Abstract
The intracellular parasite Toxoplasma gondii has the capacity to persist in the brain within neurons. In this study we demonstrated that T. gondii infected murine cerebellar neurons in vitro and replicated within these cells. Stimulation with gamma interferon (IFN-γ) and/or tumor necrosis factor (TNF) did not enable neurons to inhibit parasite invasion and replication. Cultured neurons constitutively produced interleukin 1 (IL-1), IL-6, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β but not transforming growth factor β1 (TGF-β1), IL-10, and granulocyte-macrophage colony-stimulating factor. Neuronal expression of some cytokines (IL-6, TGF-β1) and chemokines (MIP-1β) was regulated by infection and/or by IFN-γ and TNF.
Toxoplasma gondii is an obligate intracellular protozoan parasite which persists in the host central nervous system (CNS). Electron microscopic studies of animals with murine toxoplasmosis have identified neurons as the primary target cells for T. gondii, although astrocytes and microglia can also be infected by T. gondii (3, 7, 12, 15).
Studies of experimental murine toxoplamosis have identified gamma interferon (IFN-γ) production by both CD4+ and CD8+ T cells as a key factor for parasite control (11, 27, 29). In addition, tumor necrosis factor (TNF), which is produced by CD4+ and CD8+ T cells, macrophages, microglial cells, and some astrocytes in animals with Toxoplasma encephalitis (22), contributes to the control of T. gondii in cerebral toxoplasmosis (10). Although the importance of IFN-γ and TNF has been delineated, the mechanisms which control the parasite in neurons have been determined only incompletely. In particular, it is still not known whether IFN-γ and TNF directly or indirectly control intraneuronal T. gondii. In addition, the detailed immunological response of neurons to parasite infection is still not known. To gain more insight into the interaction of T. gondii with neurons, we studied parasite invasion and replication in cultivated murine CNS neurons and neuronal cytokine and chemokine production in response to infection with T. gondii, as well as the regulatory effect of IFN-γ and TNF on these processes.
Invasion and multiplication of T. gondii in neurons and modulation by IFN-γ and/or TNF.
Cerebellar granule neurons were isolated from the brains of 7-day-old BALB/c mice as described previously (19, 20). Neurons were added at a concentration of 5.2 × 105 cells per well to poly-l-lysine-coated 24-well plates. Neurons were cultured in basal medium Eagle (BME) supplemented with 10% fetal calf serum (FCS), 25 mM K+, 2 mM l-glutamine, 30 mM glucose, and 50 μg of gentamicin per ml (all obtained from Sigma, Deisenhofen, Germany). To stop the growth of nonneuronal cells, 10 μM cytosine-β-arabinoside (Sigma) was added on day 1 after plating of neurons. Immunofluorescence revealed that the cultures contained approximately 95% neurofilament-positive neurons and 5% glial fibrillary acid protein (GFAP)-positive astrocytes. Neurons that were 7 days old were infected with tachyzoites of the RH strain of T. gondii at a multiplicity of infection (MOI) of 0.1, 1, or 10. RH toxoplasms were grown on human foreskin fibroblasts in Dulbecco's modified Eagle medium supplemented with 10% FCS. For infection of neurons, parasites, which had lysed fibroblasts just before the experiments, were washed with Hanks balanced salt solution supplemented with 3% FCS and were added to neuronal cell tissue cultures. Portions of the neuronal cell cultures were treated with IFN-γ (100 U/ml; Pharmingen) and/or TNF (10 U/ml; Pharmingen) for 12 h prior to infection with T. gondii. To determine the number of T. gondii-infected neurons and the number of T. gondii cells per parasitophorous vacuole (PV), neurons were fixed with 4% paraformaldehyde either 24 or 48 h after infection. After this, neurons were stained with rabbit anti-T. gondii antiserum and then with peroxidase-coupled goat anti-rabbit immunoglobulin G F(ab)2 fragments. The reaction product was visualized by incubation with H2O2 and 3,3-diaminobenzidine (Sigma). The number of infected neurons and the number of parasites per PV were determined by examining at least 100 neurons per well.
