Abstract
An inducible promoter system provides a powerful tool for studying the genetic basis for virulence. A variety of inducible systems have been used in other organisms, including pXyl-xylR-inducible promoter, the pSpac-lacI system, and the arabinose-inducible PBAD promoter, but each of these systems has limitations in its application to Staphylococcus aureus. In this study, we demonstrated the efficacy of a tetracycline-inducible promoter system in inducing gene expression in S. aureus in vitro and inside epithelial cells as well as in an animal model of infection. Using the xyl/tetO promoter::gfpuvr fusion carried on a shuttle plasmid, we demonstrated that dose-dependant tetracycline induction, as measured by bacterial fluorescence, occurred in each of the above environments while basal activation under noninduced conditions remained low. To ascertain how the system can be used to elucidate the genetic basis of a pathogenic phenotype, we cloned the sigB gene downstream of the inducible promoter. Induction of SigB expression led to dose-dependent attachment of the tested strain to polystyrene microtiter wells. Additionally, bacterial microcolony formation, an event preceding mature biofilm formation, also increased with tetracycline induction of SigB.
Staphylococcus aureus is an important human pathogen that causes a variety of serious infections, including pneumonia, endocarditis, and sepsis (2). The recent emergence of vancomycin-resistant S. aureus strains has highlighted the need to identify potential targets for the development of novel antimicrobial therapies. Prime among these are virulence factors that contribute to pathogenesis. The evaluation of virulence genes in S. aureus has traditionally been conducted via gene knockout methods followed by complementation, often with a multicopy plasmid that overexpresses the gene product constitutively. An improvement to this method for assaying gene function is to induce gene expression with an inducible promoter system. Using such a system, gene expression can be titrated and the corresponding phenotype analyzed. Accordingly, more quantitative data on the role of a particular gene product in pathogenesis can be obtained.
A number of inducible promoter systems have been considered for use in S. aureus. These include the pXyl-xylR-inducible promoter (13) and the pSpac-lacI system (21) in Bacillus subtilis and the arabinose-inducible PBAD promoter from Escherichia coli (8). In exploring these promoters as tools to evaluate pathogenesis in S. aureus, each of these systems displayed major deficiencies that prevented their deployment. For instance, the xylose-inducible promoter system is repressible by glucose, a common constituent inside mammalian cells, thus prohibiting its use in in vivo studies. This repression cannot be readily relieved by a higher concentration of xylose, even in medium with low glucose concentration (unpublished data). The pSpac/lacI system was also not readily adaptable to S. aureus due, in part, to its high basal promoter activity (unpublished data). Additionally, induction with IPTG (isopropylthiogalactopyranoside) for the pSpac promoter renders this system less attractive in an animal model system. Finally, the arabinose-inducible PBAD promoter has not been proven useful in S. aureus, probably due to poor penetration of arabinose into staphylococci.
The tetracycline-inducible promoter system, first described in E. coli and B. subtilis (7), was recently adapted to S. aureus (11, 22). This system appears to be useful for investigating the contribution of virulence genes to S. aureus pathogenesis both in vitro and in vivo. Using the reporter gene gfpuvr cloned downstream of the xyl/tetO promoter, we report here successful and dose-dependent tetracycline induction in three different experimental conditions, including in vitro growth, bacteria within epithelial cells, and a murine airpouch model. With this expression system, we also demonstrated the role of the alternative transcription factor ςB (14, 20) in mediating microcolony formation, a prerequisite step for mature biofilm formation. Taken together, these data suggest that the tetracycline-inducible promoter system is a powerful tool, both in vitro and in vivo, for investigating the contribution of virulence genes in the pathogenesis of S. aureus infections.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth medium.
