Tg(CAG -CHRM3,-mCitrine)1Ute/J) or Ai32 (B6.Cg-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J) mice. To ablate MRGPRA3+ neurons, MrgprA3GFP-Cre mice were crossed with the ROSA26iDTR (C57BL/6-Gt(ROSA)26Sortm1(HBEGF)Awai/J) mice. To visualize the MRGPRA3+ fiber in the skin, MrgprA3GFP-Cre mice were crossed with the Ai9 (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) mice.
Mouse model of chronic dry skin
Mice were acclimated in the recording chambers for two consecutive days before treatment. To generate the mouse model of dry skin-associated chronic itch, the upper back of mouse was treated twice daily for 5 consecutive days with a mixture of acetone/ether (1:1) for 15 second followed by distilled water for 25 seconds. Scratching behaviors were videotaped every morning and blinded analyzed. The total number of bouts and time each mouse spent scratching were quantified over a 60-minute period. As been defined in References 20-21, scratching bouts was manually counted as an episode in which a mouse scratches and ends when the mouse either licks or placed its hind paw back on the floor. The total time was recorded with a stopwatch from a mouse lifts its paw and scratches continuously until the paw is returned to the floor. Tissues were harvested at Day 5 for whole mounting staining and ex vivo skin-nerve recordings.
Tail-flick test
The distal 2/3 of the mouse’s tail was subjected to 53°C water bath to perform the thermal hyperalgesia test. The latency to a rapid tail flick was recorded in both vehicle-treated and RTX-treated groups. A 20 s cut off time was utilized to avoid thermal damage to the tail. Related mice were excluded if the RTX-treated mice displayed a comparable tail flick latency when compared with that in the vehicle-treated mice.
Cre induction
To induce Cre expression, tamoxifen (Sigma) was dissolved in corn oil and made fresh daily before use. Both Cre− and Cre+ mice received intraperitoneal injection of tamoxifen at 100 mg/kg body weight for 5 consecutive days. In vivo and in vitro experiments were performed between 7 and 14 days after the final tamoxifen injection.
DTX administration
To ablate MRGPRA3+ neurons in MrgprA3Cre; DTRf/f mice, both 6-8 weeks old Cre− and Cre+ mice received intraperitoneal injections of 600 ng DTX (Sigma) every other day for 3 times. Mice were rested for at least 1 week before use. After all experiments, DRG neurons from Cre+ mice were collected for the verification of ablation efficiency by calcium imaging. Related data were excluded if the ablation efficiency was lower than 95%.
Ex vivo skin-nerve preparations
Skin-nerve preparations were harvested by dissecting right dorsal side skin as previously reported (cited as Reference 8). In brief, skin tissue (approximately 2 cm width × 3 cm length) innervated by dorsal root ganglia (T6 to T10) was separated carefully from the connective tissue. The preparation was placed corium side up in a 34°C bath perfused with carbogen-buffered synthetic interstitial fluid containing (in mM): 108 NaCl, 3.48 KCl, 0.7 MgSO4, 1.5 CaCl2, 1.7 NaH2PO4, 9.6 Na gluconate, 13 NaHCO3, 5.55 glucose, 7.6 sucrose and pH 7.2. The attached nerve bundle was then pulled into a chamber filled with mineral oil and hung on a gold recording electrode. The myelin sheath was peeled off from the nerve bundles without any further dissection. Spontaneous firing property was first recorded for 30 mins and categorized by their conduction velocity as: Aβ fibers > 10 m/s, 1.2 m/s ≤ Aδ fibers≤ 10 m/s, C fibers 1.2 m/s. Touch dome were targeted with a mechanical combined with electrical search protocol. Briefly, a functional touch dome should meet the following criteria: (1) no spontaneous SAI firing, (2) SAI fiber conduction velocity ≥10m/s, (3) in contrast to the SAⅡ firing, SAI fiber displays sustained response with irregular pattern during ramp-and-hold stimuli, and immediately ceases once the stimulation is removed, (4) respond only to pressure applied directly to a touch dome but not to the surrounding areas, (5) insensitive to hair tugging and skin stretch, (6) a typical SAI response should be composed of a dynamic firing phase and a static firing phase but only a truncated dynamic firing phase (with truncated or even without static firing phase) in the Merkel cell-deficient preps.. Once a functional touch dome was identified, the receptive field was subjected to ascending series of mechanical forces, ranging from 1 to 150 mN with an interval of 1 min, delivered by a computer-controlled dual-mode lever system (Aurora Scientific Inc.). Action potential firings to these stimuli were recorded and analyzed using Spike2 program (Cambridge Electronic Design Limited). In the vehicle-treated mice, mechanical force/blue light stimulation applied to a touch dome structure could evoke biphasic responses with a dynamic phase (high-frequency firing at touch onset) and a static phase (sustained irregular firing). According to the distribution pattern of firing frequency and firing properties, three populations of the spontaneous C fibers are classified using the following criteria: low > 0.3 Hz; 0.3 Hz≤ medium > 1 Hz; 1 Hz≤ High. Merkel cell-dependent mechanically activated C fiber firing was defined by the following criteria: (1) it is not overlapped with the spontaneous C fiber firing, (2) it is only evoked by the mechanical/optical stimuli are applied to the identified touch dome structure, but not the surrounding areas, (3) conduction velocity 1.2 m/s, (4) the C fiber firings are tightly associated with the external stimuli and firing stops once the stimulation is removed. Fiber conduction velocity was measured by electrically stimulating on the fiber-innervating area. The firing frequency is defined by the spike count in an interval of duration T divided by T.
