Abstract
Background
The leaves of V. auriculifera has been used traditionally for the treatment of inflammatory disorders, and pain in various parts of Ethiopia. However, to our knowledge, the analgesic and anti-inflammatory activity of the crude extract and solvent fractions has never been experimentally studied.
Objective
To assess the analgesic and anti-inflammatory activities of V. auriculifera leaf extract and solvent fractions in rodent models.
Material and methods
Air-dried leaves of V. auriculifera were grounded and macerated using 80% methanol. The air-dried, grounded leaves were also successively extracted with ethyl acetate, and methanol. The residue was then macerated in water for 72 hr. The extract’s peripheral analgesic activity, as well as the solvent fractions, were determined using an acetic acid-induced writhing test. The hot plate model was used to assess the central analgesic effect. Carrageenan-induced hind paw edema and cotton pellet-induced granuloma models were used to assess the anti-inflammatory effect in rats.
Results
The 80% methanol leaf extract and solvent fractions have demonstrated significant (p < 0.05) peripheral and central analgesic activity. Both 80% methanol leaf extract and solvent fractions of V. auriculifera were found to have anti-inflammatory activity in a carrageenan-induced rat paw edema model. In the cotton pellet-induced granuloma model, all concentrations of 80% methanol leaf extract (ME), methanol fraction (MEF), and aqueous fractions (AQF) of V. auriculifera inhibited exudate and granuloma formation. Although all tested doses significantly inhibited granuloma mass formation, only the medium and highest ethyl acetate fraction (EAF) doses significantly inhibited the generation of inflammatory exudate.
Conclusion
This study’s findings indicate that the solvent fractions and 80% methanol extract of V. auriculifera have analgesic and anti-inflammatory properties. This study’s findings not only confirm the plants’ traditional claim but also provide clues for further investigation of the active principles of this plant for the development of effective and safe analgesic and anti-inflammatory drugs.
Keywords: Vernonia auriculifera, analgesic, anti-inflammatory, carrageenan-induced paw edema, cotton pellet granuloma, rats
Background
Pain is an unpleasant sensory experience brought on by an actual or perceived injury. It can cause learned avoidance, and protective motor and vegetative responses, and it can also change species-specific behavior, such as social behavior.1 Even though pain is fundamentally a sensation, it is also influenced by psychological factors like emotional state and cognitive appraisal in addition to the amount of nociceptive input.2 Depression, rage, and anxiety as the emotional components of pain and expectation, attention, and appraisal as the cognitive components, are some of the most significant emotional and cognitive determinants.2 Because it can significantly interfere with daily activities and frequently results in mood disorders like anxiety and depression, pain has a negative impact on the patients’ quality of life.3
Inflammation is an inherent response of the body to a multitude of offending agents which includes pathogenic microorganisms, toxic chemical substances, immunological reactions, and physical damage to the tissue.4,5 This process can be classified as acute or chronic depending on the duration of the condition. Chronic inflammation will develop if the risk factor(s) that caused acute inflammation remains.6 Acute inflammation arises due to tissue damage as a result of microbial insult, toxic compounds, or trauma. It begins swiftly, turns out to be severe briefly, and the symptoms may well last for a few days. Unlike acute pain, chronic inflammation is slow, longstanding inflammation lasting for protracted periods of numerous months to years.7 Inflammation is a known biological process that arises in response to tissue injury. Nonetheless, disproportionate inflammation plays a role in many acute and chronic human diseases.8 It is an essential contributor to diseases (particularly chronic inflammation) such as arteriosclerosis, obesity, rheumatoid arthritis, chronic obstructive pulmonary disease, neurodegenerative diseases, diabetes, and cancer.6,9
Vernonia (Asteraceae) is the largest genus in the Vernoniae tribe, with over 1000 species.10 Several habitat types, including tropical forests, marshes, wet places, dry plains, tropical savannahs, xeric or dry locations in the desert, and even frosty portions of eastern North America, foster the development of Vernonia species.11 It can be found in humid lowland forests, wooded grassland, and scrub at elevations between 1600 and 2800 m in Ethiopia’s southwest and south, where it is widely dispersed.12 Various Vernonia species are used to manage many diseases in Ethiopian traditional medicine. Experimental research on a variety of Vernonia species has also revealed a plethora of activities, including anti-plasmodial, analgesic, anti-inflammatory, antimicrobial, antidiabetic, antioxidant, and anticancer.13 V. auriculifera locally known as “Barewa” is a well-known medicinal plant in Ethiopia for headaches, venereal diseases, gastrointestinal problems, wound healing, diabetes, and hepatitis.14
Although there are many drugs for the management of pain and inflammations they are associated with tremendous adverse effects such as gastric lesions, acute kidney injury, and adverse cardiovascular thrombotic effects that are associated with the utilization of non-steroidal anti-inflammatory drugs as well as osteoporosis, adrenal suppression, glucose intolerance, and peptic ulcer that is seen with the use of corticosteroids and tolerance and dependence induced by opiates; besides their adverse effect, they might also be insufficient to manage some cases.15 This tells us that there is a need for drugs with lessened adverse effects or different adverse effects permitting the clinician to choose the best option considering the patient’s comorbidities.16 As such, there is a continuous search for alternative anti-inflammatory and analgesic agents especially, from natural sources.15
Although V. auriculifera, the experimental plant, has traditionally been used to treat various inflammatory conditions, to the best of our knowledge there are no experimental studies to support this claim. Thus, the purpose of this study was to experimentally assess the Analgesic and Anti-inflammatory effects of 80% methanol extract and solvent fractions of the Leaves of V. auriculifera.
Materials
Plant Material
V. auriculifera leaves were collected in August 2020 from Hosanna town in the Southern Nations, Nationalities, and Peoples region, 230 kilometers south of Addis Ababa (7°33’08.0“N 37°50’56.0”E). Mr. Melaku Wondafrash, a taxonomist, identified and authenticated the plant specimens, and voucher specimens (EA001) were stored in the National Herbarium at Addis Ababa University’s College of Natural and Computational Sciences for future reference.
Experimental Animals
Healthy Swiss albino mice of both sex (8–12 weeks, weighing 25–33 grams) and healthy, adult Wistar albino rats of both sex (6–8 weeks of age) weighing 200–250 were acquired from the animal house of Addis Ababa University’s Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, and Ethiopian public health institute`s animal unit, Addis Ababa, Ethiopia. They were kept in cages under standard conditions (22 ± 3 °C, 40–70% relative humidity, and 12-hour light/dark cycles). They had free access to a pellet diet and water ad libitum. Before the trial, all animals were given a week to acclimate to the working environment. All of the research was done following internationally accepted guidelines for the use, care, and handling of laboratory animals.17 At the end of the study the animals were euthanized with high-dose anesthetics (Ketamine 150 mg/kg). The study protocol was approved on August 17, 2020, G.C. by the ethical review committee of Addis Ababa University’s College of Health Sciences, School of Pharmacy with a reference number (ERB/SOP/177/12/2020).
