Abstract
This case presents a patient with severe COVID-19 pneumonia requiring intensive care unit admission and a prolonged hospital stay. The infection resulted in long-term morbidity, functional decline, and abnormal chest CT findings. The mechanisms for long-term lung injury after COVID-19 infection, imaging appearances, and the role of imaging in follow-up are discussed.
© RSNA, 2023
Summary
The sequelae of diffuse alveolar damage and organizing pneumonia can be seen after COVID-19 infection; most findings will slowly improve or resolve, with persistent parenchymal scarring in a small subset of patients.
Teaching Points
■ The cause of lung injury with COVID-19 includes direct viral injury, host immune response, and barotrauma, with each having the potential to cause long-term lung damage.
■ Common residual CT findings after COVID-19 infection include faint ground-glass and perilobular opacities, bronchiectasis, and parenchymal bands.
■ The term fibrosis should be initially avoided, as appearances usually improve or resolve over time. Improving CT findings correlate with measures of respiratory function.
■ An increased risk of thrombosis relates to a hyperinflammatory state and can persist for 3–6 months, decreasing over time.
■ Thin-section noncontrast chest CT should be used for follow-up. Inspiratory-expiratory CT, CT pulmonary angiography, and ventilation-perfusion studies have roles in select cases.

Dr Murphy completed radiology residency in the Mater Misericordiae University Hospital in Dublin, Ireland. He is currently undertaking a fellowship in cardiothoracic imaging and intervention at Massachusetts General Hospital, Boston, where he was awarded a Ralph Schlaeger grant for research activities.

Dr Little is a cardiothoracic radiologist at Mayo Clinic, Florida. His clinical and research interests include imaging of COVID-19 and other infections, diffuse lung disease, and lung cancer screening. He also has a longstanding interest in radiology teaching and education.
Case Presentation (Dr Mark C. Murphy)
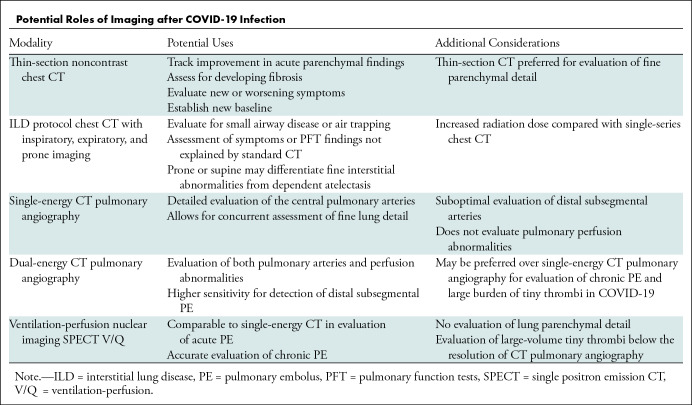
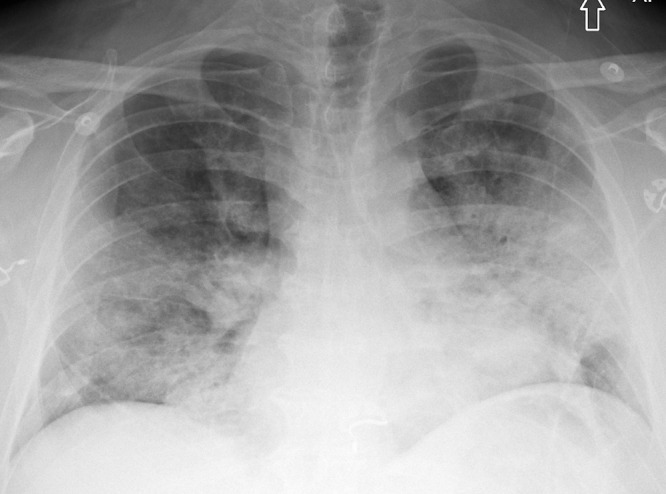
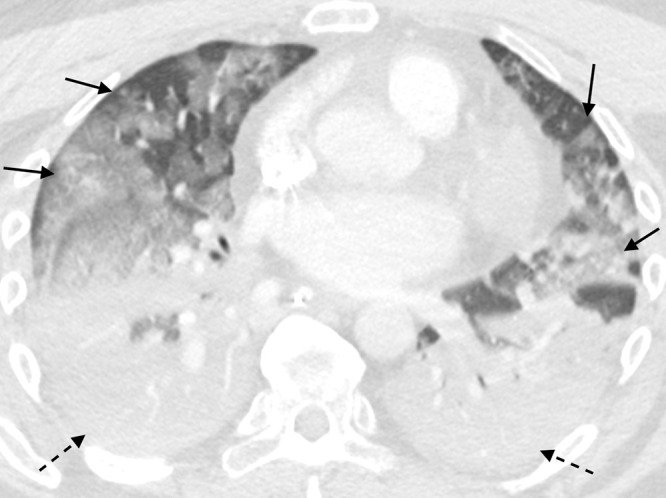
A 51-year-old man with hypertension and chronic renal impairment presented to the emergency department in late 2020 with acute hypoxemic respiratory failure. A nasopharyngeal viral polymerase chain reaction test was positive for SARS-CoV-2. Chest radiography revealed bilateral lower lung–predominant airspace disease typical for COVID-19 pneumonia (Fig 1). He developed acute respiratory distress syndrome (ARDS) and required intensive care unit transfer, mechanical ventilation, and venovenous extracorporeal membrane oxygenation. Chest CT revealed a diffuse alveolar damage (DAD) pattern with extensive ground-glass opacities and dependent consolidation (Fig 2). He received extracorporeal membrane oxygenation for 5 weeks, then tracheostomy ventilation for a further 3 weeks. Repeat CT 1 month after presentation demonstrated an organizing lung injury pattern with multifocal consolidation, mild bronchial dilation, and small-volume pneumomediastinum (Fig 3). After continued improvement, he was discharged 4 months after his initial presentation.
Figure 1:
A 51-year-old man with COVID-19 infection. Anteroposterior chest radiograph shows extensive pulmonary opacities bilaterally with a lower lung and peripheral predominance.
