ABSTRACT
Pathological effects of apoptosis associated with viral infections of the central nervous system are an important cause of morbidity and mortality. Reovirus is a neurotropic virus that causes apoptosis in neurons, leading to lethal encephalitis in newborn mice. Reovirus-induced encephalitis is diminished in mice with germ line ablation of NF-κB subunit p50. It is not known whether the proapoptotic function of NF-κB is mediated by neural-cell-intrinsic (neural-intrinsic) processes, NF-κB-regulated cytokine production by inflammatory cells, or a combination of both. To determine the contribution of cell type-specific NF-κB signaling in reovirus-induced neuronal injury, we established mice that lack NF-κB p65 expression in neural cells using the Cre/loxP recombination system. Following intracranial inoculation of reovirus, 50% of wild-type (WT) mice succumbed to infection, whereas more than 90% of mice lacking neural cell NF-κB p65 (Nsp65−/−) survived. While viral loads in brains of WT and Nsp65−/− mice were comparable, histological analysis revealed that reovirus antigen-positive areas in the brains of WT mice displayed increased immunoreactivity for cleaved caspase-3, a marker of apoptosis, relative to Nsp65−/− mice. These data suggest that neural-intrinsic NF-κB-dependent factors are essential mediators of reovirus neurovirulence. RNA sequencing analysis of reovirus-infected brain cortices of WT and Nsp65−/− mice suggests that NF-κB activation in neuronal cells upregulates genes involved in innate immunity, inflammation, and cell death following reovirus infection. A better understanding of the contribution of cell type-specific NF-κB-dependent signaling to viral neuropathogenesis could inform development of new therapeutics that target and protect highly vulnerable cell populations.
IMPORTANCE Viral encephalitis contributes to illness and death in children and adults worldwide and has limited treatment options. Identifying common host factors upregulated by neurotropic viruses can enhance an understanding of virus-induced neuropathogenesis and aid in development of therapeutics. Although many neurotropic viruses activate NF-κB during infection, mechanisms by which NF-κB regulates viral neuropathogenesis and contributes to viral encephalitis are not well understood. We established mice in which NF-κB expression is ablated in neural tissue to study the function of NF-κB in reovirus neurovirulence and identify genes activated by NF-κB in response to reovirus infection in the central nervous system. Encephalitis following reovirus infection was dampened in mice lacking neural cell NF-κB. Reovirus induced a chemokine profile in the brain that was dependent on NF-κB signaling and was similar to chemokine profiles elicited by other neurotropic viruses. These data suggest common underlying mechanisms of encephalitis caused by neurotropic viruses and potentially shared therapeutic targets.
KEYWORDS: reovirus, NF-κB, p65, RelA, encephalitis, neurovirulence
INTRODUCTION
Many neurotropic viruses activate the NF-κB pathway during infection, which can contribute to cell survival and evasion of the immune response or elicit apoptosis and mediate viral spread (1). The role of NF-κB signaling in the central nervous system (CNS) is cell type dependent. In neurons, NF-κB signaling maintains overall neuronal homeostasis, synapse growth and function, and neuroprotection in certain disease conditions. In glial cells, NF-κB signaling also contributes to maintaining neuronal cell health, although chronic NF-κB activation is neurotoxic (2). Factors that determine cell fate following virus-induced-NF-κB activation in the CNS are not well understood.
Mammalian orthoreoviruses (reoviruses) are nonenveloped viruses with a segmented, double-stranded RNA genome. These viruses infect a variety of mammalian species but cause disease only in the very young. Reovirus is transmitted by the fecal-oral route and disseminates from the intestine to virtually all organs using hematogenous or neural routes. Infection of newborn mice with serotype 3 reovirus causes neuronal apoptosis, which leads to lethal encephalitis. Following cell entry, reovirus activates NF-κB using a mechanism that requires IKKα and IKKγ (3) and subsequent nuclear translocation of the canonical p50 and p65 NF-κB subunits (3, 4). NF-κB activation results in the expression of interferon-stimulated genes (ISGs), cytokines, chemokines, and other genes involved in antiviral responses and cell proliferation (5). Mouse embryo fibroblasts (MEFs) lacking either p50 or p65 display reduced levels of apoptosis following reovirus infection compared to wild-type (WT) MEFs (4), indicating that NF-κB subunits p50 and p65 mediate reovirus-induced apoptosis.
The role of NF-κB subunit p50 in reovirus virulence in newborn mice is organ specific. In mice with germ line ablation of p50 (p50−/− mice), reovirus neurovirulence and neuronal apoptosis are diminished relative to WT mice. In contrast, p50−/− mice succumb to severe myocarditis coupled with increased viral replication in the heart (6). These results suggested that NF-κB activation produces different outcomes depending on the cell type infected, a proapoptotic function in the brain and an antiapoptotic function in the heart. It is unclear whether the proapoptotic function of NF-κB is mediated by neural-cell-intrinsic (neural-intrinsic) processes, a result of NF-κB-regulated cytokine production by inflammatory cells, or a combination of both.
In this study, we established mice lacking NF-κB in neural cells and compared reovirus neuropathogenesis in these animals and WT mice. We discovered that mice with a neural-cell-specific deletion in the NF-κB p65-encoding gene (Nsp65−/− mice) display increased survival following reovirus infection relative to WT mice and are likewise protected from brain injury. Brain regions in WT mice that harbor reovirus antigen also contain the cleaved (activated) form of the executioner apoptotic caspase, caspase-3. However, cleaved caspase-3 staining is markedly reduced in brains of Nsp65−/− mice. To elucidate how NF-κB signaling in the CNS contributes to reovirus neuropathogenesis, we compared reovirus-induced transcriptional changes in brains of WT and Nsp65−/− mice. Genes involved in innate immunity, inflammation, and cell death were identified as components of NF-κB pathways upregulated in the murine brain following reovirus infection. These data identify potential therapeutic targets for development of antiviral agents to limit virus-induced neural injury.
RESULTS
Establishment of neural-specific p65-deficient mice.
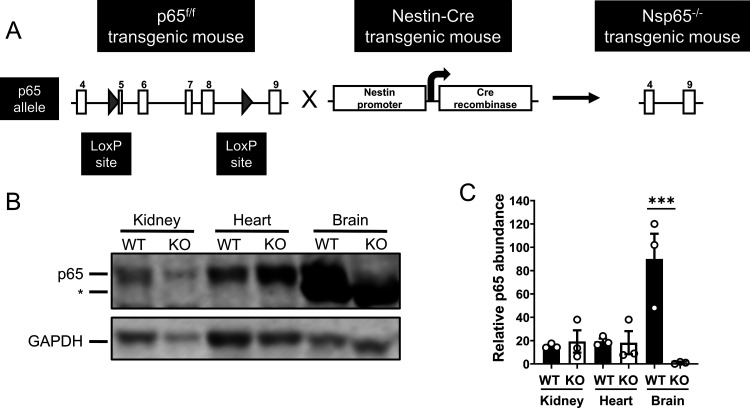
To determine the contribution of neural-cell-specific (neural-specific) NF-κB signaling in reovirus-induced encephalitis in vivo, we crossed mice expressing Cre recombinase under the control of the rat nestin promoter and enhancer to mice with loxP sites flanking exons 5 to 8 of p65/RelA (p65f/f) (7). Nestin, a type VI intermediate filament protein, is expressed in neuronal and glial precursor cells (8). Mice heterozygous for the nestin Cre transgene and homozygous for the p65 floxed allele (neural-specific p65-deficient [Nsp65−/−] mice) were present in the third generation (Fig. 1A). Immunoblot analysis revealed that p65 expression was diminished in brains of Nsp65−/− but not WT p65f/f mice (Fig. 1B and C). Expression of p65 was not diminished in tissues that do not express nestin, demonstrating that p65 expression is conditionally ablated in brain tissue of Nsp65−/− mice.
FIG 1.