Methods
Extraction of the Plant Material
Fresh leaves were picked, cleaned, and properly washed with tap water before drying in the open air at room temperature beneath a temporarily built shade for three weeks. Using a mortar and pestle, the dried leaves were coarsely ground. Thereafter, the air-dried and powdered plant components (733g) were extracted at room temperature for three days using a cold maceration method with 80% methanol. To enhance yield, this process was performed two more times with a fresh solvent added to the marc. The resultant liquid extract was then mixed and filtered with filter paper (Whatman no 1, Whatman Ltd., England). Following that, the filtrate was concentrated under reduced pressure and at 40 °C temperature in a rotary evaporator (Buchi Rotavapor R-200, Switzerland). The concentrated extract was freeze-dried at −50 °C using a lyophilizer (Operon, Korea vacuum limited, Korea) and a shiny dark green extract was obtained. The resulting extract was weighed and the yield was 10.47% (w/w). The extract was then placed in a vial and refrigerated until further use. The methanol used was analytical grade and it was purchased from its respective vendor.
Fractionation of the Plant Material
250 g of V. auriculifera leaves were sequentially extracted using a Soxhlet apparatus with ethyl acetate (99.8%), and methanol (99.8%) to get the solvent fractions. After that, the residue was macerated in water for 72 hours at room temperature. Each fraction was subsequently dried using a rotary evaporator under reduced pressure and at 40 °C temperature, with the exception of the aqueous fraction. The aqueous fraction, on the other hand, was freeze-dried at −50 °C using a lyophilizer. The yield of the resultant fractions was ethyl acetate (1.96%), methanol (11.2%), and water (8.3%). The fractions were then transferred to a vial and refrigerated until needed. The methanol used was analytical grade and it was purchased from their respective vendors. The ethyl acetate and methanol used were analytical grade and they were purchased from their respective vendors.
Grouping and Dosing of Experimental Animals
Five groups of mice (each with six) were used for the acetic acid-induced writhing model in the analgesic activity test. The first group served as the negative control and received 10 mL/kg 2% tween 80 (TW). Group II was treated with acetylsalicylic acid (ASA) (150 mg/kg) and served as a positive control. 100 mg/kg, 200 mg/kg, and 400 mg/kg of ME were used to treat Groups III, IV, and V. To study the analgesic effect of the solvent fractions, 11 groups of mice (each with six) were used for the acetic acid-induced writhing model. The first group was treated with the vehicle (10mg/kg 2% tween 80 and served as a negative control). Group II was treated with ASA (150 mg/kg) (served as a positive control) and Group III–XI was treated with different concentrations of solvent fractions [100 mg/kg (100 mg/kg AQF, 100 mg/kg MEF, 100 mg/kg), 200 mg/kg (200 mg/kg AQF, 200 mg/kg MEF, 200 mg/kg EAF), 400 mg/kg (400 mg/kg AQF, 400 mg/kg MEF, 400 mg/kg EAF)]. In the hot plate model, the grouping and dosing were similar to the acetic acid-induced writhing model but instead of ASA, morphine 10mg/kg (MOR) was given for the positive control group.
For the anti-inflammatory activity test, five groups of rats (each with six) were used for the Carrageenan-induced paw edema model. The first group was treated with the vehicle (10mL/kg 2% tween 80 and served as a negative control). Group II was treated with Indomethacin 10 mg/kg (IND) (served as a positive control). Groups III, IV, and V were treated using various doses of ME (100 mg/kg, 200 mg/kg, and 400 mg/kg). To study the anti-inflammatory effect of the solvent fractions; 11 groups of rats (each with six) were used for the Carrageenan-induced paw edema model. The first group was treated with the vehicle (10mg/kg 2% tween 80 and served as a negative control). Group II was treated with Indomethacin (10 mg/kg) (served as a positive control) and Group III–XI were treated with different concentrations of solvent fractions [100 mg/kg (100 mg/kg AQF, 100 mg/kg MEF, 100 mg/kg EAF), 200 mg/kg (200 mg/kg AQF, 200 mg/kg MEF, 200 mg/kg EAF), 400 mg/kg (400 mg/kg AQF, 400 mg/kg MEF, 400 mg/kg EAF)]. In the cotton pellet-induced granuloma model, the grouping and dosing were similar to the Carrageenan-induced paw edema model.
Determination of Analgesic and Anti-Inflammatory Activity
Acetic Acid-Induced Writhing Test
The extract’s peripheral analgesic activity, as well as the solvent fractions, were determined using the method described elsewhere.18 It was carried out by randomly allocating overnight fasted mice to their different groups with free access to water, as indicated in the grouping and dosing section. One hour before acetic acid (0.6% v/ v) (10 mL/kg, i.p) administration, the animals were given different doses of the test substances, while one group received 10mg/kg 2% tween 80 (negative control) and the other group received a standard drug ASA acid 150mg/kg (positive control). Five minutes after the acetic acid injection, the analgesic activity of the extract and solvent fractions was assessed by counting the number of writhing for 30 minutes, which involves a contraction of the abdominal muscle coupled with stretching of the hind limbs. The percentage inhibition of the number of writhes in comparison to the control group was used as an index of analgesia, and it was calculated using the formula below.
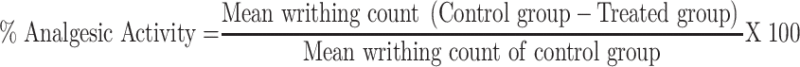 |
Hot Plate Method
Using the approach indicated elsewhere19 the anti-nociceptive potentials of the extract, as well as the solvent fraction, were investigated. The animals were introduced in an open-ended cylindrical space with a metallic plate floor that was heated by a thermode. On a plate heated to a constant temperature (55°C ± 1°C), the behavioral components that were measured in terms of reaction times, in particular paw licking, paw withdrawal, and jumping, were produced. The responses were considered supraspinally-integrated responses. Mice were randomly assigned to their respective groups before administration of the test substances as described in the grouping and dosing section. To avoid lesions to the animals’ paws, each was separately placed on a hot plate with a 15-second cut-off period. The time it took the mice to lick their paws or jump off the hot plate was used to calculate reaction time. At 0 and 30, 60, 90, and 120 minutes, after test substance administration, the reaction times were recorded and the percentage of elongation was calculated.