Figure 2:
A 51-year-old man with COVID-19 infection. Axial contrast-enhanced CT image obtained the week of initial presentation after intensive care unit transfer. There is dense consolidation within the dependent portions of the lungs bilaterally (dashed arrows) and multifocal ground-glass opacity within the nondependent portions of the lungs (solid arrows). These findings are suggestive of a diffuse alveolar damage pattern of lung injury.
Figure 3:
A 51-year-old man with COVID-19 infection. Axial contrast-enhanced CT image obtained 1 month after initial presentation. There is improved aeration of the dependent portions of the lungs; however, there is residual consolidation with contraction and architectural distortion (solid arrows). There is also persistent ground-glass opacity (dashed arrows) and new bronchial dilatation bilaterally. This appearance is suggestive of the organizing phase of acute lung injury. There is pneumomediastinum (round arrow) likely secondary to barotrauma from mechanical ventilation.
After discharge, he completed physical and respiratory rehabilitation. He returned to work but had persistent shortness of breath and hypoxemia, with oxygen saturation transiently dropping to 90% with dyspnea on exertion. Five months after presentation, pulmonary function tests showed a forced vital capacity (FVC) at the lower limit of normal; forced expiratory volume in one second (FEV1) and FEV1-to-FVC ratio were within the normal range. Outpatient CT performed 6 months after presentation revealed improvement of the previous ground-glass opacities, with mild bronchiectasis and residual lower lung–predominant parenchymal bands. Expiratory imaging showed air trapping (Fig 4).
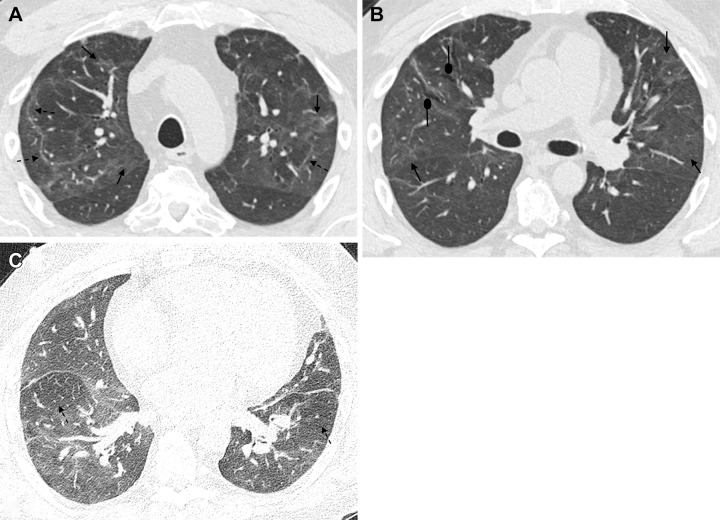
Figure 4:
A 51-year-old man 6 months after COVID-19 infection. Axial contrast-enhanced CT images from a series obtained at (A, B) inspiration and (C) expiration. (A) Persistent faint ground-glass opacities scattered throughout the lung parenchyma (solid arrows) have decreased in attenuation since prior imaging but remain extensive (tinted sign). Subpleural curvilinear opacities represent parenchymal bands and perilobular opacities (dashed arrows). (B) Mild anterior varicose bronchiectasis appreciated anteriorly in the right middle lobe (round arrows). Faint persistent ground-glass opacities (solid arrows). (C) Lobular and regional areas of persistent low attenuation (dashed arrows) on expiratory images consistent with air trapping, suggestive of small airway disease.
One week after outpatient CT, the patient presented with new chest pain, increased shortness of breath, right lower extremity edema, elevated d-dimer level, and anemia. CT pulmonary angiography demonstrated bilateral segmental and subsegmental pulmonary emboli (PE); bilateral asymmetric ground-glass opacities and consolidation were new from 1 week prior (Fig 5). FEV1 and FVC were mildly decreased, FEV1-to-FVC ratio was normal, total lung capacity was moderately decreased, and diffusing capacity of the lung for carbon monoxide (Dlco) was mildly decreased at 62%. Bronchoalveolar lavage findings were positive for Pneumocystis jiroveci (PJP) microorganisms. Treatment consisted of high-flow oxygen administered via a nasal cannula, piperacillin and tazobactam (Zosyn; Pfizer), and trimethoprim and sulfamethoxazole (Bactrim; Roche) for presumed bacterial pneumonia and PJP, and intravenous heparin for PE. Given the relatively rapid onset and asymmetric distribution of pulmonary opacities, it was unclear if the findings were secondary to PJP or if the positive culture represented colonization, with another type of pneumonia as the primary cause. His condition improved, and he was discharged on apixaban.
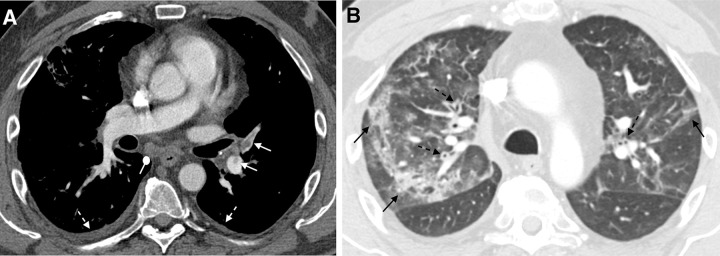
Figure 5:
A 51-year-old man 6 months after COVID-19 infection with shortness of breath. Axial CT pulmonary angiograms obtained with (A) soft-tissue and (B) lung window settings at second acute presentation to the emergency department. There are filling defects within several pulmonary artery branches (solid white arrows), consistent with acute pulmonary emboli. Multifocal patchy and linear ground-glass opacities and consolidation (solid black arrows) are new from 2 weeks prior. New bronchial wall thickening (dashed black arrows) and bilateral small pleural effusions (dashed white arrows). These findings suggest a superimposed acute process.
After discharge, shortness of breath and exercise tolerance gradually improved. FEV1-to-FVC ratio and FEV1 were normal, FVC and total lung capacity remained mildly decreased, and a mildly decreased Dlco was unchanged. Repeat chest CT was performed at this time and demonstrated mild residual parenchymal bands, bronchiectasis, and trace ground-glass opacity (Fig 6). The patient had a subsequent mild SARS-CoV-2 infection that did not require hospitalization and has been followed with plans for repeat chest CT and spirometry for 1 year.