The bacterial strains and plasmids used in this study are listed in Table 1. TSB (tryptic soy broth) was used to grow S. aureus strains, while LB (Luria-Bertani) was used for E. coli. Chloramphenicol was used at 10 μg/ml and ampicillin at 50 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Reference | Description |
|---|---|---|
| S. aureus | ||
| RN6390 | 17 | Laboratory strain that maintains its hemolytic pattern when propagated on sheep erythrocytes and has a genetic background similar to that of 8325-4 |
| RN4220 | 17 | Mutant of 8325-4 that accepts foreign DNA |
| ALC1001 | 3 | sigB mutant of RN6390 |
| ALC1497 | 3 | ALC1001 complemented with shuttle plasmid pALC1496 (with the sigB gene) |
| ALC2158 | This study | RN6390 containing pALC2073 |
| ALC2085 | This study | RN6390 containing pALC2084 (pALC2073::gfpuvr) |
| ALC2109 | This study | RN6390 containing pALC2073::sigB |
| E. coli | ||
| XL-1 Blue | Cloning strain | |
| TOP10F′ | Invitrogen | Strain for cloning PCR fragments |
| Plasmids | ||
| pCR2.1 | Invitrogen | PCR cloning vector |
| pSK236 | 6 | S. aureus-E. coli shuttle vector with pUC19 cloned into the HindIII site of pC194 |
| pWH353 | 7 | Plasmid carrying an 800-bp fragment comprising the tetR gene (encoding the TetR repressor) and the xyl/tetO promoter |
| pALC2073 | This study | pSK236 containing the tetR gene and the xyl/tetO promoter that were cleaved from pWH353 and cloned into the PstI and SmaI sites |
| pALC2084 | This study | pALC2073 with gfpuvr cloned into the EcoRI site |
| pALC2109 | This study | pALC2073 with sigB cloned into the EcoRI site |
Genetic manipulations in E. coli and S. aureus
All restriction enzymes were acquired from Gibco-BRL Scientific (Coon Rapids, Minn.). An 800-bp fragment comprising the tetR gene (encoding the TetR repressor) and the xyl/tetO promoter was cleaved from pWH353 (7) and cloned into the PstI and SmaI sites of shuttle plasmid pSK236. Correct insertion into the recombinant plasmid was confirmed by restriction mapping and sequencing. This construct was designated pALC2073.
To test the activity of the inducible promoter system, we constructed a plasmid in which the inducible xyl/tetO promoter drives the expression of the green florescent protein gene, gfpuvr. The gfpuvr gene was constructed by introducing an S65T mutation into gfpuv (Clontech, Palo Alto, Calif.), effecting a shift in the excitation maximum from 395 to 488 nm. This gene, gfpuvr, was cloned into the EcoRI site downstream from the inducible promoter in pALC2073. The correct insertion was determined by restriction mapping and sequencing. The recombinant plasmid was first electroporated into RN4220 and then finally into RN6390, as described (19).
To construct a plasmid containing sigB downstream from the inducible promoter, the sigB gene was first amplified by PCR using chromosomal DNA from strain RN6390 as the template. The following primer pairs were used for the amplification: 5′-GCTCTAGAGGGAGGTTTTAAACATGGCGAAAGAGTCGAAATCAGCT-3′ and 5′-ACGCGTCGACCTATTGATGTGCTGCTTCTTG-3′, with the XbaI and SalI sites in italic. The PCR product was ligated into pCR2.1 (Invitrogen, Carlsbad, Calif.). The sigB gene in pCR2.1 was cleaved and cloned downstream of the tetracycline-inducible promoter in pALC2073 at the EcoRI site. Correct orientation of the insert was confirmed by restriction mapping and sequencing. This plasmid was electroporated into RN4220 and then into RN6390 (19).
Analysis of tetracycline-inducible promoter in vitro
RN6390 containing pALC2073 (ALC2158) and pALC2084 (pALC2073::gfpuvr) (ALC2085) were grown overnight in TSB with chloramphenicol. The bacteria were then diluted 1:100 in TSB containing chloramphenicol and further cultured at 37°C with shaking (225 rpm) to an optical density at 650 nm (OD650) of 0.5. Tetracycline was then added at various concentrations (range, 0 to 500 ng/ml) that represented subinhibitory concentrations. The bacteria were sampled hourly (100 μl) in triplicate and analyzed in microtiter wells for fluorescence and OD650 simultaneously, using a multipurpose fluorescence spectrophotometer (FL600; BioTek Instruments, Winooski, Vt.). Results were reported as total fluorescence (FL) units/OD650 unit to minimize the variation in fluorescence due to cell densities.
Analysis of tetracycline-inducible promoter in CFT-1 epithelial cells.
