Abstract
Philadelphia (Ph)-like acute lymphoblastic leukemia (ALL) constitutes a heterogeneous subset of ALL with a uniformly unfavorable prognosis. The identification of mutations amenable to treatment with tyrosine kinase-inhibitors (TKIs) represents a promising field of investigation. We report the case of a young patient affected by relapsed/refractory Ph-like ALL treated with chimeric antigen receptor T (CAR-T) cells after successful bridging with compassionate-use ponatinib and low-dose prednisone. We restarted low-dose ponatinib maintenance three months later. Twenty months later, measurable residual disease negativity and B-cell aplasia persist. To the best of our knowledge, this is the first case reporting the use of ponatinib in Ph-like ALL as a bridge to and maintenance after CAR-T cell therapy.
Keywords: ponatinib, CAR-T, Philadelphia-like ALL, bridge therapy, maintenance therapy
Introduction
Ph-like ALL, a subtype of B-cell ALL with adverse clinical features and unfavorable prognosis (1, 2), represents up to 15% of childhood ALL and 15-25% of adolescent and young adult ALL (3). They exhibit higher rates of measurable residual disease (MRD) persistence, higher rates of relapse, even in case of a MRD clearance, and a shorter survival compared to patients with non-Ph-like ALL (4). Patients with Ph-like ALL are often young males with hyperleukocytosis and a normal karyotype (5).
The pathophysiology of Ph-like ALL accounts for a plethora of kinase-activating mutations, affecting in up to 90% of cases either the tyrosine kinase super-family (ABL1, ABL2, CSF1R and PDGFRB) or the cytokine receptor pathway (JAK1, JAK2, IL7-R, CRLF2, EPOR), but lacking a classical BCR::ABL1 rearrangement. Ph-like and Ph-positive ALL have a partly overlapping gene expression profile and are both often associated with deletions or mutations of the transcription factor IKZF1 (6, 7).
The identification of actionable lesions in Ph-like ALL has paved the way towards targeted therapies (8). The efficacy of TKIs in Ph-like ALL has already been established (9, 10). In addition, other small molecule inhibitors, such as ruxolitinib, sirolimus and gedatolisib, have shown promising results in pre-clinical models of JAK2-mutated subtypes and are under evaluation (11). Moreover, the introduction of immunotherapy and CAR-T cells in the clinical practice may represent a valuable option to impact on the negative prognosis harboured by the Ph-like signature. As the treatment paradigm in ALL is undergoing a major shift, new efforts are warranted to define the proper place for each drug within the therapeutic algorithm for different subgroups of patients.
Case presentation
We report the case of a 19-year-old female diagnosed with Ph-negative B-cell ALL in February 2019, who presented with hyperleukocytosis (WBC count 317 x 109/L). Flow cytometry on a bone marrow (BM) aspirate showed that 93% of cells were positive for CD45, CD10, CD19 and CD22, and had an aberrant expression of CD33. FISH performed using the Cytocel probe detected a deletion at 6q21/SEC63 in 43.5% of the analysed nuclei. The BCR::ABL1-like predictor was positive and showed a CRLF2 upregulation and an IKZF1 deletion (7). Molecular-cytogenetic analysis, performed using the ZytoLight® SPEC CRLF2 Dual Color Break Apart probe, and the LSI IGH Dual Color, Break Apart Rearrangement Probe (Vysis-Abbott) showed hybridization patterns consistent with the presence of an IGH : CRLF2 rearrangement. Targeted RNA sequencing detected no mutations or rearrangements.
The patient was enrolled in the chemo-immunotherapy GIMEMA LAL2317 protocol, which exploits a risk-oriented strategy based on disease characteristics and MRD evaluation at fixed time-points, intercalating a maximum of two cycles of blinatumomab into a pediatric-like chemotherapy backbone (clinicaltrial.gov NCT03367299).
