Abstract
Two genes of the sigB operon, rsbU and rsbV, were deleted in an rsbU+ strain (FDA486) to evaluate the contribution of these two genes to ςB activity in Staphylococcus aureus. The ςB protein level and the transcription of two ςB-dependent promoters (sigB and sarA P3 transcripts) were analyzed in the constructed mutants. A deletion of the first gene (rsbU) within the sigB operon led only to a partial reduction in ςβ activity. A deletion of the second gene (rsbV) resulted in a more dramatic reduction in the ςB protein level and its activity than did the deletion of rsbU, thus indicating that RsbV can be activated independent of RsbU. In the parental strain, the ςB-dependent transcript initiated upstream of rsbV was 28-fold higher than the ςA-dependent transcript originating from the rsbU promoter. The level of the ςB-dependent transcript decreased up to 50% in the rsbU mutant and up to 90% in the rsbV mutant compared with the transcript in the wild type. The yellow pigment of S. aureus colonies, a ςB-dependent phenotype, was partially reduced in the rsbU and rsbV mutants, whereas alpha-hemolysin was increased. Additionally, the sarA P3 promoter activity of the parental strain was induced to a higher level in response to pH 5.5 than was that of the rsbU or rsbV mutant, indicating that RsbU is the major activator of the ςB response to acid stress. Using a tetracycline-inducible system to modulate the expression of RsbW, we progressively repressed pigment production, presumably by reducing the free ςB level. Collectively, our data indicated that RsbU and RsbV in S. aureus contributed to different levels of ςB protein expression and varying ςB activities. Although RsbV can activate ςB independent of RsbU, RsbU remains the major activator of ςB during acid stress.
Staphylococcus aureus is one of the most common etiological agents of wound infections, which can lead to severe infections such as septicemia, osteomyelitis, or endocarditis. Eradication of the organism is extremely difficult, particularly in hospitals, due to its ability to survive in extreme conditions, both in the host and in the environment. This flexibility of S. aureus survival in response to diverse environmental cues is often attributable to global regulatory elements (e.g., sarA and agr). In some cases, an alternative sigma factor such as ςB, by virtue of its regulatory effect on target promoters (e.g., the P3 promoter of sarA), may also play a role.
In contrast to the primary sigma factor (ςA) required for the transcription of housekeeping genes, SigB is one of the alternative sigma factors (17, 19, 24, 25) that have been shown to respond to environmental stresses (15, 40), probably executing bacterial adaptive responses essential for pathogenesis and survival. Although considerable information on ςB regulation in Bacillus subtilis exists (1, 3, 4, 14, 18, 20, 33, 34, 35, 36, 37, 38, 39, 43), little is known about how ςB activity (i.e., free ςB) is controlled in S. aureus.
The regulation of ςB activity in B. subtilis is complex, requiring a cascade of events to yield the free and active forms of the ςB protein. Under normal conditions, when ςB is inactive, the ςB protein is bound tightly to the anti-ς factor, RsbW (4, 13, 27). RsbV, in its dephosphorylated form, can bind competitively to RsbW, resulting in the release of ςB to bind to its target promoters to initiate transcription. The binding of RsbW to ςB or to RsbV is dependent on the phosphorylation status of RsbV, normally modulated by the phosphatase RsbU or RsbP (36, 37, 43). In B. subtilis, the RsbR-S-T gene complex represents an environmental sensing module which requires a GTP binding protein (Obg) (33, 34, 41) and a 50S ribosome subunit protein (L13) for stress activation (34), eventually leading to RsbU activation (i.e., becoming a phosphatase). Curiously, the RsbR-S-T sensor module is absent in S. aureus.
Several studies have reported the phenotypic role of ςB in S. aureus strains, including COL, a methicillin-resistant S. aureus strain, MA12.2, RN6390, and 8325-4. RN6390 and 8325-4 are natural rsbU deletion mutants. Phenotypic changes in these strains with a sigB mutation include increased production of alpha-hemolysin in strains RN6390 and COL (8), reduction of yellow pigmentation in strains COL and Newman (21), restoration of methicillin sensitivity in methicillin-resistant S. aureus strain COL (44), and decreased ability to form biofilm in strain MA12.2 (32). Although ςB may be related to bacterial survival during stress (22), the sigB mutant did not differ significantly in pathogenicity from the parental strains (RN6390, 8325-4, and WCUH29) in several acute animal infection models (7, 28). However, the contributions of ςB to chronic infections and persistence inside host cells are not known.
The sarA locus is composed of three overlapping transcripts designated sarA P1, sarA P3, and sarA P2 initiated from the P1, P3, and P2 promoters, respectively. Due to the overlapping nature of these three transcripts, all of them encode the 372-bp sarA open reading frame. Based on Northern and in vitro transcription assay analyses, the sarA P3 promoter is a ςB-dependent promoter (12, 26, 27). Since ςB partially controls the expression of SarA, the sarA regulatory molecule, by modulating the P3 promoter activity, it was hypothesized that ςB could be indirectly involved in the regulation of virulence genes such as alpha-toxin (8, 21).