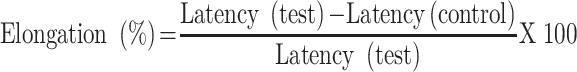 |
Carrageenan-Induced Paw Edema
The effect of the leaf extract and solvent fractions of V. auriculifera on acute inflammation was studied using the method described elsewhere.20 The rats fasted overnight with free access to water until the experiment started. A calibrated plethysmometer was used to determine the basal volume or the amount of water shifted by each rat’s left hind paw. As indicated in the grouping and dosage section, they were randomly assigned to different groups. The rats were then given the test drug through oral gavage. After 1 hour of test substance administration, inflammation in the left hind paw was induced by injecting 0.05 mL of freshly prepared 1% carrageenan suspension in normal saline into the sub-plantar surface of the left hind paw. After 1, 2, 3, and 4 hours of post-induction with carrageenan, the change in volume of the injected paw was measured using a plethysmometer. The criteria for measuring inflammation were a change in paw volume at 1, 2, 3, and 4 hours after carrageenan injection. The average foot swelling of extract and solvent fraction treated rats, as well as the standard, as compared to the negative control, and the percent inhibition of edema was calculated using the formula below.
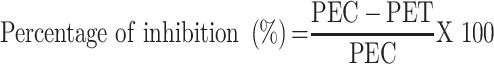 |
PEC= Paw edema of negative control and PET = paw edema of test groups including the standard.
Cotton Pellet-Induced Granuloma
The transudative and proliferative (granulomatous) components of chronic inflammation were assessed using the approach outlined elsewhere.21 Albino male Wistar rats (250–350 g) were fasted overnight and given free access to water until the experiment began. The control, standard, and test groups of rats received 2% tween 80, indomethacin, and the test substances, respectively, as described in the grouping and dosing section. In an autoclave, cotton pellets weighing 10 ± 1 mg were sterilized for 30 minutes at 120 °C & 15Ib pressure. The rats were anesthetized with ketamine hydrochloride (50 mg/kg, i.p.) 20 minutes after receiving the standard drug and test substances, and a subcutaneous tunnel was made aseptically using blunted forceps in both sides of each rat’s previously shaved genital region. For a total of seven days, mice were treated with 2% tween 80, indomethacin, and test substance (orally, once a day). The rats were euthanized on day 8 using high dosages of anesthetic, and the pellets surrounded by granuloma tissue were meticulously cut out and freed of extraneous tissue. The cotton was weighed wet after removal, then dried at 60°C for 24 hours to a constant weight, and the net dry weight, that is, after subtracting the weight of the cotton pellets, was calculated.
Measure of exudate formation = Immediate wet weight of pellet – Constant dry weight of pellet.
Measure of granuloma tissue formation = Constant dry weight – Initial weight of cotton pellet.
The exudate amount (mg), granulation tissue formation (mg), percent inhibition of exudate and granuloma tissue formation were calculated according to the formula described before.
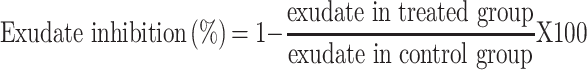 |
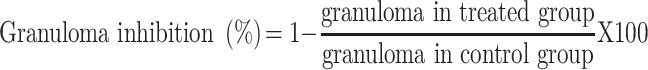 |
Data Analysis
All results of the study are expressed as mean + standard error of mean (SEM). The SPSS version 26 software was used to analyze the data. One-way analysis of variance (ANOVA) was used to analyze differences among groups. Subgroup analysis was done by Tukey’s multiple comparison tests. Statistical significance was defined as a P-value of less than 0.05.
Results
Acetic Acid-Induced Writhing Test
A decrease in writhes served as a measure of peripheral antinociceptive activity. ME (100, 200, and 400 mg/kg) significantly (p < 0.05) decreased the number of writhing animals when compared to the negative control (Table 1). The maximum inhibition was seen with the highest dose (400 mg/kg) of the ME, which displayed an increased inhibition of writhing in a dose-dependent manner. The highest dose has also provided an inhibition that is significantly higher than the lowest dose. Even though the lowest and the middle dose of ME displayed a significantly (p < 0.05) higher inhibition than that of the negative control, they demonstrated a significantly (p < 0.05) lower inhibition than that of the positive control.
Table 1.
Effect of 80% Methanolic Leaf Extracts of V. auriculifera in Acetic Acid-Induced Writhing Test
| Group | No of Writhing ± SEM | % Analgesic Activity |
|---|---|---|
| TW | 61.16 ± 1.53 | – |
| ME 100 mg/kg | 36.66 ± 1.68a | 40.05 |
| ME 200 mg/kg | 32.16 ± 1.13a | 47.41 |
| ME 400 mg/kg | 27.16 ± 0.79ab | 55.59 |
| ASA | 23.66 ± 1.05abc | 61.31 |
Notes: aAgainst negative control, bagainst ME 100mg/kg, cagainst ME 200mg/kg; TW, 2% Tween 80 10mL/kg; ME, 80% methanol extract; ASA, Acetylsalicylic acid 150mg/kg; p < 0.05.
While studying the peripheral antinociceptive effect of the fractions using the acetic acid-induced writhing test, it was observed that animals treated with solvent fractions of V. auriculifera exhibited a significantly (p < 0.05) lower acetic acid-induced writhing than the negative control group (Table 2). Furthermore, MEF’s lowest and highest doses had a significantly higher rate of inhibition than EAF’s lowest and highest doses, respectively. Moreover, even though all tested groups at all tested doses demonstrated a significantly higher inhibition than the negative control, apart from the highest dose of MEF all demonstrated a significantly lower inhibition than that of the positive control group.
Table 2.
Effect of Solvent Fractions of V. auriculifera in Acetic Acid-Induced Writhing Test
| Group | No of Writhing ±SEM | % Analgesic Activity |
|---|---|---|
| TW | 61.66 ± 1.42 | – |
| AQF100 mg/kg | 40.83 ± 1.30a | 33.78 |
| AQF200 mg/kg | 34.66 ± 0.98a | 43.78 |
| AQF400 mg/kg | 30.16 ± 1.07a | 51.08 |
| EAF100 mg/kg | 44.33 ± 0.42a | 28.1 |
| EAF200 mg/kg | 37.33 ± 1.28a | 39.45 |
| EAF400 mg/kg | 34.50 ± 1.56a | 44.04 |
| MEF100 mg/kg | 37.16 ± 1.42ac | 39.73 |
| MEF200 mg/kg | 32.50 ± 0.92a | 47.29 |
| MEF400 mg/kg | 27.00 ± 0.93ad | 56.21 |
| ASA | 24.16 ± 1.04abdef | 60.81 |
Notes: aAgainst negative control, bagainst AQF 400 mg/kg, cagainst EAF 100 mg/kg, dagainst EAF 400 mg/kg, eagainst MEF 100 mg/kg, fagainst MEF 200 mg/kg; TW, 2% Tween 80 10mL/kg; ASA, Acetylsalicylic acid 150mg/kg; p < 0.05.