Figure 6:
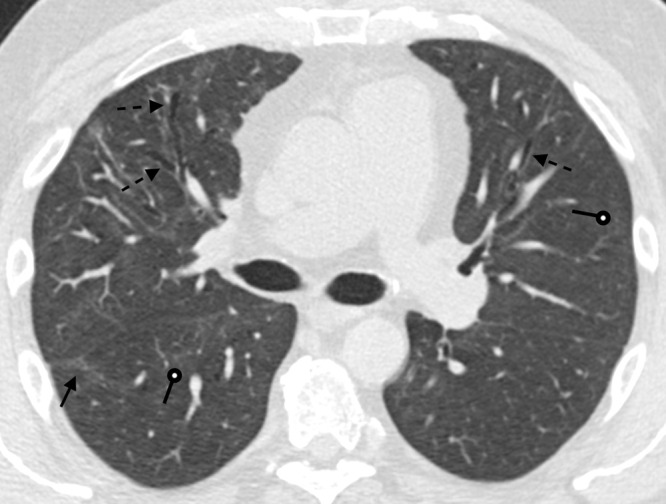
A 51-year-old man 9 months after COVID-19 infection. Axial contrast-enhanced CT image shows mild persistent anterior varicose bronchiectasis and architectural distortion best appreciated in the right middle lobe (dashed arrows). Faint patchy ground-glass opacities and reticulation are improved from 6-month imaging and barely perceptible (round arrows). Faint linear parenchymal bands have greatly improved (solid arrow). This may reflect the patient's new baseline.
Case Discussion (Dr Brent P. Little)
What is the Clinical Importance of Postacute Sequelae of COVID-19, Long COVID, and Post-COVID Syndrome?
Mortality from the ongoing COVID-19 pandemic has been devastating, with an estimated 680 million cases and 6.8 million deaths to date worldwide (1). However, many survivors have chronic symptoms and functional limitations that can be considered a pandemic within a pandemic. The variety of respiratory, neurologic, cardiac, musculoskeletal, and other signs and symptoms have been termed post-acute sequelae of COVID-19 (PASC), with the term long COVID generally used for symptoms lasting more than 30 days and the term post-COVID syndrome being used for symptoms persisting for at least 12 weeks (2). Although previous coronavirus outbreaks of SARS-CoV-1 and Middle East respiratory syndrome were also associated with long-term sequelae, many more individuals have already experienced PASC, estimated at 5%–10% of individuals with documented COVID-19 (3). Ongoing viral mutation, frequent reinfection, vaccine hesitancy, and reduced vaccine efficacy all contribute to an expanding number of individuals with long-term sequelae of COVID-19. Although post-COVID symptoms are more frequent and severe in those with severe initial infection (3), individuals who experience PASC are not limited to those with severe COVID-19; for example, PASC was reported by 15% of patients with mild infection at 8 months in one study (4) and in 4% of patients who did not require hospitalization in another (3). In addition, a substantial proportion of individuals—56% in a recent study of the COVID-19 Omicron variant—were unaware of their own previous SARS-CoV-2 infection (5) and could experience PASC without recognition of the cause.
What Is the Typical Evolution of Lung Imaging in COVID-19 and What Are the Typical Chronic Lung Findings?
Acute findings and pathology.—Typical acute findings include bilateral ground-glass opacities and consolidation with a peripheral and bronchocentric predominance, an appearance that can be seen in other viral pneumonias and organizing pneumonia (OP) (Figs 1, 2) (6,7). Ground-glass opacities appear in the first few days, followed by mixed consolidation and ground-glass opacity (8). Vaccination and SARS-CoV-2 variants can modulate this appearance (9–11). Other features that can be seen in OP, including perilobular and bandlike opacities, are also frequently reported (12,13). In severe disease, findings of DAD, such as diffuse ground-glass opacities, bronchial dilatation, and dependent consolidation, are common (Figs 2, 3) (14). Autopsy studies have shown acute fibrinous OP and edema with blends of proliferative and exudative DAD, type II pneumocyte hyperplasia, and bronchiolar epithelial necrosis (15,16). Biopsy samples from living patients have shown various patterns of OP and other forms of pneumonitis (17).
Long-term parenchymal findings.—Chronic lung abnormalities of COVID-19 likely represent a complex mixture of sequelae of direct viral damage, acute lung injury with DAD, ventilator-associated lung injury, and immune-mediated processes. The likelihood and magnitude of chronic findings increase with the severity of infection, ARDS, the duration of hospitalization and mechanical ventilation, older age (18–20), and inflammatory markers, such as C-reactive protein (CRP) and d-dimer (20).
In convalescing patients, ground-glass opacities may transiently increase in extent but take on lower attenuation, a finding known as the “tinted sign” and a feature of improving OP or DAD (21). Ground-glass opacities decrease in extent and attenuation over the next few weeks, and consolidation almost invariably resolves or evolves to thin strips of peripheral bandlike opacity (Figs 4, 6). Complete resolution of imaging abnormalities occurs in most patients. In a study of severe COVID-19 pneumonia, full resolution of pulmonary findings occurred in 58% of patients, with over half showing resolution at 3 months and the remainder showing resolution at 6 months; although 35% of these patients were deemed to have fibrotic-like findings at 6 months, the authors used a liberal definition of fibrosis, and many residual abnormalities were mild (20). However, some patients with severe COVID-19 have substantial lung abnormalities, including fibrosis, necessitating lung transplantation in some cases (22).
The severity of COVID-19 pneumonia correlates with the incidence and morphology of residual chronic lung abnormalities. In a large meta-analysis, chronic abnormalities accompanied 38% of severe COVID-19 cases and only 21% of mild or moderate cases. Ground-glass opacity was the most common abnormality in the mild or moderate disease group, while fibrotic-like findings were seen more commonly in the severe or critical group (23). In severe cases, DAD and associated ventilator-induced lung injury in the ARDS setting may be the major cause of residual lung abnormalities; an anterior predominance of bronchiectasis, reticulation, architectural distortion, and anterior air cysts is a well-known pattern of barotrauma-associated lung injury in the post-ARDS fibrosis setting and can occur in patients with severe COVID-19 (Fig 6) (24).