In this study, we scrutinized the effects of RsbU, RsbV, and RsbW on the level and activity of the ςB protein, examining in particular the sarA P3 promoter. We present evidence that an additional factor other than RsbU is required for full ςB activity in S. aureus.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains and plasmids used in this study are listed in Table 1. FDA486 is an S. aureus strain previously used to study fibrinogen binding proteins (6, 31). RN6390 is a standard laboratory S. aureus strain with a genetic background similar to that of strain 8325-4. 8325-4 and its derivatives contain an 11-bp deletion in the rsbU gene (21) but still express ςB, albeit at a lower level (8). RN4220 is a restriction-deficient S. aureus host strain used as the initial recipient for transformation of plasmids (30). Escherichia coli XL1-Blue was used for cloning isolated DNA fragments.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Origin or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| FDA486 | Wild-type strain with intact rsbU gene | 6, 31, this study |
| RN4220 | Mutant of strain 8325-4 that accepts foreign DNA | 30 |
| RN6390 | Genetic background similar to that of 8325-4, contains a natural 11-bp deletion in the rsbU gene | 21 |
| ALC2128 | ςB+ ΔrsbU::ermC from FDA486 | This study |
| ALC2129 | ςB+ ΔrsbV::ermC from FDA486 | This study |
| ALC2131 | ςB+ ΔrsbU::ermC from RN6390 | This study |
| ALC2132 | ςB+ ΔrsbV::ermC from RN6390 | This study |
| E. coli | ||
| XL-1 Blue | Highly transformable strain | Stratagene |
| Plasmids | ||
| pCL52.2 | Shuttle vector with temperature-sensitive origin of replication for S. aureus | 23 |
| pCR2.1-TOPO | E. coli vector for direct cloning of PCR fragments | Invitrogen |
| pBluescript | E. coli cloning vector | Stratagene |
| pALC552 | pBluescript containing the ermC gene | This study |
| pΔrsbU | pCL52.2 ΔrsbU::ermC | This study |
| pΔrsbV | pCL52.2 ΔrsbV::ermC | This study |
| pALC1421 | pSK236 containing sarA P3::gfpuvr | 10 |
| pALC2073 | pSK236 containing a xyl/tetO promoter under the repressive control of TetR; gene expression is inducible in the presence of tetracycline | This study |
| pALC2197 | pALC2073 containing rsbW downstream of the xyl/tetO promoter | This study |
Sequence analysis of rsbU in FDA486.
Staphylococcal chromosomal DNA was isolated by using the Qiagen genomic DNA kit (Qiagen, Valencia, Calif.). The rsbU gene was amplified from the DNA of FDA486 using primers rsbU-1 (5′-GCCGGAATTCGTGGAAGAATTTAAGCAACA-3′) and rsbU-2 (5′-TTCCCTCGAGATTTACTCTTTTTATAATCA-3′). The PCR fragment was purified with a QIAquick nucleotide removal kit (Qiagen) and subsequently sequenced with oligonucleotides rsbU-1 and U-rsbV-1 (5′-GCATGCGAATTCAACGAAACATATTTATCT-3′). Sequence data of the PCR fragment revealed an intact rsbU gene in FDA486, indicating that this S. aureus strain was suitable for our ςB studies.
Construction of plasmids pΔrsbU and pΔrsbV and inactivation of rsbU and rsbV in FDA486 and RN6390.
Regions flanking rsbU and rsbV were amplified with 5′ overhang restriction sites (EcoRI and SmaI, underlined) using primers U-rsbU-1 (5′-GCATGCGAATTCAACGAAACATATTTATCT-3′) and U-rsbU-2 (5′-AGTTGCCCCGGGACTTCCTTACA-3′) for rsbU and primers U-rsbV-1 (5′-AATGGAATTCCTTTCAGTGGCGGCACAA-3′) and U-rsbV-2 (5′-TTCCCCCGGGACTTATTAAAAATATC-3′) for rsbV. The fragments were digested with EcoRI and SmaI and cloned separately into the temperature-sensitive shuttle vector pCL52.2 (23). pCL52.2 has a temperature-sensitive origin of replication that is active in S. aureus at 32°C but not at 42°C. Downstream regions of rsbU and rsbV were amplified with flanking PstI and HindIII restriction sites (underlined) with primers D-rsbU-1 (5′-CCCAACTGCAGAAGATGATATGACTATTTTG-3′) and D-rsbU-2 (5′-TCGCCAAGCTTTAAACCTAGGCCACCTTC-3′) for rsbU and primers D-rsbV-1 (5′-AAAACTGCAGCGGAGGTCGAATAACATG-3′) and D-rsbV-2 (5′-GTGCAAGCTTTAATTCAGCGGTTAGTTC-3′) for rsbV. The downstream fragments were digested with PstI and HindIII and cloned separately into the PstI-HindIII sites of pCL52.2 already containing the upstream fragments. The ermC gene was amplified by two primers (5′-ATCCCTCAGGCTTTGGCTAACACACACGC-3′ and 5′-TGACCTGAATAAGGAAACAAGTTAAGGGATGCAG-3′). The PCR fragment encoding the ermC gene was cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.) and subcloned into pBluescript as an EcoRI-HindIII fragment, resulting in plasmid pAL552. The ermC gene was excised from pALC552 by BamHI-SalI digestion and introduced into the BamHI-SalI site of pCL52.2 between the two previously cloned fragments. The resulting plasmids were designated pΔrsbU and pΔrsbV.
pΔrsbU and pΔrsbV, initially isolated from E. coli, were introduced into S. aureus RN4220 by electroporation followed by transduction into strains FDA486 and RN6390 with phage φ11. Transductants were selected on tryptic soy agar plates containing tetracycline (3 μg ml−1) at 32°C. Transductants with pΔrsbU or pΔrsbV were grown separately in O3GL broth (29, 30) with tetracycline (3 μg ml−1) and erythromycin (5 μg ml−1) at 32°C overnight following 12 h of growth in media with erythromycin at 42°C. The cultures were diluted 1:100 in fresh O3GL broth and incubated at 32°C without antibiotics for 12 h; this step was repeated several times to yield erythromycin-resistant but tetracycline-sensitive colonies, implying plasmid loss and subsequent homologous recombination at the sequences flanking rsbU or rsbV. The deletion included the respective ribosomal binding sites of rsbU and rsbV.
The deletions of rsbU and rsbV were confirmed by PCR, sequencing, and Southern hybridization. We used a primer (5′-ATGGTCTATTTCAATGGCAGTTAC-3′) corresponding to bases 331 to 335 of the ermC gene (FASTA GenBank accession no. Y17294) in combination with a primer within the sigB gene (5′-TGCCAAGCTTTGTAATTTCTTAATTGCC-3′) to confirm the rsbU deletion or in combination with a primer downstream of the sigB operon (5′-AATATCCTTCTTTAATTCCTCAGTA-3′) to confirm the rsbV deletion. The nucleotide sequences of the PCR fragments were determined with corresponding primers to confirm the genetic construct which resulted from the insertion of ermC into the respective genes within the sigB operon. As an additional confirmation, chromosomal DNAs from the parent strain FDA486 and the mutant strains ΔrsbU::ermC and ΔrsbV::ermC were digested with EcoRV and probed in Southern hybridization experiments with a PCR fragment (using primers 5′-GCTGGAATTCCGCCTGGATATATTTATC-3′ and 5′-TTCCCCCGGGACTTATTAAAAATATTTATC-3′) within the rsbU gene or a PCR fragment (using primers 5′-TAAACTGCAGGAGCAGGTGCGAAATAAT-3′ and 5′-GTGCAAGCTTTAATTCAGCGGTTAGTTC-3′) within the sigB gene.
