Abstract
Computational models of rodent physiology implicate hippocampal theta as a key modulator of learning and memory (Buzsáki and Moser, 2013; Lisman and Jensen, 2013), yet human hippocampal recordings have shown divergent theta correlates of memory formation. Herweg et al. (2020) suggest that decreases in memory-related broadband power mask narrowband theta increases. Their survey also notes that the theta oscillations appear most prominently in contrasts that isolate memory retrieval processes and when aggregating signals across large brain regions. We evaluate these hypotheses by analyzing human hippocampal recordings captured as 162 neurosurgical patients (n = 86 female) performed a free recall task. Using the Irregular-Resampling Auto-Spectral Analysis (IRASA) to separate broad and narrowband components of the field potential, we show that (1) broadband and narrowband components of theta exhibit opposite effects, with broadband signals decreasing and narrowband theta increasing during successful encoding; (2) whereas low-frequency theta oscillations increase before successful recall, higher-frequency theta and alpha oscillations decrease, masking the positive effect of theta when aggregating across the full band; and (3) the effects of theta on memory encoding and retrieval do not differ between reference schemes that accentuate local signals (bipolar) and those that aggregate signals globally (whole-brain average). In line with computational models that ascribe a fundamental role for hippocampal theta in memory, our large-scale study of human hippocampal recordings shows that 3–4 Hz theta oscillations reliably increase during successful memory encoding and before spontaneous recall of previously studied items.
SIGNIFICANCE STATEMENT Analyzing recordings from 162 participants, we resolve a long-standing question regarding the role of hippocampal theta oscillations in the formation and retrieval of episodic memories. We show that broadband spectral changes confound estimates of narrowband theta activity, thereby accounting for inconsistent results in the literature. After accounting for broadband effects, we find that increased theta activity marks successful encoding and retrieval of episodic memories, supporting rodent models that ascribe a key role for hippocampal theta in memory function.
Keywords: fractal, hippocampus, memory, oscillations, theta
Introduction
Since the classic work of Scoville and Milner (1957), we have known that the hippocampal formation plays a crucial role in human context-dependent (episodic) memory. Whereas lesion studies reified the single-case study of H.M. (Squire et al., 1993), further advances in our understanding of hippocampal physiology arose from recording field potentials and neuronal spiking in awake behaving mammals (O'Keefe and Dostrovsky, 1971; McNaughton et al., 1983; Knierim et al., 1995). These studies led to discoveries regarding the role of theta oscillations and place cell activity in animal learning (for review, see Lisman et al., 2001). Although human scalp EEG studies had suggested some role for theta rhythms in cognitive processes (Schacter, 1977) it was only at the turn of the 21st century that depth-electrode recordings in neurosurgical patients specifically implicated theta oscillations in human spatial and verbal memory (Kahana et al., 1999; Sederberg et al., 2003; Ekstrom et al., 2005). The ability to record neural activity from indwelling electrodes synchronized with computer-controlled memory experiments spawned a series of important discoveries regarding the electrophysiology of human learning and memory (Johnson and Knight, 2015).
Despite recent progress in the neurophysiology of human memory, considerable confusion surrounds the role of hippocampal theta activity in key memory processes, such as successful encoding and retrieval. To isolate neural correlates of successful memory encoding, researchers typically sort studied items into two groups—those that are subsequently recalled or recognized and those that are subsequently forgotten. Neuroimaging studies using this contrast have frequently identified the hippocampus as a region of increased hemodynamic activity during successful encoding. To isolate neural correlates of memory retrieval, researchers often compare the period during which recollection occurs with a control period comprising either a matched deliberation interval (Burke et al., 2014b) or a period preceding a retrieval error (Long et al., 2017).
In a review, Herweg et al. (2020) identify a highly inconsistent pattern of findings, particularly with regard to data from direct recordings from the human medial-temporal lobe (MTL). They find that most studies either report negative associations between MTL theta and memory or mixed patterns of results with some electrodes exhibiting increases and others exhibiting decreases in theta power. Herweg et al. (2020) propose several possible accounts for the discrepancies across these studies. First, they suggest that estimates of theta-band spectral power are confounded with broadband power changes, with the former reflecting synchronous oscillations and the latter reflecting asynchronous broadband activity indicative of greater attentiveness or cognitive engagement (Hanslmayr et al., 2012; Miller et al., 2014; Burke et al., 2015; Voytek and Knight, 2015). In light of this confound, they claim that the standard subsequent memory effect (SME) analysis will emphasize changes in global attention rather than memory-specific encoding processes and suggest that negative effects largely reflect broadband activity that masks the positive theta effects in the data. Theta oscillations might emerge after making analytic corrections for broadband changes. Second, they suggest that studies of retrieval processes, which are not bound to external stimuli and contrast similar attentive states, may be less susceptible to broadband confounds and therefore more suited to identify increases in hippocampal theta. Memory retrieval in a free recall task also depends critically on associative processes that bind items based on a combination of their temporal and semantic relations (Kahana, 2020), which may themselves be linked to the strength of theta oscillations (Solomon et al., 2019b). Finally, they note that most magnetoencephalography (MEG) and scalp EEG studies find positive theta effects, often overlying frontal regions. Although both invasive and noninvasive studies have yielded mixed results, the noticeably greater proportion of noninvasive studies reporting positive theta effects raises questions about how theta is affected by the spatial resolution of the recording technology and the referencing scheme applied to the signal. It may be that bipolar reference schemes-used in many of the studies reporting decreases in theta-have filtered out theta increases that appear synchronously over larger brain areas.
The present article evaluates these accounts of the discrepant findings concerning hippocampal theta and memory. To do so, we analyze a large dataset comprising 162 neurosurgical patients fitted with hippocampal depth electrodes. Using standard wavelet methods we analyze spectral activity during encoding and retrieval phases of a delayed free recall task. We also separate broadband and narrowband components of spectral activity using the Irregular-Resampling Auto-Spectral Analysis (IRASA; Wen and Liu, 2016). To evaluate the hypothesized role of hippocampal theta in memory encoding, we analyze the period during which an item is studied and compare trials for which the stimulus is subsequently recalled or forgotten. At retrieval, we compare the period immediately preceding verbal recall with matched periods of deliberation, when participants are trying to recall but no items come to mind. Finally, we evaluate the hypothesis that local spatial referencing obscures the role of theta in memory by repeating the above comparisons separately using a global average reference of implanted electrodes (compared with a bipolar reference that localizes activity to the differential voltage between neighboring channels).