The incidence of residual lung abnormalities is lower for mild or moderate COVID-19 pneumonia; in a 1-year follow-up CT study, fibrotic-like abnormalities were present in 50% of patients with moderate pneumonia at 3 months, 42% of patients at 6 months, and only 5% of patients at 12 months (25). Residual thin parenchymal bands in a peripheral distribution with sparing of the subpleural lung are common (Figs 4, 6, 7); however, while some studies have considered parenchymal bands a form of fibrosis, they often resolve or become barely perceptible and should not be considered true fibrosis without long-term follow-up (26). Faint geographic ground-glass opacities that follow the pattern of initial lung involvement are frequent and can resemble the sequelae of nonspecific interstitial pneumonia or OP (Figs 4, 6, 7).
Figure 7:
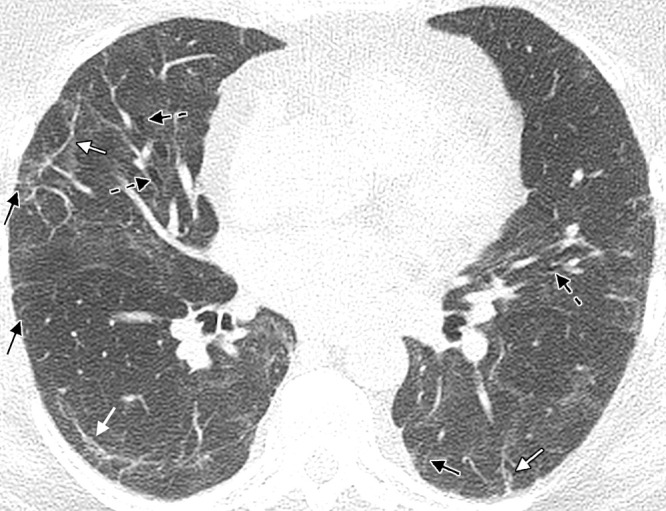
A 60-year-old woman with a history of hospitalization for moderate COVID-19 pneumonia requiring supplemental oxygen but not mechanical ventilation. Axial noncontrast CT image obtained 25 months after presentation for COVID-19 pneumonia shows bilateral thin parenchymal bands (white arrows), peripheral reticulation (black arrows), patchy ground-glass attenuation and reticulation, and traction bronchiectasis with architectural distortion (dashed black arrows). The fibrotic-like findings shown here appear to represent a new baseline given the 2-year period and have a pattern suggesting fibrotic sequelae of organizing lung injury in the setting of COVID-19. In other cases, parenchymal bands, ground-glass opacities, reticulation, and bronchial dilatation improve or resolve at follow-up imaging and cannot be interpreted as irreversible fibrosis without follow-up imaging.
A mosaic pattern of lung attenuation has been reported in some COVID-19 survivors. Although the relative contributions of small-airway and small-vessel disease remain unclear, air trapping at paired inspiratory or expiratory CT has been reported in 29%–77% of survivors, although many cases are mild (Fig 4C, 4D) (27,28). However, available studies do not compare preinfection imaging and cannot account for preexisting airway disease.
What Is Post-COVID Interstitial Lung Disease?
The term post-COVID interstitial lung disease (ILD) involves many uncertainties, as several groups have emphasized (29–31). Although many COVID-19 survivors have residual lung parenchymal abnormalities, many of these abnormalities are mild, fibrosis is uncommon except in severe pneumonia, and a progressive course is not typical (23). Longitudinal CT imaging is helpful in some patients with symptoms for (a) distinguishing findings representing fibrosis from findings that may improve or resolve (Fig 7); (b) documenting the rate of improvement and the plateau after which findings are unlikely to substantially change; (c) evaluating uncommon cases of progressive fibrosis or cases of ILD associated with COVID-19, such as OP; and (d) assessment of worsening of existing ILD due to COVID-19.
It can be problematic to distinguish irreversible fibrosis from fibrotic-like findings that may improve. Many researchers have liberally applied the term post-COVID fibrosis to a wide range of parenchymal abnormalities at CT, such as irregular bronchial dilatation, reticulation, parenchymal bands, architectural distortion, and ground-glass opacities (Fig 8), without pathologic proof (32,33). However, these findings may improve over the course of weeks or months, similar to previous long-term studies of SARS-CoV-1, Middle East respiratory syndrome, and ARDS due to other causes (34,35). Irregular bronchial dilatation is common in DAD due to fibroblastic activity but frequently resolves in convalescence, arguing against use of the term traction bronchiectasis until long-term follow-up shows irreversibility. Honeycombing, the most certain sign of fibrosis, is uncommonly reported at CT in patients with COVID-19—in most series reported as 0%–5% (18,36). Parenchymal bands and residual perilobular opacities seen in many patients may represent previous organizing lung injury and do not always represent fibrosis. These issues have likely led to an overestimation of the frequency and degree of fibrosis, leading some authors to adopt the noncommittal term fibrotic-like changes. In addition, ground-glass opacities and reticulation can be reversible but could also represent fine fibrosis, correlating with nonspecific interstitial pneumonia in some pathology studies (17,29). A primary role for serial imaging is therefore assessing improvement and establishing the timing and extent of disease at the new baseline (Figs 6, 7). Improving CT findings should prompt the expectation of potential further clinical improvement, while a plateau in findings may suggest a new baseline and prompt optimization of other clinical factors. If the trajectory of CT findings is discrepant from changes in clinical status, other contributors should be considered, including pulmonary vascular disease.
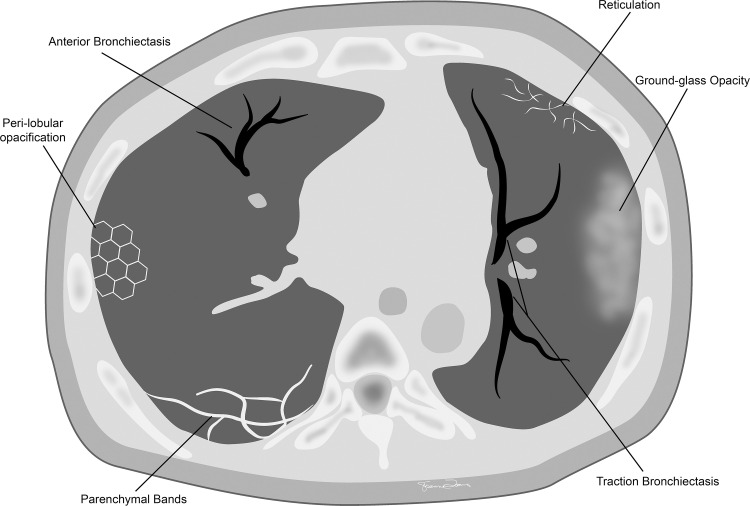
Figure 8:
Commonly seen chronic CT findings after COVID-19 infection.
