ABSTRACT
Classical swine fever (CSF), caused by classical swine fever virus (CSFV), is an important and highly infectious pig disease worldwide. Kinesin-1, a molecular motor responsible for transporting cargo along the microtubule, has been demonstrated to be involved in the infections of diverse viruses. However, the role of kinesin-1 in the CSFV life cycle remains unknown. Here, we first found that Kif5B played a positive role in CSFV entry by knockdown or overexpression of Kif5B. Subsequently, we showed that Kif5B was associated with the endosomal and lysosomal trafficking of CSFV in the early stage of CSFV infection, which was reflected by the colocalization of Kif5B and Rab7, Rab11, or Lamp1. Interestingly, trichostatin A (TSA) treatment promoted CSFV proliferation, suggesting that microtubule acetylation facilitated CSFV endocytosis. The results of chemical inhibitors and RNA interference showed that Rac1 and Cdc42 induced microtubule acetylation after CSFV infection. Furthermore, confocal microscopy revealed that cooperation between Kif5B and dynein help CSFV particles move in both directions along microtubules. Collectively, our study shed light on the role of kinesin motor Kif5B in CSFV endocytic trafficking, indicating the dynein/kinesin-mediated bidirectional CSFV movement. The elucidation of this study provides the foundation for developing CSFV antiviral drugs.
IMPORTANCE The minus end-directed cytoplasmic dynein and the plus end-directed kinesin-1 are the molecular motors that transport cargo on microtubules in intracellular trafficking, which plays a notable role in the life cycles of diverse viruses. Our previous studies have reported that the CSFV entry host cell is dependent on the microtubule-based motor dynein. However, little is known about the involvement of kinesin-1 in CSFV infection. Here, we revealed the critical role of kinesin-1 that regulated the viral endocytosis along acetylated microtubules induced by Cdc42 and Rac1 after CSFV entry. Mechanistically, once CSFV transported by dynein met an obstacle, it recruited kinesin-1 to move in reverse to the anchor position. This study extends the theoretical basis of intracellular transport of CSFV and provides a potential target for the control and treatment of CSFV infection.
KEYWORDS: classical swine fever virus, kinesin-1, Kif5B, acetylated tubulin, endocytic trafficking
INTRODUCTION
Classical swine fever (CSF), caused by classical swine fever virus (CSFV), is a significant animal health issue that causes a highly contagious hemorrhagic fever in pigs, which is deadly in its acute form (1, 2). Due to its severe economic impact, CSF is classed as notifiable by the World Organisation for Animal Health (WOAH) (3, 4). CSFV is a small enveloped virus with a positive single-stranded RNA genome and belongs to the genus Pestivirus in the Flaviviridae family (5). The genome of CSFV includes two noncoding regions, the 5′ untranslated region (UTR) and 3′ UTR, and one open reading frame (ORF) in the middle. The open ORF encodes four structural proteins (C, Erns, E1, and E2) and eight nonstructural proteins (Npro, P7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) (6–8). Of them, structural proteins E2 and Erns mediate attachment and interaction with the cellular receptors, and E1 is involved in fusion and entry (9, 10). Following entry and uncoating, a defined sequence of events in the biosynthesis of the CSFV nonstructural proteins orchestrates Pestivirus replication. Viral nonstructural proteins that are not essential for the basic life cycle of the virus have typically accessory functions such as modulation of the host's innate immune responses (11–13).
The transport of substances in eukaryotic cells along microtubules is mainly mediated by the molecular motor, which includes dynein and kinesin (14–18). Dynein transports cargo retrogradely to the negative pole of the microtubule, while kinesin transports cargo anterogradely to the positive pole of the microtubule (19, 20). Many viruses can be transported by microtubules to achieve the purpose of replication and transmission. As described by previous reports, there are over 45 different mammalian kinesin proteins arranged into 14 different families (21, 22). The founding members of the kinesin superfamily are the kinesin-1 proteins, which consist of 3 heavy chains, KIF5A, -5B, and -5C, and 2 light chains, KLC1 and KLC2. KIF5A and -5C tend to be neuron specific, while KIF5B (classic kinesin) is expressed in most cells (23, 24). Traditionally, Kif5B transports cargo toward the plus end of microtubule, which is often located in the cell periphery (25–27). Kif5B takes part in multiple processes in cells, including movement of signaling proteins, endoplasmic reticulum (ER) positioning, vesicular trafficking, transport of flagellar components, mRNA transport, spindle microtubule, and chromosomal movements (28, 29). Based on the special function of Kif5B in cells described above, many viruses hijacked Kif5B to facilitate multiple stages of virus infection, including endocytosis, uncoating, and assembly (30–38). However, whether Kif5B facilitates CSFV infection remains poorly understood.
After attachment to the cell surface receptors, CSFV enters the host cells via a pH-, dynamin-, and cholesterol-dependent, clathrin- or caveolin-mediated endocytic pathway that requires Rab5 and Rab7 (39, 40). Moreover, we have previously demonstrated that CSFV endocytosis needs dynein and microtubules (41). In this study, we further performed a systematic analysis of Kif5B that mediated CSFV endocytic trafficking associated with endosomes (the markers Rab7 and Rab11) to lysosomes (the marker Lamp-1), suggesting that kinesin-1 was involved in CSFV infection. Strikingly, the interaction of Kif5B and dynein along acetylated tubulin facilitated CSFV infection, suggesting that microtubule acetylation is essential for CSFV entry. Together, these findings shed light on the role of kinesin-1 in CSFV infection, which will provide references for understanding the pathogenesis of CSFV as well as for vaccine and drug development.
RESULTS
Kinesin-1 heavy chain Kif5B is involved in CSFV proliferation.
To determine the influence of CSFV infection on kinesin-1 expression, PK-15 cells infected with CSFV (multiplicity of infection [MOI] of 10) were collected at indicated time points and subjected to Western blotting. The result showed that Kif5B expression almost remained steady during CSFV infection (Fig. 1A), suggesting that CSFV infection did not affect Kif5B expression. Next, to define the role of Kif5B in CSFV infection, cells transfected with siKif5B were infected with CSFV for 24 h and collected for Western blotting, reverse transcriptase quantitative PCR (RT-qPCR), and virus titration. The results showed that Kif5B knockdown reduced the production of CSFV Npro protein by 53.0% (Fig. 1B), viral RNA by 38.7%, and virus titer by 23.1%, respectively (Fig. 1C). On the contrary, CSFV-infected cells were transfected with siKif5B for 24 h and collected for RT-qPCR, Western blotting, and virus titration. Interestingly, the results showed that Kif5B knockdown reduced the production of CSFV Npro protein by 25.5% (Fig. 1D), viral RNA by 18.4%, and virus titer by 8.9%, respectively (Fig. 1E). This suggests that the depletion of Kif5B played a vital role in the early stage of CSFV infection. To this end, the siKif5B-transfected cells were infected with CSFV and collected at indicated time points for RT-qPCR. The results showed that the viral RNA level of CSFV decreased by 37.3%, 49.1%, and 37.5% at 1, 6, and 24 hours postinfection (hpi), respectively (Fig. 1F), compared with the control group. To evaluate the effect of Kif5B overexpression on the proliferation of CSFV, cells were transfected with the plasmid pFLAG-Kif5B for 24 h and infected with CSFV. At 24 hpi, cells were harvested and subjected to Western blotting, RT-qPCR, and virus titration. Compared with the control group, Kif5B overexpression resulted in a significant increase of Npro protein, viral RNA, and virus titer by 71.0%, 88.2%, and 19.8%, respectively (Fig. 1G and H). Overall, Kif5B is involved in early infection of CSFV.