Materials and Methods
Participants
We analyzed hippocampal depth-electrode recordings from 162 neurosurgical patients who participated in the Restoring Active Memory project funded by the U.S. Defense Advanced Research Projects Agency (Ezzyat et al., 2018; Solomon et al., 2019a,b; Phan et al., 2019). This publicly shared dataset includes >300 patients with drug-resistant epilepsy who took part in memory testing while undergoing a neurosurgical procedure to localize seizure activity and functional tissue. These patients (n = 86 female, n = 17 left-handed) had a mean age of 38 (ranging from 18 to 64 years). Researchers obtained informed consent from each patient, and the research protocol was approved by the institutional review board at the University of Pennsylvania and each participating hospital.
Participants contributed variable numbers of trials (i.e., studied test lists) depending on the length of their hospitalization and their interest in participating. We analyzed data only from participants who recalled at least, on average, one word per list; we also limited this study to participants with at least one bipolar pair consisting of contacts localized within the hippocampus (see below, Electrode recordings: localization and preprocessing).
Experimental design
Participants completed a free recall task, in which they encoded a sequence of 12 words that appeared on a blank screen for 1600 ms each during a study phase. The spacing between words is jittered between 750 and 1000 ms. Following each study phase, participants performed a 20 s arithmetic distractor task in which they solved problems of the form X + Y + Z = ?, where X, Y, and Z were positive or negative numbers between 1 and 9. Responses were made on a keypad, with presentation of additional math problems following each response (i.e., a self-paced task). After the delay, a row of asterisks accompanied by an 800 Hz auditory tone signaled the start of the recall period. At this point, participants recalled out loud all the words they could remember from the list in 30 s. They repeat this sequence 25 times to complete the experiment, but not all participants complete a full 25 trials. Many participants repeat this process for multiple experimental sessions.
Electrode recordings: localization and preprocessing
Our study focuses specifically on neural recordings from the hippocampus, defined as including regions CA1, CA2, CA3, CA4, dentate gyrus, and subiculum. To localize the recording contacts of depth electrodes, first hippocampal subfields and MTL cortices were automatically labeled in a preimplant, T2-weighted MRI using the automatic segmentation of hippocampal subfields multiatlas segmentation method (Yushkevich et al., 2015). Next, postimplant CT images were manually annotated with the voxel coordinates of individual recording contacts in CT space. Postimplant CT images were coregistered with presurgical T1- and T2-weighted structural scans with Advanced Normalization Tools (Avants et al., 2008), aligning the locations of individual recording sites to the anatomic labels assigned through automatic segmentation. For the majority of participants in this dataset, MTL depth electrodes that were visible on overlaid CT and MRI scans were then manually annotated with localizations within MTL subregions by neuroradiologists with expertise in MTL anatomy. The electrodes used in this analysis appear in Figure 1 in transformed MNI coordinate space. Algorithms that perform automatic segmentation and coregistration naturally introduce some imprecision, especially for neurosurgical patients with lesions or otherwise altered anatomy. Moreover, surgically implanted depth electrodes displace brain tissue and further complicate this task. Our confidence in these localizations stems from the manual work of the research team in visually inspecting the alignments and segmentations for every participant and from the work of expert neuroradiologists checking the veracity of the anatomic labels.
Figure 1.
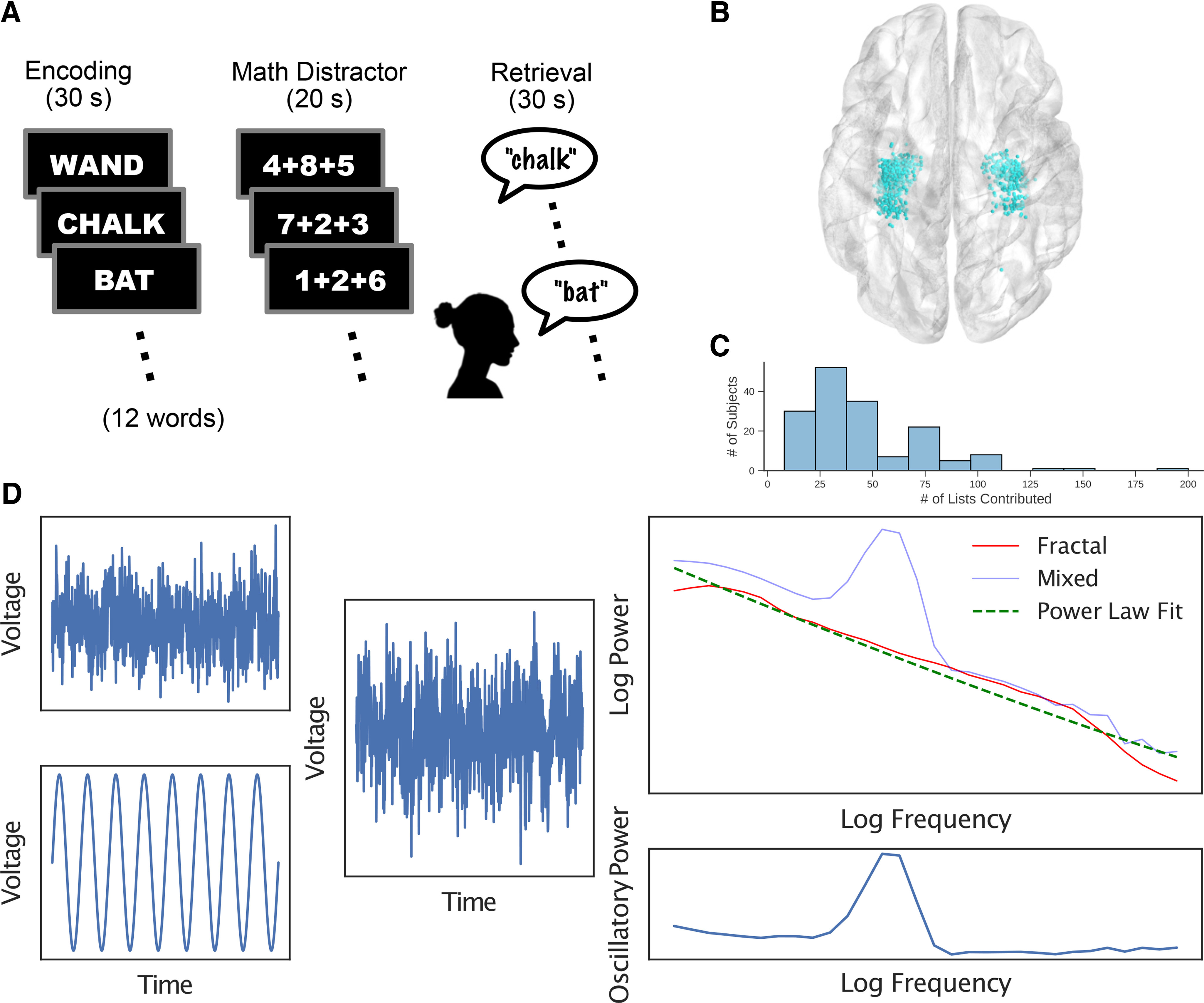
Materials and methods. A, Participants performed a free recall task in which they were presented with a list of words on a blank screen in sequence, completed a math distractor task, and were then prompted to recall as many of the presented words as possible during a 30 s free recall period. B, Data for this analysis were collected from electrodes located in the bilateral hippocampus across 162 participants. C, Distribution of the number of studied lists across participants. D, IRASA treats an EEG trace as a linear combination of an oscillatory component and a fractal pink noise component that is assumed to follow a power law distribution. IRASA capitalizes on a mathematical property of fractals called self-affinity, which causes them to behave differently under resampling from other signals, thereby allowing us to separate the components and obtain a purely oscillatory power spectrum.
The original sampling rates for these recordings vary by hospital and participant, but are all at least 500 Hz. For analysis, we resampled each recording to 500 Hz for consistency. We rereferenced the EEG using a bipolar montage to mitigate noise and increase the spatial resolution of the recordings, except for one analysis that explicitly compares this bipolar reference to a global (or whole-brain) average reference scheme. Applying a Butterworth band-stop filter of order 4 removed 60 Hz line noise from the recordings.