CFT-1 cells, an immortalized cell line derived from the tracheal epithelial cells of a cystic fibrosis patient with a ΔF508 mutation in the CFTR gene, were grown on vitronectin-treated (Cohesion, Palo Alto, Calif.) coverslips (MatTek, Ashland, Mass.) at 37°C with 5% CO2 until confluent. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Mediatech, Herndon, Va.) with 2 mM l-glutamine and 100 U of penicillin G, 100 μg of streptomycin, 250 ng of amphotericin B, 10% fetal bovine serum (FBS; Sigma, St. Louis, Mo.), 10 μg of insulin, 1 μM hydrocortisone, 3.75 μg of endothelial cell growth supplement, 25 ng of epidermal growth factor, 30 nM triiodothyronine, 5 μg of transferrin, and 10 ng of cholera toxin per ml (12). Prior to the assay, monolayers were washed three times with phosphate-buffered saline (PBS) and incubated for 1 h in DMEM/F12 plus 5 mM l-glutamine and 1% FBS.
ALC2085 from an overnight culture of TSB containing chloramphenicol was diluted 1:50 in similar medium and grown to OD650 of 0.6. These bacteria were washed twice in PBS, passed through a 5-μm filter to remove cellular aggregates, and finally resuspended in PBS. Bacteria were added to CFT-1 cells at a multiplicity of infection (MOI) of 10:1 (bacteria-host cell ratio) and incubated for 2 h at 37°C and 5% CO2. Monolayers were then washed three times with PBS to remove unattached bacteria and incubated for 4 h at 37°C with 5% CO2 in medium containing 200 μg of gentamicin (Sigma) per ml to kill extracellular ALC2085 or gentamicin plus tetracycline to induce gene expression. Induction of GFP expressed by intracellular bacteria was observed with a Leica DM IRBE microscope (Leica Microsystems, Buffalo, N.Y.) using Hamamatsu camera controller C4742-95 (Bridgewater, N.J.) and OpenLab software (Improvision, Lexington, Mass.).
Analysis of tetracycline-inducible promoter in murine airpouch model.
To assess the utility of the tetracycline-inducible promoter system in vivo, we employed a murine airpouch model in which ALC2085 was used to colonize or infect a subcutaneous airpouch. To create the airpouch, 5 ml of air in a 5-ml syringe was injected intradermally with a 26-gauge needle on the posterior side of the mouse. Three days later, the airpouch was reenforced by an additional injection of 2.5 ml of air into the same pocket. After 3 more days, 108 CFU of S. aureus strain ALC2085(pALC2084) was inoculated into the airpouch. To induce gene expression, 100 μg of tetracycline was injected introperitoneally immediately following infection and subsequently every 12 h for 48 h. This amount of tetracycline has been titrated for expression in pilot studies. PBS injection was used as the control. Twelve hours after the last tetracycline injection, the airpouch was lavaged with 1 ml of PBS (pH 7.4). The aspirant was then diluted 1:100 in sterile PBS and analyzed for the population of fluorescent bacteria with a FACScan (Becton Dickinson, Franklin Lakes, N.J.).
Preparation of cell extracts for SigB detection by Western blot.
RN6390, carrying the plasmid containing the tetracycline-inducible promoter that drives the expression of sigB (ALC2109) and the vector control (ALC2158), was grown in a 25-ml culture overnight in various concentrations of tetracycline. Following pelleting, the cells were resuspended in 1 ml of TEG buffer (25 mM Tris, 5 mM EGTA, pH 8), and cell extracts were prepared using lysostaphin (AMBI, Purchase, N.Y.) as described (4). Cell extracts were then calibrated for total cellular proteins, and 50 μg of each sample was loaded on a sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS-PAGE) gel, electrophoresed, and immunoblotted onto nitrocellulose as described (1).
To detect ςB, anti-ςB monoclonal antibody 1D1 diluted 1:1,000 was allowed to incubate with the immunoblot for 3 h. The blot was then washed and incubated with affinity-purified goat anti-mouse immunoglobulin antibody conjugated to alkaline phosphatase (Jackson ImmunoResearch, West Grove, Pa.) at a 1:10,000 dilution. Reactive bands were then detected with developing substrates as described (1). The intensities of the reactive bands were quantitated by densitometric analysis, using SigmaGel software (Jandel Scientific, San Rafael, Calif.).