Abbreviations: AQF, aqueous fraction; EAF, ethyl acetate fraction; MEF, methanol fraction.
Among the test groups, the highest percentage of analgesic activity was displayed by the highest dose of MEF at 56.21% followed by the highest dose of AQF at 51.08%. The highest dose of EAF demonstrated a percentage of analgesic activity comparable to the middle dose of MEF & AQF at 44.04%. Nevertheless, there was no significant difference between the highest doses of AQF & highest dose of EAF. Thus far, in comparison to ASA, apart from the highest dose of MEF all tested groups at all tested doses demonstrated a significantly lower inhibition than that of the positive control group.
Hot Plate Method
To assess the central antinociceptive activity of the extract, the hot plate method was used. At all-time intervals, all doses of the extract produced a central analgesic effect by increasing the latency time when compared to the negative control (Table 3). Furthermore, the lowest dose increased latency time significantly lower than the highest dose (p < 0.05) at all time intervals. It was also seen that the latency time exhibited by the lowest dose at the 60th, 90th, and 120th min was significantly lower than the middle dose (p < 0.05). In addition to that, the highest dose increased latency time significantly (p < 0.05) more than the middle and lowest doses throughout the observation period. Nevertheless, all doses of the extract had a significantly shorter (p < 0.05) latency period than the standard drug. In all doses of the extract, the maximum percent of latency was recorded at 30 minutes, and there seemed to be a decline in effect with time in all doses.
Table 3.
Analgesic Effect of 80% Methanolic Leaf Extracts of V. auriculifera in Hot Plate Test
| Mean Latency Time (sec) ± S.E.M (Percentage of Elongation) | |||||
|---|---|---|---|---|---|
| Group | 0 min | 30 min | 60 min | 90 min | 120 min |
| TW | 3.34 ± 0.12 | 3.64 ± 0.14 | 3.55 ± 0.07 | 3.42 ± 0.04 | 3.19 ± 0.05 |
| ME 100mg/kg | 3.39 ± 0.09 | 5.50 ± 0.46a (33.81) | 4.98 ± 0.03a (28.71) | 4.73 ± 0.03a (27.69) | 4.64 ± 0.12a (31.25) |
| ME 200mg/kg | 3.65 ± 0.25 | 6.41 ± 0.33a (43.21) | 6.26 ± 0.09ab (43.29) | 5.37 ± 0.17ab (36.31) | 5.18 ± 0.10ab (38.41) |
| ME 400mg/kg | 3.71 ± 0.11 | 8.01 ± 0.15abc (54.55) | 7.30 ± 0.18abc (51.36) | 6.61 ± 0.09abc (48.26) | 5.98 ± 0.08abc (46.65) |
| MOR | 3.64 ± 0.17 | 14.16 ± 0.25abcd (72.25) | 13.04 ± 0.22abcd (72.77) | 12.01 ± 0.05abcd (71.52) | 9.89 ± 0.16abcd (67.74) |
Notes: aAgainst TW, bagainst ME 100mg/kg, cagainst ME 200mg/kg, dagainst ME 400mg/kg; TW, 2% Tween 80 10mL/kg; ME, 80% methanol extract; MOR, morphine 10mg/kg; p < 0.05.
At all-time intervals, all doses of the fraction produced a central analgesic effect by increasing the latency time compared to the negative control (Table 4). Moreover, at all-time intervals, the lowest dose of all fractions showed a significantly smaller increase in latency time than the highest dose (p < 0.05). In addition, at the 60th, 90th, and 120th minutes, the highest doses of the fractions significantly increased the latency time compared to the middle doses (p < 0.05). Nevertheless, all doses of the fractions displayed a significantly lower (p < 0.05) latency period than that of the standard drug. In all doses of the fractions, the maximum percent of latency was recorded at 30 minutes, and there seemed to be a decline in effect with time in all doses.
Table 4.
Analgesic Effect of Solvent Fractions of V. auriculifera in Hot Plate Test
| Mean Latency Time (sec) ± S.E.M (Percentage of Elongation) | |||||
|---|---|---|---|---|---|
| Group | 0 min | 30 min | 60 min | 90 min | 120 min |
| TW | 3.37 ± 0.11 | 3.67 ± 0.14 | 3.55 ± 0.09 | 3.46 ± 0.03 | 3.12 ± 0.02 |
| AQF 100 mg/kg | 3.43 ± 0.08 | 6.21 ± 0.34a (40.90) | 4.85 ± 0.07a (26.80) | 4.70 ± 0.05a (26.38) | 4.57 ± 0.11a (31.72) |
| AQF 200 mg/kg | 3.44 ± 0.16 | 7.18 ± 0.26a (48.88) | 6.18 ± 0.10ab (42.55) | 5.36 ± 0.16a (35.44) | 5.08 ± 0.09ab (38.58) |
| AQF 400 mg/kg | 3.45 ± 0.12 | 8.03 ± 0.21ab (54.29) | 7.26 ± 0.16abc (51.10) | 6.49 ± 0.04abc (46.68) | 5.91 ± 0.05abc (47.20) |
| EAF 100 mg/kg | 3.67 ± 0.17 | 6.33 ± 0.46a (42.02) | 4.80 ± 0.06a (26.04) | 4.58 ± 0.07a (24.45) | 4.50 ± 0.10a (30.66) |
| EAF 200 mg/kg | 3.37 ± 0.11 | 7.12 ± 0.33a (48.87) | 6.08 ± 0.09ae (41.61) | 5.26 ± 0.14ae (34.22) | 5.01 ± 0.06ae (37.72) |
| EAF 400 mg/kg | 3.55 ± 0.10 | 8.02 ± 0.24ae (54.23) | 7.19 ± 0.14aef (50.62) | 6.41 ± 0.06aef (46.02) | 5.86 ± 0.05aef (46.78) |
| MEF 100 mg/kg | 3.44 ± 0.09 | 6.12 ± 0.37a (40.03) | 4.98 ± 0.07a (28.71) | 4.76 ± 0.04a (27.31) | 4.69 ± 0.10a (33.47) |
| MEF 200 mg/kg | 3.67 ± 0.11 | 7.69 ± 0.40ah (52.27) | 6.28 ± 0.09ah (43.47) | 5.40 ± 0.15ah (35.92) | 5.14 ± 0.12a (39.29) |
| MEF 400 mg/kg | 3.67 ± 0.12 | 8.14 ± 0.23ah (54.91) | 7.34 ± 0.15ahi (51.63) | 6.58 ± 0.07ahi (47.41) | 5.98 ± 0.13ahi (47.82) |
| MOR | 3.64 ± 0.11 | 14.15± 0.10adgj (74.06) | 13.15 ± 0.13adgj (73.00) | 12.00 ± 0.11adgj (71.16) | 9.91 ± 0.09adgj (68.51) |
Notes: aAgainst negative control, bagainst AQF 100mg/kg, cagainst AQF 200 mg/kg, dagainst AQF 400 mg/kg, eagainst EAF 100 mg/kg, fagainst EAF 200 mg/kg, gagainst EAF 400 mg/kg, hagainst MEF 100 mg/kg, iagainst MEF 200 mg/kg, jagainst MEF 400 mg/kg; TW, 2% Tween 80 10mL/kg; MOR, morphine 10mg/kg; p < 0.05.