Neural-specific p65-null mice lack p65 expression in the brain. (A) Breeding scheme for establishment of neural-specific p65-null mice (Nsp65−/−). Mice with loxP sites (gray arrows) flanking exons 5 to 8 of the p65 allele (p65f/f) were interbred with mice expressing Cre recombinase under the control of the rat nestin promoter and enhancer to establish mice heterozygous for the nestin Cre transgene and homozygous for the p65 floxed allele (Nsp65−/−). (B) p65 expression in tissue homogenates from wild-type (WT) and Nsp65−/− (KO) mice at postnatal day 8 was determined by immunoblotting with antibodies specific for p65 (upper panel) and GAPDH (lower panel) and imaged using an Odyssey fluorescence scanner. A representative image is shown. A nonspecific band is indicated by an asterisk (*). (C) Signal intensity of bands corresponding to p65 and GAPDH were quantified. The results from three independent experiments are expressed as the mean relative abundance of p65 bands normalized to GAPDH bands. Error bars indicate the SEM. ***, P < 0.001 (as determined by one-way ANOVA with Tukey’s multiple comparisons).
Apoptosis induced by reovirus infection is reduced in Nsp65–/– cortical neurons.
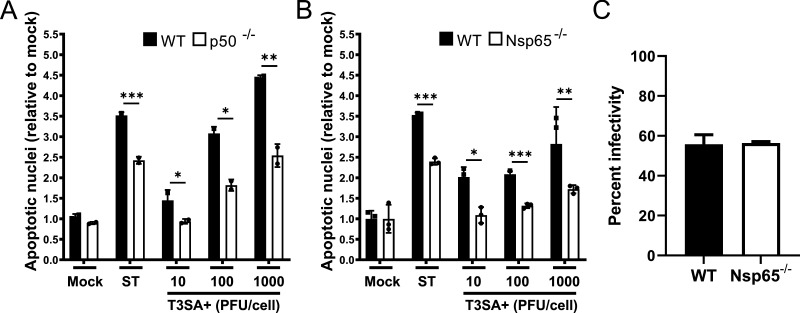
The NF-κB p50 and p65 subunits are required for reovirus-induced apoptosis in cell culture (4), and mice lacking NF-κB p50 display diminished neuronal apoptosis relative to WT mice following reovirus infection (6). To determine whether neural-intrinsic NF-κB-dependent factors contribute to reovirus-induced apoptosis, we infected cultures of WT, p50-deficient (p50−/−), and Nsp65−/− cortical neurons with potently apoptotic reovirus strain T3SA+ infectious subvirion particles (ISVPs), a reovirus disassembly intermediate that efficiently infects primary neuronal cultures (9) and quantified the percentage of apoptotic neurons following acridine orange staining (Fig. 2A and B) and viral infection by immunofluorescence staining (Fig. 2C). Reovirus infection was comparable in WT and Nsp65−/− neurons. However, a significantly higher percentage of WT neurons were observed to undergo apoptosis following reovirus infection compared with p50−/− and Nsp65−/− neurons, suggesting that the proapoptotic function of NF-κB in the CNS is at least in part determined by neuron-intrinsic factors.
FIG 2.
Reovirus-induced apoptosis is diminished in cultures of cortical neurons that lack either NF-κB p50 or p65. Wild-type (WT), p50−/− (A), and Nsp65−/− (B) cortical neurons were adsorbed with reovirus T3SA+ ISVPs at the MOIs shown. At 24 h postadsorption, the percentage of apoptotic nuclei was quantified following AO staining. Staurosporine (ST, 10 mM) was used as positive control. Each point is expressed as the mean percentage of apoptotic cells for triplicate wells. (C) The percentage of infected WT and Nsp65−/− cortical neurons was quantified 24 h postadsorption with 1,000 PFU/cell T3SA+. Error bars indicate the SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (as determined by Student t test compared to results for p50−/− or Nsp65−/− neurons at the same MOI).
Reovirus infection upregulates innate immunity genes, including interferon-stimulated genes, in primary cortical neurons.
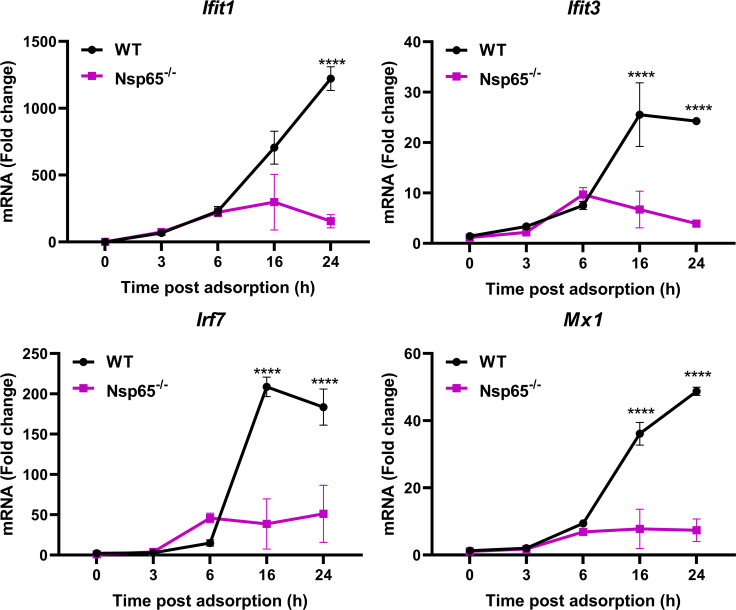
Reovirus infection upregulates genes associated with innate immunity, cytokine signaling, and apoptosis in the CNS (10). To determine whether neural-intrinsic NF-κB signaling is required for upregulation of innate immunity genes during reovirus infection, we infected WT and Nsp65−/− primary cortical neurons with T3SA+ ISVPs. Transcript levels of Ifit1, Ifit3, Irf7, and Mx1, which are interferon-stimulated genes (ISGs) upregulated in the CNS during reovirus infection (10), were detected by reverse transcription-PCR (RT-PCR) at 3, 6, 16, and 24 h postadsorption (Fig. 3). The levels of all four transcripts were comparable in primary cortical neurons derived from WT and Nsp65−/− mice until 6 h postadsorption, when a marked NF-κB-dependent increase was observed. These data demonstrate that reovirus infection of primary cortical neurons upregulates ISGs in an NF-κB-dependent manner, suggesting that upregulation of genes associated with innate immunity observed in the brains of infected mice are at least in part attributable to NF-κB activation in neurons.
FIG 3.
Reovirus-induced upregulation of ISG transcripts in cultured cortical neurons is dependent on NF-κB p65 expression. Wild-type (WT) and Nsp65−/− cortical neurons were adsorbed with 1,000 PFU of reovirus T3SA+ ISVPs or PBS. Cells were harvested at the times indicated, RNA was isolated, and transcript levels were determined by quantitative RT-PCR. Representative data from three independent experiments are shown. The data are presented as the mean values of two replicates. Error bars indicate the SD. *, P < 0.05 (as determined by two-way ANOVA with Sidak’s multiple comparisons).
Neural-intrinsic expression of NF-κB is required for reovirus virulence.
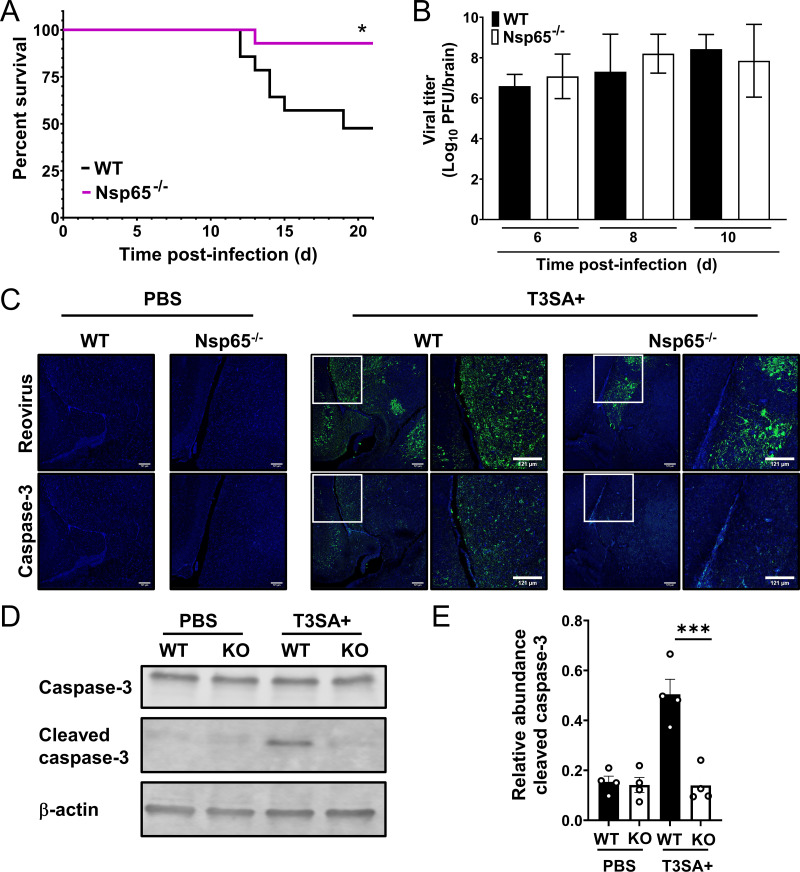
To determine whether neural-specific p65 contributes to reovirus neurovirulence, newborn WT and Nsp65−/− mice were inoculated intracranially with T3SA+ and monitored for survival for 21 days (Fig. 4A). While more than 90% of Nsp65−/− mice survived T3SA+ infection, only 50% of WT mice survived, suggesting that p65 expression in neural cells enhances reovirus neurovirulence.