Microcolony formation and adherence assays.
S. aureus strains analyzed for bacterial aggregation and microcolony formation were inoculated in TSB and grown at 37°C to an OD650 of 1.1. The bacteria were then diluted 1:100 in TSB with appropriate antibiotics (including increasing amounts of tetracycline when induction was desired), and 100 μl of each mixture was then added to the well of a polystyrene 96-well tissue culture plate (Corning, Corning, N.Y.). The bacteria were then grown at 37°C for 24 h without shaking. To assess microcolony formation, microscopy was used to examine intercellular aggregation upon induction of ςB with tetracycline, using the 10× objective of a Leica DM IRBE microscope.
To quantitate bacterial adherence, a prelude to mature biofilm formation, nonadherent bacteria from overnight cultures were removed from the microtiter wells with gentle suction. The wells were then washed three times with distilled water and air dried for 10 min. Attached bacteria were stained with 150 μl of 0.04% safranin O solution (in 20% ethanol). Staining was allowed to proceed for 10 min, after which the plate was washed four times with water and dried. For quantitation, 100 μl of 30% acetic acid was added to each well to dissolve the stain. The OD405 of the dissolved stain was then measured in a multipurpose enzyme-linked immunosorbent assay (ELISA) reader (FL600).
RESULTS
Functionality of tetracycline-inducible promoter in vitro.
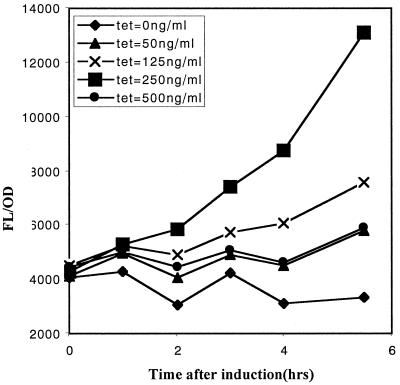
To test the inducible promoter in vitro and to ascertain the optimal concentration of tetracycline for induction, we used the gfpuvr reporter gene that we have cloned downstream of the inducible promoter in recombinant shuttle plasmid pALC2058. The promoter activity in strain RN6390 was then analyzed by measuring bacterial fluorescence as an indicator of GFPuvr expression. Our data revealed dose-dependant induction of the xyl/tetO promoter, with maximum induction at a tetracycline concentration of 250 ng/ml (Fig. 1). At tetracycline concentrations exceeding this value (e.g., 500 ng/ml), growth retardation occurred, corresponding to a decrease in promoter activity due to lower cell number. Importantly, we were not able to detect the activity of the promoter under noninduced conditions during the entire time course of this experiment (5 h), as indicated by the relatively flat FL/OD650 response of ALC2085 in the absence of tetracycline, similar to RN6390 containing the vector control when grown under identical conditions (data not shown).
FIG. 1.
Induction of the xyl/tetO promoter linked to the gfpuvr reporter with increasing concentrations of tetracycline in strain ALC2085. Following overnight culture, the cells were diluted 1:100 in fresh TSB and then grown to an OD650 of 0.5. Tetracycline was then added to the final concentrations shown above. The cells were sampled hourly following induction (100 μl each, in triplicate). Fluorescence and OD650 measurements were obtained using a multipurpose spectrophotometer (FL600; Biotek Instrument). Results are presented as reported fluorescence (FL) units/OD650 to account for variations in fluorescence due to cell density. Data are given as the mean of the triplicate measurements. Error bars are too small to be shown. The experiment was repeated twice, with one representative experiment shown.
Functionality of inducible promoter inside CFT-1 epithelial cells.