Separating broadband and narrowband effects with IRASA
IRASA, introduced by Wen and Liu (2016), is a method for separating oscillations from the pink noise background. We first assume that the EEG time series is a mixed signal containing both fractal (f(t)) and oscillatory (x(t)) components as follows:
Fractals are mathematically interesting for a number of reasons, but a property of particular importance is that they exhibit self-affinity. This means that fractals are scale free; geometrically, magnifying a portion of a fractal will produce qualitatively the same pattern. Expressed mathematically, when a fractal time series is resampled by a factor h, the following holds:
This means that the statistical distribution of the resampled time series is the same as the statistical distribution of the original time series multiplied by a scaling term (the Hurst exponent H is related to the autocorrelation of the time series.). In the frequency domain, this self-affinity manifests even more directly as follows:
which states that the Fourier transform after resampling is equal to the Fourier transform of the original time series multiplied by a scaling factor. This property is useful because resampling causes nonfractal signals to shift in the frequency domain. For an example of how signals typically shift in frequency space under resampling, consider an oscillation at 5 Hz in a recording sampled at 1000 Hz. This oscillation completes a full cycle every 200 samples. If the recording is downsampled to 500 Hz, then essentially every other sample is removed. Now, the same 5 Hz oscillation completes a cycle in only 100 samples. Without properly correcting for the change in sampling rate, it appears as if the speed of the oscillation has doubled to 10 Hz. To operationalize the property of self-affinity, we use the discrete Fourier transform to compute the autopower spectrum of the time series upsampled and downsampled by a set of noninteger factors h. Taking the median across the full range of h values removes the shifted oscillatory peaks, leaving exactly the fractal component. Subtracting this fractal component from the overall power spectrum then isolates oscillations. Figure 1 shows an example of the method applied to simulated data. The original method by Wen and Liu (2016) contains a more mathematically detailed description of IRASA.
Isolating rhythmic oscillations
To isolate the oscillatory components of the neural power spectrum, we applied IRASA to an epoch of 300–1300 ms following word presentation for every event in the encoding phase of the task. To study the electrophysiology of memory retrieval, we repeated the analysis for the epochs from 800 to 50 ms before recall vocalization. These time windows were chosen to balance the trade-off between having a sufficiently long window to assess power in the low theta band and being sufficiently specific to an individual, temporally punctate behavioral event.
IRASA decomposes the power spectrum into fractal and oscillatory components for each event and channel within every patient. The choice of resampling factors h controls the extent to which the method is robust to outliers but trades robustness for spectral smoothing that decreases our frequency resolution. As we wanted to ensure our analysis did not unintentionally include noise artifacts created by large oscillations, but were also interested in having a high-resolution spectral decomposition that can distinguish between different narrowband effects, we chose a relatively conservative set of resampling factors from 1.1 to 2.0, linearly spaced by 0.05. This is the default set of resampling factors recommended and used in the original methods article.
As IRASA explicitly extracts only the fractal component from the power spectrum, to isolate the oscillatory component we need to take the difference of the full spectrum and the fractal component. This is simple enough, but poses a challenge when log transforming to suppress extreme values and normalize the data. If the fractal estimate is greater than the mixed power spectrum, the oscillatory power will be negative and the log transform undefined. We therefore introduce a shifted symmetric log transform (SSL) to achieve the same goals without issue. This transform is defined as follows:
This function retains the useful properties of the logarithm, but it is symmetric about the x-axis and does not go to negative infinity at very small values.
Wavelet power
We computed wavelet power at both encoding and retrieval to serve as a baseline against which we can compare our results using IRASA. We computed power at logarithmically spaced frequencies using Morlet wavelets with a width of four cycles. For this analysis, we included buffers on either side, which corresponded to at least two cycles at the lowest frequency being analyzed (to avoid edge effects). At retrieval, we excluded recalls that were preceded by a vocalization during the buffer period preceding the epoch; we also implemented a mirrored buffer following the epoch to avoid contaminating our spectral estimates with vocalization artifacts from recall onset.