Our patient, classified as very high risk, underwent three cycles of chemotherapy, obtaining a complete morphologic remission (CR) after induction. Central nervous system (CNS) prophylaxis was carried out as per protocol. MRD assessment by RQ-PCR Ig gene rearrangement remained strongly positive (>10-2) after all three chemotherapy cycles. After a single cycle of blinatumomab which induced the molecular remission (<10-5), the patient underwent an allogeneic hematopoietic stem cell transplant (HSCT) from an HLA-identical sister. Conditioning consisted of treosulfan, fludarabine and TBI 4 Gy, and the graft versus host disease (GvHD) prophylaxis included post-transplantation cyclophosphamide and sirolimus (12). A post-transplant aspirate documented a CR with full donor chimerism, FISH and a molecular MRD negativity. Sirolimus was discontinued six months later. The patient never developed GvHD.
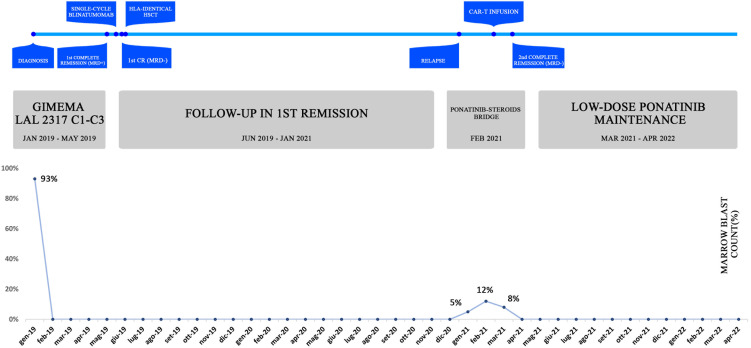
In January 2021, 18 months after the HSCT, a BM evaluation detected a relapse (5% blasts). The patient had also a palpable mass in her right breast, whose histology was compatible with an ALL localization. No CNS disease was detected.
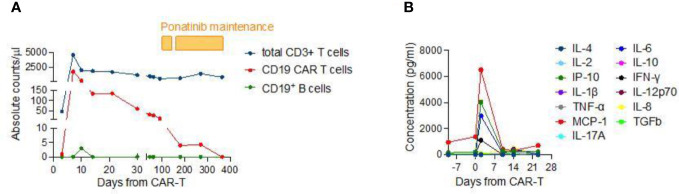
The patient was deemed fit for anti-CD19 CAR-T cell therapy with tisagenlecleucel. After lymphapheresis in early February, we started a bridging treatment with ponatinib 45 mg daily for 30 days on compassionate use and 1 mg/kg prednisone for 14 days. No cardiac, hepatic or hematologic toxicity was reported. In mid-February, we repeated a BM aspirate that confirmed a morphologic relapse (12% blasts). A third aspirate after a month of ponatinib showed a stable disease (8% blasts) and on physical examination a reduction of the palpable breast nodule was documented. We withdrew bridging drugs and started lymphodepletion with fludarabine-cyclophosphamide, followed by a CAR-T cell infusion on March 2021. The patient developed a grade 4 neutropenia and received three doses of tocilizumab for grade 1 cytokine release syndrome (CRS). No neurotoxicity occurred. Three months later the patient was in CR with a full donor chimerism and a MRD negativity. A breast ultrasound revealed a regression of her nodule. In June 2021, maintenance with ponatinib at a lower dose (15 mg/day) was initiated. In November 2022, 20 months after the CAR-T cell infusion the patient is in good clinical conditions and in persistent molecular CR. She still receives ponatinib maintenance with excellent tolerance, except for a 10-day discontinuation due to a transient G4 neutropenia. Figure 1 summarizes the case timeline. We longitudinally monitored the patient’s CAR-T cell expansion and their subsequent persistence by flow cytometry and plotted the data over time in Figure 2A . After a marked expansion peak at day 7 after infusion (1819.6/mcl), CAR-T cell counts decreased, though persisting over time. At late time-points (day 180 and 270), the patient still had circulating anti-CD19 CAR-T cells (4.0 and 4.3/mcl, respectively). At the last available follow-up (365 days, April 2022), circulating CAR-T cells were no longer detectable. However, a concomitant B-cell aplasia is ongoing and we are monitoring it as a decisional tool for future treatment. Peak levels of inflammatory cytokines and chemokines occurred within the first week after the CAR T-cell infusion, with particularly high levels of monocyte chemoattractant protein-1 (MCP-1), interferon γ-induced protein 10 kDa (IP-10), interleukin-6 (IL-6) and interferon-γ (IFN- γ, Figure 2B ).