FIG 1.
Kif5B is involved in CSFV infection. (A) Kif5B was steadily expressed during CSFV infection. Cells infected with CSFV (MOI = 1) were collected at indicated time points and subjected to Western blotting by using indicated antibodies. (B and C) Cells were transfected with siKif5B and infected with CSFV (MOI = 1). At 24 hpi, cells were collected and subjected to Western blotting, RT-qPCR, and virus titration. Data are means ± SDs from three independent experiments. **, P < 0.01. (D and E) Cells infected with CSFV (MOI = 1) were transfected with siKif5B for 24 h and harvested for Western blotting, RT-qPCR, and virus titration. Data are means ± SDs from three independent experiments. *, P < 0.05. (F) Cells transfected with siKif5B were infected with CSFV (MOI = 10) for 1, 6, and 24 h. Cells were collected and subjected to RT-qPCR. Data are means ± SDs from three independent experiments. **, P < 0.01; ***, P < 0.001. (G and H) Cells were transfected with the plasmid pFLAG-Kif5B or vector (pFLAG) and infected with CSFV (MOI = 1) for 24 h. Cells were collected and subjected to Western blotting, RT-qPCR, and virus titration. Data are means ± SDs from three independent experiments. **, P < 0.01.
Kif5B plays a vital role in early infection.
To verify whether Kif5B is involved in the early stage of CSFV infection, siKif5B-transfected cells were infected with CSFV and collected within 4 h for RT-qPCR. The results showed that compared with the control group, the viral RNA was separately decreased by 41.0%, 54.7%, and 68.2% at 1, 2, and 4 hpi, respectively (Fig. 2A). However, Kif5B depletion did not affect the binding of CSFV (Fig. 2B). Subsequently, cells transfected with the plasmid pFLAG-Kif5B were infected with CSFV (MOI = 10) and collected within 4 h for RT-qPCR. Compared with the control group, the viral RNA was increased by 41.6%, 33.7%, and 44.5% at 1, 2, and 4 hpi, respectively (Fig. 2C). However, Kif5B overexpression did not affect the binding of CSFV (Fig. 2D). To further determine the interaction of Kif5B and CSFV, cells infected with CSFV (MOI = 10) were fixed and stained with indicated antibodies. As shown in Fig. 2E, there was an obvious colocalization between CSFV particles and Kif5B. Pearson correlation and live-cell imaging analysis also confirmed the above-described results (Fig. 2F; see Movie S1 in the supplemental material). In addition, to determine whether Kif5B is involved in the late stage of CSFV infection, infected cells were fixed and stained with mouse anti-double-stranded RNA (dsRNA) antibody for confocal microscopy. The results showed that Kif5B did not colocalize with CSFV dsRNA, suggesting that Kif5B was not involved in virus replication (Fig. 2G and H). Together, these results suggest that Kif5B is involved in CSFV entry.
FIG 2.
Kif5B plays a pivotal role in the early stage of CSFV infection. (A) Cells were transfected with siKif5B or siCtrl and infected with CSFV (MOI = 10) for 1 h, 2 h, or 4 h. Viral RNA was extracted and determined by RT-qPCR. Data are means ± SDs from three independent experiments. *, P < 0.05; **, P < 0.01. (B) Cells transfected siKif5B or siCtrl were infected with CSFV (MOI = 10) at 4°C for 1 h (binding) and collected for RT-qPCR. Data are means ± SDs from three independent experiments. (C) Cells were transfected with the plasmid pFLAG-Kif5B or vector and infected with CSFV (MOI = 10) for 1 h, 2 h, or 4 h. Viral RNA was extracted and determined by RT-qPCR. Data are means ± SDs from three independent experiments. ***, P < 0.001; ****, P < 0.0001. (D) Cells were transfected with the plasmid pFLAG-Kif5B or vector and infected with CSFV (MOI = 10) at 4°C for 1 h (binding) and collected for RT-qPCR. Data are means ± SDs from three independent experiments. (E) CSFV colocalized with Kif5B. Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C. At different intervals, cells were fixed and stained with mouse anti-CSFV E2 monoclonal antibody (WH303) or rabbit anti-Kif5B antibody for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (F) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. ***, P < 0.001; ****, P < 0.0001. (G) Cells infected with CSFV (MOI = 1) for 24 h were fixed and stained with mouse anti-dsRNA antibody (red) and rabbit anti-Kif5B antibody (green) for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (H) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments.
Kif5B is associated with endosomal and lysosomal trafficking after CSFV entry.
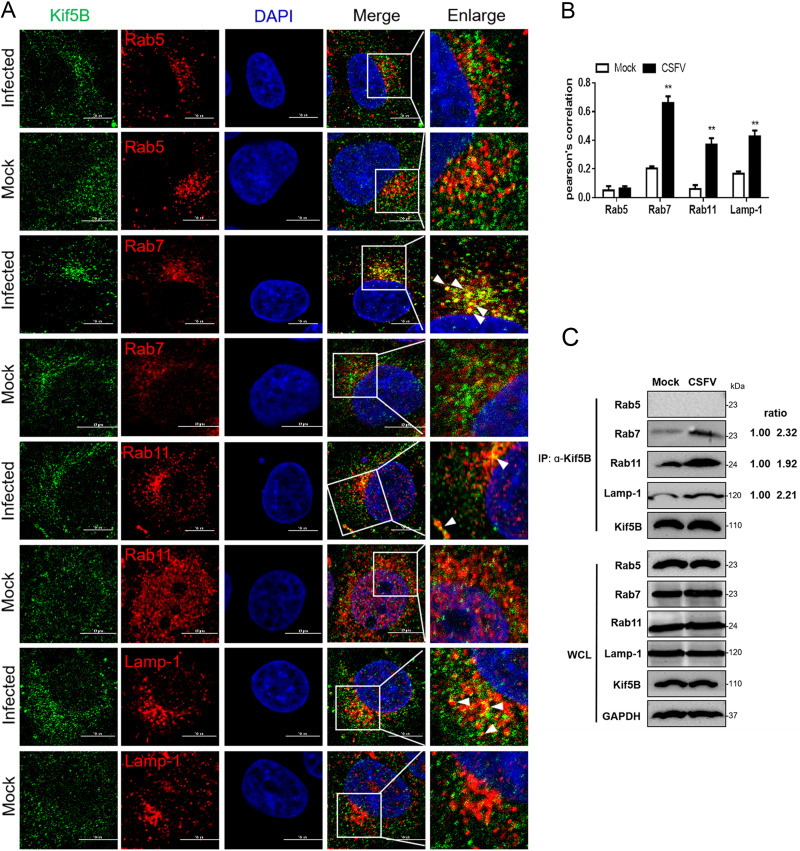
Our previous studies have confirmed that CSFV endocytosis requires different vesicles to transport virions from endosomes to lysosomes (39). To evaluate the role of Kif5B in vesicle transport, cells infected with CSFV (MOI = 10) for 4 h were fixed and stained with indicated antibodies for confocal microscopy. Compared with the mock group, Kif5B had obvious colocalization with Rab7, Rab11, and Lamp-1, but not with Rab5 (Fig. 3A), which was confirmed by Pearson coefficient analysis (Fig. 3B). Furthermore, we measured the interactions between Kif5B and Rab5, Rab7, Rab11, and Lamp-1 by using coimmunoprecipitation (co-IP) assay. As predicted, Kif5B immunoprecipitated with Rab7, Rab11, and Lamp-1 (Fig. 3C). Overall, these results suggest that Kif5B is involved in the endocytic trafficking regulated with different vesicles, including late and recycling endosomes and lysosomes.
