ABSTRACT
The envelope glycoprotein (Env) is the main focus of human immunodeficiency virus type 1 (HIV-1) vaccine development due to its critical role in viral entry. Despite advances in protein engineering, many Env proteins remain recalcitrant to recombinant expression due to their inherent metastability, making biochemical and immunological experiments impractical or impossible. Here, we report a novel proline stabilization strategy to facilitate the production of prefusion Env trimers. This approach, termed “2P,” works synergistically with previously described SOSIP mutations and dramatically increases the yield of recombinantly expressed Env ectodomains without altering the antigenic or conformational properties of near-native Env. We determined that the 2P mutations function by enhancing the durability of the prefusion conformation and that this stabilization strategy is broadly applicable to evolutionarily and antigenically diverse Env constructs. These findings provide a new Env stabilization platform to facilitate biochemical research and expand the number of Env variants that can be developed as future HIV-1 vaccine candidates.
IMPORTANCE Recent estimates have placed the number of new human immunodeficiency virus type 1 (HIV-1) infections at approximately 1.5 million per year, emphasizing the ongoing and urgent need for an effective vaccine. The envelope (Env) glycoprotein is the main focus of HIV-1 vaccine development, but, due to its inherent metastability, many Env variants are difficult to recombinantly express in the relatively large quantities that are required for biochemical studies and animal trials. Here, we describe a novel structure-based stabilization strategy that works synergistically with previously described SOSIP mutations to increase the yield of prefusion HIV-1 Env.
KEYWORDS: HIV-1, immunogen design, cryo-EM, prefusion, proline stabilization
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infection causes severe CD4+ T cell immunodeficiency that is accompanied by fever, weight loss, and opportunistic infections (1). Recent estimates place the number of new infections at approximately 1.5 million per year (2). Despite decades of rigorous research and life-saving advances in both pre- and postexposure antiretroviral drug treatments (3, 4), the development of an effective HIV-1 vaccine remains an unmet public health goal (5).
HIV-1 is a lentivirus that uses a class I viral fusion glycoprotein, called envelope (Env), to gain entry into host cells to begin the process of integration and replication (6). Like other class I viral fusion proteins, Env is proteolytically processed into two subunits, gp120 and gp41 (7). These subunits remain associated and oligomerize with other protomers to form the functional prefusion conformation of Env, which is composed of a trimer of gp120/gp41 heterodimers. The N-terminal gp120 subunit is responsible for mediating binding to both the CD4 receptor and CCR5/CXCR4 coreceptor (8–10), while the gp41 subunit contains the hydrophobic fusion peptide and the helical heptad repeats that drive membrane fusion (11).
Because Env is the sole target for neutralizing antibodies, it is currently the main focus of vaccine research. However, several characteristics of Env make it a notoriously complex and problematic immunogen. These characteristics include a dense glycan shield composed of N-linked glycans that cover the ectodomain and hamper the elicitation of neutralizing antibodies (12). Furthermore, high levels of viral replication, constant immune pressure (13), and the infidelity of the HIV-1 reverse transcriptase have resulted in extreme Env sequence diversity among viral strains (14). Another problem is the conformational heterogeneity that Env is capable of displaying. The prefusion, closed conformation that Env adopts before host cell receptor engagement is immunologically ideal for the elicitation of broadly neutralizing antibodies (bNAbs) (15). In this conformation, Env presents bNAb epitopes while simultaneously concealing many of the nonneutralizing epitopes, which elicit an unproductive immune response (16). However, Env has evolved to be metastable in this prefusion conformation, such that it is primed to rapidly and irreversibly transition to the postfusion conformation (17). This inherent metastability makes it difficult to recombinantly express the Env ectodomain in the antigenically desirable prefusion conformation. In an effort to stabilize the prefusion conformation of the Env ectodomain without altering its antigenicity, Sanders et al. developed the SOSIP mutations, composed of an engineered disulfide bond (SOS) that links the gp120 and gp41 subunits and an I559P (IP) substitution that disfavors the formation of the elongated postfusion helices that make up the six-helix bundle (18, 19). Since their initial description, the SOSIP mutations have undergone iterative improvement to enhance the thermostability and the antigenic characteristics of the prefusion trimer (15, 20, 21). Alternative and complementary protein engineering approaches have also been described as a means of yielding improved Env immunogens (22–26). However, despite these significant advances in immunogen engineering, many Env variants remain recalcitrant to recombinant in vitro expression.
Here, we describe a new combination of proline substitutions that increases the yield of recombinantly expressed prefusion Env. These substitutions, referred to as “2P,” function synergistically with previously reported SOSIP mutations and do not alter the antigenicity or the overall structure of the stabilized Env trimer. We go on to show that the mechanism by which these mutations increase yield is through enhancing the durability of the prefusion conformation rather than through boosting expression levels. Moreover, we show that the 2P mutations can effectively be applied to a broad range of antigenically and evolutionarily diverse Env constructs. By facilitating the expression and purification of soluble, near-native Env constructs, the 2P mutations should serve as a useful tool for investigating medical countermeasures against HIV-1.
RESULTS
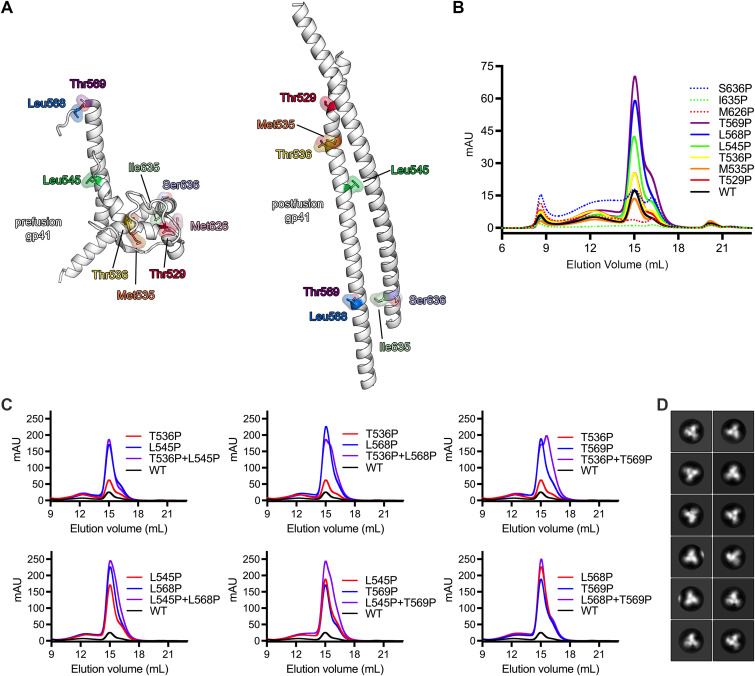
In an effort to enhance the yield of recombinantly expressed, stabilized Env trimers, we designed a series of single proline substitutions throughout the truncated gp41 subunit. Proline substitutions were specifically evaluated due to their propensity to act as “helix breakers” because their cyclized side chain prevents conventional amide backbone hydrogen bonding (27). Based on structural analysis of both the prefusion and postfusion conformations of gp41, nine positions were selected to disfavor the formation of the elongated alpha helices, which are characteristic of class I fusion proteins in the postfusion state (Fig. 1A). These substitutions were then made in the CH848 10.17DT Env, a clade C Env that has been modified to engage the unmutated common ancestor (UCA) from the DH270 V3 glycan bNAb lineage (28, 29). This Env was expressed using a chimeric gp41 subunit from BG505 and was stabilized with both SOSIP.664 and DS mutations to insert a disulfide bond between residues 201 and 433 (CH848 10.17DT DS-SOSIP) (18, 24, 30). Purification of these constructs after transient transfection revealed that several of the individual proline substitutions had a dramatic effect on Env yield (Fig. 1B). Env constructs with proline substitutions at positions 536, 545, 568, or 569 (HXB2 numbering) in particular showed a marked increase in the yield of prefusion trimer relative to the unmodified SOSIP construct. Therefore, these four mutations were selected for further analysis. Interestingly, although they were not incorporated into the final construct, proline substitutions at positions 545 and 569 were previously tested by Sanders et al. during the initial design of the SOSIP mutations. However, these mutations were deemed less effective than I559P in the context of the JRFL Env and were never evaluated in combination with the I559P substitution.