Abbreviations: AQF, aqueous fraction; EAF, ethyl acetate fraction; MEF, methanol fraction.
Carrageenan-Induced Paw Edema
All doses of the leaf extract had a statistically significant inhibitory effect (p < 0.05) on the mean increase in paw volume at all time intervals when compared to the negative control group (Table 5). At all-time intervals, the reduction produced by the higher dose was significantly greater than that produced by the middle (p < 0.05) and lower (p < 0.05) doses used in the study. However, all doses of the extracts exhibited a significantly lower (p < 0.05) reduction in mean paw edema compared to the standard drug at the first hour. After the first hour, even though the standard drug displayed better inhibition of the mean paw edema than the highest dose of the plant extract, it did not achieve statistical significance. This might be a result of a variation in the peak plasma concentration time between the standard and ME.
Table 5.
Anti-Inflammatory Effect of 80% Methanolic Leaf Extracts of V. auriculifera on Carrageenan-Induced Paw Edema in Rats
| Change in Paw Volume (mL) (Mean ±S.E.M) (Percent Inhibition %) | |||||
|---|---|---|---|---|---|
| Group | Basal Volume | 1 Hour | 2 Hours | 3 Hours | 4 Hours |
| TW | 0.61 ± 0.01 | 1.13 ± 0.02 | 1.21 ± 0.01 | 1.18 ± 0.02 | 1.16 ± 0.00 |
| ME100 | 0.65 ± 0.01 | 1.04 ± 0.01 (7.9)a | 0.86 ± 0.01 (28.9)a | 0.83 ± 0.01 (29.6)a | 0.76 ± 0.03 (34.4)a |
| ME200 | 0.64 ± 0.00 | 1.02 ± 0.01 (9.7)a | 0.85 ± 0.01 (29.7)a | 0.85 ± 0.02 (27.9)a | 0.69 ± 0.01 (40.5)a |
| ME400 | 0.65 ± 0.01 | 0.94 ± 0.01 (16.8)abc | 0.77 ± 0.00 (36.3)abc | 0.72 ± 0.00 (38.9)abc | 0.60 ± 0.01 (48.2)abc |
| IND | 0.65 ± 0.01 | 0.78 ± 0.01 (30.9)abcd | 0.76 ± 0.01 (37.1)abc | 0.67 ± 0.00 (43.2)abc | 0.56 ± 0.01 (51.7)abc |
Notes: aAgainst TW, bagainst ME 100mg/kg, cagainst ME 200mg/kg, dagainst ME 400mg/kg; TW, 2% Tween 80 10mL/kg; ME, 80% methanol extract; IND, Indomethacin 10mg/kg; p < 0.05.
After the rat’s left hind paw was injected with 0.05 mL of 1% carrageenan in the sub-plantar surface, it resulted in a progressive increase in paw thickness that reached its maximum value after 2 hours post-induction in the negative control group (Table 6). MEF, however, when compared to the negative controls, significantly (p < 0.05) inhibited paw edema at all doses used starting from the first hour. The highest percent inhibition was observed 4 hours after treatment, with values of 38.13, 44.06, and 50% for 100, 200, and 400 mg/kg, respectively. At 1, 2, and 3 hours after treatment, 400 mg/kg of MEF significantly (p < 0.05) inhibited paw edema compared to 100 and 200 mg/kg. Nevertheless, there was no significant difference between the middle and higher doses in the fourth hr., but there was a significant difference between the highest and the lowest dose. Other than the MEF, the AQF and the EAF, in comparison to the negative control, displayed significant inhibition (p < 0.05) starting from the first hr., with a maximum suppression of 8.6%, 10.43%, and 17.39% for 100, 200, and 400 mg/kg AQF, respectively. At 1, 2, and 3 hours after treatment, 400 mg/kg of the AQF significantly (p < 0.05) inhibited paw edema compared to 100 and 200 mg/kg. Nevertheless, there was no significant difference between the middle and higher doses in the fourth hour, but there was a significant difference between the highest and lowest doses. Accordingly, it can be concluded that the fractions, particularly MEF and AQF, significantly and dose-dependently suppressed acute inflammation.
Table 6.
Anti-Inflammatory Effect of the Solvent Fractions of V. auriculifera on Carrageenan-Induced Paw Edema in Rats
| Change in Paw Volume (mL) (Mean ±S.E.M) (Percent Inhibition %) | |||||
|---|---|---|---|---|---|
| Group | Basal Volume | 1 Hour | 2 Hours | 3 Hours | 4 Hours |
| TW | 0.63 ± 0.01 | 1.15 ± 0.01 | 1.23 ± 0.01 | 1.19 ± 0.01 | 1.18 ± 0.00 |
| AQF100 | 0.64 ± 0.01 | 1.05 ± 0.01a (8.6) | 0.88 ± 0.01a (28.45) | 0.86 ± 0.01a (27.73) | 0.78 ± 0.02a (33.89) |
| AQF200 | 0.66 ± 0.01 | 1.03 ± 0.009a (10.43) | 0.87 ± 0.01a (29.26) | 0.86 ± 0.03a (27.73) | 0.70 ± 0.02a (40.67) |
| AQF400 | 0.65 ± 0.01 | 0.95 ± 0.01abc (17.39) | 0.79 ± 0.007abc (35.77) | 0.75 ± 0.01abc (36.97) | 0.62 ± 0.02ab (47.45) |
| EAF100 | 0.63 ± 0.01 | 1.07 ± 0.01a (6.9) | 0.91 ± 0.01a (26.01) | 0.87 ± 0.01a (26.89) | 0.81 ± 0.02a (31.85) |
| EAF200 | 0.65 ± 0.01 | 1.04 ± 0.009a (9.5) | 0.90 ± 0.01a (26.82) | 0.83 ± 0.006a (30.25) | 0.72 ± 0.02a (38.98) |
| EAF400 | 0.63 ± 0.01 | 0.96 ± 0.009aef (16.52) | 0.81 ± 0.01aef (34.14) | 0.76 ± 0.02aef (36.13) | 0.64 ± 0.02ae (45.76) |
| MEF100 | 0.65 ± 0.01 | 1.04 ± 0.01a (9.56) | 0.88 ± 0.02a (28.45) | 0.81 ± 0.01a (31.93) | 0.73 ± 0.01a (38.13) |
| MEF200 | 0.64 ± 0.007 | 1.00 ± 0.01a (13.04) | 0.85 ± 0.01a (30.89) | 0.76 ± 0.01a (36.13) | 0.66 ± 0.01a (44.06) |
| MEF400 | 0.65 ± 0.01 | 0.91 ± 0.009ahi (20.86) | 0.75 ± 0.01ahi (38.21) | 0.70 ± 0.00ahi (41.17) | 0.59 ± 0.01ah (50) |
| IND | 0.65 ± 0.01 | 0.78 ± 0.009adgj (32.17) | 0.74 ± 0.02acfi (39.83) | 0.68 ± 0.009acfi (42.85) | 0.56 ± 0.01abeh (52.54) |
Notes: aAgainst negative control, bagainst AQF 100mg/kg, cagainst AQF 200 mg/kg, dagainst AQF 400 mg/kg, eagainst EAF 100 mg/kg, fagainst EAF 200 mg/kg, gagainst EAF 400 mg/kg, hagainst MEF 100 mg/kg, iagainst MEF 200 mg/kg, jagainst MEF 400 mg/kg; TW, 2% Tween 80 10mL/kg; IND, Indomethacin 10mg/kg; p < 0.05.