FIG 3.
Kif5B is involved in intracellular trafficking after CSFV entry. (A) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 2 h, fixed, and then stained with mouse anti-Kif5B (green), rabbit anti-Rabs, or anti-Lamp-1 (red) for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (B) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. **, P < 0.01. (C) Cells were treated as shown in panel A and then collected and subjected to a co-IP assay using rabbit anti-Kif5B antibody. Coprecipitated proteins and whole-cell lysates (WCL) were probed with antibodies against Kif5B, Rabs, Lamp-1, and GAPDH. These data are representative of three independent experiments.
Kif5B interacts with acetylated tubulin.
Previous studies demonstrated that the kinesin-1 motor preferentially moves on acetylated microtubules with stronger motility (38, 42, 43). To investigate whether the interaction between acetylated tubulin and Kif5B is associated with endocytic trafficking, cells infected with CSFV (MOI = 10) were collected at different time points and fixed and stained with indicated antibodies. Confocal microscopy showed that there was colocalization between Kif5B and acetylated tubulin within 6 h (Fig. 4A), suggesting that Kif5B interacted with acetylated tubulin in early infection, which was also reflected by Pearson correlation analysis (Fig. 4B). Next, the interactions between Kif5B and acetylated tubulin in CSFV-infected cells were analyzed by co-IP assay. The result showed that Kif5B immunoprecipitated with acetylated tubulin, consistent with the results described above (Fig. 4C). Together, these data suggest that the interplay of Kif5B and acetylated tubulin is involved in endocytic trafficking of CSFV.
FIG 4.
Kif5B interacts with acetylated tubulin. (A) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 1 h, 6 h, 12 h, and 24 h, fixed, and then stained with mouse anti-acetylated tubulin antibody (green) and rabbit anti-Kif5B antibody (red) for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (B) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. **, P < 0.01; ***, P < 0.001. (C) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and shifted to 37°C for 1 or 6 h. Cells were collected and subjected to a co-IP assay using rabbit anti-Kif5B antibody. Coprecipitated proteins and whole-cell lysates (WCL) were probed with antibodies against Kif5B, acetylated tubulin, and GAPDH. These data are representative of three independent experiments.
Microtubule acetylation promotes CSFV entry.
Acetylation is a prominent posttranslational modification to stabilize microtubules (43, 44). To investigate whether CSFV infection required microtubule stability, cells infected with CSFV (MOI = 10) were collected at indicated time points and subjected to Western blotting. The results showed that the protein expression of acetylated tubulin significantly increased after CSFV infection in a time-dependent manner (Fig. 5A). Next, cells infected with CSFV of different MOIs were harvested at 24 hpi and lysed for Western blotting. Compared with the mock group, CSFV infection resulted in a significant increase in the expression of acetylated tubulin in a dose-dependent manner (Fig. 5B). A chemical drug, trichostatin A (TSA), a histone deacetylase 6 (HDAC6) inhibitor (45), was used to further identify the role of acetylated tubulin in CSFV infection. Cells pretreated with different concentrations of TSA for 3 h were infected with CSFV (MOI = 1) for 24 h and harvested for Western blotting, RT-qPCR, and virus titration. The treatment of TSA significantly promoted the expression of acetylated tubulin in a dose-dependent manner, resulting in the increase of Npro expression; as expected, the treatment of TSA induced increasing production of viral RNA and virus titer (Fig. 5C). These results suggested TSA facilitated CSFV replication. Finally, a time-of-addition assay was performed to further identify the role of TSA. Cells infected with CSFV (MOI = 10) were treated with TSA for different time points and collected for RT-qPCR. The results showed that the earlier addition of TSA more significantly promoted CSFV infection (Fig. 5D). In addition, to determine whether acetylated tubulin is involved in the late stage of CSFV infection, infected cells were fixed and stained with mouse anti-dsRNA antibody for confocal microscopy. As shown in Fig. 5E, acetylated tubulin did not colocalize with CSFV dsRNA, suggesting that acetylated tubulin was not involved in virus replication in the ER. Pearson correlation analysis also confirmed the above-described result (Fig. 5F). These results suggest that acetylated tubulin significantly promoted the early infection of CSFV and is not involved in CSFV biosynthesis in the ER.
FIG 5.
Microtubule acetylation promotes CSFV replication. (A) CSFV-induced microtubule acetylation. Cells infected with CSFV (MOI = 1) were collected at indicated time points and subjected to Western blotting using indicated antibodies. (B) Cells infected with different MOIs of CSFV (1 and 10) were harvested at 24 hpi for Western blotting using indicated antibodies. (C) TSA facilitated CSFV infection. Cells were pretreated with different concentrations of TSA at 37°C for 3 h and then infected with CSFV (MOI = 1) for 24 h. Expression of acetylated tubulin, Npro, α-tubulin, viral RNA copies, and virus titers was measured by Western blotting, RT-qPCR, and virus titration, respectively. Data are means ± SDs from three independent experiments. **, P < 0.01; ***, P < 0.001. (D) Time-of-addition assay was performed to identify the effect of TSA on CSFV entry. Cells were pretreated with TSA for 2 h at 37°C. Alternatively, cells were infected with CSFV (MOI = 10) at 4°C for 1 h and 37°C for 1 h, and then unbound viruses were removed. Cells were shifted to 37°C, and TSA was added at 0 to 1 hpi, 1 to 2 hpi, 2 to 4 hpi, and 4 to 6 hpi. Viral RNA load was determined by RT-qPCR. (E) Cells infected with CSFV (MOI = 1) for 24 h were fixed and stained with mouse anti-dsRNA antibody (red) and rabbit anti-acetylated tubulin antibody (green) for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (F) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments.
Endosomal and lysosomal trafficking requires microtubule acetylation.
To investigate whether acetylated tubulin is involved in the endocytic trafficking of early infection, cells infected with CSFV (MOI = 10) at 37°C for 1 h, 2 h, and 4 h were fixed and stained with indicated antibodies. Confocal microscopy showed that there was a remarkable colocalization between CSFV particles and acetylated tubulin (Fig. 6A). Pearson correlation analysis supported this interaction (Fig. 6B). Furthermore, to test the role of acetylated tubulin in vesicle transport, infected cells were fixed and stained with indicated antibodies for confocal microscopy. The results showed that compared with the mock group, acetylated tubulin significantly colocalized with Rab7, Rab11, and Lamp-1, but not with Rab5 (Fig. 6C), which was confirmed by Pearson correlation analysis (Fig. 6D). Meanwhile, infected cells were lysed and subjected to co-IP assay. Compared with the mock group, acetylated tubulin immunoprecipitated with Rab7, Rab11, and Lamp-1, but not with Rab5 (Fig. 6E). Together, these results suggest that acetylated tubulin participated in endosomal and lysosomal trafficking regulated with Kif5B in the early stage of CSFV infection.