A limited but substantial percentage of patients may have persistent or new OP after COVID-19 pneumonia. A transbronchial biopsy study of 50 selected patients from a post-COVID clinic with ongoing dyspnea and CT abnormalities (median, 108 days after diagnosis) showed histology of OP in most patients (32%) (37). Case reports and imaging and pathology series have also shown findings of OP and nonspecific interstitial pneumonia (17), and there are reports of new or worsening OP in the first few weeks to months after COVID-19 (38,39). However, reports of OP attributed to COVID-19 in the longer term (>6 months) are lacking. Although corticosteroid therapy is common in the case literature, there is no consensus guideline on the timing and use of steroids in post-COVID-19 OP (39).
In a small subset of patients, preexisting ILD may be worsened by COVID-19. A small surgical lung biopsy study of 18 patients with persistent respiratory symptoms after COVID-19 infection showed usual interstitial pneumonia fibrosis in 50% of cases. Of the usual interstitial pneumonia cases, nearly half had evidence of preexisting lung disease, and many had peripheral reticulation and bronchiectasis (40). Other reports suggest that an acute exacerbation of ILD may be responsible for the worsening of existing lung disease after COVID-19 (41).
Do Chronic Imaging Findings of COVID-19 Correlate with Symptoms and Respiratory Status?
Many studies show correlations between late imaging findings, symptoms, and pulmonary function tests. In a quantitative analysis of lung parenchymal findings 3 months after discharge for severe COVID-19 pneumonia, the extent of involvement showed significant correlations with fatigue, shortness of breath, cough, memory loss, and dizziness (18). Among pulmonary function tests, a decreased Dlco has been the most frequently reported abnormality (36), found in 35% of cases in a recent meta-analysis (42), and correlates with residual ground-glass opacity, reticulation, and air trapping (27). Spirometry findings are often normal or only mildly abnormal, even for severe cases; although total lung capacity is mildly decreased in some patients with CT abnormalities at 3 months after mechanical ventilation (43), lasting substantial restriction or obstruction is uncommon (27,44), with restriction seen in only 7% and obstruction seen in 3.8% at 12 months in a multicenter prospective study of 287 patients (36) and similar frequencies in a meta-analysis (42). The functional limitations that many patients with long COVID experience may also be a result of other factors, such as skeletal, cardiac, and respiratory muscle deconditioning and pulmonary vascular changes. For example, one study showed residual lung findings in 75% of ARDS survivors at 5 years, including ground-glass opacities, septal thickening, honeycombing, and mosaic attenuation, but findings were mild and not correlated with symptoms, pulmonary function test results, 6-minute walk test results, or quality of life (45). Long-term studies of swine flu (H1N1)-associated ARDS and SARS-CoV-1 survivors showed similar findings (35,46).
Are Patients with Post-COVID-19 at Increased Risk for PE and Other Pulmonary Vascular Abnormalities?
COVID-19 infection is associated with an increased risk of venous thromboembolic disease (VTE) (Fig 5), seen in approximately 5%–15% of patients, with a cumulative incidence as high as 49% at varying time points, with higher incidence in those with severe disease (47). Thrombotic events are seen despite VTE prophylaxis, in excess of baseline risk associated with hospitalization (47). The 90-day risk of thrombosis is higher in patients with COVID-19 infection than in those with influenza (hazard ratio, 1.89) (48). The increased risk of VTE in acute COVID-19 infection decreases after infection but persists into the early and late subacute phases. A national registry self-control case series study showed a significantly increased incidence rate ratio among patients with COVID-19 for deep vein thrombosis (DVT) for up to 70 days and for PE for up to 110 days (49). The incidence rate ratio for pulmonary embolism was 46.4 for days 8–14 after infection, 20.2 for days 15–30 after infection, 4.14 for days 31–60 after infection, and 2.48 for days 61–90 after infection. The incidence rate ratio for days 91–180 after infection was 1.4. The true risk for VTE beyond this is unknown but likely even lower. Factors associated with an increased risk of VTE after discharge include prior VTE, predischarge C-reactive protein level greater than 10 mg/mL, and peak d-dimer level greater than 3 μg/mL (50). Anticoagulation is associated with a decreased incidence of postdischarge VTE in patients previously hospitalized with COVID-19, although evidence does not currently support extended prophylaxis (50).
Several causes for the incidence of VTE with COVID-19 have been proposed. Severe cytokine release can activate the clotting cascade. Markedly elevated d-dimer level correlates with disease severity (51). Incidence of PE is higher than incidence of DVT, suggesting in situ thrombus formation is more frequent than embolic disease (52). Extensive endotheliitis may contribute to in situ thrombus formation (53), and local hypoxia may play a role (54).
Although the risk of acute VTE diminishes over time, long-term morbidity can be associated with prior VTE. Some patients will subsequently develop chronic thromboembolic pulmonary hypertension, with an estimated frequency of 0.1%–9.1% in patients without COVID-19 (55). In addition to the long-term morbidity of VTE, persistent endotheliitis and microthrombotic disease associated with COVID-19 infection may contribute to PASC symptoms, with the risk related to the duration of the hyperinflammatory state (2). A recent pathology series documented chronic vascular abnormalities with dilated and tortuous vessels and inflammatory cells with background normal parenchyma (17), echoing the known vascular abnormalities of acute COVID-19 (56). Pulmonary vascular and perfusion abnormalities at single-energy CT and dual-energy CT have been well documented in acute COVID-19 (57,58); the extent to which dual-energy CT angiography imaging and ventilation-perfusion imaging may be used to detect small perfusion abnormalities due to chronic microthrombotic disease in post-COVID-19 imaging is an area of active investigation.