Figure 1.
Case report timeline. The table offers an overview of the patient’s history, treatment lines (A) and corresponding marrow blast count (B). The percentage of bone marrow blasts was evaluated at significant time-points, namely at diagnosis, post-induction, post-transplant, at disease relapse, during bridging and after CAR-T infusion. At the last follow-up, the patient is still in molecular remission and in persistent B-cell aplasia.
Figure 2.
(A) Longitudinal evaluation of total CD3+ T cells, anti-CD19 CAR-T cells, CD19+ B cells and released cytokines in the patient’s peripheral blood. Absolute counts were evaluated by flow cytometry at several time-points for up to 1 year after CAR-T cell infusion. The pharmacokinetics shows a remarkable CAR-T cell expansion in the first week following infusion (coinciding with CRS onset and resolution), with engineered cells representing nearly 40% of overall T cells, and a subsequent drop in CAR-T cells over time. At day 270, the patient still has a subset of CAR-T cells accounting for around 1% of the total T-cell count. At the time of last follow-up, circulating CAR-T cells had decreased below the detection limit of the assay but B-cell aplasia persists. (B) Evaluation of serum cytokines/chemokines concentrations in the first three weeks after CAR-T cell infusion. The analysis shows a significant peak occurring within the first week after treatment.
Discussion
The present case illustrates how ponatinib might represent a valid therapeutic option to be explored in Ph-like ALL. Even though TKIs have to date no standardized place, it seems reasonable to incorporate them in treatment schemes given their safety and potential effectiveness. The patient was initially enrolled into a sequential chemo-immunotherapy protocol (clinicaltrial.gov NCT03367299) and obtained a MRD negativity only after a cycle of blinatumomab, suggesting a possible role of this drug in Ph-like patients, whose long-term efficacy is still debatable.
Upon relapse, anti-CD19 CAR-T cells were considered the best salvage option. Considering the fast disease kinetics, the risk of major complications while waiting for the CAR-T cells and the widely accepted notion that a lower disease burden upon lymphodepletion correlates with an improved outcome, it seemed imperative to choose a safe and effective bridging option. Based on the assumption that CRLF2 hyperexpression might be amenable to treatment with ponatinib, our patient received 45 mg ponatinib daily for a month and steroids for two weeks: follow-up BM aspirate before lymphodepletion demonstrated a persistent disease stability in spite of the rapidly progressive nature of Ph-like ALL. Even though it is difficult to discriminate between the role of ponatinib and the role of steroids due to their synergistic effect, the combination proved effective.
Whereas the efficacy of TKIs in ABL1-mutated ALL is demonstrated by a growing number of studies (13), there is still uncertainty on to their role in cases lacking such mutations, the rationale of its efficacy lying in the broad-spectrum of its kinase-inhibiting activity. Interestingly, ponatinib might represent the most promising of all TKIs based on studies highlighting its efficacy regardless of the patient’s mutational status, both in vitro and in vivo (7, 14). Recently, Lunghi et al. (15) reported a patient with relapsed/refractory Ph-like ALL with BCR::JAK2 rearrangement who achieved a CR2 and a first MRD clearance with ponatinib.
Another open issue is how to consolidate and maintain the results obtained with CAR-T cells. Even though HSCT consolidation seems beneficial in specific cases, clear indications are missing. Due to the major toxicities and the poor outcome associated with a second HSCT, we decided to strictly monitor the MRD status and ongoing B-cell aplasia, while pursuing a maintenance therapy with lower-dose ponatinib. Pre-clinical data show that TKIs might affect T-cell receptor signaling. It is already established that dasatinib inhibits Src family kinase activity, potentially affecting the effectiveness of immunotherapies. More recently, it has been reported that dasatinib may also ablate CAR-mediated signaling, by interfering with LCK and inhibiting the phosphorylation of CD3z and ZAP70 (16). This activity can induce a reversible function-off state in CAR-T cells that can be exploited to mitigate CRS (16) and to improve CAR-T cell fitness by preventing exhaustion and promoting the acquisition of a memory-like phenotype (17). However, little is known about ponatinib immunomodulating properties. Small clinical series suggest that coadministration of ponatinib or dasatinib with immunotherapies do not affect their effectiveness and might be beneficial in disease control (18).