Preparation of cell extracts and immunoblot analysis for ςB protein.
Cell extracts were prepared from strains FDA486 and RN6390 and their corresponding rsbU and rsbV isogenic mutants. After pelleting, the cells were resuspended in 1 ml of buffer (100 mM Tris-HCl [pH 7.5], 100 mM KCl, 5 mM EDTA, and 1 mM dithiothreitol) for lysostaphin treatment as described previously (11). Protein samples (50 μg each) were run in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and then immunoblotted onto a nitrocellulose membrane. The membrane was blocked with 2% bovine serum albumin at room temperature for 2 h. Anti-SigB monoclonal antibody 1D1 (8) was used for the detection of ςB (1:5,000 dilution) at room temperature for 3 h, followed by another hour of incubation with a 1:5,000 dilution of goat anti-mouse antibody conjugated to alkaline phosphatase (Jackson ImmunoResearch, West Grove, Pa.). Reactive bands were detected with developing substrates as described previously (5).
Total RNA preparation.
S. aureus strain FDA486 and its corresponding mutants were cultivated in Luria-Bertani (LB) broth to mid-log phase (optical density at 650 nm [OD650], 0.7), late log phase (OD650, 1.2), and post-exponential phase (OD650, 1.7). The cells were pelleted and resuspended in 1 ml of Trizol (Gibco-BRL, Gaithersburg, Md.) in combination with 0.1-mm-diameter zirconia-silica beads in a Fast Prep reciprocating shaker (Bio 101, Inc., San Diego, Calif.) as described previously (9). Total RNAs for reverse transcription (RT)-PCR required an additional DNase I treatment step. In brief, 50 μg of the RNA samples was incubated with RNase-free DNase I (1 U of RNA/μg) (Boehringer Mannheim, Indianapolis, Ind.) in 50 μl of buffer (50 mM Tris [pH 8.2], 5 mM Mg2SO4) for 30 min at 25°C. After phenol-chloroform extraction, RNA was precipitated with 2-propanol and resuspended in diethyl pyrocarbonate-treated water.
Two-step real-time quantitative PCR.
Total cellular RNAs, isolated from the wild-type strain and the constructed mutants, were reverse transcribed to cDNA using the TaqMan Reverse Transcription kit (PE Applied Biosystems, Foster City, Calif.). PCRs were set up using SYBR Green PCR Master mix (PE Applied Biosytems) according to the manufacturer's instructions. Real-time detection and relative quantitation were achieved with the ABI-PE Sequence Detection system 7700. We analyzed the two major transcripts produced from the sigB operon. The ςA-dependent transcript was detected by PCR using a forward primer (5′-ATGGTTGGTTTAATAGGTGCC-3′) and a reverse primer (5′-ATACGTCGGAACATGTACA-3′), resulting in a 150-bp fragment that corresponded to a region inside the rsbU gene. Additional primers (5′-AAGTGATTCGTAAGGACGTCT-3′ and 5′-TCGATAACTATAACCAAAGCCT-3′) were designed to amplify a fragment of the cDNA representing sigB, which is encoded by the last gene in the operon, essentially encompassing both the ςA- and ςB-dependent transcripts since these transcripts overlap at the 3′ end. As an endogenous control, forward (5′-TTAGGTGCTGGGCAAATACA-3′) and reverse (5′-TGCATAACCAGCTAATGCTTC-3′) primers were used to amplify a 150-bp fragment of the DNA gyrase gene. The calibrator in our study was the ςA-dependent transcript of the sigB operon from the parental strain. The ΔCT value, representing the difference in threshold cycle between the target and control genes, was determined by subtracting the CT value of DNA gyrase cDNA from the CT values for cDNA derived from the ςA- and ςB-dependent transcripts of the sigB operon. The ΔΔCT value was derived from the subtraction of the resultant ΔCT values from the ΔCT value of the calibrator (ςA-dependent transcript). 2−ΔΔCT was expressed as the n-fold difference relative to the calibrator.
Northern blot hybridization.
Twenty micrograms of total cellular RNA from FDA486 and its isogenic mutants was electrophoresed through a 1.5% agarose–0.66 M formaldehyde gel in running buffer (20 mM morpholinepropanesulfonic acid [MOPS], 10 mM sodium acetate, 2 mM EDTA, pH 7.0). Blotting of RNA onto Hybond N+ membranes (Amersham, Arlington Heights, Ill.) was performed with a Turbo-blotter alkaline transfer system (Scheicher & Schuell, Inc., Keene, N.H.). A gel-purified DNA probe of the sarA gene (2) was radiolabeled with [α-32P]dCTP by the random primer method (Ready-To-Go labeling kit; Pharmacia) and used to detect the ςB-dependent sarA P3 transcript. The blot was hybridized under high-stringency conditions, washed, and autoradiographed.
Assay for alpha-hemolysin.
S. aureus strain FDA486 and the constructed mutants were tested for alpha-hemolysin production on sheep blood agar. Ten microliters of bacterial cultures harvested during the exponential phase (OD650, 0.5) was added to sheep blood agar, and the plate was incubated overnight at 37°C. The diameters of the clearing zones formed were compared between strains. In addition, alpha-hemolysin was also assayed by the microtiter method with rabbit erythrocytes. Briefly, the supernatants of overnight cultures were serially diluted in phosphate-buffered saline and incubated at 37°C for 2 h with 4% rabbit erythrocytes in a 96-well microtiter plate. Sodium dodecyl sulfate (1%) was used as a positive lysis control, and phosphate-buffered saline was used as a negative control. The plate was then read at 480 nm in a microtiter plate reader. Units of activity are expressed as the reciprocals of the dilutions giving 50% lysis.