FIG 1.
Design and evaluation of a new combination of proline stabilization mutations in gp41. (A) Left, a single monomer of gp41 in the prefusion conformation (PDB ID: 6VZI) is shown as white ribbons. Residues that were targeted for proline stabilization are shown as colored sticks surrounded by a transparent molecular surface. Right, a single monomer of gp41 in the postfusion conformation (homology model based on PDB ID 7AEJ) is shown and is colored according to the prefusion monomer. (B) Size-exclusion chromatograms from a Superose 6 Increase column for each individual mutant after affinity chromatography are overlaid; mAU, milli-absorbance unit. (C) Size-exclusion chromatograms from a Superose 6 Increase column are shown for individual proline mutants and are colored either blue or red. The combination of the two mutations is colored purple, and the unmutated CH848 10.17DT DS-SOSIP is colored black and labeled “WT.” (D) Representative negative stain EM 2D class averages of CH848 10.17DT DS-SOSIP L568P and T569P (calculated in Relion 3.0) are shown.
Individual proline mutations at these four positions were combined to form double mutants to determine whether these substitutions might be capable of functioning synergistically (Fig. 1C). Modest improvements in yield over the single substitutions were observed after combining substitutions 536 and 545 or 568 and 569, while other combinations resulted in either decreased yield (536 and 568) or the appearance of a prominent, contaminating low-molecular-weight species, which was detectable by size-exclusion chromatography (536 and 569, 545 and 568, and 545 and 569). Similarly, combining proline substitutions at all four positions did not result in enhanced yield compared to the double mutant at positions 568 and 569 (Fig. S1 in the supplemental material), which resulted in an ~8-fold increase in prefusion trimer relative to CH848 10.17DT DS-SOSIP. Therefore, the double mutant at positions 568 and 569, which yielded well-folded trimers as evaluated by negative-stain electron microscopy (EM) (Fig. 1D), was selected for subsequent characterization, and these mutations were termed “2P” to reflect their similarity to the two consecutive proline mutations, which have been shown to stabilize betacoronavirus spike proteins in the prefusion conformation (31). Intriguingly, the 2P mutations at positions 568 and 569 in gp41 are also positioned similar to the 2P mutations in the S2 subunit of the coronavirus (CoV) spike (Fig. S2A). Both sets of mutations cap the N terminus of the HIV-1 gp41 or CoV S2 central helix, which ultimately polymerizes into an elongated helix during the transition from prefusion to postfusion. Generally, the residues upstream of this helical capping position in gp41 cannot be clearly resolved during structural characterization, presumably due to a high degree of conformational flexibility. However, the recently reported structure of Env in the occluded open conformation shows these amino acids forming a 5-residue helical extension of the central helix (Fig. S2B), suggesting that the 2P mutations may be stabilizing the prefusion conformation of gp41 by disfavoring the sampling of this early intermediate (32).
We next generated an “SOS 2P” Env by reverting the I559P mutation to investigate whether the novel 2P mutations could increase the yield of prefusion Env in the absence of the stabilizing effect of the IP mutation. The SOS 2P construct yielded only slightly more prefusion trimer than the SOSIP Env, while the dramatic boost in yield that was observed previously could only be recapitulated when the IP and 2P mutations were combined (Fig. S3). This synergistic stabilizing effect is reminiscent of the phenomenon that has been reported for the HexaPro mutations in the context of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, which further enhanced the effects of the initial S-2P mutations by engineering four additional proline substitutions throughout the S2 subunit (33).
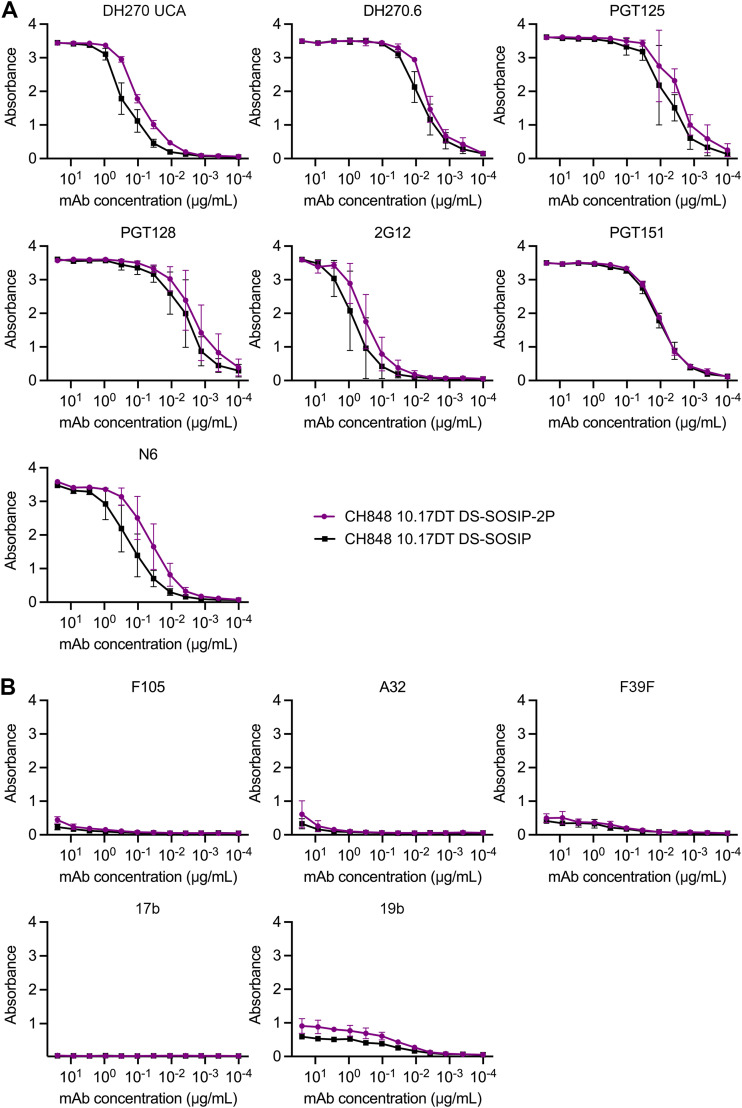
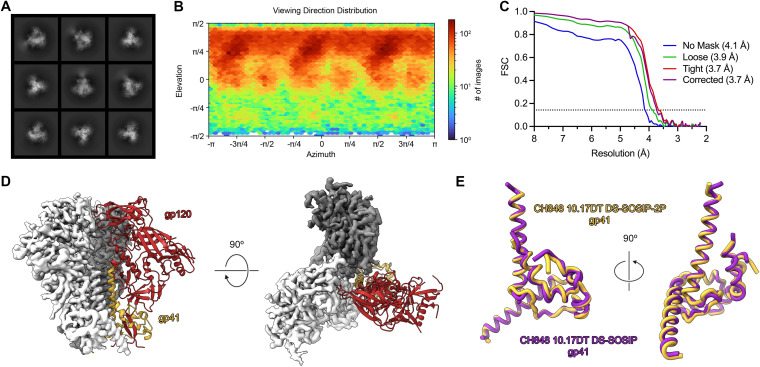
To determine whether the 2P mutations altered the antigenic landscape of the Env ectodomain, we performed an enzyme-linked immunosorbent assay (ELISA) to compare the binding profiles of CH848 10.17DT DS-SOSIP and CH848 10.17DT DS-SOSIP-2P against a panel of well-characterized monoclonal antibodies (MAbs). Both neutralizing and nonneutralizing MAbs spanning multiple epitopes were included to comprehensively evaluate what effect the 2P mutations might have on Env folding (Fig. 2A and B). Overall, the binding characteristics exhibited by the two Env constructs were very similar, although the 2P-stabilized construct appeared to bind slightly better to some neutralizing MAbs directed against the V3 glycan epitope (DH270 