Abbreviations: AQF, aqueous fraction; EAF, ethyl acetate fraction; MEF, methanol fraction.
Cotton Pellet-Induced Granuloma
As compared to 2% tween 80, ME significantly reduced the formation of granuloma mass at all doses (Table 7). At the middle and highest doses, it also significantly reduced the production of inflammatory exudate. Moreover, the highest dose significantly inhibited the development of inflammatory exudate and granuloma mass compared to the middle and lowest doses (p < 0.05).
Table 7.
Anti-Inflammatory Effect of 80% Methanolic Leaf Extracts of V. auriculifera on Cotton Pellet Induced Granuloma in Rats
| Group | Exudate (Mean ± SEM) | % Inhibition of Exudation | Granuloma (Mean ± SEM) | % Inhibition of Granulation |
|---|---|---|---|---|
| TW | 143.60 ± 1.64 | – | 151.32 ± 1.52 | – |
| ME100 | 139.14 ± 2.41 | 3.1 | 124.59 ± 3.28a | 17.66 |
| ME200 | 119.81 ± 2.25ab | 16.56 | 102.46 ± 2.32ab | 32.28 |
| ME400 | 103.56 ± 2.15abc | 27.88 | 89.88 ± 1.16abc | 40.60 |
| IND | 75.58 ± 2.40abcd | 47.36 | 71.47 ± 1.27abcd | 52.76 |
Notes: aAgainst TW, bagainst ME 100mg/kg, cagainst ME 200mg/kg, dagainst ME 400mg/kg; TW, 2% tween 80 10mL/kg; ME, 80% methanolic extract; IND, Indomethacin 10mg/kg; p < 0.05.
As shown in Table 8, AQF and MEF significantly (p < 0.05) inhibited the development of inflammatory exudate and granuloma mass at all tested doses when compared to 2% tween 80. Even though all tested doses significantly inhibited granuloma mass formation, only the middle and highest doses of the EAF significantly inhibited the formation of inflammatory exudate when compared to 2% tween 80 (p < 0.05). In terms of inhibition of granuloma mass formation, all doses of the three fractions exhibited statistically significant inhibition when compared to 2% tween 80 (P < 0.05). Despite failing to reach statistical significance, MEF was the fraction that was most effective at preventing the formation of exudate and granuloma mass.
Table 8.
Anti-Inflammatory Effect of the Solvent Fractions of V. auriculifera on Cotton Pellet Induced Granuloma in Rats
| Group | Exudate (Mean ± SEM) | % Inhibition of Exudation | Granuloma (Mean ± SEM) | % Inhibition of Granulation |
|---|---|---|---|---|
| TW | 151.56 ± 1.83 | – | 155.18 ± 1.40 | – |
| AQF100 | 141.85 ± 1.34a | 2.44 | 136.95 ± 2.45a | 11.74 |
| AQF200 | 120.87 ± 3.22ab | 20.24 | 105.28 ± 2.25ab | 32.15 |
| AQF400 | 105.66 ± 1.26ac | 30.28 | 92.34 ± 1.63abc | 40.49 |
| EAF100 | 149.32 ± 1.22 | 1.47 | 138.85 ± 1.49a | 10.52 |
| EAF200 | 123.35 ± 1.94ae | 18.61 | 107.12 ± 1.91ae | 30.97 |
| EAF400 | 106.97 ± 1.30aef | 29.42 | 95.12 ± 1.50aef | 38.70 |
| MEF100 | 138.20 ± 5.98a | 8.81 | 123.52 ± 3.95abe | 20.40 |
| MEF200 | 115.32 ± 2.18ah | 23.91 | 99.59 ± 3.13ah | 35.82 |
| MEF400 | 101.92 ± 1.71ahi | 32.75 | 90.25 ± 1.49ah | 41.84 |
| IND | 76.11 ± 2.00adgj | 49.78 | 72.17 ± 1.35adgj | 53.49 |
Notes: aAgainst negative control, bagainst AQF 100mg/kg, cagainst AQF 200 mg/kg, dagainst AQF 400 mg/kg, eagainst EAF 100 mg/kg, fagainst EAF 200 mg/kg, gagainst EAF 400 mg/kg, hagainst MEF 100 mg/kg, iagainst MEF 200 mg/kg, jagainst MEF 400 mg/kg; TW, 2% Tween 80 10mL/kg; IND, Indomethacin 10mg/kg; p < 0.05.
Abbreviations: AQF, aqueous fraction; EAF, ethyl acetate fraction; MEF, methanol fraction.
Discussion
The current study attempted to assess the analgesic and anti-inflammatory effects of 80% methanolic extract as well as solvent fractions of the leaves of V. auriculifera. In the current study, ME & MEF have demonstrated better analgesic and anti-inflammatory effects as compared to the negative control groups.