Statistical analyses
Our primary question in analyzing item encoding is the following: How does power at a given frequency (e.g., theta) behave as a function of subsequent recall status? In answering this question we want to account for individual differences as well as session-level effects; memory performance and power might differ across participants or even across different recording sessions for the same individual. Accordingly, we fit a linear mixed-effects model for each frequency of interest using the lmer4 package in R (Bates et al., 2015) which predicts channel-averaged power as a function of subsequent recall (one for success and zero for failure), with random slopes and intercepts for the effects of participant, session, and trial (studied word list). To ensure proper estimation of the effects and their standard errors, we started with a maximal model and incrementally reduced the model complexity to remove zero-variance components and avoid singularities in the estimated variance-covariance matrix (Matuschek et al., 2017; Bates et al., 2018). Our analysis of memory retrieval followed the same procedure and model, but the binary memory success variable represented successful memory retrieval (as opposed to baseline deliberation) rather than successful memory encoding (as determined by subsequent recall).
We report effects at each frequency of interest as β coefficients from this model and use a Wald test to evaluate statistical significance. We then correct for multiple comparisons by using the Benjamini–Hochberg procedure for controlling false discovery rate. This method is appropriate when tests are positively correlated, as are spectral estimates at similar frequencies.
Data availability
Raw electrophysiological data used in this study are available on request from https://memory.psych.upenn.edu/Data_Request. Analysis and data visualization code is also available for direct download from https://memory.psych.upenn.edu/files/pubs/RudoEtal22.code.tgz. The Python implementation of the IRASA method used for this study is publicly available at https://github.com/pennmem/irasa, and other custom processing scripts used for this project can be found at https://github.com/pennmem/.
Results
As our analyses sought to elucidate the role of hippocampal theta oscillations in episodic memory encoding and retrieval, we identified all participants with hippocampal electrodes in the multicenter Restoring Active Memory project (see above, Materials and Methods). Of a total of N = 281 participants who completed the same free recall task, 162 had at least one bipolar electrode pair whose contacts both fell within the hippocampal formation defined as including regions CA1, CA2, CA3, CA4, dentate gyrus, and subiculum, but excluding nonhippocampal MTL regions such as perirhinal and parahippocampal cortices. Participants performed a memory task in which they studied 12 words that they attempted to recall following a brief arithmetic distractor task (Fig. 1A, schematic of the experimental task). During the 30 s recall period, participants attempted to say as many words as they could remember from the most recent list, in any order. Each participant contributed data from multiple study-test trials (see above, Materials and Methods). As participants performed this memory task, intraparenchymal depth electrodes captured hippocampal field potentials (Fig. 1B; see above, Materials and Methods, electrode localization).
The present study seeks to clarify the role of hippocampal neural oscillations in the formation and retrieval of episodic memories. As most prior work involving direct brain recordings used standard spectral decomposition procedures (e.g., wavelet transforms, multitapers, other windowed FFT methods, etc.) these studies cannot disambiguate oscillations from broadband components of neural activity underlying successful mnemonic function. To address this limitation, we analyzed neural signals using IRASA, which exploits the fractal properties of the power law–distributed broadband component to isolate it from the mixed power spectrum (see above, Materials and Methods). Figure 1C shows how IRASA decomposes a simulated EEG trace into broadband and narrowband components. The simulated data in Figure 1C contain a single sine wave at a known frequency (narrowband) and pink noise (broadband). IRASA estimates this broadband component and subtracts it from the mixed autopower spectrum to isolate the residual oscillatory power.
The formation of episodic memories occurs when participants study words for a subsequent recall task. To identify the spectral correlates of successful encoding, we examined the 1 s interval beginning 300 ms following item presentation, thereby excluding brain signals related to perceptual processing of the presented word. Spectral analyses of hippocampal field potentials during this encoding period typically show a tilt in the power spectrum, with decreases in low-frequency power and increases in high-frequency power predicting subsequent recall (Burke et al., 2014a; Ezzyat et al., 2017; Fellner et al., 2019). This spectral tilt manifests as a flattening of the overall spectrum in log–log space, resulting in a change in the power law exponent. By isolating the power law distributed background and removing that component from the power spectrum, IRASA reveals a more accurate estimate of the narrowband oscillatory patterns that coexist with broadband changes.
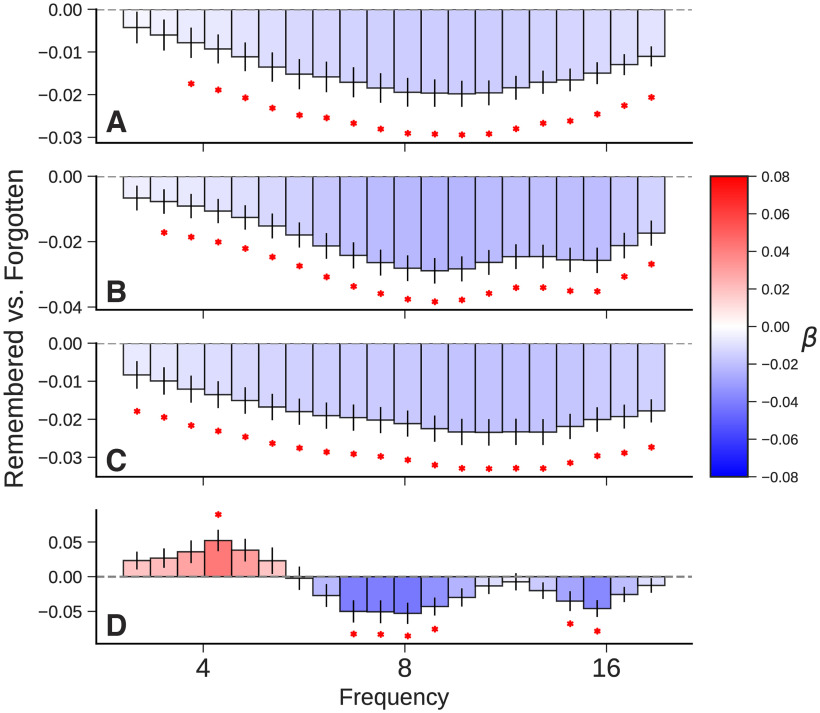
As shown in Figure 2, the mixed autopower spectrum—analogous to traditional wavelet or multitaper methods—shows the expected theta decreases during successful encoding. Likewise, the isolated broadband component shows decreases in theta power. The oscillatory spectrum, however, shows theta increases. The estimates of effect size and significance derive from the mixed-effects model (see above, Materials and Methods), which predicts power at a frequency of interest as a function of recall status while accounting for the effects of participants and session.