UCA) and the CD4-binding site (N6) (Fig. 2A). To further validate that the newly introduced 2P mutations did not disrupt the overall folding of the gp41 subunit, we determined the cryo-EM structure of the CH848 10.17DT DS-SOSIP-2P Env to a resolution of 3.7 Å (Fig. 3A to D; Fig. S4 and S5; Table S1). Our model spanned residues 32 to 664, and we were able to build N-linked glycans at 15 of the 25 putative sequons. There were several small, flexible loops in the gp120 subunit (59 to 66, 458 to 459, and 400 to 411) that could not be confidently modeled; but overall, the CH848 10.17DT DS-SOSIP-2P Env was virtually indistinguishable from the structures of previously reported CH848 10.17DT Env constructs, which lack 2P stabilization (28). Like most other previously reported structures of gp41 proteins in the prefusion conformation, the flexibility at the N terminus of the central helix precluded us from observing both of our newly introduced proline substitutions, and only Pro569 could be confidently modeled into our reconstruction. However, the overall root mean square deviation (RMSD) between our 2P-stabilized Env and a previously determined structure of CH848 10.17DT DS-SOSIP was only 0.757 Å over 1,253 Cα atoms (Fig. 3E), confirming that the 2P mutations are capable of enhancing the yield of recombinantly expressed Env ectodomain without altering the conformation of the SOSIP trimer.
FIG 2.
The 2P mutations do not alter the antigenic landscape of CH848 10.17DT DS-SOSIP. (A and B) ELISA binding curves for Env-directed monoclonal antibodies against CH848 10.17DT DS-SOSIP and CH848 10.17DT DS-SOSIP-2P are shown. CH848 10.17DT DS-SOSIP is shown in black, and CH848 10.17DT DS-SOSIP-2P is shown in purple. Data points represent the average of three replicates, and the standard deviations are plotted as error bars. The y axes show absorbance measured at 450 nm. Neutralizing antibody curves (A) and nonneutralizing antibody curves (B) are shown. DH270 UCA, DH270.6, PGT125, PGT128, and 2G12 target the V3 glycan epitope. PGT151 recognizes cleaved trimers at the gp120/gp41 interface. N6 targets the CD4-binding site (CD4bs). F105 and 17b bind to open conformations of the CD4bs and coreceptor-binding site, respectively. A32 binds to the C1C2 domain of gp120. F39F and 19b both recognize a flexible conformation of the V3 loop.
FIG 3.
The cryo-EM structure of CH848 10.17DT DS-SOSIP-2P. (A) Two-dimensional class averages of the CH848 10.17DT DS-SOSIP-2P Env ectodomain (calculated in cryoSPARC v3) are shown. (B) The viewing direction distribution plot (generated in cryoSPARC v3) is shown for the CH848 10.17DT DS-SOSIP-2P reconstruction. (C) Fourier shell correlation (FSC) curves for different masking strategies used by cryoSPARC v3 are plotted. The dotted line represents an FSC value of 0.143. (D) The 3.73-Å-resolution reconstruction is shown from side (left) and top (right) views. Two protomers are displayed as the cryo-EM map in either white or dark gray, and the third protomer is shown as a ribbon diagram of the corresponding model, with gp120 colored red and gp41 colored yellow. (E) A single monomer of gp41 from CH848 10.17DT DS-SOSIP-2P is shown in yellow and is aligned to a monomer of gp41 from a previously determined cryo-EM structure of CH848 10.17DT DS-SOSIP (PDB ID: 6UM7), colored purple.
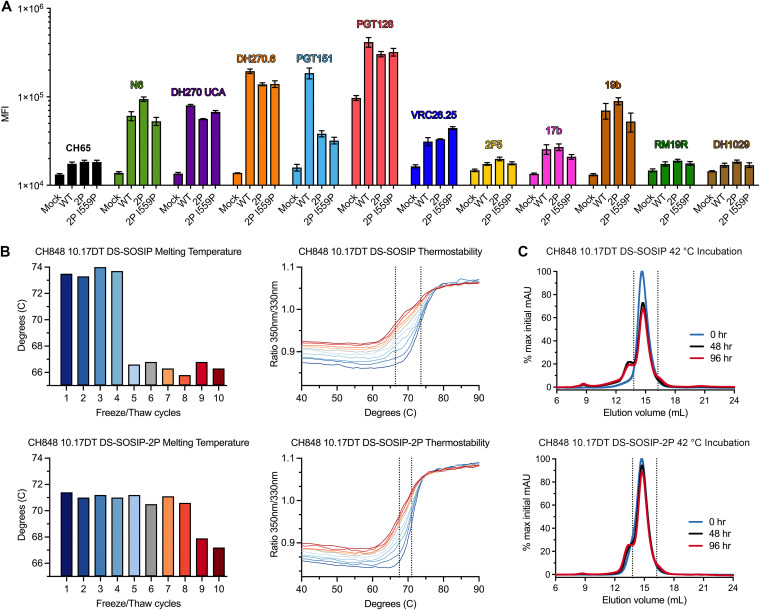
We hypothesized that this increase in the yield of prefusion trimer could be due to either an increase in Env expression or enhanced durability of the prefusion conformation after proline stabilization. To distinguish between these two possibilities, we began by investigating what impact proline stabilization might have on cell surface expression of full-length Env. FreeStyle 293-F cells were transiently transfected with CH848 10.17DT gp160 constructs containing either no proline mutations, 2P mutations, or 2P mutations in combination with the I559P mutation. After 48 h, the relative expression levels of each membrane-anchored Env construct was assessed by flow cytometry after staining with a panel of MAbs. Despite the dramatic differences in yield that could be observed when evaluating the recombinant expression of soluble ectodomains, no significant differences in surface expression levels of membrane-anchored Env constructs could be detected among the three gp160 constructs that were tested (Fig. 4A; Fig. S6). The only clear difference between the three gp160 constructs was a decrease in PGT151 binding in the 2P and the 2P + I559P constructs relative to the unstabilized gp160. However, this trend was not conserved for the rest of the antibody panel nor was there a detectable difference between the 2P-stabilized and non-2P-stabilized Env constructs when testing PGT151 binding to soluble ectodomains (Fig. 2A).