Immunofluorescence
Mouse back hairy skin was shaved and dissected. After cleaning the adipose tissue, skin was fixed overnight in 2% formalin and dehydrated in 30% sucrose at 4°C. The tissue was embedded in OCT compound and continually sectioned in 12 μm with a cryostat microtome (CM1950, Leica Biosystems). Samples were incubated with primary antibodies in PBS containing 10% donkey serum + 1% Triton X-100 at room temperature for 24 h and further incubated with secondary antibodies in PBS containing 10% donkey serum at room temperature for 1 hour. After the final wash, the tissue was mounted with Fluormount-G and examined with a confocal microscope. Primary antibodies used in this study include: Anti-dsRed (632496, 1:500, Clontech Takara), Anti-Keratin-8 (TROMA1, 1:500, DSHB), anti-Neurofilament H (N4142, 1:500, Sigma, St. Louis, MO, USA). Secondary goat AlexaFluor-conjugated antibodies (Invitrogen) against rat (Alexafluor 405, A48261, 1:100; Alexafluor 488, A11006, 1:500), rabbit (Alexafluor 488, A11034, 1:500) IgG. The Cy™3 AffiniPure donkey Anti-Rat IgG (712-165-153, 1:300) was from Jackson immune Research.
Super-resolution 3D reconstruction of the Merkel cell-neurite structure
Skin samples were first treated with commercially available clarity kit (ab243298, Abcam). In brief, skin tissue is fixed in 4% PFA and dehydrated with increasing % methanol series. After incubation with DMSO/methanol, rehydrate the tissue with decreasing % methanol series. For whole mount staining, water or AEW-treated skin area was tape-stripped until glistening. The skin tissue was then fixed overnight in 2% formalin and washed with PBS every 10 min for 2 hours. Primary antibodies were prepared in PBS containing 10% donkey serum + 20% DMSO. The skin tissue was incubated with a primary antibody at room temperature for 5 days and then washed with PBS every 10 min for 2 hours, and further incubated with the secondary antibodies in PBS containing 10% donkey serum + 20% DMSO at room temperature for 3 days. After the final wash, the tissue was mounted with Fluormount-G and examined with a THUNDER Imaging Systems (leica microsystems). It should be noted that before whole mount staining, tissue should be incubated in the pentration buffer and blocked with blocking buffer. 3D reconstruction of the Merkel-neurite structure was automatically performed with the system and exported for further analysis.
HRP conjugation of the anti-dsRed primary antibody
HRP-conjugated anti-dsRed primary antibody was prepared with commercial available lighting-link kit (ab102890, Abcam). Briefly, modifier reagent is added to each 10 μL of antibody to be labeled and mix gently. Pipette the antibody sample (with added Modifier reagent) directly onto the Vial of HRP Conjugation Mix. Resuspend gently by withdrawing and re-dispensing the liquid once or twice using a pipette and then leave standing for 3 hours in the dark at room temperature (20-25°C). After incubating for 3 hours, add 1 μL of Quencher reagent for every 10 μL of antibody used and mix gently. The conjugate is used after 30 minutes.