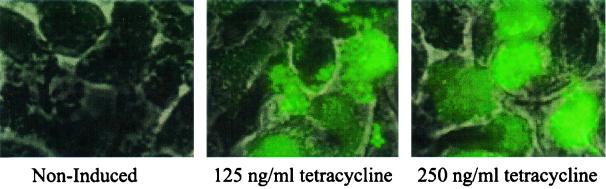
To determine the tetracycline inducibility of the xyl/tetO promoter of pALC2084 inside epithelial cells, we incubated S. aureus strain ALC2085 (RN6390 with pALC2084) with CFT-1 cells (see Materials and Methods) followed by treatment with medium containing gentamicin (to kill extracellular S. aureus) in the presence and absence of tetracycline for 4 h. In previous studies, we have shown that internalization of live S. aureus by CFT-1 cells occurred efficiently with this method (12). Following internalization, monolayers of CFT-1 cells containing intracellular ALC2085 were examined by fluorescent microscopy for GFP expression. As shown in Fig. 2, tetracycline was accessible to the intracellular compartment containing ALC2085 and able to induce the expression of GFP at concentrations ranging from 125 to 250 ng/ml, while no GFP expression was detected in the absence of tetracycline.
FIG. 2.
Tetracycline-induced GFP expression of ALC2085 inside CFT-1 epithelial cells. ALC2085, grown to an OD650 of 0.6, was added to CFT-1 monolayers at an MOI of 10:1 for 2 h. Extracellular bacteria were removed by washing followed by the addition of gentamicin (200 μg/ml) for 4 h to kill any remaining extracellular microorganisms. Tetracycline was added to some wells to facilitate induction. Cells were then examined by fluorescence microscopy. Most of the bacteria were intracellular, as evidenced by the failure of these bacteria to stain with anti-protein A antibody conjugated to Texas Red (data not shown). The experiment was repeated twice, with one representative experiment shown. Upon tetracycline induction of the xyl/tetO promoter of intracellular bacteria driving the expression of GFP, green fluorescence was observed only in tissue culture wells containing tetracycline.
Functionality of inducible promoter in murine airpouch model.
To evaluate the utility of the tetracycline-inducible promoter in vivo, we employed a murine airpouch model. Following the creation of an airpouch, mice were infected with ALC2085 at 108 CFU. Subsequently, the mice were each given an IP injection of 100 μg of tetracycline or PBS every 12 h for 48 h. The airpouch was lavaged 12 h after the last IP injection, and the lavage fluid was analyzed with a FACScan (Becton Dickinson). Ten thousand events were acquired for each sample and counts of detected events were plotted as a function of fluorescence. As shown in Fig. 3, a higher proportion of fluorescent bacteria were detected under the induced condition compared with the noninduced control.
FIG. 3.
Flow cytometry of lavaged bacteria obtained from the murine airpouch. An airpouch was created on the posterior side of the mouse. ALC2085 (RN6390 with a plasmid carrying the inducible promoter driving gfpuvr) was injected into the airpouch at 108 CFU. Tetracycline (100 μg) or a PBS injection was given every 12 h for 48 h. At 12 h after the final injection, the airpouch was lavaged with 1 ml of PBS. The aspirant was then diluted 1:1,000 in PBS (pH 7.4) and analyzed on a FACScan (Becton Dickinson). Ten thousand events were detected by forward scatter and a histogram was generated, showing the number of counts as a function of fluorescence. The experiment was repeated with two mice, with one representative experiment shown.
Employment of tetracycline-inducible system to demonstrate the role of ςB in attachment and microcolony formation.
To demonstrate how the inducible promoter system might be used to explore the regulation of a pathogenic phenotype, we examined SigB under inducible conditions in an attempt to study its role in biofilm formation. For this purpose, we cloned a ribosome-binding site together with the coding region of sigB downstream of the inducible promoter in shuttle plasmid pALC2073. The recombinant plasmid pALC2109 was electroporated into RN4220 and then into RN6390 (see Materials and Methods), a strain that is partially deficient (not absent [unpublished data]) in ςB expression due to an 11-bp deletion in rsbU.