Figure 2.
Subsequent memory effect. Comparison of spectral power for successful and unsuccessful memory encoding events (remembered/forgotten) based on hippocampal depth electrode recordings from 300 to 1300 ms following item presentation. Values represent fixed-effect coefficients for the effect of successful recall on spectral power. A red asterisk indicates that the effect size is significant after correcting for multiple comparisons (p < 0.05). A, Power computed using traditional Morlet wavelets. B, The mixed power spectrum (before separating broadband and narrowband effects) shows the expected theta and alpha decreases, analogous to the results using wavelets. C, The fractal power spectrum (broadband only) likewise shows broad decreases in low frequency. D, The oscillatory power spectrum, which is computed as the difference of the mixed and fractal power spectra, exhibits an increase in theta power while retaining the same decrease in alpha power.
Our finding of a small but reliable positive theta subsequent-memory effect in the human hippocampus contrasts with previous studies that found predominantly negative theta effects using wavelet methods and aggregate indices of hippocampal activity. These results confirm the hypothesis offered by Herweg et al. (2020) that broadband decreases in low-frequency activity mask narrowband increases in theta activity. Given that aggregating hippocampal recordings from 162 participants only yielded a modest positive theta SME, it is likely that many studies comprised of smaller samples would not be powered to detect this effect (Sederberg et al., 2007b). We next turn to the question of memory retrieval, asking whether isolation of narrowband spectral components can resolve mixed findings regarding the role of theta in retrieval processes.
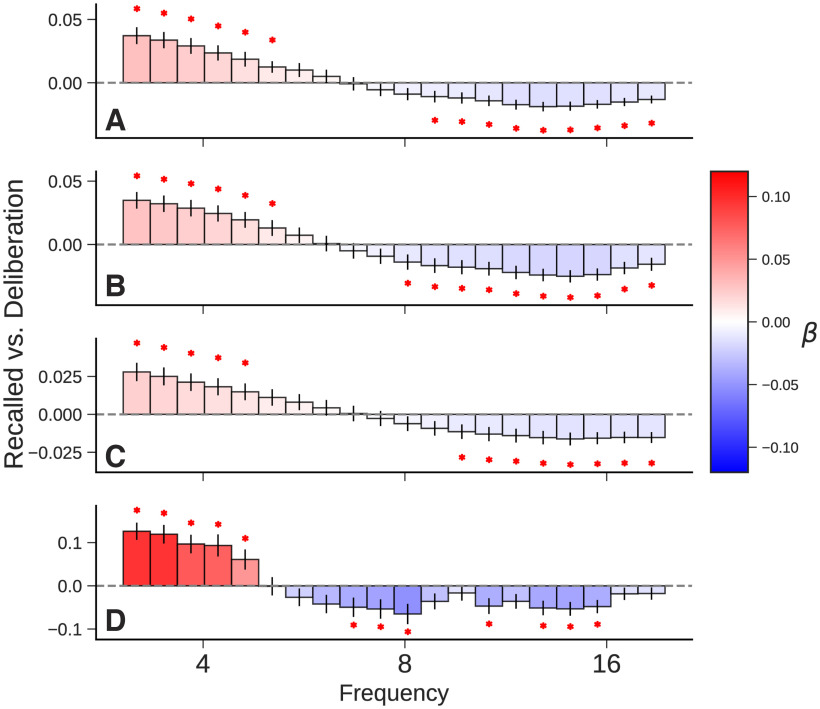
We used the same decomposition approach to estimate oscillatory power during a 750 ms epoch between 800 and 50 ms before recall onset. The theta increase observed during encoding is even more pronounced at retrieval; it even shows up in traditional power decompositions like Morlet wavelets and the mixed spectrum of IRASA (Fig. 3A,B). Positive theta during retrieval has previously been reported from intracranial electrodes in the right temporal pole (Burke et al., 2014b), but these findings were called into question by subsequent studies with far greater statistical power showing either decreases or no significant effect (Solomon et al., 2019b; Weidemann et al., 2019). We note that most prior work averaged wavelet power over the traditional theta band from either 3 or 4 Hz to 8 Hz, which blends the positive and negative effects shown in Figure 3. Assessing a more continuous spectrum instead of averaging within bands, we recover a strong positive effect at low frequencies. So, oscillatory power obtained with IRASA for the memory retrieval contrast matches the results obtained using traditional wavelet power, although we do obtain somewhat better resolution that reveals multiple distinct components in the high theta/alpha range.
Figure 3.
Memory retrieval contrast. A–D, Comparison of successful retrieval events and matched deliberation periods, events that we treat as “failed recall.” Power at logarithmically spaced frequencies were computed for the 750 ms preceding recall vocalization. Values represent fixed-effect coefficients for the effect of successful recall on spectral power. A red asterisk indicates that the effect size is significant after correcting for multiple comparisons (p < 0.05). Subplots show wavelet power (A), IRASA mixed power (B), IRASA fractal power (C), and IRASA oscillatory power (D).