Assay for sarA P3 activity in response to low pH.
To monitor the sarA P3 promoter activity in response to low pH, we used gfpuvr as the reporter gene downstream of the sarA P3 promoter in shuttle plasmid pSK236. Experimentally, we introduced the plasmid pALC1421 (pSK236 containing gfpuvr preceded by a 162-bp sarA P3 promoter fragment; Table 1) by electroporation into FDA486. We also assayed for sarA P3 promoter activity in response to acid stress. FDA486 and its isogenic mutants ΔrsbU::ermC and ΔrsbV::ermC with pALC1421 were inoculated into LB medium buffered to pH 7.0 or 5.5 with HCl, and the bacterial cultures were grown with shaking at 37°C. Samples taken at different time points of the growth cycle were analyzed in an FL600 microplate fluorescence reader (Bio-Tek Instruments, Winooski, Vt.) with 485- to 516-nm filters. The pH changes were monitored in the culture during the growth. Fluorescence values were expressed as total fluorescence/OD650 to minimize small variations in fluorescence due to cell densities. Percent differences of sarA P3 activity caused by the low pH were calculated using the following formula: [(fluorescence at pH 5.5/fluorescence at pH 7.5) − 1.0] × 100.
Pigmentation test.
S. aureus strain FDA486 and its isogenic mutants were analyzed for pigment formation. One single colony of each strain was streaked onto an O3GL agar plate, and the plate was incubated at 37°C for 24 to 36 h. To determine the effect of RsbW on pigment production, a sigB-dependent phenotype, we analyzed the pigment in strain FDA486 by overexpressing RsbW. For overexpression, we used a tetracycline-inducible system recently developed (unpublished data) to modulate the expression of RsbW and, based upon the model of RsbW binding to ςB in B. subtilis and S. aureus (4, 13, 27), to detect the resultant ςB activity attributable to the free form of ςB. Experimentally, the rsbW gene was amplified from staphylococcal chromosomal DNA by PCR (using primers 5′-AGCGTCGACAGGAGGTTATAAACATGCAATCTAAAGAAAATTTTA-3′ and 5′-CTGCAGTTAGCTGATTTCGACTCTTC-3′ [the sequence containing the ribosome binding site is underlined]). The PCR product was cloned into E. coli vector pCR2.1-TOPO (Invitrogen) and subsequently introduced into the tetracycline-inducible plasmid pALC2073 as an EcoRI fragment. The resulting plasmid was designated pALC2197 (Table 1). The orientation of the insert was verified by restriction mapping. The recombinant plasmid was introduced into S. aureus strain RN4220 prior to transformation into strain FDA486. After confirmation with restriction mapping, a selected transformant of FDA486 was streaked onto O3GL agar plates containing tetracycline (0, 80, and 200 ng/ml).
RESULTS
Construction of deletion mutants of rsbU and rsbV.
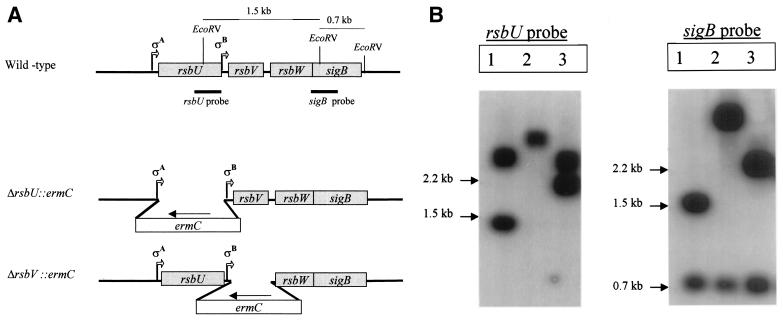
Evidence is accumulating that RsbU has an important role in activating ςB in S. aureus (16, 21). However, a recent report from our laboratory revealed that an S. aureus strain with a natural rsbU deletion (RN6390) can produce the SigB protein and activate transcription from the ςB-dependent sarA P3 promoter (8). These data suggested that ςB can be activated by additional factors, independent of RsbU. To address this question, we created deletions in the rsbU and rsbV genes (Fig. 1A) to evaluate the resultant ςB activity. PCR and sequence analysis using oligonucleotides within the ermC gene in combination with primers corresponding to regions downstream of the respective cloned fragments showed that we had correctly inserted ermC into the corresponding chromosomal regions within the sigB operon. Southern hybridization experiments, using probes corresponding to the rsbU and sigB genes, showed that the insertions had occurred correctly in the genetic constructs (Fig. 1). Neither the downstream ribosome binding sites nor the two promoters (ςA and ςB) of the sigB operon were affected by the deletions.
FIG. 1.
Inactivation of the S. aureus rsbU and rsbV genes. (A) Organization of the sigB operon in S. aureus. The rsbU, rsbV, rsbW, and sigB genes in the wild-type strain and the ΔrbU::ermC and ΔrbsV::ermC constructed mutants are shown. The vertical lines indicate the EcoRV sites on the sigB operon, and bold lines indicate the regions for the rsbU and sigB probes. (B) Southern blots of EcoRV-digested chromosomal DNAs from parent strain FDA486 (lanes 1) and its ΔrsbU::ermC (lanes 2) and ΔrsbV::ermC (lanes 3) mutants. The sizes of the bands (in kilobases) are shown to the left of each blot.
Reduced expression of the ςB protein in deletion mutants.