What Imaging Should be Considered in the Follow-up of Post-COVID-19 Lung Abnormalities?
Strong evidence-based guidelines for follow-up of chronic pulmonary findings of COVID-19 are currently lacking. The few published recommendations are largely based on expert consensus. However, understanding of the various potential clinical roles of long-term imaging can provide a rational approach. Patients requiring hospitalization for moderate and severe COVID-19 have a high frequency of chronic imaging abnormalities and symptoms, and thin-section noncontrast CT at 3 and 6 months is reasonable in patients with symptoms and in patients in whom radiographic abnormalities have not completely resolved (24,59). Follow-up can establish the trajectory of improvement in findings, potentially informing clinical expectations and guiding any alternative clinical work-up or therapy. CT can track the evolution of any fibrotic-like findings or detect persistent, worsening, or new findings of potentially treatable disease, such as OP, infection, or other inflammatory lung disease. We agree with other groups that recommend typical noncontrast thin-section ILD-protocol CT, with supine inspiratory and expiratory series (to assess for air trapping), and with prone imaging as needed (31).
An additional CT examination at 12 months seems reasonable in symptomatic patients with persistent CT abnormalities at 6 months, as findings can continue to evolve for 1 year (23,60), paralleling long-term improvement in pulmonary function test results in some studies (61). In patients hospitalized with moderate COVID-19 pneumonia, fibrotic-like findings, such as bronchiectasis, parenchymal bands, and linear opacities, improved from 6 months to 1 year, while fibrotic findings of traction bronchiectasis and reticulation remained stable at a low frequency (2%) (23).
For patients with mild to moderate COVID-19 not requiring hospitalization, the chance of long-term lung parenchymal abnormalities is lower, and imaging should be limited to those with persistent and unexplained symptoms and chest radiographic or pulmonary function test abnormalities. Some patients with persistent symptoms and decreased Dlco have normal or mildly abnormal inspiratory chest CT findings; thus, a standard thin-section ILD protocol noncontrast CT with inspiratory and expiratory series may also be prudent for these patients to detect subtle interstitial abnormalities or air trapping.
Chest CT may be appropriate at any time to evaluate acute, new, or worsening respiratory symptoms after COVID-19 if radiography is inconclusive (24). Pulmonary CT angiography with single or dual energy is appropriate for any patient with symptoms of acute PE, clinical evidence of chronic pulmonary vascular disease, or respiratory findings disproportionate to the severity of CT findings (59). Ventilation-perfusion imaging can be used as an alternative modality; although it does not enable assessment of the pulmonary parenchyma, it could have a potential advantage in evaluation for a large volume of tiny thrombi that may be below the resolution of conventional pulmonary CT angiography (62) (Fig 9, Table).
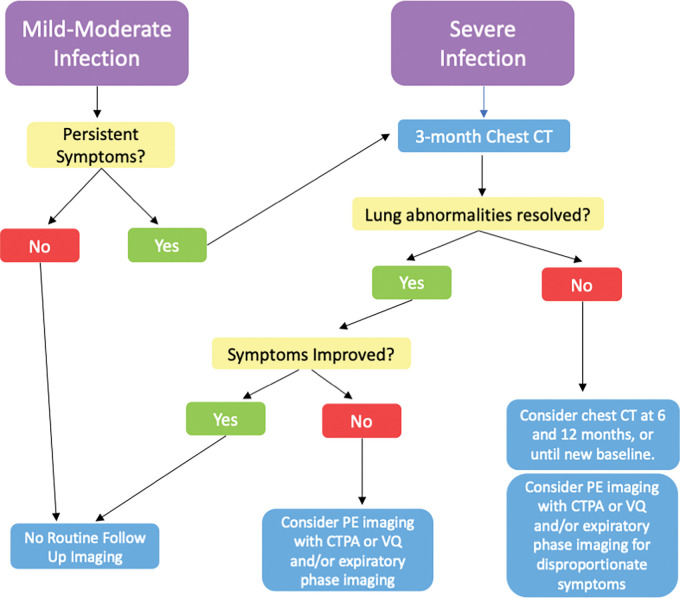
Figure 9:
Flowchart shows the timing of imaging follow-up and the role of imaging modalities after COVID-19 infection. CTPA = CT pulmonary angiography, PE = pulmonary embolus, V/Q = ventilation-perfusion.
Potential Roles of Imaging after COVID-19 Infection
Conclusion
Many patients demonstrate persistent lung abnormalities after COVID-19 infection. CT appearance can usually continue to improve for up to 1 year, and fibrosis is uncommon except in severe disease. The risk of PE is increased in the early convalescent period but decreases after several months. Imaging follow-up of symptomatic patients should include thin-section noncontrast chest CT. Inspiratory and expiratory CT, pulmonary CT angiography, and ventilation-perfusion nuclear imaging may play an important role in select cases.
Acknowledgments
Acknowledgment
The authors acknowledge Ms Susanne Loomis for her assistance with graphic design.
Disclosures of conflicts of interest: M.C.M. Internal seed grant from Ralph Schlaeger fund. B.P.L. No relevant relationships.