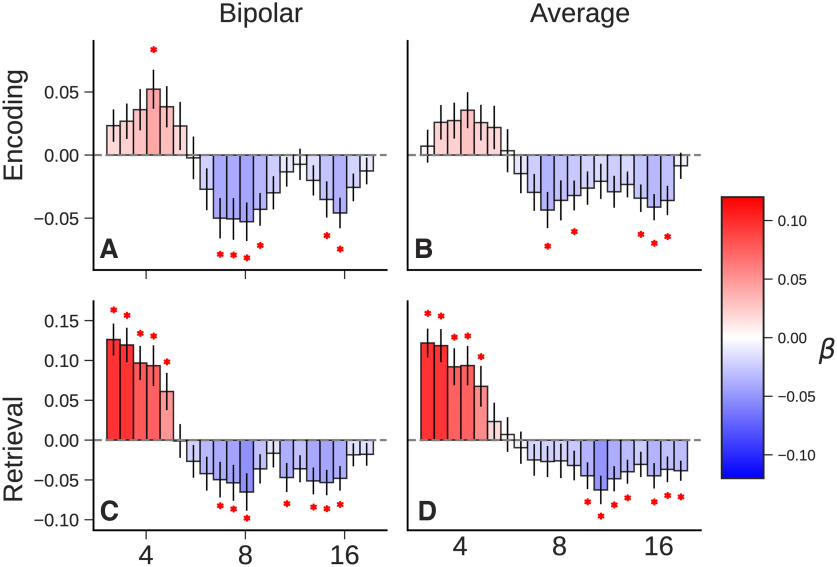
Herweg et al. (2020) proposed that the choice of referencing scheme may potentially contribute to the apparent inconsistencies between noninvasive and invasive analyses of the role of theta in memory. Scalp EEG and MEG studies often report positive theta correlates of memory encoding and retrieval (Klimesch et al., 1996; Hanslmayr et al., 2011; Kaplan et al., 2012; Fellner et al., 2013; Staudigl and Hanslmayr, 2013; Backus et al., 2016), whereas many highly powered intracranial studies have failed to show these effects. The two most commonly used, and practically distinct, methods of voltage referencing are the bipolar reference and the average reference. In a bipolar scheme, the potential difference is calculated between pairs of neighboring electrodes. This is effectively a spatial filter, as any signal shared by both electrodes will be eliminated by the differencing operation. An average reference is more sensitive to global changes in field potentials; it is calculated by averaging the potential measured at all electrodes and subtracting that average from each one. Herweg et al. (2020) observe that increases in theta power reported in scalp EEG and MEG studies with average referencing often exhibit a broad topography across the scalp, centered around frontal electrodes, and suggest that these effects might have been attenuated in intracranial studies that frequently used bipolar referencing schemes. This is because bipolar referencing acts as a spatial high-pass filter, attenuating theta effects that occur synchronously across neighboring electrodes.
Comparing the memory-related power changes measured with each referencing scheme did not reveal any reliable differences (Fig. 4). An FDR-corrected paired t test comparing bipolar with average reference (for 126 participants with monopolar recordings) did not identify significant differences between the oscillatory power estimates for the two referencing schemes at any of the frequencies of interest.
Figure 4.
Comparison of referencing schemes. A–D, Average effect sizes for successful versus unsuccessful memory contrasts with bipolar reference at encoding (A), with average reference at encoding (B), with bipolar reference at retrieval (C), and with average reference at retrieval (D). The isolated oscillatory power spectra did not differ significantly based on the spatial filter applied to the data.
Discussion
We sought to resolve long-standing controversies regarding the role of hippocampal theta in learning and memory. To do so, we reanalyzed a large dataset of human hippocampal activity recorded as neurosurgical patients performed multiple trials of a verbal delayed free recall task. Our dataset comprised 797 hippocampal recordings across 162 participants. Whereas previous research found inconsistent theta correlates of successful encoding and recall, we find narrowband 4 Hz oscillations to consistently increase during successful encoding (Fig. 2) and preceding spontaneous free recall (compared with matched deliberation periods; Fig. 3). Further, we show that increases in theta activity appear similarly whether measured using a local spatial filter (bipolar referencing) or a more global filter (referencing to the average of all electrodes; Fig. 4).
Although many studies report theta correlates of memory in broader memory regions, only a few studies specifically isolate hippocampal signals. Fell et al. (2011) analyzed hippocampal theta during memory encoding in a continuous recognition procedure. Analyzing ∼100 hippocampal electrodes, they found a significant interaction between prestimulus and poststimulus presentation changes in theta power, with significant prestimulus theta increases predicting subsequent recognition. During the poststimulus item encoding period they found a modest decrease in theta (p ∼ 0.10) for subsequently recognized items. Sederberg et al. (2007a) analyzed hippocampal subsequent memory effects in a delayed free recall task. Their study, which included 186 hippocampal recordings, detected reliable high-frequency increases during successful encoding, but they failed to observe reliable theta effects. In a much larger analysis of hippocampal recordings in delayed recall (401 hippocampal electrodes) Long et al. (2014) observed negative theta SMEs during successful encoding and null effects in the theta band during successful retrieval (Burke et al., 2014a). Lega et al. (2012) examined 237 hippocampal recordings during delayed free recall (as in Sederberg et al., 2007a). Recognizing the possibility that spectral measures confound broadband and narrowband (oscillatory) effects, Lega et al. (2012) applied an oscillation detection algorithm (Caplan et al., 2003) to filter for electrodes that exhibited narrowband oscillations in each of several frequency bands. Analyzing these channels revealed both positive and negative theta effects at different electrodes. Although Lega et al.'s (2012) study revealed striking positive theta effects at specific electrodes, it found an even larger number of hippocampal recordings that exhibited narrowband decreases, thus offering a potential explanation for the negative and null results described above. A later study (Lin et al., 2017) found hippocampal theta-band increases, but only in the posterior hippocampus.
Standard methods used to analyze spectral EEG power (such as wavelets, multitapers, and windowed FFTs) mix narrowband and broadband signals, leaving open the possibility that a negative broadband effect can mask a positive narrowband effect and vice versa. When analyzed in this manner, our study replicated a number of previously published studies in showing decreased hippocampal theta power during the encoding of subsequently forgotten items (Burke et al., 2014a; Solomon et al., 2019a). By using IRASA, however, we revealed a positive relation between 4 Hz theta and successful memory encoding that tends to be obscured by a large negative relation between broadband power and encoding success.