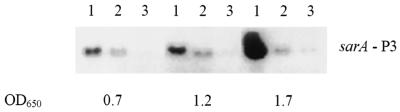
Studies of B. subtilis have shown that RsbU- or RsbP-dependent activation of ςB requires an intact rsbV allele (35). However, the gene encoding RsbP is absent in S. aureus. We blasted the amino acid sequence of the ςB regulation protein phosphatase, RsbP, against the staphylococcus N315 complete genome (available at http://www.ncbi.nlm.nih.gov). The best hits seemed to be the phosphatase RsbU (E value = 4e − 06) and a hypothetical protein with no significant similarity to RsbP (E value = 0.033). To assess whether mutations of the coding regions of the rsbU and rsbV genes affected the expression of the ςB protein differentially, we probed an immunoblot of cell extracts of overnight cultures of the respective mutants with an anti-ςB monoclonal antibody. Interestingly, inactivation of rsbU or rsbV did not cause a complete abolition of ςB protein expression. As shown in Fig. 2, the ςB protein levels in these two mutants were lower than those in their parental counterparts. However, the ςB expression was lower in the rsbV mutant than it was in the rsbU mutant. We interpreted this result with the idea that another factor, independent of RsbU, is required to activate RsbV, since the rsbV mutant had lower ςB protein expression than the rsbU mutant. The expression of sigB, the last gene in the operon, in both the rsbU and rsbV mutants is consistent with the notion that the polar effect on sigB as a result of the rsbU and rsbV mutations is likely to be minimal. Additionally, the ςA- and ςB-dependent transcripts encoded within the sigB operon were detectable in these mutants by real-time RT-PCR with primers corresponding to the sigB gene, thus confirming the relative integrity of both transcripts (see below).
FIG. 2.
ςB production in response to mutations in the rsbU or rsbV gene. Equal amounts of cell extracts (50 μg) from the parental strains and the isogenic mutants were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto nitrocellulose filters. The blots were probed with anti-ςB monoclonal antibody 1D1 (1:2,000 dilution). Lanes: 1, wild-type strain; 2, ΔrsbU::ermC mutant; 3, ΔrsbV::ermC mutant.
Reduced ςB-dependent transcription of the sigB operon in the mutants.
Studies of B. subtilis have demonstrated that the ςB protein activates a ςB-dependent promoter upstream of the rsbV gene in the sigB operon. To examine whether mRNA transcripts from the sigB operon were altered in the rsbU and rsbV mutants, we performed real-time RT-PCR analysis of total cellular RNA to detect ςA- and ςB-dependent transcripts of the sigB operon during the post-exponential phase, a time at which ςB activity is normally at its highest. Using primers for the rsbU gene, we measured the amount of the ςA transcript since the ςA-dependent promoter is found upstream of the rsbU gene; with primers for the sigB gene, we could detect a combination of the ςA- and ςB-dependent transcripts. The difference between these two transcripts yielded the amount of the ςB-dependent transcript. As shown in Fig. 3, the highest level of the ςB-dependent transcript was found in the parental strain and the lowest level was found in the rsbV mutant. The amount of the ςB-dependent transcript in parental strain FDA486 was 28-fold higher than in the corresponding ςA-dependent transcript during the post-exponential phase. Remarkably, the level of the ςA-dependent transcript was similar between the rsbU and rsbV mutants. However, the ςB-dependent transcript decreased by more than 50% with an rsbU mutation and by more than 90% with an rsbV mutation than it was in the parental control. Notably, the ςB-dependent transcript in the rsbV mutant decreased to the level of the ςA-dependent transcript within the same strain; this contrasts with the parental strain, in which the ςB-dependent transcript was much higher than its ςA counterpart. The finding that the ςB transcript continued to be detected in the rsbU and rsbV mutants implied that deletions of these genes in our study did not disrupt the sigB transcript. As a sigB primer was used to detect the ςB-dependent transcript by RT-PCR, these data also indicated that the sigB transcript was not truncated in these mutants.
FIG. 3.
Transcription of the sigB operon in response to mutations in rsbU and rsbV. The transcripts were analyzed by SYBER Green real-time RT-PCR. Total RNA from bacterial cells harvested at the early stationary phase was converted to cDNA by RT. ςB-dependent transcripts were analyzed as described in Materials and Methods. The DNA gyrase transcript was used as an endogenous control, and the comparative method was used to analyze the data. 2−ΔΔCT expresses the n-fold difference relative to the amount of ςA-dependent transcript. Results are the averages of triplicate results from a representative experiment, and the error bars show the standard deviations.
Reduction of the ςB-dependent sarA P3 transcript in rsbU and rsbV deletion mutants.
In previous studies, it has been reported that the sarA P3 promoter is recognized by ςB and the sequence of this promoter shows strong homology to ςB-dependent promoters described for B. subtilis (12, 26, 27). In our study, we showed by Northern analysis that the level of the sarA P3 transcript was decreased in the rsbU and rsbV mutants (Fig. 4) but not absent, as found in the sigB mutant (data not shown). As expected with a ςB-dependent promoter, the level of the sarA P3 transcript in FDA486 increased from the mid-log (OD650, 0.7) to post-exponential (OD650, 1.7) phases. A mutation in rsbU resulted in a dramatic reduction in sarA P3 transcription, but the diminution of this transcript was even higher in an rsbV mutant. Notably, the P3 transcript in the rsbV mutant was not discernible until the cells reached the post-exponential phase (OD650, 1.7). Based on our data, we theorize that RsbU alone cannot account for the total sarA P3 promoter activity via ςB, thus suggesting that an additional factor(s), possibly acting upon the promoter upstream of rsbV or acting directly on the RsbV protein, may be involved.
FIG. 4.
Transcription of the sarA P3 promoter with different levels of ςB. Northern blot analysis of the sarA P3 transcript in S. aureus FDA486 and the corresponding mutants. Lanes: 1, wild-type strain FDA486; 2, ΔrsbU::ermC mutant; 3, ΔrsbV::ermC mutant. The sarA P3 promoter is ςB dependent.
Reduced pigmentation in the mutants.
