Abstract
To examine the synergistic effects of alpha-toxin and perfringolysin O in clostridial myonecrosis, homologous recombination was used to construct an alpha-toxin deficient derivative of a perfringolysin O mutant of Clostridium perfringens. The subsequent strain was complemented with separate plasmids that carried the alpha-toxin structural gene (plc), the perfringolysin O gene (pfoA), or both toxin genes, and the resultant isogenic strains were examined in a mouse myonecrosis model. Synergistic effects were clearly observed in these experiments. Infection with the control strain, which did not produce either toxin, resulted in very minimal gross pathological changes, whereas the isogenic strain that was reconstituted for both toxins produced a pathology that was clearly more severe than when alpha-toxin alone was reconstituted. These changes were most apparent in the rapid spread of the disease, the gross pathology of the footpad and in the rate at which the mice had to be euthanatized for ethical reasons. Elimination of both alpha-toxin and perfringolysin O production removed most of the histopathological features typical of clostridial myonecrosis. These effects were restored when the mutant was complemented with the alpha-toxin structural gene, but reconstituting only perfringolysin O activity produced vastly different results, with regions of coagulative necrosis, apparently enhanced by vascular disruption, being observed. Reconstitution of both alpha-toxin and perfringolysin O activity produced histopathology most similar to that observed with the alpha-toxin reconstituted strain. The spreading of myonecrosis was very rapid in these tissues, and coagulative necrosis appeared to be restricted to the lumen of the blood vessels. The results of these virulence experiments clearly support the hypothesis that alpha-toxin and perfringolysin O have a synergistic effect in the pathology of gas gangrene.
Gas gangrene or clostridial myonecrosis is characterized by a rapid spread of tissue necrosis combined with a lack of leukocyte infiltration at the site of infection. Left untreated, its rapid and aggressive progression is almost always fatal. The most common causative organism, Clostridium perfringens type A, has the ability to produce numerous extracellular toxins (12) and is commonly found in the environment, in niches such as soil and sewage, and in the gastrointestinal tract of both humans and animals.
Alpha-toxin is the most toxic extracellular enzyme produced by C. perfringens type A and is essential for virulence (3, 10, 16). It is a phospholipase C that hydrolyzes both phosphatidylcholine and sphingomyelin (12, 18), both of which are important constituents of eukaryotic cell membranes. Alpha-toxin mutants are avirulent in a mouse myonecrosis model (3, 10, 17), with comparative histological examination of muscle tissue from mice infected with a series of isogenic strains indicating that alpha-toxin is required for tissue necrosis, inhibition of the influx of polymorphonuclear leukocytes (PMNL) into the lesion, and thrombosis formation (3, 10, 17). Thrombosis is a potentially important factor in the pathology of gas gangrene, since by reducing the oxygen tension within the tissues it presumably helps produce environmental conditions suitable for the growth of the invading anaerobic bacterium.
Perfringolysin O, or theta-toxin, is a cholesterol-dependent cytolysin that lyses red blood cells (19). Since perfringolysin O mutants still cause murine myonecrosis, the toxin is not essential for the disease, although it also has the ability to affect the host inflammatory response, particularly PMNL influx into the myonecrotic lesion. Perfringolysin O has been implicated in the vascular accumulation of leukocytes within blood vessels and the extracellular matrix of host tissues (5, 8, 10).