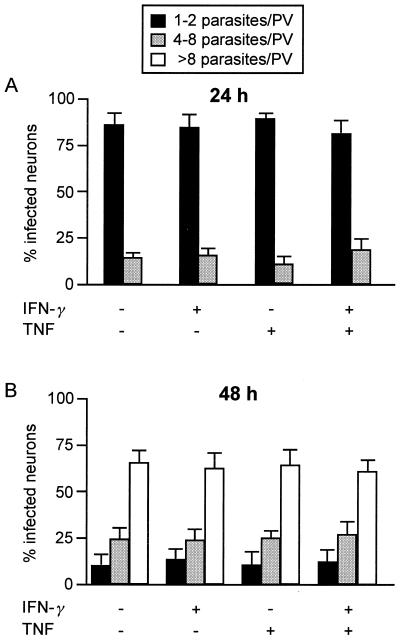
Addition of T. gondii tachyzoites to cultivated murine cerebellar granule neurons resulted in infection of neurons (Fig. 1). When an MOI of 0.1 was used, only a small number of neurons (3%) was infected 24 h after infection, whereas an MOI of 10 resulted in destruction of the neuronal cell layer within 48 h after infection. In contrast, an MOI of 1 resulted in infection of 23.5% ± 4.8% of the neurons 24 h after infection (Table 1), and 48 h after infection neurons were still alive and the typical network of finely branched axons was well preserved. Thus, an MOI of 1 was found to be optimal for studying the interaction of T. gondii with neurons and was used for further experiments, although 72 h after infection the neuronal cell layer was partially destroyed by replicating parasites and uninfected neurons died due to loss of their axonal contacts. Pretreatment of neurons with either IFN-γ or TNF or with both cytokines did not reduce the percentage of infected neurons (Table 1). At 24 h postinfection, most infected neurons harbored one or two parasites per PV, and only a low percentage of neurons contained more parasites per PV (Fig. 2A). Again, pretreatment of neurons with IFN-γ or TNF or with both cytokines did not significantly change the number of parasites per PV. At 48 h postinfection, the numbers of parasites per PV had increased equally in all experimental groups (Fig. 2B) (P < 0.01 for each experimental group at 48 h postinfection compared to the corresponding group 24 h postinfection, as determined by the Wilcoxon test), and the numbers of parasites per PV in untreated and cytokine-treated neurons did not differ significantly. An increase in the concentration of IFN-γ from 100 to 500 U/ml also did not inhibit parasite invasion and multiplication in neurons, whereas a TNF dose greater than 10 U/ml (i.e., 100 U/ml) was found to be toxic for neurons, especially when it was combined with IFN-γ (data not shown). Thus, neurons were not able to restrict invasion and growth of T. gondii, and treatment of neurons with IFN-γ and/or TNF did not induce toxoplasmastatic activity in these cells, although neurons are known to express the receptors for IFN-γ and TNF and respond to this stimulation by induction of major histocompatibility complex class I expression (18, 21, 24) as well as cytokine and chemokine production (see below).
FIG. 1.
Cultured cerebellar neurons after infection with T. gondii RH (MOI, 1) for 48 h: PV containing numerous tachyzoites in the cytoplasm of a neuron with a prominent nucleolus. Note the intimate contact of the PV with the nuclear membrane. Anti-T. gondii immunostaining and slight counterstaining with hemalum were used. Magnification, ×625.
TABLE 1.
Levels of infection of neurons
| Treatmenta | % of T. gondii-infected neuronsb |
|---|---|
| Medium alone | 23.5 ± 4.8 |
| IFN-γ (100 U/ml) | 22.8 ± 4.1c |
| TNF (10 U/ml) | 27.0 ± 6.1c |
| IFN-γ (100 U/ml) + TNF (100 U/ml) | 20.3 ± 3.9c |
Neurons were treated with cytokines for 12 prior to infection with T. gondii.
