TO THE EDITOR:
Balikagala et al. (Sept. 23 issue)1 report evidence that Plasmodium falciparum malaria parasite resistance to first-line artemisinin derivatives has emerged in northern Uganda and is associated with an increased prevalence of kelch13 A675V and C469Y mutations. We edited these mutations into the P. falciparum Dd2 (Southeast Asia) and MAS-136 (eastern Uganda) strains and measured survival among dihydroartemisinin-treated intraerythrocytic ring-stage parasites cultured in vitro. Dd2 parasites expressing the kelch13 A675V or C469Y mutation showed marginally reduced susceptibility to dihydroartemisinin at the reference concentration of 700 nmol per liter as compared with parental Dd2 expressing wild-type kelch13, and no significant difference was observed at lower concentrations of dihydroartemisinin (Fig. 1A). As a control, we also tested recombinant Dd2 parasites expressing the kelch13 R539T mutation, which was identified in Southeast Asia, and observed substantially reduced susceptibility to dihydroartemisinin — findings consistent with the results of previously published data.2 MAS-136 parasites that were edited to express the kelch13 A675V or C469Y mutation showed no reduced susceptibility to dihydroartemisinin at all concentrations tested, as compared with wild-type MAS-136 parasites (Fig. 1B).
Figure 1. Ring-Stage Parasite Survival in CRISPR-Cas9 Gene–Edited Plasmodium falciparum Lines Expressing kelch13 Mutations Associated with Artemisinin Resistance in Northern Uganda.
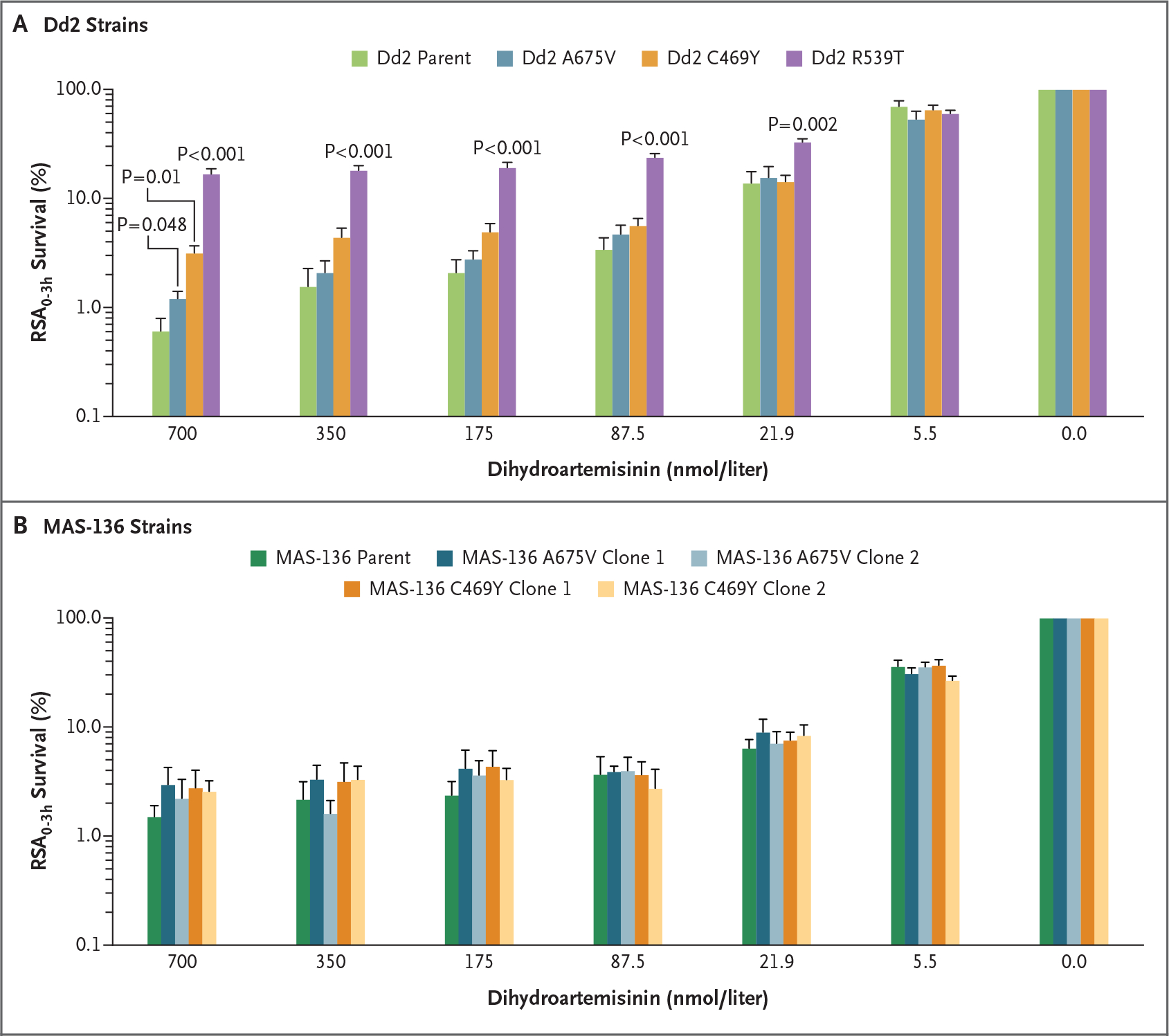
The mean survival among ring-stage parasites is plotted as a function of dihydroartemisinin concentration in parental or mutant Dd2 (Panel A) and MAS-136 (Panel B) strains. Error bars indicate the standard error. The strains were assayed in duplicate on six occasions for the Dd2 strains and on four occasions for the MAS-136 strains. Synchronized early ring-stage parasites (0 to 3 hours after the invasion of red cells) were exposed in ring-stage survival assays (RSA0–3h) to dihydroartemisinin for 6 hours, the drug was removed by extensive washing, and cultures were continued for 66 hours. The numbers of live intraerythrocytic parasites were measured by flow cytometry, and survival after drug treatment was calculated relative to dimethylsulfoxide (vehicle control)–treated parasites. Unpaired t-tests with Welch’s correction were used to compare edited kelch13 mutant strains with wild-type unedited parental strains. CRISPR denotes clustered regularly interspaced short palindromic repeats.
These data suggest that an A675V or C469Y mutation alone may not ensure high-level resistance to treatment with an artemisinin derivative and that additional genetic determinants may be required. Further work is needed to assess the effects of these mutations across multiple genetic backgrounds and to identify other factors that contribute to resistance.
Footnotes
No potential conflict of interest relevant to this letter was reported.
References
- 1.Balikagala B, Fukuda N, Ikeda M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 2021;385:1163–71. [DOI] [PubMed] [Google Scholar]
- 2.Stokes BH, Dhingra SK, Rubiano K, et al. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. Elife 2021;10:e66277. [DOI] [PMC free article] [PubMed] [Google Scholar]