An acetic acid-induced writhing model was used to assess the peripheral analgesic activity of 80% methanolic extract and solvent fractions of V. auriculifera. The intraperitoneal injection of acetic acid results in peritoneal inflammation characterized by writhing and an increase in prostaglandin E2 and prostaglandin F2a levels in the peritoneal fluid.22 The abdominal writhing responses following acetic acid injection were significantly reduced by the oral administration of all test dosages of 80% methanol leaf extract and solvent fractions. These results suggest that the 80% methanolic extract and solvent fractions of V. auriculifera inhibit lipoxygenase and/or cyclooxygenase enzymes in peripheral tissues, thereby interfering with the mechanism of transduction in the primary afferent nociceptor. The hot-plate test demonstrated that the solvent fractions and 80% methanolic extract of V. auriculifera increased the nociceptive threshold significantly in a manner that was quantified by increased latencies. Animals given the solvent fractions of V. auriculifera and the 80% methanolic extract had a longer latency time than those in the control group during the hot-plate test. As such, it can be concluded that the 80% methanolic extract and solvent fractions of V. auriculifera are endowed with a central analgesic effect based on the lengthened mice response times in the hot-plate test.
Carrageenan-induced edema is a well-studied biphasic experimental animal model for acute inflammation. The early phase (1–2 hours) of the carrageenan model is primarily mediated by histamine, serotonin, and increased prostaglandin synthesis in the injured tissue environment.23 The late phase is maintained by prostaglandin release, which is mediated by bradykinin, leukotrienes, polymorphonuclear cells, and prostaglandins produced by tissue macrophages.24 80% methanol leaf extract and solvent fractions of V. auriculifera were found to have anti-inflammatory activity in a carrageenan-induced paw edema model in rats. All test dosages of 80% methanol leaf extract and solvent fractions demonstrated significant (p < 0.05) anti-inflammatory activity at all periods; nevertheless, the maximum percentage edema inhibition was found with 400mg/kg MEF (50%) at 4 hours post-induction. As with the effects of nonsteroidal anti-inflammatory drugs like indomethacin, the observed edema inhibition was highest in the later stages of inflammation, which suggests that the anti-inflammatory activity is mediated through the inhibition of the cyclooxygenase enzyme. Prostaglandin synthesis is known to be inhibited by flavonoids.25 As such, the polyphenols that have been reported in the leaves of V. auriculifera might be responsible for the demonstrated anti-inflammatory and analgesic effects.14,26 In the cotton pellet-induced granuloma model, ME, MEF, and AQF inhibited both exudate and granuloma formation at all investigated concentrations. Even though all tested doses significantly prevented granuloma mass formation, only the middle and highest doses of the EAF significantly inhibited the generation of inflammatory exudate when compared to 2% tween 80 (p < 0.05). The results of the carrageenan-induced acute inflammation model are backed by the significant (p < 0.05) inhibitory effect of V. auriculifera on exudate formation, demonstrating that both outcomes support the efficiency of the 80% methanolic extract and solvent fractions of V. auriculifera in inhibiting both the exudative and proliferative components of inflammation. Experimental studies on β-sitosterol demonstrated various activities like immunomodulatory, antioxidant, antimicrobial, angiogenic, anti-inflammatory, antifertility, anticancer, antinociceptive, and antidiabetic with no overt toxicity.27–29 And β-sitosterol has been reported in V. auriculifera.30 As such, it might be responsible for the demonstrated analgesic and anti-inflammatory activities of the leaf extract and solvent fractions.
Reactive oxygen species (ROS) are by-products of stress stimuli (biotic and abiotic) and intrinsic oxygen metabolism. By inactivating enzymes and damaging essential cellular organelles and membranes, their generation causes cancer, aging, and chronic inflammation, and contributes to diabetes, and other diseases. They are also known to cause inflammation and pain associated with tissue injury.31 Because they donate hydrogen to free radicals, phenolic compounds and flavonoids are antioxidant substances that act as reducing agents and free-radical scavengers.32 The hydroxyl group in flavonoids appears to have high reactivity, as a result, they tend to stabilize reactive oxygen species by interacting with the reactive component of the radical.33 The leaves of V. auriculifera are also known to contain flavonoids and phenols.14,26 As a result, the demonstrated analgesic and anti-inflammatory activity of the extract could be attributed to the presence and antioxidant action of phenolic compounds and flavonoids.
A wide variety of biological activities, including antibacterial, antifungal, antiviral, anti-inflammatory, and antioxidant ones, have been linked to flavonoids, which are extensively prevalent in plants.32,34,35 Quercetin, which is a flavonoid, inhibits the activities of both cyclooxygenase and lipoxygenase, reducing the generation of inflammatory metabolites.36 The ability of flavonoids to prevent neutrophil degranulation, which is a direct method for neutrophils and other immune cells to produce less arachidonic acid, is thought to be another anti-inflammatory property of flavonoids.33 Thus, the extracts’ anti-inflammatory activity could be due to flavonoids or other secondary metabolites such as saponins37 or tannins38 which have been reported in the leaves of V. auriculifera elsewhere.26,30
Conclusion
The finding of this study revealed 80% methanol extract as well as the solvent fractions of V. auriculifera have analgesic, and anti-inflammatory activities compared with negative control which validates the folkloric uses. Moreover, the findings of this study not only verify the plants’ traditional claim but also provides clues for further investigation of the active principles of this plant for the development of effective and safe analgesic and anti-inflammatory drugs. The findings of this study should be taken as a basis for further investigation of the analgesic and anti-inflammatory action of V. auriculifera. Henceforth, further studies should be conducted on the identification of the exact mechanism(s) of action of analgesic and anti-inflammatory activity of the 80% methanolic extract as well as the solvent fractions should be carried out. Isolation and identification of pharmacologically active compounds from the active fractions should also be done.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Lalan BK, Hiray R, Ghongane B. Evaluation of analgesic and anti-inflammatory activity of extract of Holoptelea integrifolia and Argyreia speciosa in animal models. J Clin Diagnostic Res. 2015;9(7):FF01. doi: 10.7860/JCDR/2015/12059.6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters ML. Emotional and cognitive influences on pain experience. Pain Psychiatric Disorders. 2015;30:138–152. [DOI] [PubMed] [Google Scholar]