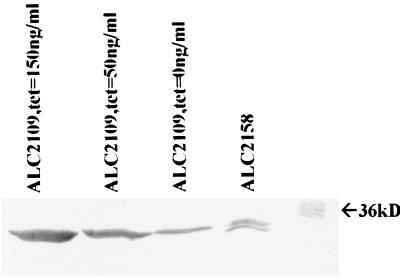
To ensure that the RN6390 clone carrying pALC2109 was synthesizing ςB in a dose-dependent manner upon induction, the strain was grown in various amounts of tetracycline in overnight cultures from which cell extracts were prepared, and 50 μg of total protein from each extract was run on an SDS gel and immunoblotted. The blot was probed with 1D1 anti-ςB monoclonal antibody at a dilution of 1:1,000, followed by an appropriate conjugate and developing substrates. As shown in Fig. 4, the expression of ςB correlated well with tetracycline induction in a dose-dependant fashion. Densitometric analysis revealed 719 densitometric units for the noninduced culture, 1,800 units for tetracycline induction at 50 ng/ml, and 3,297 units for induction at 150 ng/ml. As a control, we used RN6390 containing the vector pALC2073 alone (ALC2158). As anticipated from a SigB-deficient strain, SigB was also expressed from ALC2158, but the level of expression was low (556 densitometric units for the intact Sigma and 248 units for the degradative product); this level of expression was similar to that of ALC2109 without induction.
FIG. 4.
Immunoblot of cell extracts of ALC2109 (RN6390 with a plasmid containing the inducible promoter driving sigB) and ALC2158 (RN6390 with the vector control) as detected by anti-SigB monoclonal antibody 1D1. The strain was grown overnight in TSB with the amount of tetracycline indicated. Cell extracts were prepared, and 50 μg of total protein each was resolved by SDS-PAGE and blotted onto nitrocellulose. SigB protein was detected with anti-SigB monoclonal antibody 1D1 (1:1,000 dilution). The dose-dependent induction of SigB was confirmed by densitometry of the reactive bands (see text). The experiment was repeated twice, with one representative experiment shown.
Having established the induction of ςB expression by tetracycline in strain ALC2109, we proceeded to ascertain the effect of ςB expression on staphylococcal attachment to microtiter wells, a simulated event that mirrors early biofilm formation in vitro (23). Bacterial attachment was assessed by staining with safranin the adherent colonies on the bottom of the microtiter wells with overnight cultures. The safranin stain was then solubilized by the addition of 30% acetic acid. The OD405 for each well was then determined using a multipurpose spectrophotometer (FL600). As controls, we tested RN6390 (ςB deficient), ALC1001 (RN6390 with a ςB mutation), and ALC1497 (ALC1001 with a multicopy plasmid encoding the ςB operon) (3).
Although both RN6390 and ALC1001 attached poorly, ALC1497, a strain that hyperexpresses ςB, attached well. Interestingly, bacterial attachment of ALC2109 to microtiter wells was proportional to the induction of ςB (Fig. 5 and 4), with maximal adherence occurring at a tetracycline concentration of 150 ng/ml. At higher concentrations, growth was inhibited under the nonshaking and oxygen-limiting conditions of microtiter wells.
FIG. 5.
Bacterial attachment to polystyrene microtiter wells. Cells were grown in TSB for 24 h in a 96-well polystyrene tissue culture plate. The plate was then washed three times gently with distilled water. The wells were stained with safranin O for 10 min, washed, and air dried. Then 100 μl of 30% acetic acid was added to each well to solubilize the stain. The OD405 for each well was then determined. Each of the conditions was replicated in eight wells. The mean OD405 values for each condition are displayed, with the error bar showing the standard deviation from the mean.
To ensure that biofilm formation was attributable to ςB induction and not a consequence of antibiotic stress, ALC2158 (RN6390 with pALC2073), a strain with just the inducible promoter, we also tested for biofilm formation after growth in medium containing various amounts of tetracycline (0 to 150 ng/ml). At all tetracycline concentrations, ALC2158 failed to produce biofilm above parental levels (data not shown).
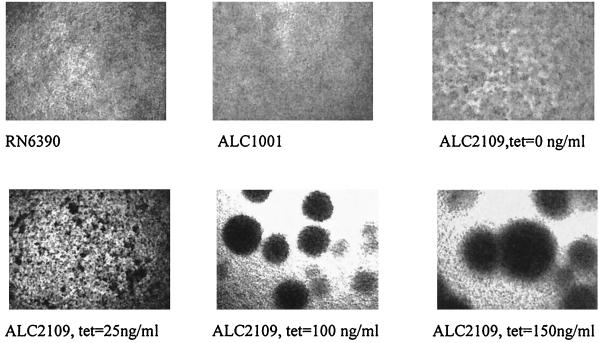
It has been suggested that bacterial attachment and subsequent microcolony formation or bacterial aggregation precedes biofilm formation (23). Following overnight growth under identical conditions as for the attachment assay, we observed increasing microcolony formation as a result of bacterial aggregation by microscopy. As shown in Fig. 6, the size of the autoaggregates was proportional to the ςB level attributable to tetracycline induction.