There is now good evidence that exotoxin-mediated functional modulation of the inflammatory cascade contributes significantly to the ineffective host response to C. perfringens infection (5, 8); vascular leukostasis and leukocyte paucity cannot be explained by the myonecrotic actions of the exotoxins alone (10). This hypothesis is supported by studies (5, 9) which showed that exposure to these toxins upregulated the production of cell adhesion molecules, important factors involved in leukocyte migration across the endothelium during inflammation. More recently, purified alpha-toxin was shown to strongly induce homotypic and heterotypic platelet aggregation, which would promote leukostasis within the vasculature (6, 7). Therefore, alpha-toxin seems to be of major importance with respect to reducing blood flow and impeding the migration of leukocytes to the site of infection. While work carried out by using isogenic sets of genetically modified strains of C. perfringens that were defective in the production of either alpha-toxin or perfringolysin O did not provide evidence to support these claims, it did show that a perfringolysin O-deficient strain produced no detectable leukocyte aggregation within the infected tissue (10). Since thrombosis was present in many blood vessels and thrombotic occlusions in these tissues were completely free of leukocyte accumulation, these studies provided further evidence that leukocyte paucity is not purely due to cytotoxic effects.
Previous studies have suggested that these toxins may work synergistically to produce effects on the inflammatory system (5, 8, 10, 17). To gain a better understanding of this phenomenon, we decided to construct a C. perfringens strain mutated in both the alpha-toxin (plc) and perfringolysin O (pfoA) structural genes and then to complement this mutant with various combinations of the wild-type genes.
To isolate a plc pfoA double mutant, the pfoA mutant JIR4069 (3) was transformed (14) to tetracycline resistance with the plc-suicide vector pJIR1774 (Fig. 1). This plasmid is a pUK21 (20) derivative that carries an internal 0.53-kb Sau3A-derived plc fragment from pTOX6 (13) and the tet(M) gene from Tn916. The resultant tetracycline-resistant transformants were screened on egg yolk agar and horse blood agar for their ability to produce alpha-toxin and perfringolysin O, respectively, and a mutant that was unable to produce either toxin was chosen for further study. This mutant, JIR4444, was also resistant to rifampin, nalidixic acid, and erythromycin, as expected for a derivative of JIR4069.
FIG. 1.
Construction of a plc pfoA double mutant by homologous recombination. A single crossover event between the plc gene located on the chromosome of the pfoA mutant JIR4069 (A) and the truncated plc gene located on the suicide vector pJIR1774 led to the construction of the plc pfoA double mutant JIR4444 (B). The insert shows the results of Southern hybridization analysis of EcoRI-digested chromosomal DNA from the wild-type strain JIR325 and mutant strains JIR4069 and JIR4444 (lanes 1, 2, and 3, respectively), probed as indicated. Molecular size standards are also shown (lane S). The arrow indicates the position of the faint 1.5-kb band detected in lanes 2 and 3 with the pfoA-specific probe.
Southern hybridization analysis was used to confirm that JIR4444 had the genotype expected from a single crossover between the plc fragment carried on pJIR1774 and the plc gene located on the JIR4069 chromosome (Fig. 1). Chromosomal DNA was purified (1, 3) from wild-type strain JIR325 and mutant strains JIR4069 and JIR4444, digested with EcoRI, subjected to electrophoresis on 0.8% agarose gels, and transferred to nylon membranes (Hybond N; Amersham). Southern blots were hybridized overnight at 65°C and washed at high stringency (65°C with 0.1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) with either a 0.95-kb BamHI-HindIII fragment from pTOX6 (internal to the plc gene,); a 0.9-kb SpeI-HindIII fragment from pTS302 (internal to pfoA gene) (15); the 3.0-kb EcoRI fragment from pVB101 (V. Burdett, unpublished), which contains the tet(M) gene; or a 0.7-kb PCR product generated from pJIR870, which is internal to the regulatory virR gene (11). Each probe was labeled by using digoxigenin-labeled dUTP by random priming as specified by the manufacturer (Boehringer Mannheim). As expected, the plc probe hybridized to a 4.6-kb band in both JIR325 and the pfoA mutant JIR4069, whereas two bands (4.7 and 3.3 kb) were observed with JIR4444 (Fig. 1). This result was consistent with the predicted single crossover event. When probed with the pfoA-specific fragment, the expected 6.7-kb hybridizing band was observed with JIR325, while JIR4069 and JIR4444 each had a 3.6-kb band and a 1.5-kb band, as predicted. To confirm that the chromosomal rearrangements were specific for the plc gene, the DNA was probed with a virR-specific fragment. In all three chromosomal preparations, a 2.5-kb band hybridized with the probe (data not shown). Finally, only JIR4444 hybridized with the tet(M)-specific probe, with the expected 3.0-kb band being observed (Fig. 1). These results confirmed that JIR4444 was a plc mutant derived from homologous recombination between pJIR1774 and the JIR4069 chromosome.