FIG 6.
CSFV endocytosis requires acetylated microtubules. (A) CSFV colocalized with acetylated tubulin. Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C. At different intervals, cells were fixed and stained with mouse anti-CSFV E2 monoclonal antibody (WH303) or rabbit anti-acetylated tubulin antibody for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (B) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. (C) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 2 h, fixed, and then stained with mouse anti-acetylated tubulin antibody (red), rabbit anti-Rabs, or anti-Lamp-1 antibody (green) for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (D) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. *, P < 0.05; **, P < 0.01. (E) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 2 h. Cells were collected and subjected to a co-IP assay using mouse anti-acetylated tubulin antibody. Coprecipitated proteins and whole-cell lysates (WCL) were probed with antibodies against Kif5B, Rabs, Lamp-1, and GAPDH. These data are representative of three independent experiments.
Microtubule acetylation requires the activation of Rac1/Cdc42.
Our previous study reported that CSFV entry into PK-15 cells was regulated by the epidermal growth factor receptor (EGFR)-PI3K/MAPK-RhoA/Rac1/Cdc42 signaling pathway, and EGFR served as a help receptor (41). In addition, previous studies have shown that the tubulin acetylation induced by influenza A virus (IAV) and equid alphaherpesvirus 1 (EHV-1) needed the activation of Rac1/Cdc42, which were members of the Rho family of small GTPases and have been shown to promote the formation of lamellipodia and filopodia at the leading edge of motile cells and thus affect cell migration (46, 47). To investigate whether Rac1/Cdc42 was associated with the promotion of microtubule acetylation after CSFV infection, cells pretreated with different concentrations of Rac1 inhibitor NSC23766 and Cdc42 inhibitor ML141 were infected with CSFV and collected for Western blotting and RT-qPCR. The results showed that the treatment of NSC23766 and ML141 significantly inhibited the acetylated tubulin expression in a dose-dependent manner. The viral RNA copies of NSC23766- or ML141-treated cells were reduced by 26.7%, 37.0%, and 51.7% or 24.3%, 40.3%, and 47.0%, respectively, compared to that of dimethyl sulfoxide (DMSO)-treated cells (Fig. 7A and B). Next, we further investigated whether TSA could rescue the reduced level of microtubule acetylation and inhibition of viral replication induced by ML141 and NSC23766. As shown in Fig. 7C, the reduction of microtubule acetylation in infected cells pretreated with ML141 or NSC23766 was restored with the addition of TSA. The treatment of TSA restored CSFV proliferation, which was inhibited by ML141 or NSC23766. Furthermore, When TSA was added to siRac1- or siCdc42-treated infected cells, the reduction of microtubule acetylation was also restored, and addition of TSA in siRac1- or siCdc42-transfected infected cells resulted in recovery from viral infection (Fig. 7D). Additionally, indirect immunofluorescence assay showed that the treatments of the drugs or small interfering RNAs (siRNAs) caused an obvious reduction of the fluorescent spots, suggesting the role of Rac/Cdc42 signaling in CSFV infection (Fig. 7E and F). Overall, these results suggest that Rac1/Cdc42 activation is essential to induce microtubule acetylation upon CSFV infection.
FIG 7.
The activation of Rac1/Cdc42 signaling induces microtubule acetylation. (A and B) NSC23766 and ML141 inhibited the level of microtubule acetylation induced by CSFV infection. Cells pretreated with different concentrations of NSC23766 (A) or ML141 (B) at 37°C for 3 h were infected with CSFV (MOI = 10) at 4°C for 1 h and then shifted to 37°C for 1 h. Protein expression of acetylated tubulin and viral RNA load was detected by Western blotting and RT-qPCR. Data are means ± SDs from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C and D) TSA restored tubulin acetylation and CSFV proliferation. Cells pretreated with ML141 and NSC23766 for 3 h (C) or transfected with siCdc42 or siRac1 (D) were infected with CSFV for 1 h and then cultured in the presence of TSA. At 24 hpi, cells were collected and subjected to Western blotting and RT-qPCR. Protein expression of acetylated tubulin, Cdc42/Rac1, and α-tubulin was measured by Western blotting, and viral RNA copy number was detected by RT-qPCR. Data are means ± SDs from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (E and F) Cells treated with ML141 and NSC23766 for 3 h (E) or transfected with siCdc42 or siRac1 (F) were infected with CSFV for 24 h and then collected for indirect immunofluorescence assay. The fixed cells were stained with mouse anti-E2 antibody (WH303) and observed by fluorescence microscope.
Kinesin/dynein-mediated bidirectional CSFV movement along acetylated tubulin.
After reaching the cytoplasm, the virus is transported from the plasma membrane to dock the site of viral replication, which is correlated with microtubule-based motors such as kinesin and dynein (48). To investigate the interplay of Kif5B and dynein in early infection, cells infected with CSFV (MOI = 10) were collected at different time points, fixed, and stained with indicated antibodies. Confocal microscopy showed that there was a notable colocalization between Kif5B and dynein at 1 and 6 hpi (Fig. 8A and B). Meanwhile, the interaction between Kif5B and dynein in infected cells was also detected by co-IP assay. As shown in Fig. 8C, compared with the mock group, Kif5B was immunoprecipitated by dynein. These data confirmed our hypothesis that Kif5B interacted with dynein. To further monitor the movement of dynein along acetylated tubulin, cells infected with CSFV (MOI = 10) were fixed and stained with indicated antibodies for confocal microscopy. It was found that, compared with the mock group, dynein had obvious colocalization with acetylated tubulin (Fig. 8D and E). Consistently, compared with the mock group, dynein immunoprecipitated with acetylated tubulin (Fig. 8F). Hence, we reasoned that kinesin and dynein mediated bidirectional movement along acetylated tubulin. Finally, to clarify the network of Kif5B, dynein, and acetylated tubulin in early infection of CSFV, siKif5B, siDynein, and siCtrl-transfected cells were infected for 1 h, fixed, and stained with indicated antibodies for confocal microscopy. As shown in Fig. 8G, CSFV particles did not colocalize with acetylated tubulin upon the knockdown of Kif5B or dynein, while there was colocalization of CSFV particles with acetylated tubulin in the siCtrl group (Fig. 8G). Pearson correlation analysis also confirmed the result described above (Fig. 8H). Together, these results indicate that the bidirectional movement mediated by Kif5B and dynein along acetylated tubulin is involved in CSFV intercellular transport.
FIG 8.