FIG 4.
Investigating the underlying mechanism of enhanced protein yield after proline stabilization. (A) Four cell populations were stained with a panel of Env-directed monoclonal antibodies. The average of three independent experiments is plotted ± standard error of mean. Each antibody has been assigned a color and is labeled above the corresponding bars; mock, untransfected; WT, CH848 10.17DT gp160; 2P, CH848 10.17DT 2P gp160; 2P I559P, CH848 10.17DT 2P I559P gp160; MFI, mean fluorescence intensity. (B) Top left, the CH848 10.17DT DS-SOSIP Tm is plotted for each round of freeze/thaw. Top right, DSF melting curves used to calculate CH848 10.17DT DS-SOSIP Tm are plotted and colored according to the bar graph on the left. The dotted line at 73.6°C represents the average Tm of rounds 1 to 4, and the dotted line at 66.4°C represents the average Tm for rounds 5 to 10. Bottom left, CH848 10.17DT DS-SOSIP-2P Tm is plotted for each round of freeze/thaw. Bottom right, DSF melting curves used to calculate CH848 10.17DT DS-SOSIP-2P Tm are plotted and colored according to the bar graph on the left. The dotted line at 71.0°C represents the average Tm of rounds 1 to 8, and the dotted line at 67.5°C represents the average Tm for rounds 9 to 10. (C) Top, size-exclusion chromatograms for three aliquots of CH848 10.17DT DS-SOSIP, incubated at 42°C for 0, 48, or 96 hr, are overlaid. The 0 hr sample is colored blue, the 48 hr sample is colored black, and the 96 hr sample is colored red. Data are plotted as a percentage of the maximum mAU value from the 0 hr sample. Bottom, size-exclusion chromatograms for three aliquots of CH848 10.17DT DS-SOSIP-2P, incubated at 42°C for 0, 48, or 96 hr, are overlaid. The 0 hr sample is colored blue, the 48 hr sample is colored black, and the 96 hr sample is colored red. Data are plotted as a percentage of the maximum mAU value from the 0 hr sample. Dashed lines at 13.80 and 16.25 mL correspond to area under the concentration time curve (AUC) measurements in Table S3 in the supplemental material.
Based on the cell surface expression results, we next evaluated the durability of recombinantly expressed, proline-stabilized Env ectodomains. Aliquots of CH848 10.17DT DS-SOSIP and CH848 10.17DT DS-SOSIP-2P were subjected to 10 rounds of rapid freezing and thawing. Thermostability measurements of both Env constructs were collected by differential scanning fluorimetry (DSF) during each round (Fig. 4B). The melting temperature (Tm) of CH848 10.17DT DS-SOSIP began at 73.6°C, a value that was maintained until after four freeze/thaw cycles, when it abruptly dropped to 66.4°C where it remained throughout the rest of the experiment. The Tm of CH848 10.17DT DS-SOSIP-2P was initially measured to be 71.0°C, a value that was maintained until after eight freeze/thaw cycles, when the Tm dropped to 67.5°C. To further investigate this phenomenon, we performed a forced degradation assay in which aliquots of CH848 10.17DT DS-SOSIP and CH848 10.17DT DS-SOSIP-2P were incubated at 42°C for either 48 or 96 hr. The integrity of the incubated trimers was then evaluated by size-exclusion chromatography (Fig. 4C). After 48 hr at 42°C, we observed the appearance of a high-molecular-weight aggregate peak centered around 13.5 mL of elution volume in the non-2P-stabilized sample. The emergence of this peak corresponded with a decrease in the peak previously shown to contain properly folded trimer (Fig. 1D) centered around 14.8 mL of elution volume. A similar, albeit less prominent, peak was not observed in the 2P-stabilized sample until after 96 hr of incubation at 42°C, and the decrease of the peak at 14.8 mL was less pronounced, as measured by quantifying the area under the curve (Table S2). These data, in conjunction with our evaluation of cell surface expression levels, suggest that proline stabilization of the gp41 subunit has little impact on the level of protein expression and that observed increases in the yield of recombinant ectodomain after proline stabilization are instead due to the increased durability of the prefusion conformation of Env.
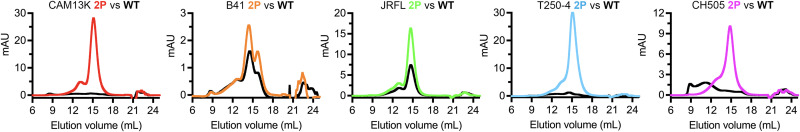
Leu568 and Thr569 are 99.8% and 98.9% conserved among all of the 6,599 Env sequences curated in the Los Alamos National Laboratory (LANL) HIV database (Fig. S7), likely reflecting their functional importance during the transition from prefusion to postfusion and their insulation from antibody-mediated selective pressure. This high degree of sequence conservation at positions 568 and 569 prompted us to evaluate whether our 2P stabilization strategy might also be effective outside the context of the CH848 10.17DT Env. A panel of multiclade HIV-1 and simian immunodeficiency virus (SIV) Env constructs (Table S3) was selected, and the yield of recombinantly expressed prefusion trimer after transient transfection of a 2P-stabilized SOSIP construct was compared to that of a non-2P-stabilized SOSIP construct (Fig. 5). Overall, 2P stabilization improved the yield of soluble Env trimers from HIV-1 clades B and C, subgroup 02_AG, and even the SIVcpz Env that was tested from CAM13K. However, the degree to which 2P stabilization enhanced Env yield was highly variable for the constructs that were evaluated. B41 and JRFL Env constructs exhibited fairly modest ~2-fold increases in yield, whereas fold increases for CAM13K, T250-4, and CH505 Env constructs could not be reliably calculated due to an inability to purify the non-2P-stabilized construct. During the review of the manuscript, a report from Gorman et al, was published describing the cryo-EM structure of an Env trimer from rhesus macaque SIV (34). In an effort to reliably express this trimer in the prefusion conformation, Gorman et al. also independently identified the T569P mutation, which reportedly aided in their purification of SIVmac Env. These findings suggest that 2P stabilization is a broadly applicable approach to stabilizing the prefusion conformation of Env, albeit to various degrees depending on the Env variant in question. Furthermore, the 2P mutations are compatible with a variety of alternative Env stabilization strategies, including the SOSIP, DS, and F14 mutations (Table S3).
FIG 5.
Applying 2P mutations to evolutionarily diverse Env constructs. Size-exclusion chromatograms are shown for each construct after PGT145 affinity chromatography. Curves for the 2P-stabilized construct are colored, and the curves for the corresponding non-2P-stabilized constructs (“WT”) are overlaid in black. Chromatograms were generated using a Superose 6 Increase column. Full details about these Env constructs can be found in Table S3 in the supplemental material.