The mutated pfoA and plc genes located on the JIR4444 chromosome were complemented by transformation with separate C. perfringens-Escherichia coli shuttle vectors that each carried a chloramphenicol resistance gene. These plasmids included the negative control, pJIR750 (4); pJIR871, which carries the wild-type pfoA gene (3); pJIR1642, a pJIR750 derivative that contains the wild-type plc gene from pTOX6; and pJIR1720, a pJIR871 derivative that carries the same fragment from pTOX6 and therefore harbors the wild-type plc and pfoA genes. Each of the JIR4444-derived transformants had the correct phenotypes when grown on egg yolk agar and horse blood agar . Quantitative assays were then performed to determine the toxin levels produced by each strain. The results (Table 1) were in agreement with the qualitative data. No detectable perfringolysin O or hemolytic activity was observed in culture filtrates derived from the pfoA mutants, unless they had been complemented with the wild-type pfoA gene, in which case hemolytic activity was similar to wild-type levels. Similar results were observed for alpha-toxin activity, although alpha-toxin levels in JIR4069 and JIR4444(pJIR1642) were a little lower than in the wild type (Table 1), for reasons not understood at this time. Taken together, these data indicate that the shuttle plasmids were able to complement the respective plc and pfoA mutations. In particular, the double complementation plasmid pJIR1720 was found to restore both perfringolysin O and alpha-toxin activity back to wild-type levels.
TABLE 1.
Complementation of the plc pfoA mutant JIR4444a
| Strain | Genotype | Plasmid-encoded toxin gene(s) | Perfringolysin O level (log2 titer) | Mean alpha-toxin level ± SD (U/mg of protein [102]) |
|---|---|---|---|---|
| JIR325 | Rifr Nalr | 7.2 ± 0.5 | 1.97 ± 0.20 | |
| JIR4069 | JIR325 ΔpfoA::ermB | <1 | 0.61 ± 0.11 | |
| JIR4444 | JIR4069 plcΩpJIR1774 | <1 | <0.09 ± 0.01 | |
| JIR4459 | JIR4444(pJIR750) | <1 | <0.09 ± 0.01 | |
| JIR4460 | JIR4444(pJIR871) | pfoA+ | 6.7 ± 0.3 | <0.10 ± 0.01 |
| JIR4461 | JIR4444(pJIR1642) | plc+ | <1 | 0.60 ± 0.17 |
| JIR4462 | JIR4444(pJIR1720) | pfoA+plc+ | 6.7 ± 0.3 | 1.90 ± 0.06 |
Perfringolysin O and phospholipase C assays were performed as previously described (3). The protein concentration was determined by using a microtiter plate protocol from the BCA Protein Assay Reagent Kit (Pierce), with bovine serum albumin as the standard. The results represent the average of duplicate assays carried out on preparations from three separate cultures of each strain.