The bidirectional movement of CSFV along acetylated tubulin requires kinesin and dynein. (A) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 1 or 6 h. Cells were fixed and stained with mouse anti-Kif5B antibody (red) and rabbit anti-dynein antibody (green) for confocal microscopy. Bars, 10 μm. (B) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. ***, P < 0.001; ****, P < 0.0001. (C) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 1 or 6 h. Cells were collected and subjected to a co-IP assay using mouse anti-dynein antibody. Coprecipitated proteins and whole-cell lysates (WCL) were probed with antibodies against Kif5B, dynein, and GAPDH. (D) Cells infected with CSFV (MOI = 10) were incubated at 4°C for 1 h and then shifted to 37°C for 2 h. Cells were fixed and stained with mouse anti-acetylated tubulin (red) and rabbit anti-dynein (green) for confocal microscopy. Bars, 10 μm. (E) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. ****, P < 0.0001. (F) The cells described above were collected and subjected to a co-IP assay using mouse anti-dynein antibody. Coprecipitated proteins and whole-cell lysates (WCL) were probed with antibodies against dynein, acetylated tubulin, and GAPDH. These data are representative of three independent experiments. (G) siKif5B-, siDynein-, and siCtrl-transfected cells were infected with CSFV (MOI = 10) and collected at 2 hpi. Cells were fixed with 4% PFA and stained with rabbit anti-acetylated tubulin antibody (green) and mouse anti-E2 antibody (red) for confocal microscopy. Bars, 10 μm. These data are representative of three independent experiments. (H) The colocalization analysis was expressed as Pearson’s correlation coefficient, measured for individual cells. Data are means ± SDs from three independent experiments. **, P < 0.01.
DISCUSSION
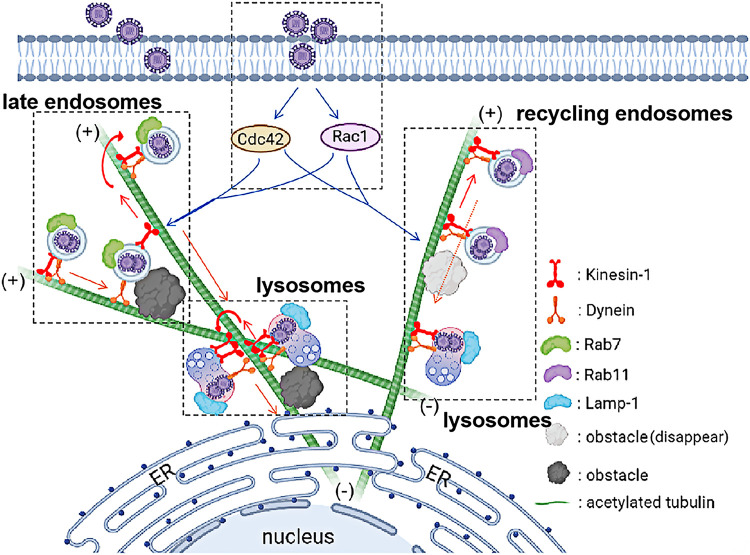
Successful infection of viruses requires the microtubule system (MT) that holds host cells hostage to transport virions, viral proteins, and intracellular complexes to complete the entire virus replication process (14, 48). The microtubule motor proteins dynein and one or more kinesins are involved in this behavior (16, 43, 49, 50). Dynein moves toward the MT minus end, and many kinesins move toward the MT plus end. Intracellular transport occurs in a highly crowded and dynamic cytoplasm (14). The microtubule network is densely decorated with barriers such as microtubule defects, microtubule-associated proteins (MAPs), protein aggregates, quiescent organelles, reverse motion traffic, and other cytoskeletal filaments (51, 52). This bidirectional transport may help viruses avoid obstacles on microtubules such as other cargoes, which ensures they eventually reach the correct cellular location. During the infection cycles of adenovirus, herpes simplex virus 1 (HSV-1), and pseudorabies virus (PRV), viruses undergo both retrograde and cis-motion traffic and frequently change direction (14, 16, 30, 33, 35, 48). Our previous study reported that CSFV was trafficked through the late endosomes to the lysosomes along the microtubules, which require dynein (41). However, intracellular trafficking toward the MT minus end before docking at the ER is blocked due to crowded microtubule traffic, resulting in the inhibition of CSFV replication. To this end, we assume whether CSFV is moved bidirectionally along the MT to the ER. Besides defining the role of dynein during CSFV infection described in the previous study, we systematically provided a new schematic about kinesin-1 in the CSFV life cycle, as shown in Fig. 9, showing (i) CSFV infection activates the Rac1/Cdc42 signaling, resulting in microtubule acetylation; (ii) kinesin-1 was recruited during CSFV infection to transport late and recycling endosomes and lysosomes along acetylated tubulin; and (iii) kinesin-1 and dynein are synergistically involved in the bidirectional transport of CSFV. Kinesin-1 regulates the endocytic trafficking of CSFV toward the MT plus end for avoiding obstacles.
FIG 9.
Schematic model depicting CSFV bidirectional transport along acetylated microtubules in early infection. Kinesin-1 and dynein are synergistically involved in the intracellular transport of CSFV as follows: (i) CSFV promotes the acetylation of microtubules via the activation of Rac1/Cdc42 signaling; (ii) dynein transports late endosomes containing CSFV particles, circulating endosomes, or lysosomes along acetylated microtubules to the negative pole; (iii) when encountering obstacles, kinesin-1 transports late endosomes, circulating endosomes, or lysosomes along acetylated microtubules to bypass obstacles; and (iv) once the obstacles on the microtubule disappear, dynein continues to transport along the microtubules to the negative pole before docking on the ER.
The acetylation modification of tubulin is important to the stability of microtubules (42–44). It has been shown that kinesin-1 preferentially transports cargo on acetylated microtubules. Previous studies have shown that acetylated microtubules facilitate the infection of IAV, HIV-1, human papillomavirus 3 (HPV3), and HSV-1 (46, 53–55). We have found that microtubules provide intracellular transport tracks for CSFV infection. Here, we further show that tubulin acetylation promotes CSFV infection. The results showed that the bidirectional transportation mediated by kinesin-1 and dynein requires acetylated microtubules as a highway. In addition, an earlier report has shown that virus infection induces Rho GTPases to modulate tubulin acetylation and promote microtubule stabilization for the establishment of effective infection (46). We previously found that after virus infection, rearrangements of the actin cytoskeleton are generally regulated by Rho GTPase signaling pathways, including RhoA, Rac1, and Cdc42. Herein, we also found that the treatment of Rac1/Cdc42 inhibitors impaired tubulin acetylation and resulted in the decrease of CSFV viral RNA, suggesting that the Rac1/Cdc42 signaling pathway induced by CSFV promotes MT acetylation. Therefore, we reasoned that the Rho GTPase signaling pathways play a variety of regulatory functions in the life cycle of CSFV infection.
In conclusion, we first found that Kif5B is essential for CSFV infection. Furthermore, Kif5B was involved in the transport of multiple vesicles after endocytosis, including late and recycling endosomes and lysosomes. Mechanistically, Kif5b is mainly responsible for transporting virus particles in the opposite direction of dynein, bypassing the crowded acetylated microtubules. Our finding provides not only a theoretical basis for the early infection mechanism of CSFV but also a new target for the development of effective antiviral drugs.
MATERIALS AND METHODS
Cells, virus, and plasmids.