Abbreviations:
- ARDS
- acute respiratory distress syndrome
- DAD
- diffuse alveolar damage
- DLco
- diffusing capacity of the lung for carbon monoxide
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- ILD
- interstitial lung disease
- OP
- organizing pneumonia
- PASC
- post-acute sequelae of COVID-19
- PE
- pulmonary emboli
- VTE
- venous thromboembolic disease
References
- 1. Dong E , Du H , Gardner L . An interactive web-based dashboard to track COVID-19 in real time . Lancet Infect Dis 2020. ; 20 ( 5 ): 533 – 534 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nalbandian A , Sehgal K , Gupta A , et al . Post-acute COVID-19 syndrome . Nat Med 2021. ; 27 ( 4 ): 601 – 615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Y , Bowe B , Al-Aly Z . Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status . Nat Commun 2021. ; 12 ( 1 ): 6571 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havervall S , Rosell A , Phillipson M , et al . Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers . JAMA 2021. ; 325 ( 19 ): 2015 – 2016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joung SY , Ebinger JE , Sun N , et al . Awareness of SARS-CoV-2 Omicron Variant Infection Among Adults With Recent COVID-19 Seropositivity . JAMA Netw Open 2022. ; 5 ( 8 ): e2227241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung M , Bernheim A , Mei X , et al . CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) . Radiology 2020. ; 295 ( 1 ): 202 – 207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernheim A , Mei X , Huang M , et al . Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection . Radiology 2020. ; 295 ( 3 ): 685 – 691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan F , Ye T , Sun P , et al . Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19) . Radiology 2020. ; 295 ( 3 ): 715 – 721 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JE , Hwang M , Kim YH , et al . Imaging and Clinical Features of COVID-19 Breakthrough Infections: A Multicenter Study . Radiology 2022. ; 303 ( 3 ): 682 – 692 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoon SH , Lee JH , Kim BN . Chest CT Findings in Hospitalized Patients with SARS-CoV-2: Delta versus Omicron Variants . Radiology 2022. . 10.1148/radiol.220676 . Published online June 28, 2022 [Published correction appears in Radiology 2022;305(2):E66.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polyakov NJ , Som A , Mercaldo ND , Di Capua J , Little BP , Flores EJ . True-Positive Rate of RSNA Typical Chest CT Findings for COVID-19 Pneumonia in an Increasingly Vaccinated Population . Radiology 2022. . 10.1148/radiol.220680 . Published online September 6, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Som A , Lang M , Yeung T , et al . Implementation of the Radiological Society of North America Expert Consensus Guidelines on Reporting Chest CT Findings Related to COVID-19: A Multireader Performance Study . Radiol Cardiothorac Imaging 2020. ; 2 ( 5 ): e200276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson S , Kay FU , Abbara S , et al . Radiological Society of North America Expert Consensus Document on Reporting Chest CT Findings Related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA . Radiol Cardiothorac Imaging 2020. ; 2 ( 2 ): e200152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi H , Itoh T , Sasaki Y , Konishi J . Diagnostic imaging of idiopathic adult respiratory distress syndrome (ARDS)/diffuse alveolar damage (DAD) histopathological correlation with radiological imaging . Clin Imaging 1996. ; 20 ( 1 ): 1 – 7 . [DOI] [PubMed] [Google Scholar]
- 15. Sofizan NMFBN , Rahman AFBA , Soon LP , Ly CK , Abdullah NZB . Autopsy findings in COVID-19 infection-related death: a systematic review . Egypt J Forensic Sci 2022. ; 12 ( 1 ): 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kianzad A , Meijboom LJ , Nossent EJ , et al . COVID-19: Histopathological correlates of imaging patterns on chest computed tomography . Respirology 2021. ; 26 ( 9 ): 869 – 877 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ravaglia C , Doglioni C , Chilosi M , et al . Clinical, radiological and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection . Eur Respir J 2022. ; 60 ( 4 ): 2102411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazar M , Barbu EC , Chitu CE , et al . Interstitial Lung Fibrosis Following COVID-19 Pneumonia . Diagnostics (Basel) 2022. ; 12 ( 8 ): 2028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hama Amin BJ , Kakamad FH , Ahmed GS , et al . Post COVID-19 pulmonary fibrosis; a meta-analysis study . Ann Med Surg (Lond) 2022. ; 77 : 103590 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han X , Fan Y , Alwalid O , et al . Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia . Radiology 2021. ; 299 ( 1 ): E177 – E186 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu D , Zhang W , Pan F , et al . The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study . Respir Res 2020. ; 21 ( 1 ): 125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bharat A , Querrey M , Markov NS , et al . Lung transplantation for patients with severe COVID-19 . Sci Transl Med 2020. ; 12 ( 574 ): eabe4282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe A , So M , Iwagami M , et al . One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis . Respirology 2022. ; 27 ( 8 ): 605 – 616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Treggiari MM , Romand JA , Martin JB , Suter PM . Air cysts and bronchiectasis prevail in nondependent areas in severe acute respiratory distress syndrome: a computed tomographic study of ventilator-associated changes . Crit Care Med 2002. ; 30 ( 8 ): 1747 – 1752 . [DOI] [PubMed] [Google Scholar]
- 25. Bocchino M , Lieto R , Romano F , et al . Chest CT-based Assessment of 1-year Outcomes after Moderate COVID-19 Pneumonia . Radiology 2022. ; 305 ( 2 ): 479 – 485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martini K , Larici AR , Revel MP , et al . European Society of Thoracic Imaging (ESTI), the European Society of Radiology (ESR). COVID-19 pneumonia imaging follow-up: when and how? A proposition from ESTI and ESR . Eur Radiol 2022. ; 32 ( 4 ): 2639 – 2649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jia X , Han X , Cao Y , et al . Quantitative inspiratory-expiratory chest CT findings in COVID-19 survivors at the 6-month follow-up . Sci Rep 2022. ; 12 ( 1 ): 7402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franquet T , Giménez A , Ketai L , et al . Air trapping in COVID-19 patients following hospital discharge: retrospective evaluation with paired inspiratory/expiratory thin-section CT . Eur Radiol 2022. ; 32 ( 7 ): 4427 – 4436 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wells AU , Devaraj A , Desai SR . Interstitial Lung Disease after COVID-19 Infection: A Catalog of Uncertainties . Radiology 2021. ; 299 ( 1 ): E216 – E218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanne JP , Little BP , Schulte JJ , Haramati A , Haramati LB . Long-Term Lung Abnormalities Associated with COVID-19 Pneumonia . Radiology 2022. . 