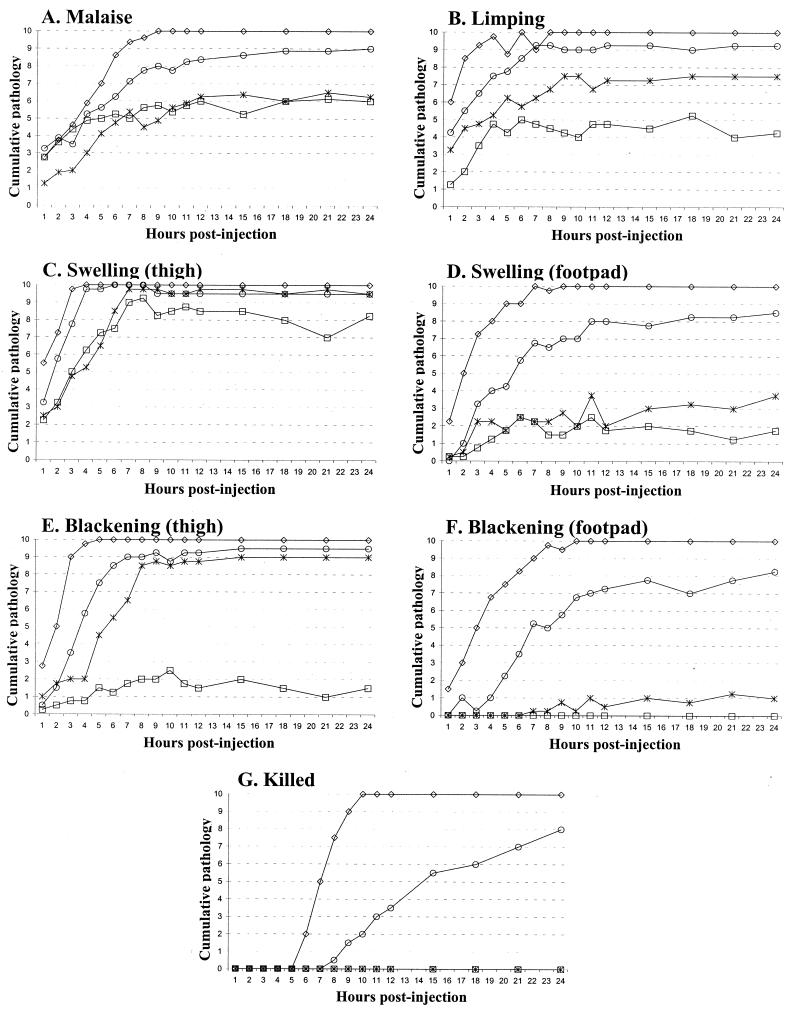
To quantify the relative contributions of alpha-toxin and theta-toxin to the pathology of clostridial myonecrosis, BALB/c mice were injected with this isogenic series of JIR4444-derived C. perfringens strains (Fig. 2). Based upon previous work with single toxin knockout strains (3), it was expected that complementation of the double mutant with the alpha-toxin structural gene would result in a more severe pathology than complementation with the perfringolysin O gene. The results (Fig. 2) were in agreement with this prediction except that blackening of the thigh was observed to be equally as severe when either alpha-toxin or perfringolysin O was produced (Fig. 2E). Synergistic effects of alpha-toxin and perfringolysin O were clearly observed in these experiments. Infection with the control strain, which did not produce either toxin, resulted in very minimal pathological changes, whereas the isogenic strain that was reconstituted for both toxins produced a pathology that was clearly more severe than when alpha-toxin alone was reconstituted. These changes were most apparent in the rapid spread of the disease pathology to the footpad and in the rate at which the mice had to be euthanatized for ethical reasons (Fig. 2).
FIG. 2.