Porcine kidney (PK-15) cells were cultured in Dulbecco’s modified essential medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS) (Sigma), 0.2% NaHCO3, 100 μg/mL streptomycin, and 100 IU/mL penicillin (Gibco, Invitrogen) at 37°C with 5% CO2. The virulent CSFV Shimen strain (GenBank accession number AF092448) was obtained from the National Institute of Veterinary Drug Control (Beijing, China). The plasmid pFLAG-Kif5B was constructed in the laboratory following standard molecular cloning procedures.
Cell infection and drug treatments.
To test the effects of these drugs on CSFV infection, cells seeded in 24-well plates were treated with ML141 (TargetMoI), NSC237666 (TargetMoI), or TSA (APExBIO) for 3 h at 37°C. For viral entry, cells pretreated with different drugs were inoculated at an MOI of 10 in the presence of the drug at 4°C for 1 h and then shifted to 37°C for 1 h (entry), cells were then lysed by three freeze-thaw cycles, and the total RNA was extracted using TRIzol reagent. The viral RNA was measured using RT-qPCR as described previously. Data are presented as threshold cycle (2−ΔΔCT) values from quadruplicate samples. For viral replication, cells pretreated with the indicated drugs were infected at an MOI of 1 in the presence of the drug at 4°C for 1 h. Cells were washed with phosphate-buffered saline (PBS) and incubated in maintenance medium without the drug for 24 h at 37°C. Cells were treated as described above, and the viral RNA was measured as described above.
Plasmids and siRNA transfection.
Cells grown to 70% confluence on coverslip dishes were transfected with indicated plasmids using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. At 8 h posttransfection (hpt), the transfection mixture was replaced with DMEM containing 2% FBS, and cells were incubated for an additional 48 h. For siRNA knockdown, cells were transfected with 100 nM siRNA using Lipofectamine 3000 according to the manufacturer’s instructions. The siRNA duplexes used in the study were as follows: siKif5B (UKHC) (catalog no. sc-37007; Santa Cruz, USA), as well as siCdc42 (5-AGAGGAUUAUGACAGAUUATT-3) and siRac1 (5-GGAGAUCGGUGCUGUGAAATT-3), which were designed and synthesized by Shanghai GenePharma Biotechnology. At 48 hpt, cells were infected with CSFV and collected at 24 hpi for RT-qPCR.
RT-qPCR.
Total RNA was extracted from infected cells using TRIzol reagent (Invitrogen, USA), and viral RNA was measured using RT-qPCR as described above. Data are presented as 2−ΔΔCT from quadruplicate samples.
Virus titration.
The transfected cells were infected with the CSFV Shimen strain for 1 h. Cells were lysed by three freeze-thaw cycles. Virus yields were determined as described previously (39). Data are presented as 50% tissue culture infective doses (TCID50s) from quadruplicate samples.
Confocal microscopy.
Cells grown on dishes were infected with CSFV (MOI = 10) at 4°C for 1 h and rinsed and then shifted to 37°C for indicated time points. After incubation, the monolayers were fixed with 4% paraformaldehyde (PFA) in PBS and permeabilized with 0.1% Triton X-100. For visualization of CSFV and Kif5B, cells were stained with mouse anti-CSFV E2 antibody (WH303) and rabbit anti-Kif5B antibody (catalog no. 21632-1-AP; Proteintech). To visualize the colocalization of Kif5B and dsRNA, cells were stained with rabbit anti-Kif5B antibody and dsRNA monoclonal antibody J2 (Scicons). To visualize the colocalization of Kif5B and Rab proteins or Lamp-1, cells were stained with mouse anti-Kif5B antibody (catalog no. sc-133184; Santa Cruz, USA) and rabbit anti-Rab5 antibody (catalog no. 3547; CST, USA), rabbit anti-Rab7 antibody (catalog no. 9367; CST, USA), rabbit anti-Rab11 antibody (catalog no. 5589; CST, USA), or rabbit anti-Lamp-1 antibody (catalog no. 9091; CST, USA). For visualization of Kif5B and acetylated tubulin, cells were stained with rabbit anti-Kif5B antibody and mouse acetylated tubulin antibody (catalog no. T7451; Sigma, USA). To visualize the colocalization of acetylated tubulin and dsRNA, cells were stained with rabbit anti-acetylated tubulin antibody (catalog no. 5335; CST, USA) and mouse anti-dsRNA antibody. For visualization of acetylated tubulin and CSFV, cells were stained with rabbit anti-acetylated tubulin antibody and mouse anti-CSFV E2 antibody. To visualize the colocalization of acetylated tubulin and Rab proteins or Lamp-1, cells were stained with mouse anti-acetylated tubulin antibody and rabbit anti-Rabs antibody or rabbit anti-Lamp-1 antibody. To visualize the colocalization of Kif5B and dynein, cells were stained with mouse anti-Kif5B antibody and rabbit anti-dynein antibody (catalog no. 12345-1-AP; Proteintech, USA). To visualize the colocalization of acetylated tubulin and dynein, cells were stained with mouse anti-acetylated tubulin antibody and rabbit anti-dynein antibody. After being washed three times with PBS, cells were stained with corresponding secondary antibodies. Then, cells were stained with DAPI (4′,6-diamidino-2-phenylindole). Finally, the colocalization coefficients were calculated using the professional quantitative colocalization analysis software included with a Nikon A1 confocal microscope and expressed as Pearson’s correlation coefficients.
Live-cell imaging and analysis.
The assays were performed based on a previously described method with some minor modifications (48). In brief, CSFV-infected cell supernatants were centrifuged at 10,000 rpm for 30 min at 4°C, and we added polyethylene glycol 6000 (PEG 6000) (10%, wt/vol) slowly to the supernatants while stirring at 4°C for 8 to 10 h. Then, the mixture was centrifuged at 40,000 × g for 2 h at 4°C, and the pellet was resuspended in PBS overnight. The virus was purified by a 10% to 60% gradient of sucrose at 40,000 × g at 4°C for 4 h. The pellets were resuspended in PBS, and the purified virions were incubated with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil) (Sigma, USA) at room temperature for 1 h. Unbound dye was removed by gel filtration on a NAP-10 column (GE Healthcare, USA). Cells transfected with pEGFP-Kif5B construct (Addgene; catalog no. 172203) were infected with Dil-labeled CSFV particles (MOI = 20) at 4°C for 1 h for virus attachment and then cultured in a microscopic incubation system (Tokai Hit, Japan) maintained at 37°C and supplied with 5% CO2. Finally, three-color fluorescence images were recorded at intervals of 1.5 s using a fluorescence microscope (Nikon A1 Si).
Co-IP.
Cells were incubated with CSFV (MOI = 10) at 37°C for 2 h and were lysed in NP-40 lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM NaF, and 1 mM Na3O4, pH 7.4) for 30 min at 4°C. Lysates were cleared by centrifugation at 1,000 × g for 10 min at 4°C. The 20% aliquot of the supernatant (whole-cell lysate) was removed from all samples for later use. The remaining 80% of the lysates was incubated with 0.5 mg of the appropriate control IgG and 20 μL of a protein A/G Plus-agarose slurry (catalog no. sc-2003; Santa Cruz, USA) for 4 h at 4°C with rotation. Agarose beads were removed by centrifugation at 1,000 × g for 5 min at 4°C. The lysates were then treated with mouse anti-Kif5B antibody, rabbit anti-Kif5B antibody, or mouse anti-acetylated tubulin antibody and incubated with rotation for 8 to 12 h at 4°C. Protein A/G Plus-agarose slurry was added to each sample and incubated for 2 h more under the same conditions. The agarose beads were collected by centrifugation, washed with NP-40 lysis buffer at least three times, and then resuspended in 2×SDS loading buffer for SDS-PAGE and Western blotting.