DISCUSSION
Conventional vaccination efforts have thus far been unable to elicit broadly neutralizing antibodies, which are correlated with protection against HIV-1 acquisition. These failures have prompted the development of new mosaic immunogens and sequential immunization strategies, the latter of which are intended to guide the immune system from germline targeting through the process of antibody maturation (35, 36). These proposed vaccination regimens require the production of numerous, diverse Env immunogens, many of which are recalcitrant to recombinant expression despite modification with previously reported stabilization strategies. Here, we report a new combination of proline stabilization mutations that facilitate the purification of antigenically diverse Env constructs by enhancing the durability of the prefusion conformation.
By targeting the region in gp41 that connects the central helix to the rest of heptad repeat 1 (HR1), the 2P mutations are predicted to disfavor the formation of the elongated alpha helices that drive the transition to the occluded, open and postfusion conformations. In this sense, they are mechanistically similar to the previously reported “HR1-redesigned” trimers (23), in which residues 548 to 568 of gp41 are replaced with a flexible linker. However, because the 2P mutations only require two amino acid substitutions at the highly conserved 568 and 569 positions, rather than removal of the HR1 and the introduction of an exogenous 8- to 10-amino-acid-long loop, they present a less intensive engineering feat during the design and creation of new Env constructs. Moreover, we have shown that the 2P mutations can be used in conjunction with many existing stabilization strategies, including SOSIP (18), DS (24), and F14 (22) mutations. Additional studies are required to determine whether the 2P mutations are also compatible with alternative stabilization strategies, such as the recently described repair-and-stabilize approach (25, 37). Perhaps the most interesting opportunities to combine 2P with existing stabilization strategies will involve approaches that have already altered the residues at positions 568 or 569, such as the aforementioned HR1-redesigned trimers (23) or the MD2 trimer (38), which makes use of a L568D mutation to enhance expression of the BG505 Env. It should also be noted that although the alternative proline substitutions at positions 536 and 545 proved less effective than the 2P combination at positions 568 and 569 in the CH848 10.17DT Env, it is possible that in the context of other Env variants, alternative or additional proline mutations may also prove beneficial, such as the 555P mutation that has previously been described in the 16055 Env (39).
Having developed this stabilization strategy and confirmed that it did not alter the antigenicity or structure of soluble Env trimers, we also show that its mechanism of increasing the yield of Env trimers is through enhancing the durability of the prefusion conformation rather than increasing overall expression. During the relatively harsh prolonged conditions of transient transfection, Env ectodomains that more reliably retain the prefusion conformation are presumably less likely to spontaneously and irreversibly trigger to the postfusion conformation and be lost as insoluble aggregates (30). In addition to facilitating the purification of recombinantly expressed prefusion Env constructs for use as biochemical reagents in the laboratory, the enhanced durability of 2P Env may also have implications for future vaccine design and production efforts.
Many proposed vaccination regimens currently rely on extensive priming and boosting schedules, which would inevitably complicate vaccine adherence among the general population (40, 41). One reason for this approach is the difficulty associated with eliciting even autologous neutralizing antibodies (42, 43). By enhancing the durability of the prefusion conformation of Env, it may be possible to increase the residence time of Env immunogens in B cell germinal centers, theoretically mimicking the conditions of natural infection where natively expressed Env is continually present (15). Reliable presentation of the prefusion Env is thought to be strictly required to elicit a polyclonal, broadly neutralizing response because the UCAs of many bNAbs, such as DH270 and CH01, are only capable of recognizing Env in the prefusion conformation (28, 44). The enhanced durability observed in our thermostability and forced degradation assays suggests that 2P-stabilized Env proteins may not require the same restrictive cold-chain storage conditions that are necessary for unstabilized Env immunogens. In addition to the advantages that this poses in a laboratory environment, it is also a critical consideration when thinking about administering vaccines in regions that may lack sufficient infrastructure to reliably maintain a vaccine cold chain.
Finally, we also show that the 2P stabilization strategy is effective in multiple HIV-1 and SIV isolates. By introducing these mutations into a diverse panel of different Env ectodomains, we were able to enhance the yield of multiple prefusion trimers, albeit to differing degrees of success depending on the Env variant. This improvement in Env yield underscores the key importance of the HR1 region in trimer stability (23). But given the variant-dependent levels of efficacy that were observed with the 2P stabilization strategy, it is likely that other factors, such as glycan networking or conformational heterogeneity within the gp120 subunit, also have a significant impact on the overall yield of a given trimer (45, 46). The complex chemical environment generated by the tertiary and quaternary structure of the Env trimer makes it difficult to pinpoint what precise features might be contributing to this variant-dependent efficacy. However, as mosaic and sequential vaccination strategies continue to be developed, it is our hope that having the 2P stabilization strategy as a broadly applicable means to enhance the yield of antigenically diverse prefusion trimers will prove useful for immunogen design.
MATERIALS AND METHODS
Protein production and purification.
Plasmids encoding Env ectodomains (CH848 10.17DT DS-SOSIP, CH848 10.17DT DS-SOS-2P, CH848 10.17DT DS-SOSIP-2P, CAM13 Q171K DS-SOSIP, CAM13 Q171K DS-SOSIP-2P, B41 SOSIP, B41 SOSIP-2P, JRFL SOSIPv6, JRFL SOSIPv6-2P, T250-4 DS-SOSIP, T250-4 DS-SOSIP-2P, CH505w24 F14 DS-SOSIP, and CH505w24 F14 DS-SOSIP-2P) were mixed with a plasmid encoding furin at a ratio of 4:1. All Env constructs contained a C-terminal HRV3C cleavage site, a TwinStrepTag, and an 8 × His tag. These plasmid mixtures were transfected into FreeStyle 293-F cells (Thermo Fisher) using polyethylenimine. Transfected supernatants were harvested and filtered 5 days after transfection. Because CH848 Env does not bind to PGT145, all CH848 constructs were purified by StrepTactin resin (IBA). All other Env constructs were purified by PGT145 affinity chromatography, as described previously (20). After affinity chromatography, Env constructs were further purified by size-exclusion chromatography using a Superose 6 Increase 10/300 GL column (Cytiva) in 2 mM Tris (pH 8.0), 200 mM NaCl, and 0.02% NaN3.
Plasmids encoding the heavy and light chains of mAbs used for ELISA or flow cytometric analysis (CH65, N6, DH270 UCA, DH270.6, PGT151, PGT128, VRC26.25, 2F5, 17b, 19b, RM19R, DH1029, 2G12, F39F, PGT125, F105, and A32) were combined at a ratio of 1:1 and used to transiently transfect Expi293 cells (Thermo Fisher) with ExpiFectamine (Thermo Fisher). Transfected supernatants were harvested and filtered 5 days after transfection, and antibodies were purified using Protein A resin (Thermo Fisher). Eluted antibodies were then buffer exchanged into phosphate-buffered saline (PBS).
Negative stain electron microscopy.
CH848 10.17DT DS-SOSIP-2P was diluted to 0.2 mg/mL with buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 8 mM glutaraldehyde, and 5 g/dL glycerol. After 5 min of incubation, excess glutaraldehyde was quenched by the addition 1 M Tris (pH 8.0) for a final concentration of 80 mM Tris and incubated for an additional 5 min. Quenched sample was applied to a glow-discharged, carbon-coated EM grid for 8 to 10 s, blotted, and stained with 2 g/dL uranyl formate for 1 min before being blotted and allowed to air dry. The stained grid was examined on a Philips EM420 electron microscope operating at 120 kV and a nominal magnification of ×49,000. Twelve images were collected on a 76-megapixel charge-coupled-device (CCD) camera at 2.4 Å/pixel. Images were analyzed, and two-dimensional (2D) class averaging was performed using standard protocols within Relion 3.0 (47).