- 3.Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–444. doi: 10.1080/00325481.2019.1663705 [DOI] [PubMed] [Google Scholar]
- 4.Agnihotri S, Wakode S, Agnihotri A. An overview on anti-inflammatory properties and chemo-profiles of plants used in traditional medicine. Indian J Natural Products Resources. 2010;1:150. [Google Scholar]
- 5.Achar KC, Hosamani KM, Seetharamareddy HR. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur J Med Chem. 2010;45(5):2048–2054. doi: 10.1016/j.ejmech.2010.01.029 [DOI] [PubMed] [Google Scholar]
- 6.Lande AA, Ambavade SD, Swami US, Adkar PP, Ambavade PD, Waghamare AB. Saponins isolated from roots of Chlorophytum borivilianum reduce acute and chronic inflammation and histone deacetylase. J Integr Med. 2015;13(1):25–33. doi: 10.1016/S2095-4964(15)60157-1 [DOI] [PubMed] [Google Scholar]
- 7.Pahwa R, Goyal A, Jialal I. Chronic Inflammation. StatPearls [Internet]; 2021. [PubMed] [Google Scholar]
- 8.Deng J-S, Chi C-S, Huang -S-S, Shie P-H, Lin T-H, Huang G-J. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J Ethnopharmacol. 2011;137(3):1161–1171. doi: 10.1016/j.jep.2011.07.041 [DOI] [PubMed] [Google Scholar]
- 9.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflammation Res. 2009;58(9):537–552. doi: 10.1007/s00011-009-0037-3 [DOI] [PubMed] [Google Scholar]
- 10.Keeley SC, Jones SB. Distribution of pollen types in Vernonia (Vernonieae: compositae). Systematic Botany. 1979;4(3):195–202. doi: 10.2307/2418418 [DOI] [Google Scholar]
- 11.Toyang NJ, Verpoorte R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J Ethnopharmacol. 2013;146(3):681–723. doi: 10.1016/j.jep.2013.01.040 [DOI] [PubMed] [Google Scholar]
- 12.Wado TE, Suleman S, Mohammed T. Antimicrobial Evaluation of Sequentially extracted Leaf of Vernonia auriculifera Hiern (Rejicho). BMC Complementary Med Therapies. 2022;22(1):1–10. doi: 10.1186/s12906-022-03690-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobrinho ACN, de Souza EB. A review on antimicrobial potential of species of the genus Vernonia (Asteraceae). J Med Plants Res. 2015;9(31):838–850. doi: 10.5897/JMPR2015.5868 [DOI] [Google Scholar]
- 14.Albejo B, Endale M, Kibret B, Anza M. Phytochemical investigation and antimicrobial activity of leaves extract of Vernonia auriculifera Hiern. J Pharm Pharmacognosy Res. 2015;3(6):141–147. [Google Scholar]
- 15.Alam B, Akter F, Parvin N, et al. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna j Phytomed. 2013;3(2):112. [PMC free article] [PubMed] [Google Scholar]
- 16.Ferraz CR, Carvalho TT, Manchope MF, et al. Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules. 2020;25(3):762. doi: 10.3390/molecules25030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NRCoN A. Guide for the Care and Use of Laboratory Animals Eighth Edition. Washington: The; 2011. [Google Scholar]
- 18.Koster R. Acetic Acid for Analgesic Screening. InFed Proc. 1959;18:412. [Google Scholar]
- 19.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J Pharmacol Exp Therapeutics. 1953;107(3):385–393. [PubMed] [Google Scholar]
- 20.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proce Soc Exp Biol Med. 1962;111(3):544–547. doi: 10.3181/00379727-111-27849 [DOI] [PubMed] [Google Scholar]
- 21.Winter CA, Porter CC. Effect of alterations in side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J Am Pharmaceutical Assoc. 1957;46(9):515–519. doi: 10.1002/jps.3030460902 [DOI] [PubMed] [Google Scholar]
- 22.Tatiya AU, Saluja AK, Kalaskar MG, Surana SJ, Patil PH. Evaluation of analgesic and anti-inflammatory activity of Bridelia retusa (Spreng) bark. J Traditional Complementary Med. 2017;7(4):441–451. doi: 10.1016/j.jtcme.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansouri MT, Hemmati AA, Naghizadeh B, Mard SA, Rezaie A, Ghorbanzadeh B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J Pharmacol. 2015;47(3):292. doi: 10.4103/0253-7613.157127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laavola M, Leppänen T, Eräsalo H, Hämäläinen M, Nieminen R, Moilanen E. Anti-inflammatory effects of nortrachelogenin in murine J774 macrophages and in carrageenan-induced paw edema model in the mouse. Planta Med. 2017;234(06):519–526. [DOI] [PubMed] [Google Scholar]
- 25.Md Idris MH, Mohd Amin SN, Mohd Amin SN, et al. Flavonoids as dual inhibitors of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX): molecular docking and in vitro studies. Beni-Suef Univ J Basic Appl Sci. 2022;11(1):1–9. doi: 10.1186/s43088-022-00296-y [DOI] [Google Scholar]
- 26.Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of Wound Healing Activity of Methanolic Crude Extract and Solvent Fractions of the Leaves of Vernonia auriculifera Hiern (Asteraceae) in Mice. J Exp Pharmacol. 2021;13:677. doi: 10.2147/JEP.S308303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nirmal SA, Pal SC, Mandal SC, Patil AN. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology. 2012;20(4):219–224. doi: 10.1007/s10787-011-0110-8 [DOI] [PubMed] [Google Scholar]
- 28.Ambavade SD, Misar AV, Ambavade PD. Pharmacological, nutritional, and analytical aspects of β-sitosterol: a review. Orient Pharm Exp Med. 2014;14(3):193–211. doi: 10.1007/s13596-014-0151-9 [DOI] [Google Scholar]
- 29.Villaseñor IM, Angelada J, Canlas AP, Echegoyen D. Bioactivity studies on β‐sitosterol and its glucoside. Phytotherapy Res. 2002;16(5):417–421. doi: 10.1002/ptr.910 [DOI] [PubMed] [Google Scholar]
- 30.Joyce JK, Neil AK, Hafizah C. Triterpenoids from Vernonia auriculifera Hiern exhibit antimicrobial activity. Af J Pharmacy Pharmacol. 2011;5(8):1150–1156. [Google Scholar]
- 31.Kayani WK, Dilshad E, Ahmed T, Ismail H, Mirza B. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complement Altern Med. 2016;16(1):1–13. doi: 10.1186/s12906-016-1363-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira RN, Mancini MC, Oliveira F, et al. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria. 2016;21:767–779. doi: 10.1590/S1517-707620160003.0072 [DOI] [Google Scholar]
- 33.Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418–425. doi: 10.1093/ajcn/74.4.418 [DOI] [PubMed] [Google Scholar]
- 34.Garelnabi EA, Mohammed MS, Osman WJ, et al. Secondary metabolites as anti-inflammatory agents. J Phytopharmacol. 2014;3(4):275. [Google Scholar]
- 35.Govindappa M, Poojashri M. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. J Pharmacognosy Phytother. 2011;3(3):43–51. [Google Scholar]
- 36.Yang D, Wang T, Long M, Li P. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. 2020;2020. doi: 10.1155/2020/8825387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar KP, Naik VS, Chandra VB, Lavanya R, Narendra K. Evaluation of in vitro and in vivo anti-inflammatory activity of aqueous extract of Gliricidia sepium flowers in rats. Int J Pharmacognosy Phytochem Res. 2014;6:477–481. [Google Scholar]
- 38.Muthu S, Durairaj B. Evaluation of antioxidant and free radical scavenging activity of Annona muricata. Eur J Exp Biol. 2015;5(3):39–45. [Google Scholar]