FIG. 6.
Microcolony formation with various levels of ςB induction in response to increasing tetracycline concentrations. Strains were grown in a 96-well polystyrene tissue culture plate under the same conditions used in the attachment assay (see Materials and Methods). Microscopy was done at 10× magnification. Strain RN6390 disclosed a very low level of intercellular aggregation, while the isogenic sigB mutant ALC1001 did not exhibit any tendency toward cellular aggregation. Upon induction of ςB expression by increasing concentrations of tetracycline, intercellular aggregation, as reflected by the size of the microcolony, was found to increase.
DISCUSSION
In evaluating the genetic basis for virulence in S. aureus, there is a need for an inducible promoter system that can be employed both in vitro and in vivo. Using such a system, gene expression can be titrated and the corresponding phenotype analyzed, thereby providing insights into the pathogenesis of specific genes. We have previously attempted to deploy a number of inducible systems in S. aureus, including pXyl-xylR, pSpac-lacI, and PBAD, and found each of them to have major deficiencies. In contrast, the tetracycline-inducible system, consisting of the tetR gene (encoding the TetR repressor) and the xyl/tetO promoter, has proven to be highly useful for evaluating S. aureus gene expression in vitro and in vivo, including those found in cultured epithelial cells and in an animal model of infection.
The tetracycline-inducible promoter system possesses several characteristics that make it ideally suited for genetic studies in S. aureus. First, the basal level of expression of the promoter is extremely low, as evidenced by the failure of the strain carrying the inducible promoter-gfpuvr construct to fluoresce above background levels under noninducing conditions. This finding allows meaningful comparison of phenotypes under induced and noninduced conditions while avoiding the problem of leaky expression at basal levels. Second, induction is dose dependent (Fig. 1), making it possible to titrate gene expression to wild-type levels. This system clearly represents an advance in the induction of gene expression over a multicopy plasmid that provides constitutive and, at times, uncontrollable expression. Third, a high level of promoter activity, if required, can be achieved with this system, as evidenced by the high level of GFPuvr production with the GFP reporter (Fig. 1), as well as by augmented ςB expression achieved with the inducible promoter-sigB fusion (Fig. 4).
Besides in vitro studies, this inducible promoter system has proven to be useful for studies of S. aureus inside epithelial cells. There is now substantial evidence that S. aureus is internalized into human epithelial cells as well as cultured osteoblasts (9, 10, 15, 16). Indeed, it was recently shown that S. aureus is not an innocent bystander during the internalization process by a pulmonary epithelial cell line, but rather replicates and induces apoptosis in host cells (12). Given that S. aureus, as an adaptive intracellular pathogen inside epithelial cells, may contribute toward chronicity in human infections (e.g., osteomyelitis and chronic abscesses), it will be essential to identify the S. aureus genes that facilitate this pathogenic process.
We argue that the tetracycline-inducible promoter can be a powerful tool for this purpose, because high levels of gene induction were achievable in internalized S. aureus cells (inside CFT-1 epithelial cells), while basal promoter activity was undetectable in the absence of tetracycline (Fig. 2). In addition to cell cultures, we were able to show that high levels of promoter activity can be induced in a murine airpouch model, while the promoter remained quiescent under noninduced conditions. We thus propose that this promoter system can be useful in ascertaining the role of various pathogenic genes in cultured epithelial cells as well as in animal models of infections.