FIG 4.
Neural-specific NF-κB p65 is required for reovirus neurovirulence but not virus replication. (A) Survival following intracranial inoculation. Three-day-old wild-type (WT) and Nsp65−/− mice (n = 14 WT and 16 Nsp65−/−) were inoculated intracranially with 10 PFU of reovirus T3SA+ and monitored for disease for 21 days. Mice were euthanized when moribund. Values that differ from WT by log rank test are indicated (*, P < 0.05). (B and C) Viral titers (B) and immunohistopathology (C) in the brain following intracranial inoculation. Independent cohorts of identically inoculated mice (n = 5 to 6 mice for each strain) were euthanized at 6, 8, and 10 days postinoculation. (B) Viral titers of the homogenized left-brain hemisphere were determined by plaque assay. The data are log transformed and displayed with a linear x axis scale. (C) Titer-matched right brain hemispheres at 8 days postinoculation were fixed in formalin and embedded in paraffin. Serial coronal sections were immunostained with reovirus polyclonal antiserum (green) or antibody specific for activated caspase-3 (green). Nuclei were stained with DAPI (blue). Regions corresponding to high-magnification insets are indicated by white boxes in the low-magnification images. Representative sections are shown. (D) Titer-matched brain homogenates at 6 days postinoculation were resolved by SDS-PAGE, and immunoblotted with antiserum specific for caspase-3 (upper panel), activated caspase-3 (middle panel), and β-actin (lower panel), and imaged using an Odyssey fluorescence scanner. A representative image is shown. (E) Signal intensity of bands corresponding to activated caspase-3 and β-actin were quantified. Results from four independent experiments are expressed as the mean relative abundance of activated caspase-3 bands normalized to β-actin bands. Error bars indicated the SD. ***, P < 0.001 (as determined by one-way ANOVA with Tukey’s multiple comparisons).
To determine whether neural-specific NF-κB influences reovirus replication and pathology, newborn WT and Nsp65−/− mice were inoculated intracranially with T3SA+. At 6, 8, and 10 days postinoculation, mice were euthanized, and the brains were resected. Viral loads detected in the left hemispheres of WT and Nsp65−/− mice were comparable at all time points tested (Fig. 4B). Serial coronal sections from embedded, titer-matched right hemispheres at 8 days postinoculation were immunostained with reovirus-specific polyclonal antiserum or an antibody specific for the cleaved form of caspase-3 to monitor for cell death (Fig. 4C). Areas in the brains of WT mice positive for reovirus antigen displayed enhanced cleaved caspase-3 immunoreactivity, whereas reovirus antigen-positive areas in the brains of Nsp65−/− mice displayed low levels of cleaved caspase-3 immunoreactivity. Immunoblot analysis of brain homogenates of WT and Nsp65−/− mice 6 days postinoculation showed a significant increase in cleaved caspase-3 levels in reovirus-infected WT mice relative to reovirus-infected Nsp65−/− mice (Fig. 4D and E). Together, these data suggest that neural-intrinsic NF-κB-dependent factors are not required for reovirus replication in the brain but are essential mediators of reovirus apoptosis and neurovirulence.
NF-κB-dependent changes in mRNA expression in neural cells following reovirus infection.
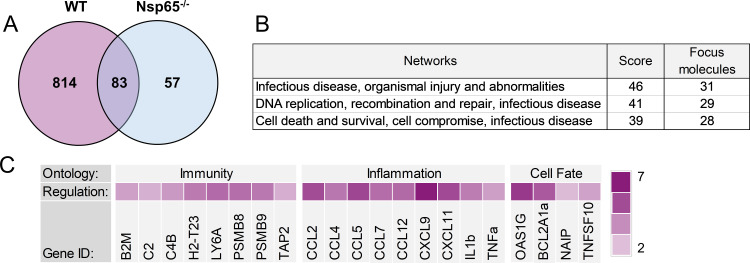
To identify additional mediators of NF-κB-dependent, reovirus-induced apoptosis in neurons, we inoculated 2-day-old WT and Nsp65−/− mice intracranially with reovirus T3SA+ or PBS (mock). At 6 days postinoculation, mice were euthanized, and cortices were microdissected, homogenized, and processed for viral titer via plaque assay and RNA purification. Transcript abundance in samples from infected WT and Nsp65−/− mice, matched for viral titer, as well as those from phosphate-buffered saline (PBS)-inoculated controls, was determined by mRNA sequencing. Three samples of RNA for each genotype were subjected to paired-end RNA sequencing. We identified genes that were significantly up or downregulated in a reovirus (897 genes)-, p65 (140 genes)-, and reovirus and p65 (814 genes)-dependent manner and ranked the genes for abundance using DESeq2 (Fig. 5A; see also Table S1 in the supplemental material). The top 789 genes were uploaded to Ingenuity Software (Qiagen, Inc.), which considers the adjusted P values of the analyses, and gene networks containing more than one candidate in the analysis were identified (Fig. 5B). Examples of gene networks that contained multiple candidates were related to inflammation, antimicrobial response, immune cell development, and cell death (Fig. 5B). Representative genes under the control of p65 that were differentially expressed in infected brain tissue were predominantly in groups involved in innate immunity, inflammation, and cell death (Fig. 5C).
FIG 5.
Expression of genes influenced by reovirus infection and NF-κB p65 in the brain. Wild-type (WT) and Nsp65−/− newborn mice were inoculated intracranially with 10 PFU of reovirus T3SA+ or PBS. Cortices were removed at 6 days postinoculation and processed for RNA purification and mRNA sequencing. RNA samples prepared from infected mice were matched for viral titer. (A) Venn diagram of differential expression analysis using DeSeq2 package in R between WT infected versus mock infected (pink) and Nsp65−/− infected versus mock infected (blue). (B) Networks with the most differentially expressed genes were identified using Ingenuity Pathway Analysis. (C) Log2-fold change heat map indicating the expression levels of selected genes. The log2-fold change was calculated based on the DESeq2 analysis of RNA levels in WT-infected brains relative to Nsp65−/−-infected brains.