To the best of our knowledge, this is one of the first reports of successful treatment of non ABL-mutated Ph-like ALL with ponatinib and the very first report of ponatinib being used as a bridge to and maintenance after CAR-T cell therapy.
The current treatment landscape in ALL is rapidly evolving. Unfortunately, some subsets of ALL are lagging behind and still retain a poor prognosis. Several open issues require settling in Ph-like ALL, such as the role of TKIs, which inhibitor to prefer, the appropriate timing of its introduction, and the outcomes of combination therapies. There is also an urgent need to define a standardized bridging strategy to CAR-T cells and post-CAR-T cell management in ALL. Future studies, preferably prospective and randomized, are warranted in order to re-define the appropriate therapeutic algorithm for patients with Ph-like ALL.
Data availability statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the patient has been treated according to current institutional guidelines, upon written informed consent for all the treatment procedures, the review of medical records and the use of immunological monitoring for patients undergoing allogeneic HSCT within the non-interventional “ALMON study”, approved by San Raffaele Institutional Ethical Committee on 19/10/2007. Bone marrow and peripheral blood samples were collected and stocked at San Raffaele Hospital upon written informed consent for future potential studies within our Institutional observational “biobanking protocol”, approved by San Raffaele Institutional Ethical Committee on 04/05/2006.The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FG conceived the study and wrote the paper; EC wrote the paper and provided all clinical and hematological data; FL, EX, RG, LL, AB, ML-S, MGC, JP, FC provided all clinical and hematological data; MN and CB performed and evaluated CAR-T cell monitoring; RS performed and evaluated cytogenetic analysis; MC performed and evaluated serum cytokines/chemokines concentrations; SC evaluated and performed the BCR/ABL1-like predictor. RLS, SC, RF, FC supervised and wrote the paper. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all medical, nursing and administrative personnel of the Hematology Department of our Institute. We thank Prof. Cristina Mecucci for guidance in the cytogenetic analysis. We thank Elena Tassi, Veronica Valtolina and Valeria Beretta for technical assistance in CAR-T cell monitoring. We thank Deborah Cardinali for technical assistance in evaluation of the BCR/ABL1-like predictor. We thank Laura Falcone for technical assistance in evaluation of serum cytokines/chemokines concentrations.
Funding Statement
This work was partially supported by Associazione Italiana per la Ricerca sul Cancro (AIRC-IG 18458 and AIRC 5 per Mille 22737), the Italian Ministry of Education, University and Research (PRIN 2017WC8499) to CB and the Italian Ministry of Health and Alliance Against Cancer (Ricerca Corrente CAR T project: RCR-2019-23669115). European Union (T2Evolve IMI) to CB and FC. This work was partially supported by Associazione Italiana Ricerca sul Cancro (AIRC), Special 5x1000 Program Metastases (21198), Milan (Italy) to RF.
Conflict of interest
CB is inventor on different patents on cancer immunotherapy and genome editing Use of common g-chain cytokines for the visualization, isolation and genetic modification of memory T lymphocytes, Patent family PCT/IT2006/000600; Targeted disruption of T cell receptor genes using engineered zinc finger protein nucleases, Patent family US N. 12/927,292 and PCT/US2014/031360; WT1-TCRs, Patent family N. PCT/EP2018/060477 and N. PCT/EP2019/079916; Compositions and methods for immunotherapy, PCT/US2019/056399. CB has been a member of Advisory Boards and a Consultant for Intellia Therapeutics, TxCell, Novartis, GSK, Allogene, Kite/Gilead, Miltenyi, Kiadis, Janssen and received research support from Intellia Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol (2009) 10(2):125–34. doi: 10.1016/S1470-2045(08)70339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain N, Roberts KG, Jabbour E, Patel K, Eterovic AK, Chen K, et al. Ph-like acute lymphoblastic leukemia: A high-risk subtype in adults. Blood (2017) 129(5):572–81. doi: 10.1182/blood-2016-07-726588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiaretti S, Messina M, Foà R. BCR/ABL1–like acute lymphoblastic leukemia: How to diagnose and treat? Cancer (2019) 125:194–204. doi: 10.1002/cncr.31848 [DOI] [PubMed] [Google Scholar]