One hundred neurons were examined 24 h postinfection, and the data are means ± standard deviations based on three wells per experimental group. In two additional experiments similar data were obtained.
P > 0.05 for cytokine-treated neurons compared to untreated neurons (Wilcoxon test).
FIG. 2.
Proliferation of T. gondii in neurons 24 h (A) and 48 h (B) after infection. After 7 days of cultivation neurons were treated with the cytokines indicated for 12 h. After this, RH toxoplasms were added at an MOI of 1. At 24 and 48 h after infection neurons were fixed with 4% paraformaldehyde, and T. gondii was stained immunohistochemically. The number of toxoplasms per PV was determined microscopically for 100 infected neurons per group; the data are means ± standard deviations based on three wells per group. Similar data were obtained in two repeat experiments.
IFN-γ and TNF modulate cytokine production in T. gondii-infected neurons.
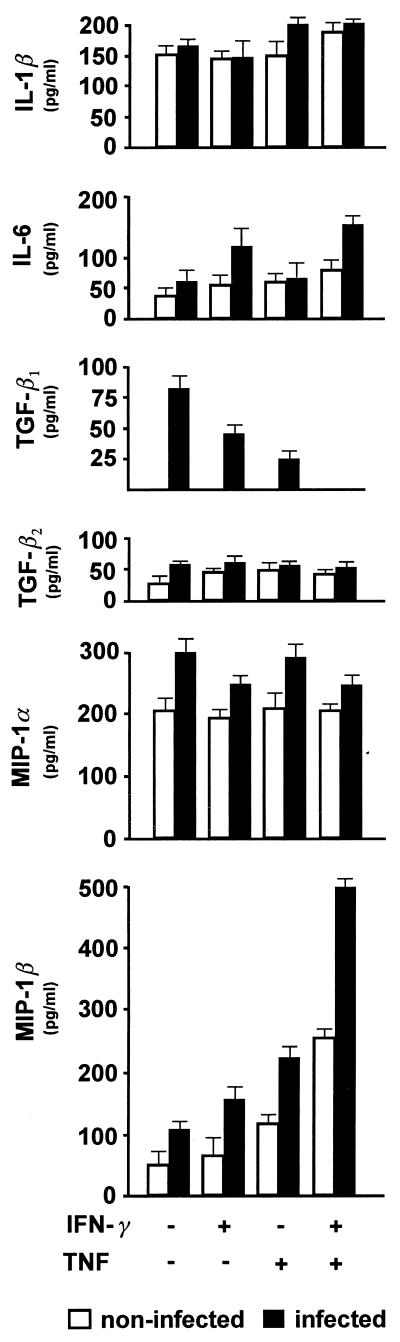
Selective isolation and cultivation of neurons enabled us to study for the first time cytokine and chemokine production in T. gondii-infected neurons. To analyze whether T. gondii infection of neurons induces production of cytokines and chemokines in neurons, a panel of these mediators was studied 48 h postinfection (Fig. 3). To detect cytokines and chemokines, commercially available enzyme-linked immunosorbent assays were used as recommended by the manufacturers. Interleukin 1β (IL-1β) and IL-6 assays were obtained from Biosource, Lucerne Chem AG, Lucerne, Switzerland; IL-10 and granulocyte-macrophage colony-stimulating factor (GM-CSF) assays were obtained from Endogen Inc., Boston, Mass.; and macrophage inflammatory protein 1α (MIP-1α), MIP-1β, transforming growth factor β1 (TGF-β1), and TGF-β2 assays were obtained from R&D Systems, RNT Systems, London, United Kingdom. The Wilcoxon test was used for statistical evaluation of the cytokine and chemokine data. Uninfected murine cerebellar neurons produced significant amounts of IL-1β, IL-6, and TGF-β2, which is consistent with the results of previous studies (5, 22). Infection of neurons with T. gondii and treatment with IFN-γ and/or TNF did not modulate IL-1β and TGF-β2 production. The low basal level of production of IL-6 was slightly upregulated by infection and by treatment with IFN-γ and IFN-γ plus TNF (P < 0.05 for infected and IFN-γ- and IFN-γ–TNF-stimulated neurons compared to the corresponding noninfected groups). In contrast, TGF-β1 production was significantly induced by infection of neurons (P < 0.01 for both infected nonstimulated neurons and infected IFN-γ-treated neurons compared to the corresponding uninfected groups; P < 0.05 for infected TNF-stimulated neurons compared to uninfected TNF-stimulated neurons) and declined in response to treatment with IFN-γ and/or TNF (P < 0.05 for infected IFN-γ-stimulated neurons compared to infected nonstimulated neurons; P < 0.01 for infected TNF-stimulated neurons compared to infected nonstimulated neurons). In addition to cytokines, neurons also produced MIP-1α, which was upregulated by infection with T. gondii (P < 0.05 for all groups of infected neurons compared to the corresponding groups of uninfected neurons), but production was not further increased by cytokine application. In contrast, MIP-1β expression in uninfected neurons was gradually upregulated by treatment with