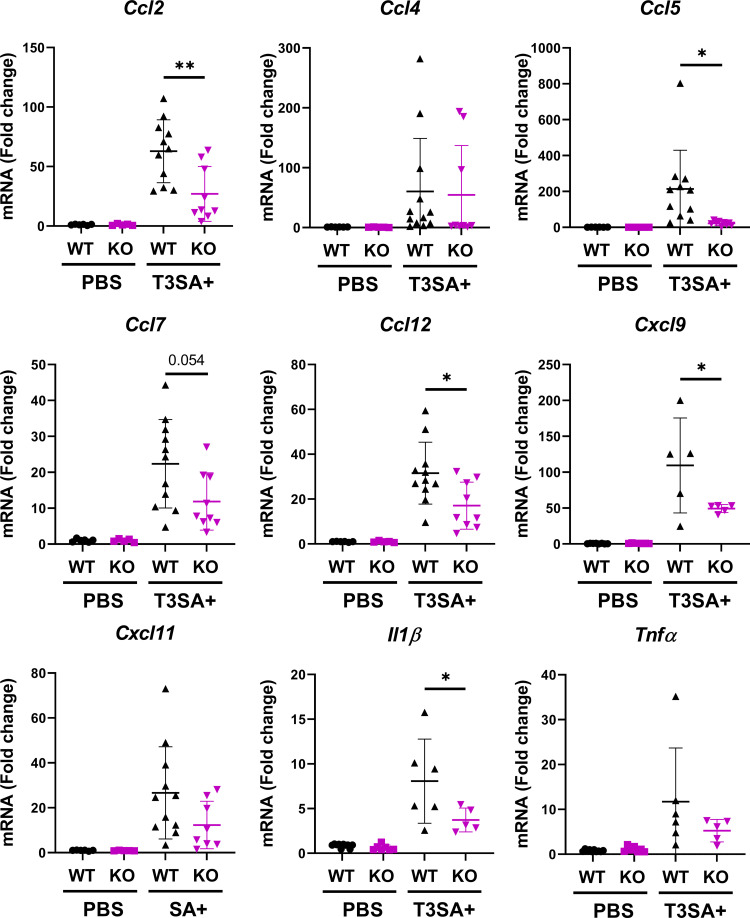
Reovirus infection in the brain induces a p65-dependent upregulation of proinflammatory cytokines.
Proinflammatory cytokines are important mediators of neuropathogenesis associated with neurotropic viral infections (11, 12). We first validated at the transcript and protein level the proinflammatory cytokines differentially expressed in a reovirus- and NF-κB-dependent manner (Fig. 5). To validate at the transcript level, 3-day-old WT and Nsp65−/− mice were inoculated intracranially with T3SA+ or PBS. At 6 days postinoculation, brains were harvested and processed for quantitative RT-PCR and plaque assay. RNA isolated from brain homogenates from WT and Nsp65−/− mice, matched for viral titer, were analyzed for Ccl2, Ccl4, Ccl5, Ccl7, Ccl12, Cxcl9, Cxcl11, Il1β, and Tnfα transcript levels (Fig. 6). The levels of all chemokines tested were upregulated in a reovirus- and p65-dependent manner, with statistically significant differences between WT and Nsp65−/− mice observed for Ccl2, Ccl5, Ccl12, and Cxcl9, as well as Il1β.
FIG 6.
Reovirus-induced upregulation of cytokine transcripts in the brain is dependent on NF-κB p65 expression in neural cells. Wild-type (WT) and Nsp65−/− (KO) newborn mice were inoculated intracranially with 10 PFU of reovirus T3SA+ or PBS. Cortices were removed at 6 days postinoculation, RNA was isolated, and transcript levels were determined by quantitative RT-PCR. Samples were matched for viral titer. The data are presented as the mean values of 5 to 11 mice. *, P < 0.05; **, P < 0.005 (as determined by one-way ANOVA with Tukey’s multiple comparisons).
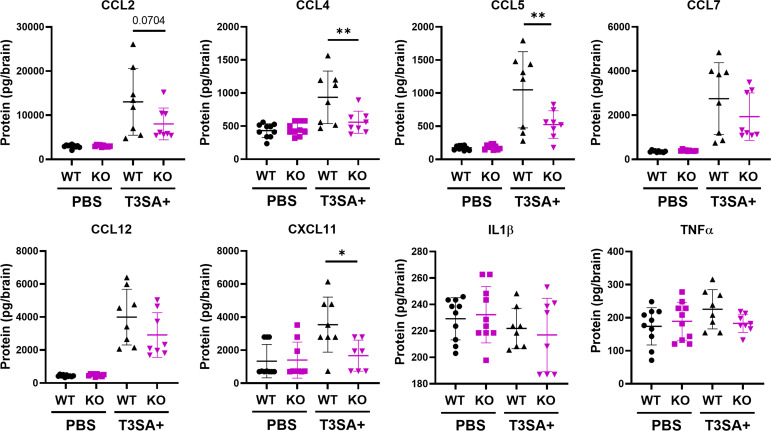
To validate at the protein level the proinflammatory cytokines that were upregulated at the transcript level in the presence of neural p65, 3-day-old WT and Nsp65−/− mice were inoculated intracranially with T3SA+ or PBS. At 6 days postinoculation, the brains were harvested, homogenized, and processed for Luminex protein assay and plaque assay. Brain homogenates from WT and Nsp65−/− mice, matched for viral titer, were analyzed for CCL2, CCL4, CCL5, CCL7, CCL12, CXCL11, interleukin-1β (IL-1β), and tumor necrosis factor alpha (TNF-α) protein levels (Fig. 7). The levels of CCL2, CCL4, CCL5, CCL7, CCL12, and CXCL11 were upregulated in a reovirus- and p65-dependent manner, with statistically significant differences between WT and Nsp65−/− mice observed for CCL4, CCL5, and CXCL11. These data validate our gene expression data and demonstrate that cytokine production during reovirus infection is dependent on neural-intrinsic NF-κB expression.
FIG 7.
Reovirus-induced cytokine expression in the brain is dependent on NF-κB p65 expression in neural cells. Wild-type (WT) and Nsp65−/− (KO) newborn mice were inoculated intracranially with 10 PFU of reovirus T3SA+ or PBS. Cortices were removed at 6 days postinoculation and processed for Luminex protein analysis and plaque assay. Samples were matched for viral titer. The data are presented as the mean values of 8 to 10 mice. *, P < 0.05; **, P < 0.005 (as determined by one-way ANOVA with Tukey’s multiple comparisons).
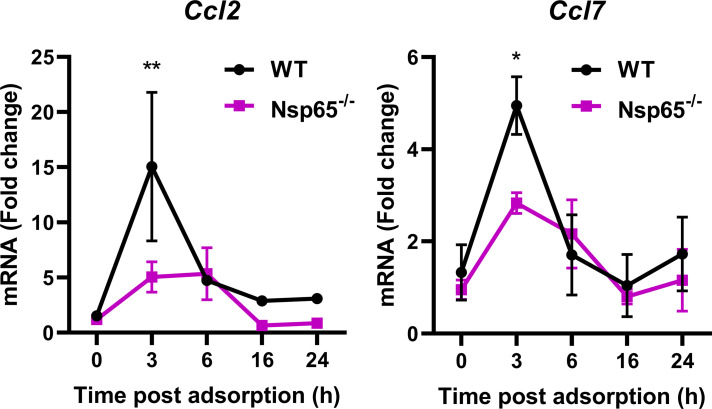
While p65 expression is diminished in cells containing nestin in the brains of Nsp65−/− mice, p65 is expressed in cells that lack nestin, raising the possibility that surrounding cells in the brain contribute to the observed differences in cytokine production. To determine whether reovirus infection of neurons is sufficient to induce an increase in cytokine transcript levels in an NF-κB-dependent manner, we infected WT and Nsp65−/− primary cortical neurons with T3SA+ ISVPs. Transcript levels for Ccl2, Ccl4, Ccl5, Ccl7, Ccl12, Cxcl9, and Cxcl11 were detected by RT-PCR at 3, 6, 16, and 24 h postadsorption. Although we observed an increase in transcript levels of all chemokines, only Ccl2 and Ccl7 transcript levels were dependent on NF-κB expression in neurons (Fig. 8). These data demonstrate that reovirus infection of primary cortical neurons upregulates gene expression of a subset of cytokines in an NF-κB-dependent manner, suggesting that differences in cytokine levels observed in the brains of infected mice are at least in part attributable to NF-κB activation in neurons.
FIG 8.
Reovirus-induced expression of Ccl2 and Ccl7 in cultured cortical neurons is dependent on NF-κB p65. Wild-type (WT) and Nsp65−/− cortical neurons were adsorbed with 1,000 PFU of reovirus T3SA+ ISVPs or PBS. Cells were harvested at the times indicated, RNA was isolated, and transcript levels were determined by quantitative RT-PCR. Representative data from three independent experiments are shown. The data are presented as the mean values of two replicates. Error bars indicate the SD. *, P < 0.05; **, P < 0.01 (as determined by two-way ANOVA with Sidak’s multiple comparisons).