Western blotting.
Cells grown on dishes were infected with CSFV (MOI = 10) at 4°C for 1 h and shifted to 37°C for indicated time points. After incubation, cells were washed three times with ice-cold PBS and then lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (catalog no. R0020; Solarbio) for 30 min at 4°C. Lysates were clarified by centrifugation at 12,000 × g for 10 min at 4°C. A 120-μL aliquot of the supernatant was removed from all samples for later use and then mixed with 5× SDS loading buffer. Proteins in the lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with the indicated antibodies. β-Actin, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), and α-tubulin were used as a loading control. To determine the levels of indicated proteins, the corresponding protein/β-actin or α-tubulin quantity was used to calculate grayscale using ImageJ 7.0 software.
Statistical analysis.
All data were presented as means and standard deviations (SDs) as indicated. Student’s t test was used to compare the data from pairs of treated and untreated groups. Asterisks in the figures indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001). All statistical analyses and calculations were performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA).
ACKNOWLEDGMENT
This work was supported by grants from the National Natural Science Foundation of China (31872471 and 32172840).
Footnotes
Supplemental material is available online only.
Contributor Information
Bin Zhou, Email: zhoubin@njau.edu.cn.
Susana López, Instituto de Biotecnologia/UNAM.
REFERENCES
- 1.Moennig V. 2015. The control of classical swine fever in wild boar. Front Microbiol 6:1211. 10.3389/fmicb.2015.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B. 2019. Classical swine fever in China-an update minireview. Front Vet Sci 6:187. 10.3389/fvets.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moennig V. 2000. Introduction to classical swine fever: virus, disease and control policy. Vet Microbiol 73:93–102. 10.1016/s0378-1135(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 4.Ganges L, Crooke HR, Bohórquez JA, Postel A, Sakoda Y, Becher P, Ruggli N. 2020. Classical swine fever virus: the past, present and future. Virus Res 289:198151. 10.1016/j.virusres.2020.198151. [DOI] [PubMed] [Google Scholar]
- 5.Meyers G, Thiel HJ, Rumenapf T. 1996. Classical swine fever virus: recovery of infectious viruses from cDNA constructs and generation of recombinant cytopathogenic defective interfering particles. J Virol 70:1588–1595. 10.1128/JVI.70.3.1588-1595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan J, Liao Y, Zhang M, Liu C, Li Z, Li Y, Li X, Wu K, Yi L, Ding H, Zhao M, Fan S, Chen J. 2021. Anti-classical swine fever virus strategies. Microorganisms 9:761. 10.3390/microorganisms9040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamp B, Riedel C, Roman-Sosa G, Heimann M, Jacobi S, Becher P, Thiel H-J, Rümenapf T. 2011. Biosynthesis of classical swine fever virus nonstructural proteins. J Virol 85:3607–3620. 10.1128/JVI.02206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rijnbrand R, van der Straaten T, van Rijn PA, Spaan WJ, Bredenbeek PJ. 1997. Internal entry of ribosomes is directed by the 5' noncoding region of classical swine fever virus and is dependent on the presence of an RNA pseudoknot upstream of the initiation codon. J Virol 71:451–457. 10.1128/JVI.71.1.451-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Nie Y, Wang P, Ding M, Deng H. 2004. Characterization of classical swine fever virus entry by using pseudotyped viruses: E1 and E2 are sufficient to mediate viral entry. Virology 330:332–341. 10.1016/j.virol.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Zheng G, Li L, Zhang Y, Qu L, Wang W, Li M, Yu S, Zhou M, Luo Y, Sun Y, Munir M, Li S, Qiu H. 2020. MERTK is a host factor that promotes classical swine fever virus entry and antagonizes innate immune response in PK-15 cells. Emerg Microbes Infect 9:571–581. 10.1080/22221751.2020.1738278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauhofer O, Summerfield A, Sakoda Y, Tratschin J, Hofmann MA, Ruggli N. 2007. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J Virol 81:3087–3096. 10.1128/JVI.02032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Li S, Sun Y, Dong H, Li Y, Zhao B, Guo D, Weng C, Qiu H. 2013. Poly(C)-binding protein 1, a novel Npro-interacting protein involved in classical swine fever virus growth. J Virol 87:2072–2080. 10.1128/JVI.02807-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Chen J, Zhang XM, Gao ZC, Liu CC, Zhang YN, Hou JX, Li ZY, Kan L, Li WL, Zhou B. 2018. Porcine Mx1 protein inhibits classical swine fever virus replication by targeting nonstructural protein NS5B. J Virol 92:e2117–e2147. 10.1128/JVI.02147-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodding MP, Way M. 2011. Coupling viruses to dynein and kinesin-1. EMBO J 30:3527–3539. 10.1038/emboj.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arriagada G. 2017. Retroviruses and microtubule-associated motor proteins. Cell Microbiol 19:e12759. 10.1111/cmi.12759. [DOI] [PubMed] [Google Scholar]
- 16.Lukic Z, Dharan A, Fricke T, Diaz-Griffero F, Campbell EM. 2014. HIV-1 uncoating is facilitated by dynein and kinesin 1. J Virol 88:13613–13625. 10.1128/JVI.02219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horníková L, Bruštíková K, Forstová J. 2020. Microtubules in polyomavirus infection. Viruses 12:121. 10.3390/v12010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis RJ, Minton AP. 2003. Cell biology: join the crowd. Nature 425:27–28. 10.1038/425027a. [DOI] [PubMed] [Google Scholar]
- 19.Welte MA. 2010. Bidirectional transport: matchmaking for motors. Curr Biol 20:R410–R413. 10.1016/j.cub.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Gelfand VI. 2017. Moonlighting motors: kinesin, dynein, and cell polarity. Trends Cell Biol 27:505–514. 10.1016/j.tcb.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirokawa N, Noda Y, Tanaka Y, Niwa S. 2009. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10:682–696. 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 22.Verhey KJ, Kaul N, Soppina V. 2011. Kinesin assembly and movement in cells. Annu Rev Biophys 40:267–288. 10.1146/annurev-biophys-042910-155310. [DOI] [PubMed] [Google Scholar]