Electron microscopy
For transmission electron microscopy (TEM), skin samples were fixed by immersion in 4% paraformaldehyde in 1x phosphate buffered saline (PBS) for 24 hours at 4°C. Samples were then rinsed in PBS 3 times for 10 minutes each and incubated in 1% sodium borohydride for 30 minutes to quench aldehydes. Following this, samples were again rinsed in PBS 3 times for 10 minutes each and then incubated in 0.5% Triton X-100 for 30 minutes. The samples were rinsed again in PBS 3 times for 10 minutes each and incubated in 1:300 HRP-conjugated anti-dsRed antibody in PBS for 72 hours at room temperature with agitation. Samples were then rinsed in 0.15M cacodylate buffer with 2 mM CaCl2 3 times for 10 minutes each and DAB labeled using DAB Peroxidase Substrate kit (SK-4100, VWR International). Samples were then rinsed in cacodylate buffer 3 times for 10 minutes each and subjected to a secondary fixation step for one hour in 1% osmium tetroxide/1.5% potassium ferrocyanide in the dark. Following this, samples were washed in ultrapure water 3 times for 10 minutes each and en bloc stained overnight with 1% aqueous uranyl acetate. After staining was complete, samples were briefly washed in ultrapure water, dehydrated in a graded ethanol series (10%, 30%, 50%, 70%, 90%, 100% x2) for 10 minutes in each step, infiltrated into LX112 resin, and cured in an oven at 60˚C for 48 hours. 70 nm thin sections were then cut, post-stained with uranyl acetate and Reynold’s lead and imaged on a Transmission Electron Microscope (JEOL JEM-1400 Plus) at 120 KeV. Imaging was performed in the Washington University Center for Cellular Imaging (WUCCI).
Statistics
All data are presented as mean ± SEM for n independent observations. Differences between population means were assessed with unpaired Student t test (two tail) for normally distributed data. Two-way ANOVA and repeated measures tests were used to test hypotheses about effects in multiple groups. p<0.05 was considered significantly different.
Supplementary Material
Acknowledgments:
We thank S.M. Carlton, A. Beyder, R. Hill for helpful discussion. Atoh1CreERT mouse line was kindly provided by M. Hoshinoa, E. A. Lumpkin, and B. U. Hoffman. Atoh1f/f mouse line was kindly provided by N. F. Shroyer and R. D. Newberry. Dr. Qin Liu is also affiliated with Departments of Ophthalmology & Visual Sciences and Neuroscience of Washington University School of Medicine. This study did not generate new unique reagents.
Funding:
National Institutes of Health grant R01AA027065 to HH (NINDS)
National Institutes of Health grant R01AR077183 to HH (NIAMS)
National Institutes of Health grant R01DK103901 to HH (NIDDK)
National Institutes of Health grant R01NS106289 to GFW (NINDS)
The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital grant CDI-CORE-2015-505 to JAF
The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital grant CDI-CORE-2019-813 to JAF
The Foundation for Barnes-Jewish Hospital grant 3770 to JAF
Footnotes
Competing interests: B.S.K. has served as a consultant for AbbVie, Almirall S.A., Amagma, Argenx, Astra Zeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim Corporation, Bristol-Myers Squibb, Cara Therapeutics, Daewoong Pharmaceutical, Eli Lilly and Company, Guidepoint Global, Janssen Pharmaceuticals, Incyte Corporation, Kiniksa Pharmaceuticals, LectureLinx, LEO Pharma, Maruho, Novartis, OM Pharma, Pfizer, Sanofi Genzyme, Shaperon, Third Rock Ventures, and Trevi Therapeutics; is a stockholder of Recens Medical and Locus Biosciences; serves on the scientific advisory boards for Abrax Japan, Granular Therapeutics, Recens Medical, National Eczema Association, Cell Reports Medicine, and Journal of Allergy and Clinical Immunology; B.S.K. is an inventor on patent/patent application (WO2017143014A1) held/submitted by Washington University that covers the use of JAK inhibitors for chronic pruritus.
Data and materials availability: All data associated with this study are in the paper or supplementary materials Correspondence and requests for materials should be addressed to JF or HH.