As a demonstration of how the inducible promoter can be used to probe the regulation of a pathogenic phenotype, we examined the role of ςB in two key components of biofilm formation, attachment and microcolony formation. Biofilms are purported to play a role in the pathogenesis of persistent infections, presumably by minimizing the exposure of the bacteria to antimicrobial agents and the host defenses (5). It has been suggested that two antecedent but distinct events leading to mature biofilm formation are attachment and intercellular aggregation (23). By placing sigB under the control of the inducible promoter on a shuttle plasmid in RN6390, we were able to achieve titratable ςB expression. We found, as have others (18), that the presence of ςB correlated with increased attachment to polystyrene. More specifically, we have found that increasing expression of SigB (Fig. 4) in response to tetracycline induction correlated with higher levels of bacterial attachment to the polystyrene surface. Whether attachment of staphylococcal cells to polystyrene directly mimics the early step in mature biofilm formation is not clear. Further experiments have to be done to demonstrate if ςB plays a role in regulating attachment to relevant biological surfaces (catheters in vivo, biological tissues, etc.).
A second step in biofilm formation, recognized previously in Staphylococcus epidermidis, is microcolony formation or intercellular aggregation (23). By microscopy, we were able to demonstrate that increased ςB induction correlated with the size of bacterial autoaggregates of S. aureus grown in liquid medium. Whether S. aureus can produce ςB to a high enough level to mediate autoaggregation in vivo, as found in our in vitro studies, is not clear. Further studies are therefore needed to ascertain the significance of S. aureus microcolony formation in vivo.
ACKNOWLEDGMENTS
We thank George O'Toole for assistance with fluorescence microscopy and Susham Ingavale for critically evaluating the manuscript.
This work was supported in part by NIH grant AI47441 to A.L.C.
REFERENCES
- 1.Blake M S, Johnston K H, Russell-Jones G J, Gotschlich E C. A rapid sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J M. Epidemiology and prevention of nosocomial infections. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 309–329. [Google Scholar]
- 3.Cheung A L, Chien Y T, Bayer A S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67:1331–1337. doi: 10.1128/iai.67.3.1331-1337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien Y, Cheung A L. Molecular interactions between two global regulators. sar and agr, in Staphylococcus aureus. J Biol Chem. 1998;274:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 5.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 6.Gaskill M E, Khan S A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988;263:6276–6280. [PubMed] [Google Scholar]
- 7.Geissendoefer M, Hillen W. Regulated expression of heterologous genes in Bacillus subtilis using the Tn10 encoded tet regulatory elements. Appl Micriobiol Biotechnol. 1990;33:657–663. doi: 10.1007/BF00604933. [DOI] [PubMed] [Google Scholar]
- 8.Guzman L, Belin D, Carson M, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamill R J, Vann J M, Proctor R A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986;54:833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by culture osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Marra A, Rosenberg M, Woodnut G. Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J Bacteriol. 1999;181:6585–6590. doi: 10.1128/jb.181.21.6585-6590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahl B C, Goulian M, van Wamel W, Herrmann M, Simon S M, Kaplan G, Peters G, Cheung A L. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun. 2000;68:5385–5392. doi: 10.1128/iai.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim L, Mogk A, Schumann W. A xylose-inducible Bacillus subtilis integration vector and its application. Gene. 1996;181:71–76. doi: 10.1016/s0378-1119(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 14.Kullik I, Giachino P, Fuchs T. Deletion of alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy F. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 16.Lowy F D, Fant J, Higgins L L, Ogawa S K, Hatcher V B. Staphylococcus aureus-human endothelial cells interactions. J Ultrastruct Mol Struct Res. 1988;98:137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- 17.Novick R P. The staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 1–40. [Google Scholar]
- 18.Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. Alternative transcription factor ςB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol. 2000;182:6824–6826. doi: 10.1128/jb.182.23.6824-6826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenk S, Laddaga R A. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett. 1992;73:133–138. doi: 10.1016/0378-1097(92)90596-g. [DOI] [PubMed] [Google Scholar]
- 20.Wu S, de Lencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yansura D, Henner D J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci USA. 1984;81:439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Fan F, Palmer L M, Lonetto M A, Petit C, Voelker L L, St. John A, Bankosky B, Rosenberg M, McDevitt D. Regulated gene expression in Staphylococcus aureus for identifying conditional lethal phenotypes and antibiotic mode of action. Gene. 2000;255:297–305. doi: 10.1016/s0378-1119(00)00325-5. [DOI] [PubMed] [Google Scholar]
- 23.Ziebuhr W, Heilmann C, Gotz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermis blood culture strains and mucosal isolate. Infect Immun. 1997;60:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]