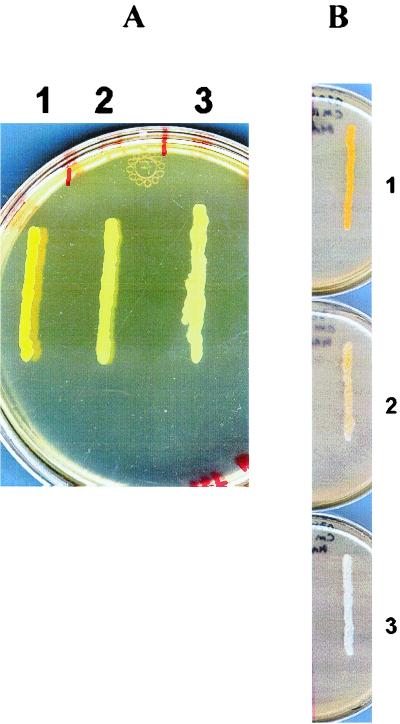
In previous studies, it was reported that a sigB deletion reduced pigment production in S. aureus (21, 28). In addition, a ςB-dependent promoter has been found in the operon containing genes that encode the enzymes involved in the carotenoid synthesis, CrtM and CrtN (42). We compared the pigment production of FDA486 with that of the rsbU and rsbV mutants to ascertain whether pigment synthesis correlates with ςB activity. Upon analyzing these two strains on O3GL agar plates after 24 h of incubation at 37°C, an intense yellow pigment was observed in the wild-type strain, whereas the pigments were reduced in the rsbU and rsbV mutants (Fig. 5A).
FIG. 5.
Pigment formation in response to different levels of ςB (A) or RsbW (B). The bacterial strains were streaked onto O3GL agar plates and incubated overnight at 37°C. Streaks: 1, wild-type strain FDA486; 2, FDA486 ΔrsbU::ermC mutant; 3, FDA486 ΔrsbV::ermC mutant. (B) Strain FDA486 carrying pALC2073 with the rsbW gene. The strains were streaked onto O3GL agar plates without tetracycline (streak 1), with 80 ng of tetracycline ml−1 (streak 2), and with 200 ng of tetracycline ml−1 (streak 3). The plates were incubated overnight at 37°C.
Increased alpha-hemolysin activity in the mutants.
In previous studies, it was reported that a sigB deletion could lead to hyperproduction of alpha-hemolysin in S. aureus (8). With FDA486 and its corresponding mutants, we could also observe an increased zone of alpha-hemolysis in the mutants compared to the wild-type strain. The diameters of the zone of alpha-hemolysis surrounding colonies of isogenic mutants were bigger than those of the parental strain (Table 2). This result was corroborated by quantitative assays in microtiter wells in which the hemolytic titers of the supernatants of the mutant and parental strains were compared. The alpha-hemolytic titer was lowest for the parental strain and highest for the rsbV mutant (Table 2), as one would predict from the reduced ςB activity in this mutant.
TABLE 2.
Alpha-hemolysin activity of S. aureus FDA486 and its isogenic mutants
| Strain | Alpha-hemolysin activity measured by:
|
|
|---|---|---|
| Clearing on blood agar platesa | Hemolytic titerb | |
| FDA486 | + | 2 |
| ΔrsbU::ermC | ++ | 4 |
| ΔrsbV::ermC | +++ | 8 |
Assayed by measuring the zones of hemolysin on sheep erythrocyte agar plates. +, weak; ++, moderate; +++, strong.
Units of activity are expressed as the reciprocals of the highest dilutions of the original culture supernatants yielding 50% hemolysis of rabbit erythrocytes.
Induction of sarA P3 activity can be mediated by low pH.
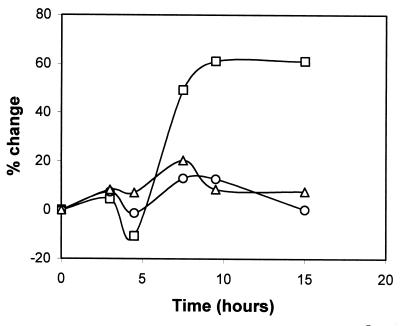
To assess whether ςB activity could be induced upon exposure to low pH, we measured GFPuvr activity driven by the sarA P3 promoter in media at pH 7.5 or 5.5. Our results indicated that the activity of this promoter was significantly enhanced when the culture was grown at pH 5.5 compared with identical medium calibrated to pH 7.5 (Fig. 6). This phenomenon was clearly much stronger in the wild-type strain than in the two mutants. The sarA P3 activity increased more than 60% in the parental strain with the lower pH, whereas promoter activity only increased 15 to 20% in the rsbU and rsbV mutants in response to a low pH. These data imply that activation of ςB by acid stress is likely to be mainly an RsbU-dependent phenomenon. In measuring the pH of the bacterial culture during growth, we confirmed that the culture's pH was almost constant during the sampling duration (data not shown).
FIG. 6.
Effect of pH 5.5 relative to pH 7.5 on sarA P3 promoter activity. S. aureus strain FDA486 and its corresponding mutants containing plasmid pALC1421 were grown in LB at pH 7.5 or 5.5. Expression of gfp driven by the sarA P3 promoter was measured during the growth cycle and the percent differences were calculated using the formula [(fluorescence at pH 5.5/fluorescence at pH 7.5) − 1.0] × 100. Symbols: squares, wild-type strain FDA486; circles, ΔrsbU::ermC mutant; triangles, ΔrsbV::ermC mutant.
Reduced ςB activity with induction of RsbW.
Based upon previous data (4, 13, 27), it has been speculated that RsbW may have sequestered SigB to modulate free ςB activity in S. aureus. To confirm this interaction, we used a tetracycline-inducible system to modulate RsbW expression with various doses of tetracycline. The effects of increasing RsbW induction with tetracycline on bacterial pigment are illustrated in Fig. 5B. With the assumption that pigment production responds positively to varying levels of free ςB protein, the gradual induction of RsbW, concomitant with the steady loss of pigment production in the induced strain, supported previous reports that the expression of RsbW could sequester free ςB and, hence, block its activity. The reduced binding of ςB to the ςB-dependent promoter of the staphyloxanthin biosynthesis operon will thus diminish the ability of the induced strain to produce pigment.