DISCUSSION
The NF-κB pathway is central to many cellular processes, including the regulation of cell cycle and cell fate, initiation of innate immune responses, and production of cytokines and chemokines. Several neurotropic viruses, including enterovirus 71 (13, 14), herpes simplex virus 1 (HSV-1) (15), human immunodeficiency virus type 1 (16), reovirus (4, 6), SARS-CoV-2 (17), Sindbis virus (18), Venezuelan equine encephalitis virus (19), vesicular stomatitis virus (20), and West Nile virus (WNV) (21), activate NF-κB, which can lead to cell survival or apoptosis depending on the virus strain and cell type infected. Based on studies using mice with germ line ablation of NF-κB p50, apoptosis induced by serotype 3 reovirus contributes to neuropathogenesis and is dependent on NF-κB activation (6). However, the contribution of neural-intrinsic NF-κB to reovirus neuropathogenesis, as well as the contribution of genes activated by NF-κB in response to reovirus infection in the CNS, is unknown. In this study, we used mice in which NF-κB p65 expression is ablated in neural cells to discover that neural-intrinsic NF-κB signaling is required for full reovirus neurovirulence and to identify neural-specific, reovirus-induced changes in the expression of genes under NF-κB control. We found that reovirus infection in the brain leads to upregulation of NF-κB-dependent host pathways involved in innate immunity, inflammation, and cell death.
Mice with germ line ablation of NF-κB p50 are protected from severe encephalitis but are more susceptible to reovirus-induced myocarditis (6), indicating tissue-specific roles for NF-κB. To determine whether reovirus-induced neural injury is mediated by the activation of NF-κB in neurons, immune cells, or a combination of both, we established Nsp65−/− mice in which NF-κB p65 is deleted in neural cells (Fig. 1). Expression of p65 in neurons was required for maximal cell death (Fig. 2) and ISG expression (Fig. 3) following reovirus infection of WT and Nsp65−/− primary cortical neurons. In addition, Nsp65−/− mice displayed increased survival following reovirus infection relative to WT mice and were protected from severe brain injury (Fig. 4). These data demonstrate that neural-intrinsic NF-κB-dependent factors are essential for reovirus neurovirulence and suggest that Nsp65−/− mice can be used to study the role of neural-intrinsic NF-κB signaling in neuropathogenesis induced by other neurotropic viruses.
An NF-κB-dependent gene network induced by reovirus infection was identified using a HeLa cell culture system, revealing temporal expression of distinct gene clusters, a majority of which are involved in innate immune responses and IFN signaling (5). However, genes activated by NF-κB can differ depending on the agonist or cell type (1, 2). Here, we defined an NF-κB-dependent gene network induced by reovirus in the brain and observed that a majority of the genes identified are involved in innate immune responses and cell death (Fig. 5), similar to findings made using HeLa cells (5). We also demonstrated that the temporal expression of NF-κB-dependent genes in primary cortical neurons differs from that in HeLa cells. In neurons, chemokines are rapidly upregulated following reovirus adsorption (Fig. 7), while ISG upregulation is delayed (Fig. 3). However, upregulation of chemokines and ISGs is delayed in HeLa cells (5). These data suggest that the pro- or antiapoptotic effect of NF-κB signaling during viral infection may not be dictated solely by cell type but may be dependent on the agonist or site of infection.
In the Nsp65−/− mice used in our study, p65 expression is diminished in cells containing nestin, which includes neurons, microglia, and reactive astrocytes. The proinflammatory response induced in the CNS following injury or disease is highly regulated by NF-κB signaling in neurons and glial cells (2). NF-κB signaling in neurons primarily serves a protective role through regulation of immune response and inflammation genes, growth factors, and antiapoptotic genes, while prolonged signaling in activated astrocytes and microglia is associated with neurotoxicity (2). We found that NF-κB signaling in neurons is required for reovirus-induced neuronal cell death in vitro (Fig. 2) and neuropathogenesis in vivo (Fig. 4), suggesting that reovirus activation of NF-κB in neurons is proapoptotic. Whether NF-κB signaling in activated astrocytes and microglia contributes to reovirus neuropathogenesis is not known.
Neurotropic viral infections lead to elaboration of chemokines and recruitment of immune cells, which contribute to neuropathology (22, 23). Using RNA sequence analysis of brain cortices, we identified several chemokine genes that are expressed in response to reovirus infection and NF-κB activation, including Ccl2, Ccl4, Ccl5, Ccl7, Ccl12, CxclL9, and Cxcl11 (Fig. 5). To validate these data, we found that several chemokines are upregulated in response to reovirus infection and NF-κB activation, with statistically significant increases observed at the transcript level for Ccl2, Ccl5, Ccl12, Cxcl9, and Il1β (Fig. 6) and at the protein level for CCL4, CCL5, and CXCL11 (Fig. 7). Reovirus infection also upregulates CXCL10 both at the transcript and protein level, but this effect is independent of NF-κB expression (data not shown) and may be due to STAT1 signaling (24). Since transcript and protein levels were quantified at 6 days postinoculation, it is possible that peak levels of CCL2, CCL7, and CCL12 are produced at earlier or later intervals in the infectious course and thus were not significantly increased in our analysis. Chemokines upregulated in the brain following reovirus infection also are upregulated by other neurotropic viruses (22, 23). Following CNS infection, HSV-1 induces CCL2, CCL3, CCL5, CXCL9, and CXCL10 and Japanese encephalitis virus and WNV induce CCL2, CCL7, CCL5, CXCL9, and CXCL10 (12). The similarities in chemokine responses elicited by neurotropic viruses suggest similar underlying pathological mechanisms and potentially common therapeutic targets.
Chemokines released during neurotropic viral infections can be produced by resident CNS cells, including neurons, microglia, and astrocytes (11, 22). Since neurons are the primary target of neurotropic viruses, they are thought to initiate immune signaling through chemokine release. Neurons are a source of chemokines following measles virus, Theiler’s murine encephalomyelitis virus (TMEV), and WNV infection (12). Neuronal release of CCL5 and CXCL10 during measles virus infection and CXCL10 during WNV infection correlates with T cell recruitment (25, 26), while the release of CCL2 during TMEV infection (27) and CCL2 and CCL7 during WNV infection (28, 29) results in inflammatory monocyte recruitment (28, 29). We found that reovirus infection in the brain upregulates CCL2, CCL7, and CXCL10 (Fig. 6 and 7), suggesting a similar role for infiltrating T cells and inflammatory monocytes in reovirus infection. However, the source of the chemokines, timing of release, and dependence on NF-κB signaling are not known. Using primary cortical neuron cultures prepared from WT and Nsp65−/− mice, we found that transcript levels of Ccl2 and Ccl7 are increased in an NF-κB-dependent manner during reovirus infection (Fig. 8), while increased Ccl4, Ccl5, Ccl7, Ccl12, Cxcl9, and Cxcl11 transcript levels were independent of NF-κB. These data suggest that while reovirus-infected neurons contribute to the observed increase in chemokines in the brain, only CCL2 and CCL7 are dependent on NF-κB expression in neurons. Therefore, NF-κB signaling in other cell types contributes to chemokine responses in the brain during reovirus infection.
Astrocytes and microglia maintain neuronal health in both disease and nondisease states. During neurotropic viral infections, proinflammatory factors released by infected neurons activate astrocytes and microglia, triggering production of chemokines, such as CCL2, CCL4, CCL5, CXCL10, IL-1β, and TNF-α (11, 22, 28, 30). Reovirus infection of the CNS activates astrocytes and microglia (31, 32). In the spinal cord, reovirus infection upregulates chemokines associated with glial activation, including CCL5, CXCL10, IL-1β, IFN-γ, and TNF-α, and leads to an infiltration of T cells (32). We observed upregulation of CCL5, CXCL10, IL-1β, and TNF-α in the reovirus-infected brain dependent on neural-intrinsic expression of NF-κB (Fig. 6 and 7). These data suggest that NF-κB signaling in astrocytes and microglia also may contribute to chemokine expression induced by reovirus infection in the CNS and may augment reovirus neuropathogenesis. Defining the pattern of chemokine release and the relevant neural cell types will aid in understanding how the immune response controls reovirus infection in the CNS.