References and Notes
- 1.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK, TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proceedings of the National Academy of Sciences of the United States of America 106, 11330–11335 (2009); published online EpubJul 7 ( 10.1073/pnas.0905605106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM, TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature neuroscience 14, 595–602 (2011); published online EpubMay ( 10.1038/nn.2789). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, Yu W, Qian A, Zhang Y, Liu S, Yin S, Xu A, Cheng J, Liu Q, O’Neil RG, Xia Y, Ma L, Carlton SM, Kim BS, Renner K, Liu Q, Hu H, Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. The Journal of allergy and clinical immunology 141, 608–619 e607 (2018); published online EpubFeb ( 10.1016/j.jaci.2017.05.051). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Yang P, Mack MR, Dryn D, Luo J, Gong X, Liu S, Oetjen LK, Zholos AV, Mei Z, Yin S, Kim BS, Hu H, Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nature communications 8, 980 (2017); published online EpubOct 30 ( 10.1038/s41467-017-01056-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M, A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. The Journal of allergy and clinical immunology 133, 448–460 (2014); published online EpubFeb ( 10.1016/j.jaci.2013.10.048). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X, Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365 (2009); published online EpubDec 24 ( 10.1016/j.cell.2009.11.034). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X, A subpopulation of nociceptors specifically linked to itch. Nature neuroscience 16, 174–182 (2013); published online EpubFeb ( 10.1038/nn.3289). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X, Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2015); published online EpubMar 12 ( 10.1038/nature14022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele HR, Xing Y, Zhu Y, Hilley HB, Lawson K, Nho Y, Niehoff T, Han L, MrgprC11(+) sensory neurons mediate glabrous skin itch. Proceedings of the National Academy of Sciences of the United States of America 118, (2021); published online EpubApr 13 ( 10.1073/pnas.2022874118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun YG, Chen ZF, A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007); published online EpubAug 9 ( 10.1038/nature06029). [DOI] [PubMed] [Google Scholar]
- 11.Mishra SK, Hoon MA, The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013); published online EpubMay 24 ( 10.1126/science.1233765). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishiuji Y, Addiction and the itch-scratch cycle. What do they have in common? Experimental dermatology 28, 1448–1454 (2019); published online EpubDec ( 10.1111/exd.14029). [DOI] [PubMed] [Google Scholar]
- 13.Fourzali KM, Golpanian RS, Chan YH, Yosipovitch G, Average daily itch vs. worst daily itch in chronic itch evaluation. The British journal of dermatology 183, 957–958 (2020); published online EpubNov ( 10.1111/bjd.19228). [DOI] [PubMed] [Google Scholar]
- 14.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, Luo J, Gao X, Yang L, Hamilton SL, Wang PL, Brestoff JR, Council ML, Brasington R, Schaffer A, Brombacher F, Hsieh CS, Gereau RWT, Miller MJ, Chen ZF, Hu H, Davidson S, Liu Q, Kim BS, Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171, 217–228 e213 (2017); published online EpubSep 21 ( 10.1016/j.cell.2017.08.006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang TB, Kim BS, Scratching Beyond the Surface of Itchy Wounds. Immunity 53, 235–237 (2020); published online EpubAug 18 ( 10.1016/j.immuni.2020.07.016). [DOI] [PubMed] [Google Scholar]
- 16.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A, Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 (2014); published online EpubMay 29 ( 10.1038/nature13251). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA, Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 (2014); published online EpubMay 29 ( 10.1038/nature13250). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Luo J, Yang P, Du J, Kim BS, Hu H, Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 360, 530–533 (2018); published online EpubMay 4 ( 10.1126/science.aar5703). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y, Itch-associated response induced by experimental dry skin in mice. Japanese journal of pharmacology 88, 285–292 (2002); published online EpubMar ( 10.1254/jjp.88.285). [DOI] [PubMed] [Google Scholar]
- 20.Nojima H, Carstens E, Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. Journal of neuroscience methods 126, 137–143 (2003); published online EpubJun 30 ( 10.1016/s0165-0270(03)00074-8). [DOI] [PubMed] [Google Scholar]
- 21.Wimalasena NK, Milner G, Silva R, Vuong C, Zhang Z, Bautista DM, Woolf CJ, Dissecting the precise nature of itch-evoked scratching. Neuron 109, 3075–3087 e3072 (2021); published online EpubOct 6 ( 10.1016/j.neuron.2021.07.020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ru F, Sun H, Jurcakova D, Herbstsomer RA, Meixong J, Dong X, Undem BJ, Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. The Journal of physiology 595, 3651–3666 (2017); published online EpubJun 1 ( 10.1113/JP273795). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly S, Dunham JP, Murray F, Read S, Donaldson LF, Lawson SN, Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthritis and cartilage 20, 305–313 (2012); published online EpubApr ( 10.1016/j.joca.2012.01.002). [DOI] [PubMed] [Google Scholar]