Although we used IRASA to isolate narrowband power, a number of other methods have been developed to address this problem, usually by modeling the 1/f background and considering deviations or residuals to be true narrowband, synchronous oscillations. The Better Oscillation Detection Method (Caplan et al., 2001) characterizes oscillations by measuring when narrowband power exceeds a power threshold above the fitted 1/f spectrum for a specified number of cycles at the frequency of interest; a newer method called FOOOF (Fitting Oscillations and One-Over-F; Donoghue et al., 2020) identifies oscillations by assuming they are Gaussians superposed on top of a 1/f distribution and selecting oscillatory peaks through an iterative fitting algorithm. We expect that using these related methods would lead to similar results regarding the increase in theta with successful memory encoding.
Comparing the period immediately preceding correct recall of a studied item with matched deliberation intervals revealed that although low-frequency (4 Hz) theta increased, higher theta band power decreased. In this case, separating narrowband and broadband power did not prove necessary to uncover the positive correlation between theta activity and successful recall. Finally, Herweg et al. (2020) hypothesized that bipolar referencing may obscure theta increases by filtering out activity correlated across multiple neighboring electrodes. Our comparison of bipolar and average references reveals clear theta increases regardless of referencing scheme.
This study does not discuss, nor directly account for, differences in epilepsy etiologies across patients or in epileptic activity across trials. Although these and other clinical factors are outside the scope of this article as they do not bear directly on our hypotheses, they may bear on the study of memory in patients with epilepsy more generally (Quon et al., 2021; Camarillo-Rodriguez et al., 2022).
Hanslmayr et al. (2012) and (2016) propose that broadband and narrowband spectral activity have distinct and complementary roles in memory encoding; broad low-frequency desynchronization across the brain supports increased representation of information content, whereas narrowband theta power increases reflect the hippocampus organizing and encoding that information. Our data are consistent with this theory as we observe simultaneous decreases in broadband, fractal power and increases in narrowband, oscillatory theta power during memory encoding. Numerous other computational models of memory, mostly informed by studies in rodents, assign a prominent role for theta in both memory formation and retrieval. Our decomposition of narrowband and broadband components of human hippocampal field potentials reveals increases in narrowband theta during both successful encoding and retrieval, supporting the applicability of these models to human episodic memory.
Footnotes
This work was supported by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant NS1113198. We thank Daniel Schonhaut, Josh Jacobs, John Sakon, David Halpern, and Noa Herz for comments on previous versions of this manuscript.
The authors declare no competing financial interests.
References
- Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus AR, Schoffelen J-M, Szebényi S, Hanslmayr S, Doeller CF (2016) Hippocampal-prefrontal theta oscillations support memory integration. Curr Biol 26:450–457. 10.1016/j.cub.2015.12.048 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bates D, Kliegl R, Vasishth S, Baayen H (2018) Parsimonious mixed models. arXiv:1506.04967. 10.48550/arXiv.1506.04967. 10.48550/arXiv.1506.04967 [DOI] [Google Scholar]
- Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, Kahana MJ (2014a) Human intracranial high-frequency activity maps episodic memory formation in space and time. Neuroimage 85:834–843. 10.1016/j.neuroimage.2013.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JF, Sharan AD, Sperling MR, Ramayya AG, Evans JJ, Healey MK, Beck EN, Davis KA, Lucas TH, Kahana MJ (2014b) Theta and high-frequency activity mark spontaneous recall of episodic memories. J Neurosci 34:11355–11365. 10.1523/JNEUROSCI.2654-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JF, Ramayya AG, Kahana MJ (2015) Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Curr Opin Neurobiol 31:104–110. 10.1016/j.conb.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Moser E (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 16:130–138. 10.1038/nn.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarillo-Rodriguez L, Leenen I, Waldman Z, Serruya M, Wanda PA, Herweg NA, Kahana MJ, Rubinstein D, Orosz I, Lega B, Podkorytova I, Gross RE, Worrell G, Davis KA, Jobst BC, Sheth SA, Weiss SA, Sperling MR (2022) Temporal lobe interictal spikes disrupt encoding and retrieval of verbal memory: a subregion analysis. Epilepsia 63:2325–2337. 10.1111/epi.17334 [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ (2001) Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol 86:368–380. 10.1152/jn.2001.86.1.368 [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ (2003) Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci 23:4726–4736. 10.1523/JNEUROSCI.23-11-04726.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, Noto T, Lara AH, Wallis JD, Knight RT, Shestyuk A, Voytek B (2020) Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci 23:1655–1665. 10.1038/s41593-020-00744-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan J, Ho E, Shattuck K, Fried I, Kahana M (2005) Human hippocampal theta activity during virtual navigation. Hippocampus 15:881–889. 10.1002/hipo.20109 [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Kragel JE, Burke JF, Levy DF, Lyalenko A, Wanda P, O'Sullivan L, Hurley KB, Busygin S, Pedisich I, Sperling MR, Worrell GA, Kucewicz MT, Davis KA, Lucas TH, Inman CS, Lega BC, Jobst BC, Sheth SA, Zaghloul K (2017) Direct brain stimulation modulates encoding states and memory performance in humans. Curr Biol 27:1251–1258. 10.1016/j.cub.2017.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Wanda PA, Levy DF, Kadel A, Aka A, Pedisich I, Sperling MR, Sharan AD, Lega BC, Burks A, Gross RE, Inman CS, Jobst BC, Gorenstein MA, Davis KA, Worrell GA, Kucewicz MT, Stein JM, Gorniak R, Das SR (2018) Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun 9:365. 10.1038/s41467-017-02753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Staresina B, Wagner T, Kranz T, Elger CE, Axmacher N (2011) Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J Neurosci 31:5392–5397. 10.1523/JNEUROSCI.3668-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner M-C, Bäuml K-HT, Hanslmayr S (2013) Brain oscillatory subsequent memory effects differ in power and long-range synchronization between semantic and survival processing. Neuroimage 79:361–370. 10.1016/j.neuroimage.2013.04.121 [DOI] [PubMed] [Google Scholar]