ELISA.
Env protein containing a C-terminal StrepTag was bound in wells of a 384-well plate, which was previously coated with streptavidin (Thermo Fisher Scientific) at 2 μg/mL and blocked with PBS containing 4% (wt/vol) whey protein, 15% normal goat serum, 0.5% Tween 20, and 0.05% sodium azide. Proteins were incubated at room temperature for 1 hr and washed with PBS and 0.1% Tween 20. mAbs were then added in serial dilutions beginning at 25 μg/mL. Antibodies were incubated at room temperature for 1 hr and washed, and binding was detected with goat anti-human horseradish peroxidase (HRP; Jackson ImmunoResearch) and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sera Care Life Sciences).
Cryo-EM sample preparation and data collection.
Purified CH848 10.17DT DS-SOSIP-2P was diluted to a final concentration of 1.8 mg/mL in 10 mM Tris (pH 8.0). To prevent interaction of the trimer with the air-water interface during vitrification, the sample was incubated in 0.085 mM n-dodecyl β-d-maltoside (DDM). A 3.5-μL drop of protein was deposited on a Quantifoil-1.2/1.3 grid (Electron Microscopy Sciences) that had been glow discharged for 10 s using an easiGlow glow discharge cleaning system (PELCO). After a 30-s incubation in >95% humidity, excess protein was blotted away for 2.5 s before being plunge frozen into liquid ethane using a Leica EM GP2 plunge freezer (Leica Microsystems). Frozen grids were imaged using a Titan Krios (Thermo Fisher) microscope equipped with a K3 detector (Gatan). Data were collected using the Gatan Latitude software.
Cryo-EM data analysis and refinement.
Movies were imported into cryoSPARC v3.3.1 (48) and aligned using patch-based motion correction. Patch-based contrast transfer function (CTF) estimation was then performed before 5,780,212 particles were selected using a nontemplated blob-picking strategy. Junk particles were removed by 2D classification, leaving a stack of 683,406 particles that were subjected to iterative rounds of ab initio volume calculation and heterogeneous three-dimensional (3D) classification, leaving a final stack of 111,026 particles. These particles yielded a 3.95-Å reconstruction after performing asymmetrical (C1) nonuniform refinement, with the resulting map exhibiting C3 symmetry. Nonuniform refinement (49) was then performed again using the same particle stack and applying C3 symmetry, which improved the resolution of the resulting reconstruction to 3.73 Å. This map was then subjected to postprocessing using DeepEMhancer (50). A full description of the cryo-EM data-processing workflow can be found in Fig. S4 in the supplemental material. Chains A, D, E, H, I, and 1 from Protein Data Bank ID (PDB ID) 6UM7 were used as a starting model that was docked into the sharpened map and remodeled through iterative rounds of building and refinement in Coot (51), PHENIX (52), and ISOLDE (53).
gp160 cell surface expression and characterization.
The 293-F cells (Thermo Fisher, R79007) were diluted to 1.25 × 106 cells/mL and seeded in 12-well plates 2 to 3 hr before transfection. Transient transfection of DNA plasmids was performed with jetPRIME transfection reagent (Polyplus, 101000046) following the manufacturer’s instructions. Transfected cells were cultured in an incubator at 37°C with 8% CO2 and shaking at 125 rpm for 24 hr before flow cytometry staining. Twenty-four hours after transfection, 293-F cells were harvested, counted, and rinsed with 1% bovine serum albumin (BSA)/PBS and pelleted at 500 × g for 5 min. Next, cells were resuspended to a density of 1 × 106 cells/mL in 1% BSA/PBS, and 50,000 cells were aliquoted to each well of U-bottom 96-well plates. An equal volume of recombinant anti-HIV-1 Env antibodies at 4 μg/mL was added to cells to reach a working concentration of 2 μg/mL. Antibodies were incubated with cells at 4°C for 30 min. Cells were then washed once with 150 μL of 1% BSA/PBS and then incubated with 50 μL of goat anti-human IgG Fc secondary antibody phycoerythrin (PE; Thermo Fisher, 12-4998-82) at a final concentration of 2.5 μg/mL in 1% BSA/PBS. After a 30-min incubation at 4°C while protected from light, cells were washed once with 1× PBS and incubated with 100 μL of live/dead fixable aqua dead cell stain (Thermo Fisher, L34966; 1:1,000 in PBS) for 20 min at room temperature protected from light. Next, cells were washed once with 100 μL of 1% BSA/PBS and resuspended in 50 μL of 1% BSA, 2 mM EDTA, and 1% paraformaldehyde (PFA) in PBS. Flow cytometric data were acquired on an iQue 3 high-throughput flow cytometry system (Sartorius). Data were analyzed using FlowJo v10 (FlowJo).
Freeze/thaw thermostability analysis.
Purified CH848 10.17DT DS-SOSIP and CH848 10.17DT DS-SOSIP-2P were diluted to 0.2 mg/mL in 2 mM Tris (pH 8.0), 200 mM NaCl, and 0.02% NaN3. Samples were rapidly frozen in liquid nitrogen and thawed by incubation at 30°C for 5 min. Thermostability measurements were collected using a Tycho NT.6 by increasing the temperature from 35°C to 95°C at a rate of 30.0°C/min. Tm was determined as the inflection temperature using the Tycho Nanotemper data-processing software.
Forced degradation assay.
Aliquots (250 μg) of purified CH848 10.17DT DS-SOSIP or CH848 10.17DT DS-SOSIP-2P were incubated at 42°C for 0, 48, or 96 hr. Aliquots were then run over a Superose 6 Increase 10/300 GL column (Cytiva Life Sciences) to evaluate Env integrity. Area under the curve was calculated using UNICORN 7.0 Evaluation software (Cytiva Life Sciences).
Data availability.
The cryo-EM map and corresponding atomic model have been deposited in the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB) under accession codes EMD-28608 and 8EU8, respectively. All flow cytometry data are available upon request.
ACKNOWLEDGMENTS
We thank Aja Sanzone, Alecia Brown, and Beth Bryan for their assistance with cell culture. We thank Rory Henderson for helpful discussions regarding the putative mechanisms of 2P stabilization. We also thank Mitchell Martin, Jessica Martinson, and Bhavna Hora of the Duke Human Vaccine Institute Sequencing Core. Flow cytometry was performed in the Duke Human Vaccine Institute Research Flow Cytometry Facility (Durham, NC). This work was supported by the Consortia for HIV/AIDS Vaccine Development grant UM1AI144371 from the Division of AIDS, NIAID, as well as R01 AI050529 (B.H.H.), R37 AI 150590 (B.H.H.), and NIAID grant number 5T32AI007392-32 (D.W.).
D.W., Z.M., B.H.H., P.A., K.O.S., and B.F.H. designed the research. D.W., Z.M., B.T., K.J., O.A., R.P., K.M., R.J.E., and M.B. performed research. D.W., Z.M., K.M., and R.J.E. analyzed data. D.W., K.O.S., and B.F.H. wrote the manuscript with input from all authors.