IFN-γ, TNF, and IFN-γ plus TNF and was further increased by T. gondii infection (P < 0.05 for infected nonstimulated, IFN-γ-stimulated, and TNF-stimulated neurons compared to the corresponding uninfected neurons; P < 0.01 for infected IFN-γ–TNF-stimulated neurons compared to uninfected IFN-γ–TNF-stimulated neurons; P < 0.05 for infected IFN-γ-stimulated neurons compared to infected nonstimulated neurons; P < 0.05 for both uninfected and infected TNF-stimulated neurons compared to the corresponding groups of nonstimulated neurons; P < 0.01 for both infected and uninfected IFN-γ–TNF-stimulated neurons compared to the corresponding groups of nonstimulated neurons). In contrast to the production of cytokines and chemokines mentioned above, neurons did not produce IL-10 and GM-CSF (data not shown). Since IL-10 and GM-CSF are produced by T. gondii-infected microglial cells and astrocytes, respectively (9), these findings further illustrate the purity of the neuronal cell cultures.
FIG. 3.
Cytokine and chemokine production by neurons. Neurons were treated as indicated with IFN-γ and/or TNF for 12 h before RH toxoplasms were added at an MOI of 1. At 48 h after infection the supernatant was harvested, and IL-1β, IL-6, IL-10, GM-CSF, TGF-β1, TGF-β2, MIP-1α, and MIP-1β contents were determined by enzyme-linked immunosorbent assays. IL-10 and GM-CSF were not detected (data not shown). The data are means ± standard deviations based on three wells per group. A second experiment yielded comparable results.
This study showed that T. gondii invades and replicates in murine cerebellar granule neurons and that IFN-γ and/or TNF does not inhibit parasite invasion and replication in neurons. The level of infected neurons was relatively high compared to data reported by Fagard et al. (6) for rat hippocampal neurons. However, as discussed by these authors, murine neurons may be better target cells for toxoplasms than rat neurons. The lack of IFN-γ and/or TNF activity that inhibits parasite invasion and replication in neurons is in marked contrast to the findings obtained for other CNS resident cells (i.e., astrocytes and microglia), which effectively restrict multiplication of T. gondii following stimulation with either IFN-γ or a combination of IFN-γ and TNF (4, 12). Interestingly, studies performed with murine bone marrow chimeras have revealed that expression of IFN-γ receptors and TNF receptors on both hematopoietic and radioresistant host cells, including microglia, astrocytes, and neurons, is required for efficient control of T. gondii (30). However, infection of mixed cultures of neurons, astrocytes, microglia, and oligodendrocytes with T. gondii resulted in spontaneous, IFN-γ-independent encystation of parasites within neurons and astrocytes (8, 16). Collectively, these findings and our observations indicate that IFN-γ and TNF play an important role in control of T. gondii in microglia and astrocytes but are not enough to control this parasite in neurons. The mechanisms that underlie astrocyte–microglia-mediated control of intraneuronal parasite replication have not been determined yet but may include soluble factors as diverse as neurotrophins like nerve growth factor, which has a strong immunoregulatory capacity (25, 26), and microglia-derived leukotrienes (17), which have been shown to have toxoplasmastatic activity in mast cells and macrophages (13, 31).