- 23.DuRaine G, Wisner TW, Howard P, Johnson DC. 2018. Kinesin-1 proteins KIF5A, -5B, and -5C promote anterograde transport of herpes simplex virus enveloped virions in axons. J Virol 92:e01269-18. 10.1128/JVI.01269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirokawa N, Noda Y. 2008. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev 88:1089–1118. 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 25.Serra-Marques A, Martin M, Katrukha EA, Grigoriev I, Peeters CA, Liu Q, Hooikaas PJ, Yao Y, Solianova V, Smal I, Pedersen LB, Meijering E, Kapitein LC, Akhmanova A. 2020. Concerted action of kinesins KIF5B and KIF13B promotes efficient secretory vesicle transport to microtubule plus ends. Elife 9:e61302. 10.7554/eLife.61302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhmanova A, Hammer JA. 2010. Linking molecular motors to membrane cargo. Curr Opin Cell Biol 22:479–487. 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirokawa N, Tanaka Y. 2015. Kinesin superfamily proteins (KIFs): various functions and their relevance for important phenomena in life and diseases. Exp Cell Res 334:16–25. 10.1016/j.yexcr.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Verhey KJ, Hammond JW. 2009. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10:765–777. 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 29.Schliwa M, Woehlke G. 2003. Molecular motors. Nature 422:759–765. 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- 30.Strunze S, Engelke MF, Wang IH, Puntener D, Boucke K, Schleich S, Way M, Schoenenberger P, Burckhardt CJ, Greber UF. 2011. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe 10:210–223. 10.1016/j.chom.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Ni Y, Zhou N, Xue W, Rong L, Yung W, Lin R, Kao RY, Duan Z, Sun H, Gong H, Tang X, Liu M, Zhang W, Qi S, Chung S, Song Y, Huang J. 2018. A new role of anterograde motor Kif5b in facilitating large clathrin-coated vesicle mediated endocytosis via regulating clathrin uncoating. Cell Discov 4:65. 10.1038/s41421-018-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guardia CM, Farías GG, Jia R, Pu J, Bonifacino JS. 2016. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep 17:1950–1961. 10.1016/j.celrep.2016.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diwaker D, Murray JW, Barnes J, Wolkoff AW, Wilson DW. 2020. Deletion of the pseudorabies virus gE/gI-US9p complex disrupts kinesin KIF1A and KIF5C recruitment during egress, and alters the properties of microtubule-dependent transport in vitro. PLoS Pathog 16:e1008597. 10.1371/journal.ppat.1008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malikov V, Naghavi MH. 2017. Localized phosphorylation of a kinesin-1 adaptor by a capsid-associated kinase regulates HIV-1 motility and uncoating. Cell Rep 20:2792–2799. 10.1016/j.celrep.2017.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furey C, Jovasevic V, Walsh D. 2020. TACC3 regulates microtubule plus-end dynamics and cargo transport in interphase cells. Cell Rep 30:269–283.e6. 10.1016/j.celrep.2019.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zila V, Difato F, Klimova L, Huerfano S, Forstova J. 2014. Involvement of microtubular network and its motors in productive endocytic trafficking of mouse polyomavirus. PLoS One 9:e96922. 10.1371/journal.pone.0096922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schepis A, Stauber T, Krijnse Locker J. 2007. Kinesin-1 plays multiple roles during the vaccinia virus life cycle. Cell Microbiol 9:1960–1973. 10.1111/j.1462-5822.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 38.Ravindran MS, Engelke MF, Verhey KJ, Tsai B. 2017. Exploiting the kinesin-1 molecular motor to generate a virus membrane penetration site. Nat Commun 8:15496. 10.1038/ncomms15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Liu Y, Xiao F, Liu C, Liang X, Chen J, Zhou J, Baloch AS, Kan L, Zhou B, Qiu H, Pfeiffer JK. 2018. Rab5, Rab7, and Rab11 are required for caveola-dependent endocytosis of classical swine fever virus in porcine alveolar macrophages. J Virol 92:e718–e797. 10.1128/JVI.00797-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi B, Liu C, Zhou J, Wang S, Gao Z, Zhang X, Zhou B, Chen P. 2016. Entry of classical swine fever virus into PK-15 cells via a pH-, dynamin-, and cholesterol-dependent, clathrin-mediated endocytic pathway that requires Rab5 and Rab7. J Virol 90:9194–9208. 10.1128/JVI.00688-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y, Lou JX, Liu CC, Liu YY, Chen XN, Liang XD, Zhang J, Yang Q, Go YY, Zhou B. 2021. Microfilaments and microtubules alternately coordinate the multi-step endosomal trafficking of classical swine fever virus. J Virol 95:e2420–e2436. 10.1128/JVI.02436-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balabanian L, Berger CL, Hendricks AG. 2017. Acetylated microtubules are preferentially bundled leading to enhanced kinesin-1 motility. Biophys J 113:1551–1560. 10.1016/j.bpj.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. 2006. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16:2166–2172. 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A. 2014. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157:1405–1415. 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyake Y, Keusch JJ, Wang L, Saito M, Hess D, Wang X, Melancon BJ, Helquist P, Gut H, Matthias P. 2016. Structural insights into HDAC6 tubulin deacetylation and its selective inhibition. Nat Chem Biol 12:748–754. 10.1038/nchembio.2140. [DOI] [PubMed] [Google Scholar]
- 46.Husain M, Harrod KS. 2011. Enhanced acetylation of alpha-tubulin in influenza A virus infected epithelial cells. FEBS Lett 585:128–132. 10.1016/j.febslet.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 47.Kolyvushko O, Kelch MA, Osterrieder N, Azab W. 2020. Equine alphaherpesviruses require activation of the small GTPases Rac1 and Cdc42 for intracellular transport. Microorganisms 8:1013. 10.3390/microorganisms8071013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou W, Kang W, Li Y, Shan Y, Wang S, Liu F. 2021. Dynamic dissection of dynein and kinesin-1 cooperatively mediated intercellular transport of porcine epidemic diarrhea coronavirus along microtubule using single virus tracking. Virulence 12:615–629. 10.1080/21505594.2021.1878748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirokawa N. 1998. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279:519–526. 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 50.Reck-Peterson SL, Redwine WB, D R, Vale PA. 2018. The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol 19:382–398. 10.1038/s41580-018-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixit R, Ross JL, Goldman YE, Holzbaur ELF. 2008. Differential regulation of dynein and kinesin motor proteins by Tau. Science 319:1086–1089. 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferro LS, Can S, Turner MA, ElShenawy MM, Yildiz A. 2019. Kinesin and dynein use distinct mechanisms to bypass obstacles. Elife 8:e48629. 10.7554/eLife.48629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong M, Zheng K, Chen M, Xiang Y, Jin F, Ma K, Qiu X, Wang Q, Peng T, Kitazato K, Wang Y. 2014. Heat-shock protein 90 promotes nuclear transport of herpes simplex virus 1 capsid protein by interacting with acetylated tubulin. PLoS One 9:e99425. 10.1371/journal.pone.0099425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabo Y, Walsh D, Barry DS, Tinaztepe S, de Los Santos K, Goff SP, Gundersen GG, Naghavi MH. 2013. HIV-1 induces the formation of stable microtubules to enhance early infection. Cell Host Microbe 14:535–546. 10.1016/j.chom.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S, Jiang Y, Cheng Q, Zhong Y, Qin Y, Chen M. 2017. Inclusion body fusion of human parainfluenza virus type 3 regulated by acetylated alpha-tubulin enhances viral replication. J Virol 91:e01802-16. 10.1128/JVI.01802-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Download jvi.01929-22-s0001.mp4, MP4 file, 0.5 MB (475.9KB, mp4)
Legend of Movie S1. Download jvi.01929-22-s0002.pdf, PDF file, 0.06 MB (59.1KB, pdf)