Effective host immune responses clear pathogenic microorganisms while limiting tissue damage, a goal that can be achieved only with a precise balance of pro- and anti-inflammatory mediators. Chemokine expression at early times following neurotropic viral infection is required to control viral replication by promoting immune cell infiltration and activating astrocytes and microglia (11). However, prolonged chemokine expression can be neurotoxic and contribute to neural injury (28, 30). We found that while chemokine levels were upregulated in both WT and Nsp65−/− mice following reovirus infection, the level of chemokines released during infection was dependent on NF-κB expression. In addition, lower levels of chemokines observed in Nsp65−/− mice correlated with increased survival and decreased neuronal apoptosis (Fig. 4). We propose that early in infection, reovirus-infected neurons express a subset of chemokines in an NF-κB-dependent manner resulting in immune cell recruitment and activation of astrocytes and microglia, both of which are necessary for controlling viral infection. Following activation, astrocytes and microglia express additional chemokines in an NF-κB-dependent manner resulting in prolonged activation and neurotoxicity, which in turn might contribute to reovirus-induced apoptosis of neurons. In the absence of NF-κB signaling, we think that reovirus infection induces low levels of chemokine expression through alternate transcription factors, such as STAT1 and AP1 (33–35), resulting in immune cell infiltration and limited astrocyte and microglia activation. Low levels of chemokines and lack of NF-κB signaling in astrocytes and microglia may limit additional chemokine expression, resulting in an environment conducive to virus clearance with minimal tissue damage.
Our studies enhance an understanding of the function of NF-κB in viral neuropathogenesis. We identified NF-κB-dependent pathways induced by reovirus infection in the CNS and discovered that, like other neurotropic viruses, reovirus infection leads to the expression of genes involved in immune signaling. In addition, we identified a pattern of chemokine expression activated by reovirus in the brain that was similar to chemokine expression patterns observed following other neurotropic viral infections. The similarities in chemokine profiles induced by several neurotropic viruses suggest that therapeutic interruption of the chemokine networks activated by reovirus may have broad utility against neurotropic viruses.
MATERIALS AND METHODS
Mice.
Control p50+/+ (B6129PF1/J-AW-J/AW) and p50−/− (B6129P-Nfkb1tm1Bal) mice (6, 36) and nestin Cre [B6.Cg-Tg(Nes-cre)1Kln/J] mice were obtained from Jackson Laboratory. RelAF/F (p65f/f) mice were provided by Albert Baldwin (7). Experiments were conducted using animal biosafety 2 (ABSL2) facilities and guidelines. All animal husbandry and experimental procedures were performed in accordance with U.S. Public Health Service policy and approved by the Institutional Animal Care and Use Committees at Vanderbilt University and the University of Pittsburgh.
Establishment of neural-specific p65-deficient mice.
RelAF/F mice containing loxP sites flanking exons 5 to 8 of RelA were interbred with mice expressing Cre recombinase under the control of the rat nestin promoter and enhancer [stock no. 003771; B6.Cg-Tg(Nes-cre)1Kln/J (37)] to establish mice heterozygous for floxed RelA and the nestin Cre transgene (RelA+/F; nestin-Cre+/–). Mice carrying the nestin Cre transgene and homozygous for the RelA floxed allele (neural-specific p65-deficient [Nsp65−/−] mice) were present in the third generation. For experimental breeding, Nsp65−/− males were crossed with RelAF/F females to establish litters with 50% of pups carrying the nestin Cre transgene (Nsp65−/−) and 50% without the nestin Cre transgene (WT). All experimental mice were genotyped with respect to RelA and Cre following necropsy.
Cells and viruses.
Spinner-adapted murine L929 (L) cells were maintained in Joklik’s modified Eagle minimal essential medium (US Biological, catalog no. M3867) supplemented to contain 5% fetal bovine serum (FBS; VWR, 97068-085), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 25 ng/mL amphotericin B. Primary neuronal cultures were derived from cortices of p50+/+ (B6129PF1/J-AW-J/AW), p50−/− (B6129P-Nfkb1tm1Bal), Nsp65−/− (RelAF/F; nestin-Cre+/–), or WT (RelAF/F; nestin-Cre−/−) day 15 embryos, as described previously (38). Neurons were plated at a density of 105 cells/well in 24-well plates (Greiner) pretreated with 10 mg/mL poly-d-lysine hydrobromide (Sigma-Aldrich, catalog no. P0899) and 1.64 mg/mL laminin (Corning, catalog no. 354232) diluted in Neurobasal medium (Gibco, catalog no. 21103049). Cultures were incubated for 24 h in Neurobasal medium supplemented to contain 10% FBS, 0.6 mM GlutaMAX, 50 U/mL penicillin, and 50 μg/mL streptomycin. Cultures thereafter were maintained in Neurobasal medium supplemented to contain 1× B-27 (Gibco, catalog no. 35050079), 0.6 mM GlutaMAX, 50 U/mL penicillin, and 50 μg/mL streptomycin. One-half of the medium volume was replaced with fresh medium every 3 to 4 days. Neurons were cultivated for 5 to 7 days prior to use.
Purified reovirus strain T3SA+ was prepared from second- or third-passage L-cell lysate stocks (39). Viral particles were extracted from infected cell lysates using Vertrel XF, layered onto 1.2- to 1.4-g/cm3 CsCl gradients, and centrifuged at 62,000 × g for 16 h. Bands corresponding to virions (1.36 g/cm3) were collected and dialyzed in virion-storage buffer (150 mM NaCl, 15 mM MgCl2, 10 mM Tris-HCl [pH 7.4]) (40). The concentration of reovirus virions in purified preparations was determined from an equivalence of one optical density unit at 260 nm equals 2.1 × 1012 virions (40). Viral titer was determined by plaque assay using L cells (41). ISVPs were prepared by treating 2 × 1011 virion particles with 20 μg of α-chymotrypsin (Sigma-Aldrich) in a 100-μL volume of virion storage buffer at 35°C for 60 min (42). Reactions were terminated by the addition of 2 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). The ISVP titer was determined by plaque assay using L cells.
SDS-PAGE and immunoblotting.
Samples for SDS-PAGE were diluted in 5× Laemmli sample buffer (Bio-Rad) containing 10% β-mercaptoethanol, incubated at 95°C for 5 min, and resolved in 10% Mini-Protean TGX gels (Bio-Rad). Proteins were transferred to nitrocellulose membranes (Bio-Rad) at 100 V at 4°C for 1 h and immunoblotted using rabbit anti-p65 (Abcam, ab16502), rabbit anti-cleaved caspase-3, (Cell Signaling Technology, catalog no. 9664), and mouse anti-GAPDH (Sigma-Aldrich, catalog no. G8795). Secondary antibodies IRDye 680RD goat anti-rabbit IgG and IRDye 800CW goat anti-mouse IgG (Li-Cor) were used for detection. Membranes were scanned using an Odyssey CLx imaging system (Li-Cor), and the pixel intensities of the bands were quantified using Image Studio Software (Li-Cor).
Quantification of apoptosis by acridine orange staining.
Neurons were adsorbed with a multiplicity of infection (MOI) of 1,000 reovirus ISVPs per cell diluted in PBS at 37°C for 1 h. The inoculum was removed, fresh medium was added, and neurons were incubated at 37°C for 24 or 48 h. The percentage of apoptotic cells was determined using acridine orange (AO) staining, as described previously (43). Images were collected from >600 cells in two to three fields of view per well by epi-illumination fluorescence microscopy using a fluorescein filter set (Zeiss Photomicroscope III).
Infection of mice.
Two- to three-day-old WT or Nsp65−/− mice weighing 1.6 to 2.3 g were inoculated intracranially in the right cerebral hemisphere with 5 μL containing 2 PFU/μL of purified T3SA+ diluted in PBS using a 30-gauge needle and syringe (Hamilton). Titers of virus in the inoculum were determined to confirm the number of infectious particles in the final dilution. The results of these assays are reported as the inoculating viral dose. For analysis of virulence, mice were monitored for disease signs for 21 days postinoculation and euthanized when moribund, as defined by severe lethargy or seizures, paralysis, or loss of 25% of body weight. Death was not used as an endpoint in our experiments.