D.W., K.O.S., and B.F.H. are inventors on US patent application number 63/418,720 (“Stabilization of Human Immunodeficiency Virus [HIV] Envelopes”), and K.O.S. and B.F.H. are inventors on international patent application PCT/US2018/020788 (“Compositions and Methods for Inducing HIV-1 Antibodies”). All other authors declare no competing interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Daniel Wrapp, Email: daniel.wrapp@duke.edu.
Kevin O. Saunders, Email: kevin.saunders@duke.edu.
Barton F. Haynes, Email: barton.haynes@duke.edu.
Guido Silvestri, Emory University.
REFERENCES
- 1.Centers for Disease Control. 1981. Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. MMWR Morb Mortal Wkly Rep 30:305–308. [PubMed] [Google Scholar]
- 2.Eisinger RW, Fauci AS. 2018. Ending the HIV/AIDS pandemic. Emerg Infect Dis 24:413–416. 10.3201/eid2403.171797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, iPrEx Study Team . 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860. 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Fauci AS. 2017. An HIV vaccine is essential for ending the HIV/AIDS pandemic. JAMA 318:1535–1536. 10.1001/jama.2017.13505. [DOI] [PubMed] [Google Scholar]
- 6.Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091–1094. 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- 7.Willey RL, Bonifacino JS, Potts BJ, Martin MA, Klausner RD. 1988. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci USA 85:9580–9584. 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767. 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 9.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 10.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 11.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh WC, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. 1987. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science 237:1351–1355. 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 12.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 13.Roark RS, Li H, Williams WB, Chug H, Mason RD, Gorman J, Wang S, Lee FH, Rando J, Bonsignori M, Hwang KK, Saunders KO, Wiehe K, Moody MA, Hraber PT, Wagh K, Giorgi EE, Russell RM, Bibollet-Ruche F, Liu W, Connell J, Smith AG, DeVoto J, Murphy AI, Smith J, Ding W, Zhao C, Chohan N, Okumura M, Rosario C, Ding Y, Lindemuth E, Bauer AM, Bar KJ, Ambrozak D, Chao CW, Chuang GY, Geng H, Lin BC, Louder MK, Nguyen R, Zhang B, Lewis MG, Raymond DD, Doria-Rose NA, Schramm CA, Douek DC, Roederer M, Kepler TB, Kelsoe G, et al. 2021. Recapitulation of HIV-1 Env-antibody coevolution in macaques leading to neutralization breadth. Science 371:eabd2638. 10.1126/science.abd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel PH, Preston BD. 1994. Marked infidelity of human immunodeficiency virus type 1 reverse transcriptase at RNA and DNA template ends. Proc Natl Acad Sci USA 91:549–553. 10.1073/pnas.91.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Moral-Sanchez I, Russell RA, Schermer EE, Cottrell CA, Allen JD, Torrents de la Pena A, LaBranche CC, Kumar S, Crispin M, Ward AB, Montefiori DC, Sattentau QJ, Sliepen K, Sanders RW. 2022. High thermostability improves neutralizing antibody responses induced by native-like HIV-1 envelope trimers. NPJ Vaccines 7:27. 10.1038/s41541-022-00446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz JA, Bar-On Y, Lu CL, Fera D, Lockhart AAK, Lorenzi JCC, Nogueira L, Golijanin J, Scheid JF, Seaman MS, Gazumyan A, Zolla-Pazner S, Nussenzweig MC. 2017. Non-neutralizing antibodies alter the course of HIV-1 infection in vivo. Cell 170:637–648. 10.1016/j.cell.2017.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissenhorn W, Wharton SA, Calder LJ, Earl PL, Moss B, Aliprandis E, Skehel JJ, Wiley DC. 1996. The ectodomain of HIV-1 Env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J 15:1507–1514. 10.1002/j.1460-2075.1996.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, Kalyanaraman R, Paluch M, Berkhout B, Maddon PJ, Olson WC, Lu M, Moore JP. 2002. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol 76:8875–8889. 10.1128/jvi.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol 74:627–643. 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, Crampton J, Hu J, Bontjer I, Torres JL, Arendt H, DeStefano J, Koff WC, Schuitemaker H, Eggink D, Berkhout B, Dean H, LaBranche C, Crotty S, Crispin M, Montefiori DC, Klasse PJ, Lee KK, Moore JP, Wilson IA, Ward AB, Sanders RW. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163:1702–1715. 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torrents de la Pena A, Julien JP, de Taeye SW, Garces F, Guttman M, Ozorowski G, Pritchard LK, Behrens AJ, Go EP, Burger JA, Schermer EE, Sliepen K, Ketas TJ, Pugach P, Yasmeen A, Cottrell CA, Torres JL, Vavourakis CD, van Gils MJ, LaBranche C, Montefiori DC, Desaire H, Crispin M, Klasse PJ, Lee KK, Moore JP, Ward AB, Wilson IA, Sanders RW. 2017. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep 20:1805–1817. 10.1016/j.celrep.2017.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson R, Lu M, Zhou Y, Mu Z, Parks R, Han Q, Hsu AL, Carter E, Blanchard SC, Edwards RJ, Wiehe K, Saunders KO, Borgnia MJ, Bartesaghi A, Mothes W, Haynes BF, Acharya P, Munir Alam S. 2020. Disruption of the HIV-1 envelope allosteric network blocks CD4-induced rearrangements. Nat Commun 11:520. 10.1038/s41467-019-14196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong L, He L, de Val N, Vora N, Morris CD, Azadnia P, Sok D, Zhou B, Burton DR, Ward AB, Wilson IA, Zhu J. 2016. Uncleaved prefusion-optimized gp140 trimers derived from analysis of HIV-1 envelope metastability. Nat Commun 7:12040. 10.1038/ncomms12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon YD, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang GY, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O'Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GB, Stuckey J, Thomas PV, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schon A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, et al. 2015. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol 22:522–531. 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutten L, Lai YT, Blokland S, Truan D, Bisschop IJM, Strokappe NM, Koornneef A, van Manen D, Chuang GY, Farney SK, Schuitemaker H, Kwong PD, Langedijk JPM. 2018. A universal approach to optimize the folding and stability of prefusion-closed HIV-1 envelope trimers. Cell Rep 23:584–595. 10.1016/j.celrep.2018.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leaman DP, Zwick MB. 2013. Increased functional stability and homogeneity of viral envelope spikes through directed evolution. PLoS Pathog 9:e1003184. 10.1371/journal.ppat.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiao H, Pelletier SL, Hoffman L, Hacker J, Armstrong RT, White JM. 1998. Specific single or double proline substitutions in the “spring-loaded” coiled-coil region of the influenza hemagglutinin impair or abolish membrane fusion activity. J Cell Biol 141:1335–1347. 10.1083/jcb.141.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders KO, Wiehe K, Tian M, Acharya P, Bradley T, Alam SM, Go EP, Scearce R, Sutherland L, Henderson R, Hsu AL, Borgnia MJ, Chen H, Lu X, Wu NR, Watts B, Jiang C, Easterhoff D, Cheng HL, McGovern K, Waddicor P, Chapdelaine-Williams A, Eaton A, Zhang J, Rountree W, Verkoczy L, Tomai M, Lewis MG, Desaire HR, Edwards RJ, Cain DW, Bonsignori M, Montefiori D, Alt FW, Haynes BF. 2019. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science 366:eaay7199. 10.1126/science.aay7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fera D, Lee MS, Wiehe K, Meyerhoff RR, Piai A, Bonsignori M, Aussedat B, Walkowicz WE, Ton T, Zhou JO, Danishefsky S, Haynes BF, Harrison SC. 2018. HIV envelope V3 region mimic embodies key features of a broadly neutralizing antibody lineage epitope. Nat Commun 9:1111. 10.1038/s41467-018-03565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyce MG, Georgiev IS, Yang Y, Druz A, Geng H, Chuang GY, Kwon YD, Pancera M, Rawi R, Sastry M, Stewart-Jones GBE, Zheng A, Zhou T, Choe M, Van Galen JG, Chen RE, Lees CR, Narpala S, Chambers M, Tsybovsky Y, Baxa U, McDermott AB, Mascola JR, Kwong PD. 2017. Soluble prefusion closed DS-SOSIP.664-Env trimers of diverse HIV-1 strains. Cell Rep 21:2992–3002. 10.1016/j.celrep.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W, Kong WP, Andres EL, Kettenbach AN, Denison MR, Chappell JD, Graham BS, Ward AB, McLellan JS. 2017. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci USA 114:E7348–E7357. 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Dam KA, Bridges MD, Hoffmann MAG, DeLaitsch AT, Gristick HB, Escolano A, Gautam R, Martin MA, Nussenzweig MC, Hubbell WL, Bjorkman PJ. 2022. Neutralizing antibodies induced in immunized macaques recognize the CD4-binding site on an occluded-open HIV-1 envelope trimer. Nat Commun 13:732. 10.1038/s41467-022-28424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, Le KC, Wrapp D, Lee AG, Liu Y, Chou CW, Byrne PO, Hjorth CK, Johnson NV, Ludes-Meyers J, Nguyen AW, Park J, Wang N, Amengor D, Lavinder JJ, Ippolito GC, Maynard JA, Finkelstein IJ, McLellan JS. 2020. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 369:1501–1505. 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorman J, Wang C, Mason RD, Nazzari AF, Welles HC, Zhou T, Bess JW, Jr, Bylund T, Lee M, Tsybovsky Y, Verardi R, Wang S, Yang Y, Zhang B, Rawi R, Keele BF, Lifson JD, Liu J, Roederer M, Kwong PD. 2022. Cryo-EM structures of prefusion SIV envelope trimer. Nat Struct Mol Biol 29:1080–1091. 10.1038/s41594-022-00852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes BF, Burton DR, Mascola JR. 2019. Multiple roles for HIV broadly neutralizing antibodies. Sci Transl Med 11:eaaz2686. 10.1126/scitranslmed.aaz2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes BF, Wiehe K, Borrrow P, Saunders KO, Korber B, Wagh K, McMichael AJ, Kelsoe G, Hahn BH, Alt F, Shaw GM. 2022. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat Rev Immunol 12:1–17. 10.1038/s41577-022-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawi R, Rutten L, Lai YT, Olia AS, Blokland S, Juraszek J, Shen CH, Tsybovsky Y, Verardi R, Yang Y, Zhang B, Zhou T, Chuang GY, Kwong PD, Langedijk JPM. 2020. Automated design by structure-based stabilization and consensus repair to achieve prefusion-closed envelope trimers in a wide variety of HIV strains. Cell Rep 33:108432. 10.1016/j.celrep.2020.108432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, Stanfield RL, McCoy LE, Ozorowski G, Hu X, Kalyuzhniy O, Briney B, Schiffner T, Garces F, Freund NT, Gitlin AD, Menis S, Georgeson E, Kubitz M, Adachi Y, Jones M, Mutafyan AA, Yun DS, Mayer CT, Ward AB, Burton DR, Wilson IA, Irvine DJ, Nussenzweig MC, Schief WR. 2016. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity 45:483–496. 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, Sharma SK, Cottrell C, Guenaga J, Tran K, Wilson R, Behrens AJ, Crispin M, de Val N, Wyatt RT. 2018. Structure-guided redesign improves NFL HIV Env trimer integrity and identifies an inter-protomer disulfide permitting post-expression cleavage. Front Immunol 9:1631. 10.3389/fimmu.2018.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barouch DH, O'Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16:319–323. 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. 2012. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 30:423–433. 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Y, Tran K, Bale S, Kumar S, Guenaga J, Wilson R, de Val N, Arendt H, DeStefano J, Ward AB, Wyatt RT. 2016. Thermostability of well-ordered HIV spikes correlates with the elicitation of autologous tier 2 neutralizing antibodies. PLoS Pathog 12:e1005767. 10.1371/journal.ppat.1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guttman M, Cupo A, Julien JP, Sanders RW, Wilson IA, Moore JP, Lee KK. 2015. Antibody potency relates to the ability to recognize the closed, pre-fusion form of HIV Env. Nat Commun 6:6144. 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crooks ET, Almanza F, D'Addabbo A, Duggan E, Zhang J, Wagh K, Mou H, Allen JD, Thomas A, Osawa K, Korber BT, Tsybovsky Y, Cale E, Nolan J, Crispin M, Verkoczy LK, Binley JM. 2021. Engineering well-expressed, V2-immunofocusing HIV-1 envelope glycoprotein membrane trimers for use in heterologous prime-boost vaccine regimens. PLoS Pathog 17:e1009807. 10.1371/journal.ppat.1009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, Kong R, Thomas PV, Wagh K, Zhou T, Behrens AJ, Bylund T, Choi CW, Davison JR, Georgiev IS, Joyce MG, Kwon YD, Pancera M, Taft J, Yang Y, Zhang B, Shivatare SS, Shivatare VS, Lee CC, Wu CY, Bewley CA, Burton DR, Koff WC, Connors M, Crispin M, Baxa U, Korber BT, Wong CH, Mascola JR, Kwong PD. 2016. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell 165:813–826. 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodge EA, Naika GS, Kephart SM, Nguyen A, Zhu R, Benhaim MA, Guo W, Moore JP, Hu SL, Sanders RW, Lee KK. 2022. Structural dynamics reveal isolate-specific differences at neutralization epitopes on HIV Env. iScience 25:104449. 10.1016/j.isci.2022.104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJ, Lindahl E, Scheres SH. 2018. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7:e42166. 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. 2017. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14:290–296. 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 49.Punjani A, Zhang H, Fleet DJ. 2020. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat Methods 17:1214–1221. 10.1038/s41592-020-00990-8. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Garcia R, Gomez-Blanco J, Cuervo A, Carazo JM, Sorzano COS, Vargas J. 2021. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun Biol 4:874. 10.1038/s42003-021-02399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 52.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58:1948–1954. 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 53.Croll TI. 2018. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr D Struct Biol 74:519–530. 10.1107/S2059798318002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S7 and Tables S1 to S3. Download jvi.01673-22-s0001.pdf, PDF file, 8.8 MB (8.8MB, pdf)
Data Availability Statement
The cryo-EM map and corresponding atomic model have been deposited in the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB) under accession codes EMD-28608 and 8EU8, respectively. All flow cytometry data are available upon request.