- 4. Chiaretti S, Messina M, Della SI, Piciocchi A, Cafforio L, Cavalli M, et al. Philadelphia-Like acute lymphoblastic leukemia is associated with minimal residual disease persistence and poor outcome. first report of the minimal residual disease-oriented GIMEMA LAL1913. Haematologica (2021) 106(6):1559–68. doi: 10.1182/blood.2019001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen IM, et al. High frequency and poor outcome of Philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol (2017) 35(4):394–401. doi: 10.1200/JCO.2016.69.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mullighan CG, Su X, Zhang J, Radtke I, Phillips LAA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med (2009) 360(5):470–80. doi: 10.1056/nejmoa0808253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiaretti S, Messina M, Grammatico S, Piciocchi A, Fedullo AL, Di Giacomo F, et al. Rapid identification of BCR/ABL1-like acute lymphoblastic leukaemia patients using a predictive statistical model based on quantitative real time-polymerase chain reaction: Clinical, prognostic and therapeutic implications. Br J Haematol (2018) 181(5):642–52. doi: 10.1111/bjh.15251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang Y-L, Pei D, et al. Targetable kinase-activating lesions in ph-like acute lymphoblastic leukemia. N Engl J Med (2014) 371(11):1005–15. doi: 10.1056/NEJMoa1403088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weston BW, Hayden MA, Roberts KG, Bowyer S, Hsu J, Fedoriw G, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1-PDGFRB-positive acute lymphoblastic leukemia. J Clin Oncol (2013) 31(25). doi: 10.1200/JCO.2012.47.6770 [DOI] [PubMed] [Google Scholar]
- 10. Perwein T, Strehl S, König M, Lackner H, Panzer-Grümayer R, Mann G, et al. Imatinib-induced long-term remission in a relapsed RCSD1-ABL1-positive acute lymphoblastic leukemia. Haematologica (2016) 101(8):e332–5. doi: 10.3324/haematol.2015.139568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tasian SK, Teachey DT, Li Y, Shen F, Harvey RC, Chen IM, et al. Potent efficacy of combined PI3K/mTOR and JAK or ABL inhibition in murine xenograft models of ph-like acute lymphoblastic leukemia. Blood (2017) 129(2):177–87. doi: 10.1182/blood-2016-05-707653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greco R, Lorentino F, Albanese S, Lupo Stanghellini MT, Giglio F, Piemontese S, et al. Posttransplantation cyclophosphamide- and sirolimus-based graft-Versus-Host-Disease prophylaxis in allogeneic stem cell transplant. Transplant Cell Ther (2021) 27(9):776. doi: 10.1016/j.jtct.2021.05.023 [DOI] [PubMed] [Google Scholar]
- 13. Tanasi I, Ba I, Sirvent N, Braun T, Cuccuini W, Ballerini P, et al. Blood efficacy of tyrosine kinase inhibitors in ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood (2019) 134(16):1351–5. doi: 10.1182/blood.2019001244 [DOI] [PubMed] [Google Scholar]
- 14. Ansuinelli M, Cesini L, Chiaretti S, Foà R. Emerging tyrosine kinase inhibitors for the treatment of adult acute lymphoblastic leukemia. Expert Opin Emerg Drugs (2021) 26(3):281–94. doi: 10.1080/14728214.2021.1956462 [DOI] [PubMed] [Google Scholar]
- 15. Lunghi M, Patriarca A, Greco M, Taherinasab A, Della Starza I, Cavalli M, et al. Ponatinib for the treatment of ph-like acute lymphoblastic leukemia. Leuk Lymphoma (2021) 62(3):755–7. doi: 10.1080/10428194.2020.1842401 [DOI] [PubMed] [Google Scholar]
- 16. Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Trans Med (2019) 11. doi: 10.1126/scitranslmed.aau5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science (2021) 372. doi: 10.1126/science.aba1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badar T, Szabo A, Advani A, Wadleigh M, Arslan S, Khan MA, et al. Real-world outcomes of adult b-cell acute lymphocytic leukemia patients treated with blinatumomab. Blood Adv (2020) 4(10):2308–16. doi: 10.1182/bloodadvances.2019001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.