Viral replication and immunohistopathology were analyzed at 6 and 8 days postinoculation, respectively. Mice were euthanized, and brains were removed and hemisected along the longitudinal fissure. The left hemisphere was collected in 1 mL of PBS, frozen and thawed once, and homogenized using a TissueLyser (Qiagen, Inc.). Viral titers in brain homogenates were determined by plaque assay using L929 cells (44). The right hemisphere was submerged in 10% buffered formalin at room temperature for 24 to 120 h, embedded in paraffin, and cut into consecutive 6-μm sections. Consecutive sections were deparaffinized, incubated with sodium citrate antigen retrieval buffer (10 mM sodium citrate, 0.05% Tween 20 [pH 6]) at 100°C for 45 min, blocked with 5% bovine serum albumin in PBS at room temperature for 1 h, and incubated with either reovirus polyclonal antiserum (1:1,000) or antibody specific for the cleaved (active) form of capsase-3 (Cell Signaling, catalog no. 9664; 1:300) at room temperature for 1 h. Sections were incubated with secondary antibody Alexa Fluor 488-conjugated IgG (Invitrogen, catalog no. A11034; 1:1,000) at room temperature for 1 h. Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes, catalog no. D3571; 1:2,000) at room temperature for 10 min. Sections were mounted with Aqua Poly Mount (Polysciences, catalog no. 18606-20) and imaged using a Leica TCS SP8 microscope.
Infection of primary cortical neurons.
Neurons were adsorbed with T3SA+ ISVPs at an MOI of 1,000 PFU/cell at 37°C for 1 h, washed with PBS, and incubated at 37°C for various intervals. Cells were washed with PBS and lysed in 1 mL of TRIzol for RNA isolation.
RNA sequencing.
Two- to three-day-old WT or Nsp65−/− mice were inoculated intracranially with T3SA+ and euthanized at 6 days postinoculation. Cortices were microdissected, homogenized, and processed for plaque assay and RNA purification. Tails were harvested for genotyping. Total RNA was extracted from brain cortex homogenates using TRIzol, digested with DNase, and further purified using the High Pure RNA isolation kit (Roche). RNA quantity was assessed using the Quant-iT RiboGreen RNA assay kit (Invitrogen), and RNA quality was assessed using an Agilent bioanalyzer. A total of 1 mg of RNA was used for library preparation. Transcript abundance in cortical samples from infected WT and Nsp65−/− mice matched for viral titer as well as those from PBS-inoculated controls were determined by mRNA sequencing using a HiSeq 2000 instrument (Illumina). Three samples of RNA for each genotype per time point were subjected to paired-end RNA sequencing. Statistical analysis identified genes that were significantly increased or decreased in a reovirus-, p65-, or reovirus-/p65-dependent manner.
Data analysis.
Quality-controlled FASTQ files were aligned to the Ensemble Mus musculus genome (GRCm38) using STAR aligner (version 2.5.1). HTSeq-count was used to quantify counts of reads uniquely mapped to annotated genes using the GRCm38 annotation gtf file (45). Differential gene expression by age, brain regions, and p65 expression, and infection was analyzed using DESeq2 (46) using a model based on the negative binomial distribution. The resulting P values were adjusted using the Benjamini-Hochberg approach for controlling the false discovery rate, and differentially expressed genes were identified at the 5% threshold. Gene-set enrichment analysis was used to assess the statistical enrichment of gene ontologies and pathways (47).
Luminex assays.
Brain homogenates were prepared from mice 6 days postinoculation as described for viral titer determination. Lysates were analyzed by Luminex profiling (Luminex Corporation) using the Bio-Plex Pro Mouse Chemokine 31-plex panel (Bio-Rad, catalog no. 12009159) at a 1:2 dilution according to the manufacturer’s instructions. Cytokine abundance was determined using the laboratory multianalyte profiling system (LabMAP; Luminex).
Quantitative PCR and cDNA synthesis.
Total RNA was extracted from brain tissue of mice 6 days postinoculation or infected cortical neurons at various intervals using TRIzol and an RNeasy minikit (Qiagen) according to the manufacturer’s instructions, followed by DNase digestion. RNA was reverse-transcribed with the SuperScript IV first-strand synthesis system (Thermo Fisher) using 0.5 to 1 μg of RNA. Quantitative RT-PCR was conducted using SsoAdvanced Universal Probes Supermix (Bio-Rad) with an Applied Biosystems ViiA7. Gene expression was determined using the ΔΔCq method in which cDNA abundance was normalized to mouse HPRT. Primers from Thermo Fisher include CCL2 (Mm00441242), CCL7 (Mm00443113), HPRT (Mm00446968), IFIT1 (Mm00515153), IFIT3 (Mm01704846), IRF7 (Mm00516788), and MX1 (Mm00487796).
Statistical analysis.
Experiments were conducted in triplicate and repeated at least twice. Representative results of single experiments are shown in the figures. Mean values were compared using Student t test, one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons, two-way ANOVA with Sidak’s multiple comparisons, or the log-rank test. Error bars denote the data ranges, the standard errors of the means (SEM), or the standard deviations (SD). P values of <0.05 were considered to be statistically significant.
ACKNOWLEDGMENTS
We thank Danica Sutherland for review of the manuscript and members of the Dermody laboratory for insightful discussions about this work. We thank Albert Baldwin at the University of North Carolina, Chapel Hill, for sharing the strain of mice containing floxed p65/RelA developed in his laboratory. We acknowledge the staff of the Vanderbilt Translational Pathology Shared Resource and the Research Pathology/Health Sciences Tissue Bank at UPMC Hillman Cancer Center.
This study was supported by U.S. Public Health Service awards T32 AI060525 (P.H.B.), T32 HL007751 and F31 DK108562 (J.J.B.), R01 AI050800 (T.S.D.), and R01 AI038296 (T.S.D.); the Elizabeth B. Lamb Center for Pediatric Research; and the Heinz Endowments. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that no conflicts of interest exist.
Footnotes
Supplemental material is available online only.
Contributor Information
Terence S. Dermody, Email: terence.dermody@chp.edu.
Susana López, Instituto de Biotecnologia/UNAM.
REFERENCES
- 1.Hiscott J, Kwon H, Genin P. 2001. Hostile takeovers: viral appropriation of the NF-κB pathway. J Clin Invest 107:143–151. 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dresselhaus EC, Meffert MK. 2019. Cellular specificity of NF-κB function in the nervous system. Front Immunol 10:1043. 10.3389/fimmu.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansberger MW, Campbell JA, Danthi P, Arrate P, Pennington KN, Marcu KB, Ballard DW, Dermody TS. 2007. IκB kinase subunits α and γ are required for activation of NF-κB and induction of apoptosis by mammalian reovirus. J Virol 81:1360–1371. 10.1128/JVI.01860-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly JL, Rodgers SE, Clarke P, Ballard DW, Kerr LD, Tyler KL, Dermody TS. 2000. Reovirus-induced apoptosis requires activation of transcription factor NF-κB. J Virol 74:2981–2989. 10.1128/jvi.74.7.2981-2989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell SM, Holm GH, Pierce JM, Tian B, Watson MJ, Chari RS, Ballard DW, Brasier AR, Dermody TS. 2006. Identification of an NF-κB-dependent gene network in cells infected by mammalian reovirus. J Virol 80:1077–1086. 10.1128/JVI.80.3.1077-1086.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donnell SM, Hansberger MW, Connolly JL, Chappell JD, Watson MJ, Pierce JM, Wetzel JD, Han W, Barton ES, Forrest JC, Valyi-Nagy T, Yull FE, Blackwell TS, Rottman JN, Sherry B, Dermody TS. 2005. Organ-specific roles for transcription factor NF-κB in reovirus-induced apoptosis and disease. J Clin Invest 115:2341–2350. 10.1172/JCI22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. 2008. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol 180:2588–2599. 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 8.Lendahl U, Zimmerman LB, McKay RD. 1990. CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595. 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 9.Pruijssers AJ, Hengel H, Abel TW, Dermody TS. 2013. Apoptosis induction influences reovirus replication and virulence in newborn mice. J Virol 87:12980–12989. 10.1128/JVI.01931-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyler KL, Leser JS, Phang TL, Clarke P. 2010. Gene expression in the brain during reovirus encephalitis. J Neurovirol 16:56–71. 10.3109/13550280903586394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosking MP, Lane TE. 2010. The role of chemokines during viral infection of the CNS. PLoS Pathog 6:e1000937. 10.1371/journal.ppat.1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrant DM, Klein RS. 2011. Chemokines and viral infections of the CNS. In Pathogenesis of Encephalitis. IntechOpen. https://www.intechopen.com/chapters/24792.