DISCUSSION
Comprehensive studies previously reported have elucidated the mechanism of ςB regulation in B. subtilis (1, 3, 4, 14, 18, 20, 33, 34, 35, 36, 37, 38, 39, 43). Contrary to that observed for S. aureus, activation of ςB in B. subtilis is complex, requiring a cascade of events for the release of free ςB from the ςB-RsbW complex. Importantly, the activity of ςB in B. subtilis is dependent on the phosphorylation status of RsbV, controlled primarily by two separate phosphatases, RsbU and RsbP (36, 37, 43). The effect of rsbU, a gene lying upstream of rsbV, on ςB activity was also reported by Wise and Price (43) and further substantiated by Voelker et al. (37). In the case of S. aureus, recent studies have shown that a lack of RsbU resulted in dramatic changes in ςB activity compared to an rsbU+ strain (16). As the existence of an RsbP homolog was not confirmed in the recently released S. aureus genome (http://www.ncbi.nlm.nih.gov), activation of ςB by a second pathway independent of RsbU in S. aureus is not clearly defined.
In this study, we addressed this question by investigating the effect of rsbU or rsbV mutation on the ςβ protein level and its activity. We used two different approaches to evaluate the level of ςB activity. In our first approach, we measured the extent of sigB transcription and translation. Using quantitative real-time RT-PCR analyses with oligonucleotides corresponding to sigB (i.e., combined ςA- and ςB-dependent transcripts) and comparing them to those corresponding to rsbU (i.e., a ςA-dependent transcript), we discovered that the level of ςB-dependent transcripts was highest in the parental strain FDA486 and lowest in the rsbV mutant. Importantly, the ςB-dependent transcript level of the parental strain was much higher than that of the ςA-dependent transcript during the post-exponential phase. Additionally, the ςB transcript continued to be transcribed despite mutations in rsbU and rsbV, indicating that the polar effect on downstream genes was likely to be minimal. More specifically, the ςB-dependent transcript decreased by more than 50% in the rsbU mutant and by greater than 90% in the rsbV mutant than it did in the parental strain, thus indicating the additional control in ςB activity via RsbV (Fig. 3). Immunoblot analysis disclosed that the wild-type strain produced the greatest amounts of the ςB protein while the ςB protein level in the rsbU mutant was lower but still higher than that in the rsbV mutant. The amounts of the ςB protein correlated quite well with the levels of the sigB transcript. Collectively, these data imply that the expression of SigB during the post-exponential phase is largely controlled by the ςB-dependent promoter upstream of rsbV.
In our second approach, we determined ςB activity by measuring transcription from the sarA P3 promoter. Studies by Deora et al. (12) and Bishai et al. (27) as well as from our lab (8, 26) have shown that the sarA P3 promoter of S. aureus is a ςB-dependent promoter. Northern blot analysis with a sarA probe revealed that the level of the P3 transcript was dramatically reduced in the isogenic mutants compared to that of FDA486. In particular, the rsbV mutant has a lower level of P3 transcription than that of the RsbU mutant (Fig. 4). In addition, the markedly reduced transcription from the sarA P3 promoter in the isogenic mutants correlated well with reductions in ςB protein levels and transcription from the ςB-dependent promoter upstream of rsbV (Fig. 2 and 3). Taken together, and applying the working model of B. subtilis (38), there appear to be at least two pathways for ςB activation in S. aureus, one which is dependent on RsbU and another which is independent of RsbU but dependent on RsbV.
We also investigated two functions, pigment and alpha-hemolysin production, phenotypes that are controlled directly (pigment) or indirectly (alpha-hemolysin) by ςB. The positive influence of ςB on staphylococcal pigmentation was originally reported by Kullik et al. (21) and recently substantiated by Giachino et.al. (16). In our study, we also observed a progressive loss of pigment production from parent to rsbU and rsbV mutants (Fig. 5). This finding, coupled with those of the ςB protein level (Fig. 2) and their associated activities (Fig. 4), clearly implied a correlation in ςB levels and the degree of pigment production. Previous work from our laboratory demonstrated that the production of alpha-hemolysin is negatively controlled by ςB (8), probably indirectly controlled by regulating transcription from the sarA P3 promoter. A reduction in P3 activity as a result of declining ςB expression or activity leads to increased expression of SarA which, in turn, modulates the alpha-hemolysin gene via an SarA-dependent pathway (8). Taking advantage of the cytolytic effects of alpha-hemolysin upon rabbit erythrocytes, we performed two functional assays for alpha-hemolysin production, with both disclosing increased hemolytic activity in the rsbU and rsbV mutants compared with the parental strain. The hemolytic activity appeared to be higher for the rsbV mutant than for the rsbU mutant, hence confirming the negative impact of ςB activity on alpha-hemolysin expression.
Finally, we found that the ςB activity responded positively to acid stress and that the staphylococcal pigment could be negatively controlled by different levels of the RsbW protein. The response to acid stress was strongest in the wild-type strain, with sarA P3 activity increased by more than 60%, whereas the analogous response in the two mutants resulted in a 15 to 20% increase in activity. Thus, the activation of ςB in response to acid stress is dependent mainly on RsbU. However, we did not rule out the possibility that RsbV may mediate ςB activation in response to other environmental stresses (e.g., antibiotic or drug stress). We also analyzed the pigment of FDA486 in response to different levels of RsbW provided under inducible conditions. With the idea that RsbW may function as an anti-ς factor (4, 13, 27), we modulated rsbW expression using a tetracycline-inducible system and analyzed the pigment as an indication of free ςB activity. Our data clearly confirmed the ability of RsbW to sequester ςB to block its free-form activity. More specifically, colonies of the wild-type strain produced a strong yellow or orange pigment in the absence of tetracycline while the pigment was completely lost in 200 ng of tetracycline/ml, a concentration that has been shown to significantly induce RsbW expression.
In conclusion, this study ascertained that the expression and activity of ςB protein are only partially dependent on RsbU during bacterial growth, indicating that additional factors are required for full ςB activity in S. aureus. Based on our data, we propose an additional but complementary model of ςB regulation involving an RsbU-independent pathway to convert RsbV-P to RsbV.
ACKNOWLEDGMENTS
This work was partially supported by NIH grant AI37142. We thank the Office of International Affairs at Karolinska Institutet for fellowship support.
We thank TIGR and the University of Oklahoma Genome Center for access to the staphylococcal genome. Willem Van Wamel is acknowledged for fruitful discussions and comments.