10.1148/radiol.221806 . Published online August 30, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solomon JJ , Heyman B , Ko JP , Condos R , Lynch DA . CT of Post-Acute Lung Complications of COVID-19 . Radiology 2021. ; 301 ( 2 ): E383 – E395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu M , Liu Y , Xu D , Zhang R , Lan L , Xu H . Prediction of the Development of Pulmonary Fibrosis Using Serial Thin-Section CT and Clinical Features in Patients Discharged after Treatment for COVID-19 Pneumonia . Korean J Radiol 2020. ; 21 ( 6 ): 746 – 755 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gulati A , Lakhani P . Interstitial lung abnormalities and pulmonary fibrosis in COVID-19 patients: a short-term follow-up case series . Clin Imaging 2021. ; 77 : 180 – 186 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu X , Dong D , Ma D . Thin-Section Computed Tomography Manifestations During Convalescence and Long-Term Follow-Up of Patients with Severe Acute Respiratory Syndrome (SARS) . Med Sci Monit 2016. ; 22 : 2793 – 2799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ong KC , Ng AWK , Lee LSU , et al . Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome . Eur Respir J 2004. ; 24 ( 3 ): 436 – 442 . [DOI] [PubMed] [Google Scholar]
- 36. Faverio P , Luppi F , Rebora P , et al . One-year pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study . Respir Res 2022. ; 23 ( 1 ): 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Culebras M , Loor K , Sansano I , et al . Se-COVID-19 team. Histological Findings in Transbronchial Cryobiopsies Obtained From Patients After COVID-19 . Chest 2022. ; 161 ( 3 ): 647 – 650 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ng BH , Ban AYL , Nik Abeed NN , Faisal M . Organising pneumonia manifesting as a late-phase complication of COVID-19 . BMJ Case Rep 2021. ; 14 ( 10 ): e246119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vadász I , Husain-Syed F , Dorfmüller P , et al . Severe organising pneumonia following COVID-19 . Thorax 2021. ; 76 ( 2 ): 201 – 204 . [DOI] [PubMed] [Google Scholar]
- 40. Konopka KE , Perry W , Huang T , Farver CF , Myers JL . Usual Interstitial Pneumonia is the Most Common Finding in Surgical Lung Biopsies from Patients with Persistent Interstitial Lung Disease Following Infection with SARS-CoV-2 . EClinicalMedicine 2021. ; 42 : 101209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fonseca M , Summer R , Roman J . Acute Exacerbation of Interstitial Lung Disease as a Sequela of COVID-19 Pneumonia . Am J Med Sci 2021. ; 361 ( 1 ): 126 – 129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee JH , Yim JJ , Park J . Pulmonary function and chest computed tomography abnormalities 6-12 months after recovery from COVID-19: a systematic review and meta-analysis . Respir Res 2022. ; 23 ( 1 ): 233 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Gassel RJJ , Bels JLM , Raafs A , et al . High Prevalence of Pulmonary Sequelae at 3 Months after Hospital Discharge in Mechanically Ventilated Survivors of COVID-19 . Am J Respir Crit Care Med 2021. ; 203 ( 3 ): 371 – 374 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bellan M , Baricich A , Patrucco F , et al . Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19 . Sci Rep 2021. ; 11 ( 1 ): 22666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilcox ME , Patsios D , Murphy G , et al . Radiologic outcomes at 5 years after severe ARDS . Chest 2013. ; 143 ( 4 ): 920 – 926 . [DOI] [PubMed] [Google Scholar]
- 46. Luyt CE , Combes A , Becquemin MH , et al. ; REVA Study Group . Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS . Chest 2012. ; 142 ( 3 ): 583 – 592 . [DOI] [PubMed] [Google Scholar]
- 47. Al-Ani F , Chehade S , Lazo-Langner A . Thrombosis risk associated with COVID-19 infection. A scoping review . Thromb Res 2020. ; 192 : 152 – 160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lo Re V 3rd , Dutcher SK , Connolly JG , et al . Association of COVID-19 vs Influenza With Risk of Arterial and Venous Thrombotic Events Among Hospitalized Patients . JAMA 2022. ; 328 ( 7 ): 637 – 651 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katsoularis I , Fonseca-Rodríguez O , Farrington P , et al . Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study . BMJ 2022. ; 377 : e069590 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li P , Zhao W , Kaatz S , Latack K , Schultz L , Poisson L . Factors Associated With Risk of Postdischarge Thrombosis in Patients With COVID-19 . JAMA Netw Open 2021. ; 4 ( 11 ): e2135397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao Y , Cao J , Wang Q , et al . D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study . J Intensive Care 2020. ; 8 ( 1 ): 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Birocchi S , Manzoni M , Podda GM , Casazza G , Cattaneo M . High rates of pulmonary artery occlusions in COVID-19. A meta-analysis . Eur J Clin Invest 2021. ; 51 ( 1 ): e13433 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ackermann M , Verleden SE , Kuehnel M , et al . Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19 . N Engl J Med 2020. ; 383 ( 2 ): 120 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ten VS , Pinsky DJ . Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction . Curr Opin Crit Care 2002. ; 8 ( 3 ): 242 – 250 . [DOI] [PubMed] [Google Scholar]
- 55. Lang IM , Madani M . Update on chronic thromboembolic pulmonary hypertension . Circulation 2014. ; 130 ( 6 ): 508 – 518 . [DOI] [PubMed] [Google Scholar]
- 56. Villalba JA , Hilburn CF , Garlin MA , et al . Vasculopathy and Increased Vascular Congestion in Fatal COVID-19 and Acute Respiratory Distress Syndrome . Am J Respir Crit Care Med 2022. ; 206 ( 7 ): 857 – 873 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Patel BV , Arachchillage DJ , Ridge CA , et al . Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations . Am J Respir Crit Care Med 2020. ; 202 ( 5 ): 690 – 699 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lang M , Som A , Mendoza DP , et al . Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT . Lancet Infect Dis 2020. ; 20 ( 12 ): 1365 – 1366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. George PM , Barratt SL , Condliffe R , et al . Respiratory follow-up of patients with COVID-19 pneumonia . Thorax 2020. ; 75 ( 11 ): 1009 – 1016 . [DOI] [PubMed] [Google Scholar]
- 60. Vijayakumar B , Tonkin J , Devaraj A , et al . CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge . Radiology 2022. ; 303 ( 2 ): 444 – 454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steinbeis F , Thibeault C , Doellinger F , et al . Severity of respiratory failure and computed chest tomography in acute COVID-19 correlates with pulmonary function and respiratory symptoms after infection with SARS-CoV-2: An observational longitudinal study over 12 months . Respir Med 2022. ; 191 : 106709 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dhawan RT , Gopalan D , Howard L , et al . Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19 . Lancet Respir Med 2021. ; 9 ( 1 ): 107 – 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]