The neuronal production of the CC chemokines MIP-1α and MIP-1β, which can attract T cells, macrophages, and granulocytes (1), indicates that tachyzoite-infected neurons contribute to the recruitment of inflammatory leukocytes to the site of the offending pathogen. In fact, intracellular toxoplasms are generally surrounded by an infiltrate composed of T cells, macrophages, and some granulocytes (23). However, immunohistochemical studies of murine Toxoplasma encephalitis have shown that some bradyzoite-containing cysts are not accompanied by inflammatory leukocytes (23), which suggests that neuronal production of chemokines may depend on the growth stage of T. gondii. The production of IL-1β and IL-6 by neurons indicates that neurons contribute to intracellular parasite control, since both of these cytokines are important for the control of cerebral toxoplasms (14, 28). In addition, neuron-derived TGF-β2, an immunosuppressive cytokine which is also produced after infection of peritoneal macrophages with T. gondii (2), may contribute to regulation of the intracellular immune response. The regulatory activities of both IFN-γ and TNF during neuronal cytokine and chemokine production illustrate that neurons are also integrated via this pathway into the complex neuroimmunological network that provides protection against offending toxoplasms.
Acknowledgments
This work was supported by grant Schl 392/2-3 from the Deutsche Forschungsgemeinschaft to D. Schlüter.
We thank N. Kaefer for expert technical assistance and H. Klatt for photographic help.
REFERENCES
- 1.Asensio V C, Campbell I L. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez L E, Covaro G, Remington J. Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor beta and downregulation of expression of tumor necrosis factor receptors. Infect Immun. 1993;61:4126–4130. doi: 10.1128/iai.61.10.4126-4130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao C C, Anderson W R, Hu S, Gekker G, Martella A, Peterson P K. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin Immunol Immunopathol. 1993;67:178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- 4.Chao C C, Hu S, Gekker G, Novick W J, Jr, Remington J S, Peterson P K. Effects of cytokines on multiplication of Toxoplasma gondii in microglial cells. J Immunol. 1993;150:3404–3410. [PubMed] [Google Scholar]
- 5.Constam D B, Schmid P, Aguzzi A, Schachner M, Fontana A. Transient production of TGF-beta 2 by postnatal cerebellar neurons and its effect on neuroblast proliferation. Eur J Neurosci. 1994;6:766–778. doi: 10.1111/j.1460-9568.1994.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 6.Fagard R, Van Tan H, Creuzet C, Pelloux H. Differential development of Toxoplasma gondii in neural cells. Parasitol Today. 1999;15:504–507. doi: 10.1016/s0169-4758(99)01568-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson D J, Hutchison W M. The host-parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Arch A Pathol Anat Histopathol. 1987;411:39–43. doi: 10.1007/BF00734512. [DOI] [PubMed] [Google Scholar]
- 8.Fischer H G, Nitzgen B, Reichmann G, Gross U, Hadding U. Host cells of Toxoplasma gondii encystation in infected primary culture from mouse brain. Parasitol Res. 1997;83:637–641. doi: 10.1007/s004360050311. [DOI] [PubMed] [Google Scholar]
- 9.Fischer H G, Nitzgen B, Reichmann G, Hadding U. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur J Immunol. 1997;27:1539–1548. doi: 10.1002/eji.1830270633. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 12.Halonen S K, Chiu F, Weiss L M. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun. 1998;66:4989–4993. doi: 10.1128/iai.66.10.4989-4993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson W R, Jr, Chi E Y. The importance of leukotrienes in mast cell-mediated Toxoplasma gondii cytotoxicity. J Infect Dis. 1998;177:1437–1443. doi: 10.1086/517833. [DOI] [PubMed] [Google Scholar]