REFERENCES
- 1.Akbar S, Kang C M, Gaidenko T A, Price C W. Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 2.Bayer M G, Heinrichs J H, Cheung A L. The molecular architecture of the sar locus in Staphylococcus aureus. J Bacteriol. 1996;178:4563–4570. doi: 10.1128/jb.178.15.4563-4570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson A K, Haldenwang W G. Characterization of a regulatory network that controls ςB expression in Bacillus subtilis. J Bacteriol. 1992;174:749–757. doi: 10.1128/jb.174.3.749-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson A K, Haldenwang W G. Bacillus subtilis ςB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA. 1993;90:2330–2334. doi: 10.1073/pnas.90.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake M S, Johnston K H, Russell-Jones G J, Gotschlich E C. A rapid sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 6.Bodén M, Flock J-I. Cloning and characterization of a gene for a 19 kDa fibrinogen binding protein from Staphylococcus aureus. Mol Microbiol. 1994;12:599–606. doi: 10.1111/j.1365-2958.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 7.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Chien Y-T, Bayer A S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67:1331–1337. doi: 10.1128/iai.67.3.1331-1337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Eberhardt K, Fischetti V A. A method to isolate RNA from gram-positive bacteria and mycobacteria. Anal Biochem. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- 10.Cheung A L, Nast C C, Bayer A S. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect Immun. 1998;66:5988–5993. doi: 10.1128/iai.66.12.5988-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien Y-T, Cheung A L. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J Biol Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 12.Deora R, Tseng T, Misra T K. Alternative transcription factor ςSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J Bacteriol. 1997;179:6355–6359. doi: 10.1128/jb.179.20.6355-6359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufour A, Haldenwang W G. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV) J Bacteriol. 1994;176:1813–1820. doi: 10.1128/jb.176.7.1813-1820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufour A, Voelker U, Voelker A, Haldenwang W G. Relative levels and fractionation properties of Bacillus subtilis ςB and its regulators during balanced growth and stress. J Bacteriol. 1996;178:3701–3709. doi: 10.1128/jb.178.13.3701-9sigma.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaidenko T A, Price C W. General stress transcription factor ςB and sporulation transcription factor ςH each contribute to survival of Bacillus subtilis under extreme growth conditions. J Bacteriol. 1998;180:3730–3733. doi: 10.1128/jb.180.14.3730-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giachino P, Engelmann S, Bischoff M. ςB activity depends on RsbU in Staphylococcus aureus. J Bacteriol. 2001;183:1843–1852. doi: 10.1128/JB.183.6.1843-1852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: Yamamoto K, McKnight S, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 18.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmann J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 20.Kalman S, Duncan M L, Thomas S M, Price C W. Similar organization of the sigB and spoIIA operons encoding alternative sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullik I, Giachino P. The alternative sigma factor ςB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 23.Lee C Y. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol Microbiol. 1992;6:1515–1522. doi: 10.1111/j.1365-2958.1992.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 24.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna A C, Bayer M G, Cheung A L. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180:3828–3836. doi: 10.1128/jb.180.15.3828-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki E, Chen J-M, Ko C, Bishai W R. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J Bacteriol. 1999;181:2846–2851. doi: 10.1128/jb.181.9.2846-2851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholas R O, Li T, McDevitt D, Marra A, Sucoloski S, Demarsh P L, Gentry D R. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect Immun. 1999;67:3667–3669. doi: 10.1128/iai.67.7.3667-3669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novick R. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 30.Novick R P. The staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 1–40. [Google Scholar]
- 31.Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock J. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect Immun. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. Alternative transcription factor ςB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol. 2000;182:6824–6826. doi: 10.1128/jb.182.23.6824-6826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott J M, Haldenwang W G. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor ςB. J Bacteriol. 1999;181:4653–4660. doi: 10.1128/jb.181.15.4653-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott J M, Ju J, Mitchell T, Haldenwang W G. The Bacillus subtilis GTP binding protein Obg and regulators of the ςB stress response transcription factor cofractionate with ribosomes. J Bacteriol. 2000;182:2771–2777. doi: 10.1128/jb.182.10.2771-2777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smirnova N, Scott J, Voelker U, Haldenwang W G. Isolation and characterization of Bacillus subtilis sigB operon mutations that suppress the loss of the negative regulator RsbX. J Bacteriol. 1998;180:3671–3680. doi: 10.1128/jb.180.14.3671-3680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijay M, Brody M S, Fredlund E, Price C W. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the ςB transcription factor of Bacillus subtilis. Mol Microbiol. 2000;35:180–188. doi: 10.1046/j.1365-2958.2000.01697.x. [DOI] [PubMed] [Google Scholar]
- 37.Voelker U, Dufour A, Haldenwang W G. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of ςB. J Bacteriol. 1995;177:114–122. doi: 10.1128/jb.177.1.114-122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voelker U, Voelker A, Maul B, Hecker M, Dufour A, Haldenwang W. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voelker U, Luo T, Smirnova N, Haldenwang W G. Stress activation of Bacillus subtilis ςB can occur in the absence of the ςB negative regulator RsbX. J Bacteriol. 1997;179:1980–1984. doi: 10.1128/jb.179.6.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voelker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welsh, K. M., K. A. Trach, C. Folger, and J. A. Hoch. 1994. Biochemical characterization of the essential GTP-binding protein Obg of Bacillus subtilis.176:7161–7168. [DOI] [PMC free article] [PubMed]
- 42.Wieland B, Feil C, Gloria-Maercker E, Thumm G, Lechner M, Bravo J-M, Poralla K, Gotz F. Genetic and biochemical analysis of the biosynthesis of the yellow carotenoid 4,4′-diaponeurosporene of Staphylococcus aureus. J Bacteriol. 1994;176:7719–7726. doi: 10.1128/jb.176.24.7719-7726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise A A, Price C W. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor ςB in response to environmental signals. J Bacteriol. 1995;177:123–133. doi: 10.1128/jb.177.1.123-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, de Lencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]