- 24.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN, Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 1281–1292 (2006); published online EpubJan 25 ( 10.1523/JNEUROSCI.3388-05.2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao WH, Bennett GJ, Persistent low-frequency spontaneous discharge in A-fiber and C-fiber primary afferent neurons during an inflammatory pain condition. Anesthesiology 107, 813–821 (2007); published online EpubNov ( 10.1097/01.anes.0000286983.33184.9c). [DOI] [PubMed] [Google Scholar]
- 26.Kleggetveit IP, Namer B, Schmidt R, Helas T, Ruckel M, Orstavik K, Schmelz M, Jorum E, High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain 153, 2040–2047 (2012); published online EpubOct ( 10.1016/j.pain.2012.05.017). [DOI] [PubMed] [Google Scholar]
- 27.Woo SH, Stumpfova M, Jensen UB, Lumpkin EA, Owens DM, Identification of epidermal progenitors for the Merkel cell lineage. Development 137, 3965–3971 (2010); published online EpubDec ( 10.1242/dev.055970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG, Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proceedings of the National Academy of Sciences of the United States of America 113, E5491–5500 (2016); published online EpubSep 13 ( 10.1073/pnas.1610176113). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA, Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron 100, 1401–1413 e1406 (2018); published online EpubDec 19 ( 10.1016/j.neuron.2018.10.034). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall KL, Clary RC, Baba Y, Orlowsky RL, Gerling GJ, Lumpkin EA, Touch Receptors Undergo Rapid Remodeling in Healthy Skin. Cell reports 17, 1719–1727 (2016); published online EpubNov 8 ( 10.1016/j.celrep.2016.10.034). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moayedi Y, Duenas-Bianchi LF, Lumpkin EA, Somatosensory innervation of the oral mucosa of adult and aging mice. Scientific reports 8, 9975 (2018); published online EpubJul 2 ( 10.1038/s41598-018-28195-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Hu H, TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals 11, (2018); published online EpubOct 6 ( 10.3390/ph11040100). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kittaka H, Tominaga M, The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergology international : official journal of the Japanese Society of Allergology 66, 22–30 (2017); published online EpubJan ( 10.1016/j.alit.2016.10.003). [DOI] [PubMed] [Google Scholar]
- 34.Akiyama T, Carstens E, Neural processing of itch. Neuroscience 250, 697–714 (2013); published online EpubOct 10 ( 10.1016/j.neuroscience.2013.07.035). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain A, Hakim S, Woolf CJ, Unraveling the Plastic Peripheral Neuroimmune Interactome. Journal of immunology 204, 257–263 (2020); published online EpubJan 15 ( 10.4049/jimmunol.1900818). [DOI] [PubMed] [Google Scholar]
- 36.Talagas M, Lebonvallet N, Leschiera R, Sinquin G, Elies P, Haftek M, Pennec JP, Ressnikoff D, La Padula V, Le Garrec R, L’Herondelle K, Mignen O, Le Pottier L, Kerfant N, Reux A, Marcorelles P, Misery L, Keratinocytes Communicate with Sensory Neurons via Synaptic-like Contacts. Annals of neurology 88, 1205–1219 (2020); published online EpubDec ( 10.1002/ana.25912). [DOI] [PubMed] [Google Scholar]
- 37.Yosipovitch G, Berger T, Fassett MS, Neuroimmune interactions in chronic itch of atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 34, 239–250 (2020); published online EpubFeb ( 10.1111/jdv.15973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwendinger-Schreck J, Wilson SR, Bautista DM, Interactions between keratinocytes and somatosensory neurons in itch. Handbook of experimental pharmacology 226, 177–190 (2015) 10.1007/978-3-662-44605-8_10). [DOI] [PubMed] [Google Scholar]
- 39.Reinisch CM, Tschachler E, The touch dome in human skin is supplied by different types of nerve fibers. Annals of neurology 58, 88–95 (2005); published online EpubJul ( 10.1002/ana.20527). [DOI] [PubMed] [Google Scholar]
- 40.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM, The ion channel TRPA1 is required for chronic itch. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 9283–9294 (2013); published online EpubMay 29 ( 10.1523/JNEUROSCI.5318-12.2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH, Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain : a journal of neurology 137, 1039–1050 (2014); published online EpubApr ( 10.1093/brain/awu007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prato V, Taberner FJ, Hockley JRF, Callejo G, Arcourt A, Tazir B, Hammer L, Schad P, Heppenstall PA, Smith ES, Lechner SG, Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell reports 21, 3102–3115 (2017); published online EpubDec 12 ( 10.1016/j.celrep.2017.11.066). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Hanson CE, Liu Q, Han L, Mrgprs activation is required for chronic itch conditions in mice. Itch 2, (2017); published online EpubDec ( 10.1097/itx.0000000000000009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.