- 14.Jebbari H, Roberts C W, Ferguson D J, Bluethmann H, Alexander J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol (Oxford) 1998;20:231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones T C, Bienz K A, Erb P. In vitro cultivation of Toxoplasma gondii cysts in astrocytes in the presence of gamma interferon. Infect Immun. 1986;51:147–156. doi: 10.1128/iai.51.1.147-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luder C G, Giraldo-Velasquez M, Sendtner M, Gross U. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp Parasitol. 1999;93:23–32. doi: 10.1006/expr.1999.4421. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo M, Hamasaki Y, Fujiyama F, Miyazaki S. Eicosanoids are produced by microglia, not by astrocytes, in rat glial cell cultures. Brain Res. 1995;685:201–204. doi: 10.1016/0006-8993(95)00490-h. [DOI] [PubMed] [Google Scholar]
- 18.Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-d-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol. 1992;22:2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- 20.Rensing-Ehl A, Malipiero U, Irmler M, Tschopp J, Constam D, Fontana A. Neurons induced to express major histocompatibility complex class I antigen are killed via the perforin and not the Fas (APO-1/CD95) pathway. Eur J Immunol. 1996;26:2271–2274. doi: 10.1002/eji.1830260945. [DOI] [PubMed] [Google Scholar]
- 21.Robertson B, Kong G, Peng Z, Bentivoglio M, Kristensson K. Interferon-gamma-responsive neuronal sites in the normal rat brain: receptor protein distribution and cell activation revealed by Fos induction. Brain Res Bull. 2000;52:61–74. doi: 10.1016/s0361-9230(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 22.Schluter D, Kaefer N, Hof H, Wiestler O D, Deckert-Schluter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol. 1997;150:1021–1035. [PMC free article] [PubMed] [Google Scholar]
- 23.Schluter D, Lohler J, Deckert M, Hof H, Schwendemann G. Toxoplasma encephalitis of immunocompetent and nude mice: immunohistochemical characterisation of Toxoplasma antigen, infiltrates and major histocompatibility complex gene products. J Neuroimmunol. 1991;31:185–198. doi: 10.1016/0165-5728(91)90040-e. [DOI] [PubMed] [Google Scholar]
- 24.Sipe K J, Srisawasdi D, Dantzer R, Kelley K W, Weyhenmeyer J A. An endogenous 55 kDa TNF receptor mediates cell death in a neural cell line. Brain Res Mol Brain Res. 1996;38:222–232. doi: 10.1016/0169-328x(95)00310-o. [DOI] [PubMed] [Google Scholar]
- 25.Stanisz A M, Stanisz J A. Nerve growth factor and neuroimmune interactions in inflammatory diseases. Ann N Y Acad Sci. 2000;917:268–272. doi: 10.1111/j.1749-6632.2000.tb05392.x. [DOI] [PubMed] [Google Scholar]
- 26.Susaki Y, Shimizu S, Katakura K, Watanabe N, Kawamoto K, Matsumoto M, Tsudzuki M, Furusaka T, Kitamura Y, Matsuda H. Functional properties of murine macrophages promoted by nerve growth factor. Blood. 1996;88:4630–4637. [PubMed] [Google Scholar]
- 27.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 28.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen T A, Dalrymple S A, Murray R, Remington J S. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997;65:2339–2345. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki Y, Remington J S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 30.Yap G S, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong E C, Chi E Y, Henderson W R., Jr Toxoplasma gondii alters eicosanoid release by human mononuclear phagocytes: role of leukotrienes in interferon gamma-induced antitoxoplasma activity. J Exp Med. 1994;180:1637–1648. doi: 10.1084/jem.180.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]