- 13.Tung WH, Lee IT, Hsieh HL, Yang CM. 2010. EV71 induces COX-2 expression via c-Src/PDGFR/PI3K/Akt/p42/p44 MAPK/AP-1 and NF-κB in rat brain astrocytes. J Cell Physiol 224:376–386. 10.1002/jcp.22133. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao HB, Chou AH, Lin SI, Chen IH, Lien SP, Liu CC, Chong P, Liu SJ. 2014. Toll-like receptor 9-mediated protection of enterovirus 71 infection in mice is due to the release of danger-associated molecular patterns. J Virol 88:11658–11670. 10.1128/JVI.00867-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory D, Hargett D, Holmes D, Money E, Bachenheimer SL. 2004. Efficient replication by herpes simplex virus type 1 involves activation of the IκB kinase-IκB-p65 pathway. J Virol 78:13582–13590. 10.1128/JVI.78.24.13582-13590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Tan J, Lin Y, Jia R, Yang W, Liang C, Geng Y, Qiao W. 2013. HIV-1 Vpr activates both canonical and noncanonical NF-κB pathway by enhancing the phosphorylation of IKKα/β. Virology 439:47–56. 10.1016/j.virol.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Su CM, Wang L, Yoo D. 2021. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci Rep 11:13464. 10.1038/s41598-021-92941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin KI, Lee SH, Narayanan R, Baraban JM, Hardwick JM, Ratan RR. 1995. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol 131:1149–1161. 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaya M, Voss K, Sampey G, Senina S, de la Fuente C, Mueller C, Calvert V, Kehn-Hall K, Carpenter C, Kashanchi F, Bailey C, Mogelsvang S, Petricoin E, Narayanan A. 2014. The role of IKKβ in Venezuelan equine encephalitis virus infection. PLoS One 9:e86745. 10.1371/journal.pone.0086745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulares AH, Ferran MC, Lucas-Lenard J. 1996. NF-κB activation is delayed in mouse L929 cells infected with interferon suppressing, but not inducing, vesicular stomatitis virus strains. Virology 218:71–80. 10.1006/viro.1996.0167. [DOI] [PubMed] [Google Scholar]
- 21.Kesson AM, King NJ. 2001. Transcriptional regulation of major histocompatibility complex class I by flavivirus West Nile is dependent on NF-κB activation. J Infect Dis 184:947–954. 10.1086/323603. [DOI] [PubMed] [Google Scholar]
- 22.Clarke P, Leser JS, Bowen RA, Tyler KL. 2014. Virus-induced transcriptional changes in the brain include the differential expression of genes associated with interferon, apoptosis, interleukin 17 receptor A, and glutamate signaling as well as flavivirus-specific upregulation of tRNA synthetases. mBio 5:e00902-14. 10.1128/mBio.00902-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandwani MN, Creisher PS, O’Donnell LA. 2019. Understanding the role of antiviral cytokines and chemokines on neural stem/progenitor cell activity and survival. Viral Immunol 32:15–24. 10.1089/vim.2018.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke SJ, Goff MR, Lu D, Proud D, Karlstad MD, Collier JJ. 2013. Synergistic expression of the CXCL10 gene in response to IL-1β and IFN-γ involves NF-κB, phosphorylation of STAT1 at Tyr701, and acetylation of histones H3 and H4. J Immunol 191:323–336. 10.4049/jimmunol.1300344. [DOI] [PubMed] [Google Scholar]
- 25.Patterson CE, Daley JK, Echols LA, Lane TE, Rall GF. 2003. Measles virus infection induces chemokine synthesis by neurons. J Immunol 171:3102–3109. 10.4049/jimmunol.171.6.3102. [DOI] [PubMed] [Google Scholar]
- 26.Bardina SV, Lim JK. 2012. The role of chemokines in the pathogenesis of neurotropic flaviviruses. Immunol Res 54:121–132. 10.1007/s12026-012-8333-3. [DOI] [PubMed] [Google Scholar]
- 27.Howe CL, LaFrance-Corey RG, Goddery EN, Johnson RK, Mirchia K. 2017. Neuronal CCL2 expression drives inflammatory monocyte infiltration into the brain during acute virus infection. J Neuroinflammation 14:238. 10.1186/s12974-017-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein RS, Garber C, Funk KE, Salimi H, Soung A, Kanmogne M, Manivasagam S, Agner S, Cain M. 2019. Neuroinflammation during RNA viral infections. Annu Rev Immunol 37:73–95. 10.1146/annurev-immunol-042718-041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardina SV, Michlmayr D, Hoffman KW, Obara CJ, Sum J, Charo IF, Lu W, Pletnev AG, Lim JK. 2015. Differential roles of chemokines CCL2 and CCL7 in monocytosis and leukocyte migration during West Nile virus infection. J Immunol 195:4306–4318. 10.4049/jimmunol.1500352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waltl I, Kalinke U. 2022. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci 45:158–170. 10.1016/j.tins.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Schittone SA, Dionne KR, Tyler KL, Clarke P. 2012. Activation of innate immune responses in the central nervous system during reovirus myelitis. J Virol 86:8107–8118. 10.1128/JVI.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke P, Zhuang Y, Berens HM, Leser JS, Tyler KL. 2019. Interferon β contributes to astrocyte activation in the brain following reovirus infection. J Virol 93:e02027-18. 10.1128/JVI.02027-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD, Collier JJ. 2014. NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab 306:E131–E149. 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauch I, Müller M, Decker T. 2013. The regulation of inflammation by interferons and their STATs. Jakstat 2:e23820. 10.4161/jkst.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singha B, Gatla HR, Vancurova I. 2015. Transcriptional regulation of chemokine expression in ovarian cancer. Biomolecules 5:223–243. 10.3390/biom5010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sha WC, Liou HC, Tuomanen EI, Baltimore D. 1995. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80:321–330. 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 37.Luo L, Ambrozkiewicz MC, Benseler F, Chen C, Dumontier E, Falkner S, Furlanis E, Gomez AM, Hoshina N, Huang WH, Hutchison MA, Itoh-Maruoka Y, Lavery LA, Li W, Maruo T, Motohashi J, Pai EL, Pelkey KA, Pereira A, Philips T, Sinclair JL, Stogsdill JA, Traunmüller L, et al. 2020. Optimizing nervous system-specific gene targeting with cre driver lines: prevalence of germline recombination and influencing factors. Neuron 106:37–65. 10.1016/j.neuron.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antar AAR, Konopka JL, Campbell JA, Henry RA, Perdigoto AL, Carter BD, Pozzi A, Abel TW, Dermody TS. 2009. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 5:59–71. 10.1016/j.chom.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furlong DB, Nibert ML, Fields BN. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol 62:246–256. 10.1128/JVI.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RE, Zweerink HJ, Joklik WK. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 39:791–810. 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- 41.Virgin HW, IV, Bassel-Duby R, Fields BN, Tyler KL. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol 62:4594–4604. 10.1128/JVI.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baer GS, Dermody TS. 1997. Mutations in reovirus outer-capsid protein σ3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J Virol 71:4921–4928. 10.1128/JVI.71.7.4921-4928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyler KL, Squier MK, Rodgers SE, Schneider SE, Oberhaus SM, Grdina TA, Cohen JJ, Dermody TS. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein σ1. J Virol 69:6972–6979. 10.1128/JVI.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland DM, Aravamudhan P, Dietrich MH, Stehle T, Dermody TS. 2018. Reovirus neurotropism and virulence are dictated by sequences in the head domain of the viral attachment protein. J Virol 92:e00974-18. 10.1128/JVI.00974-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anders S, Pyl PT, Huber W. 2015. HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545–15550. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jvi.01442-22-s0001.xlsx, XLSX file, 0.1 MB (97.1KB, xlsx)