- Fellner M-C, Gollwitzer S, Rampp S, Kreiselmeyr G, Bush D, Diehl B, Axmacher N, Hamer H, Hanslmayr S (2019) Spectral fingerprints or spectral tilt? Evidence for distinct oscillatory signatures of memory formation. PLoS Biol 17:e3000403. 10.1371/journal.pbio.3000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Volberg G, Wimber M, Raabe M, Greenlee MW, Bäumel KHT (2011) The relationship between brain oscillations and bold signal during memory formation: a combined EEG-fMRI study. J Neurosci 31:15674–15680. 10.1523/JNEUROSCI.3140-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner M (2012) Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front Hum Neurosci 6:74. 10.3389/fnhum.2012.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Staresina BP, Bowman H (2016) Oscillations and episodic memory: addressing the synchronization/desynchronization conundrum. Trends Neurosci 39:16–25. 10.1016/j.tins.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweg NA, Solomon EA, Kahana MJ (2020) Theta oscillations in human memory. Trends Cogn Sci 24:208–227. 10.1016/j.tics.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Knight RT (2015) Intracranial recordings and human memory. Curr Opin Neurobiol 31:18–25. 10.1016/j.conb.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ (2020) Computational models of memory search. Annu Rev Psychol 71:107–138. 10.1146/annurev-psych-010418-103358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR (1999) Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399:781–784. 10.1038/21645 [DOI] [PubMed] [Google Scholar]
- Kaplan R, Doeller CF, Barnes GR, Litvak V, Düzel E, Bandettini PA, Burgess N (2012) Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS Biol 10:e1001267. 10.1371/journal.pbio.1001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T (1996) Theta band power in the human scalp EEG and the encoding of new information. Neuroreport 7:1235–1240. 10.1097/00001756-199605170-00002 [DOI] [PubMed] [Google Scholar]
- Knierim J, Kudrimoti H, McNaughton B (1995) Place cells, head direction, cells, and the learning of landmark stability. J Neurosci 15:1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega B, Jacobs J, Kahana M (2012) Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22:748–761. 10.1002/hipo.20937 [DOI] [PubMed] [Google Scholar]
- Lin JJ, Rugg MD, Das S, Stein J, Rizzuto DS, Kahana MJ, Lega BC (2017) Theta band power increases in the posterior hippocampus predict successful episodic memory encoding in humans. Hippocampus 27:1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Jensen O (2013) The theta-gamma neural code. Neuron 77:1002–1016. 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Jensen O, Kahana MJ (2001) Toward a physiologic explanation of behavioural data on human memory. In: Neuronal mechanisms of memory formation (Hölscher C, ed), pp 195–223. Cambridge, UK: Cambridge UP. [Google Scholar]
- Long NM, Burke JF, Kahana MJ (2014) Subsequent memory effect in intracranial and scalp EEG. Neuroimage 84:488–494. 10.1016/j.neuroimage.2013.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Sperling MR, Worrell GA, Davis KA, Gross RE, Lega BC, Jobst BC, Sheth SA, Zaghloul K, Stein JM, Kahana MJ (2017) Contextually mediated spontaneous retrieval is specific to the hippocampus. Curr Biol 27:1074–1079. 10.1016/j.cub.2017.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschek H, Kliegl R, Vasishth S, Baayen H, Bates D (2017) Balancing Type 1 error and power in linear mixed models. J Mem Lang 94:305–315. 10.1016/j.jml.2017.01.001 [DOI] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52:41–49. 10.1007/BF00237147 [DOI] [PubMed] [Google Scholar]
- Miller KJ, Honey CJ, Hermes D, Rao RP, den Nijs M, Ojemann JG (2014) Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. Neuroimage 85:711–720. 10.1016/j.neuroimage.2013.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- Phan TD, Wachter JA, Solomon E, Kahana MJ (2019) Multivariate stochastic volatility modeling of neural data. Elife 8:e42950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon RJ, Camp EJ, Meisenhelter S, Song Y, Steimel SA, Testorf ME, Andrew AS, Gross RE, Lega BC, Sperling MR, Kahana MJ, Jobst BC (2021) Features of intracranial interictal epileptiform discharges associated with memory encoding. Epilepsia 62:2615–2626. 10.1111/epi.17060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL (1977) EEG theta waves and psychological phenomena: a review and analysis. Biol Psychol 5:47–82. 10.1016/0301-0511(77)90028-x [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR (2003) Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23:10809–10814. 10.1523/JNEUROSCI.23-34-10809.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ (2007a) Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 17:1190–1196. 10.1093/cercor/bhl030 [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, Kahana MJ (2007b) Gamma oscillations distinguish true from false memories. Psychol Sci 18:927–932. 10.1111/j.1467-9280.2007.02003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon EA, Stein JM, Das S, Gorniak R, Sperling MR, Worrell G, Inman CS, Tan RJ, Jobst BC, Rizzuto DS, Kahana MJ (2019a) Dynamic theta networks within the human medial temporal lobe support episodic encoding and retrieval. Curr Biol 29:1100–1111.e4. 10.1016/j.cub.2019.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon EA, Lega BC, Sperling MR, Kahana MJ (2019b) Hippocampal theta codes for distances in semantic and temporal spaces. Proc Natl Acad Sci U S A 116:24343–24352. 10.1073/pnas.1906729116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G (1993) The structure and organization of memory. Annu Rev Psychol 44:453–495. 10.1146/annurev.ps.44.020193.002321 [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S (2013) Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr Biol 23:1101–1106. 10.1016/j.cub.2013.04.074 [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT (2015) Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry 77:1089–1097. 10.1016/j.biopsych.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann CT, Kragel JE, Lega BC, Worrell GA, Sperling MR, Sharan AD, Jobst BC, Khadjevand F, Davis KA, Wanda PA, Kadel A, Rizzuto DS, Kahana MJ (2019) Neural activity reveals interactions between episodic and semantic memory systems during retrieval. J Exp Psychol Gen 148:1–12. 10.1037/xge0000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Liu Z (2016) Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr 29:13–26. 10.1007/s10548-015-0448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA (2015) Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp 36:258–287. 10.1002/hbm.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw electrophysiological data used in this study are available on request from https://memory.psych.upenn.edu/Data_Request. Analysis and data visualization code is also available for direct download from https://memory.psych.upenn.edu/files/pubs/RudoEtal22.code.tgz. The Python implementation of the IRASA method used for this study is publicly available at https://github.com/pennmem/irasa, and other custom processing scripts used for this project can be found at https://github.com/pennmem/.