Gross pathology of infected mice. BALB/c mice were injected with an isogenic series of JIR4444-derived C. perfringens strains. For each strain, 10 6- to 8-week-old mice were injected intramuscularly in the right thigh with 100 μl of a washed cell suspension that contained ca. 109 CFU (10). The identity of each strain was independently encoded prior to injection to ensure that the study was carried out blind. After injection, the mice were monitored regularly and detailed observations of the gross pathological changes were noted over a 24-h period, as previously described (2). These observations involved scoring each mouse for outward signs of illness, which included matting of the fur and lack of response to environmental stimuli, as well as for the severity of swelling and blackening (scored for both the thigh and foot as an indicator of the spread of infection) and for the ability to move the infected limb (limping). The severity of each pathology parameter was scored as either 0 (no discernible pathology), ½ (moderate pathology), or 1 (marked pathology apparent). The number of severely affected mice killed for animal ethics reasons was also recorded. Note that cumulative pathology on the y axis refers to the sum of the individual pathology scores from 10 mice, the number 10 therefore represents a maximal score. Key: □, JIR4444(pJIR750), the plc pfoA double mutant carrying a vector plasmid; ×, JIR4444(pJIR871), the double mutant carrying a pfoA+ plasmid; ○, JIR4444(pJIR1642), the double mutant carrying a plc+ plasmid; and ⋄, JIR4444(pJIR1720), the double mutant carrying a plc+ pfoA+ plasmid.
In separate experiments, mice were killed at different times after infection, and the histopathology of the infected muscle tissue was examined (Fig. 3). Infection with the wild-type strain produced the typical pathology of advanced myonecrosis, thrombosis and vascular leukostasis (Fig. 3A). Leukocyte infiltration of these tissues was minimal and was typically restricted to small aggregates at the borders of myonecrosis. Elimination of both alpha-toxin and perfringolysin O production removed most of the features typical of clostridial myonecrosis (Fig. 3B). Little or no vascular leukostasis was apparent, and numerous leukocytes infiltrated these tissues and migrated fully to the seat of infection. Indeed, tissues from mice infected with these strains presented almost identical histopathology to that observed in previous experiments from mice injected with washed, heat-killed C. perfringens cells (data not shown).
FIG. 3.
Hematoxylin-and-eosin-stained sections from infected tissues. Mice were injected as described in the legend to Fig. 2, with the strains indicated (see Fig. 2). Two mice were randomly chosen from each infected group at various times after injection. After each mouse was killed, the infected limb was exposed by blunt dissection, allowing observation of the severity and spread of necrosis. Samples of the muscle were then removed and fixed overnight in a solution of 0.5% tannic acid (wt/vol) in methanol before paraffin embedding and sectioning for hematoxylin and eosin staining and analysis. Symbols and abbreviations: arrows, leukocyte aggregates; asterisks, bacterial aggregates; mn, myonecrosis; cn, coagulative necrosis; th, thrombosis; bv, blood vessel tissue.
Reconstituting only perfringolysin O activity in the double mutant produced vastly different results (Fig. 3C and D). While some areas of these tissues displayed the expected myonecrosis (Fig. 3C), this pathology was quickly replaced or obscured by vast regions of coagulative necrosis, apparently enhanced by vascular disruption (Fig. 3D). As a result, the presence or absence of vascular leukostasis was difficult to determine. In contrast, tissue samples from mice infected with the alpha-toxin reconstituted double mutant displayed pathology more typical of clostridial myonecrosis (Fig. 3E and F). Extensive myonecrosis and thrombosis were observed in areas surrounding the seat of infection. Some vascular leukostasis was apparent but was primarily restricted to arterioles and not to capillaries and venules (Fig. 3E). Numerous leukocytes were observed to infiltrate the tissue by 8 h (Fig. 3F), although this infiltration appeared to be restricted to nonnecrotic areas. Note that, in a recent study, leukostasis was found to predominate in the venular system, particularly early after injection of crude toxin preparations or purified alpha-toxin, and was found to be triggered by the alpha-toxin-mediated aggregation of activated platelets (6, 7).
Reconstitution of both alpha-toxin and perfringolysin O activity produced histopathology most similar to that observed with the alpha-toxin reconstituted strain (Fig. 3G and H). Some vascular leukostasis was observed (Fig. 3G), although it was not as severe as that observed when infecting with the wild-type strain. The spreading of myonecrosis was very rapid in these tissues, and the coagulative necrosis previously observed upon infection with the perfringolysin O reconstituted strain appeared to be restricted to the lumen of the blood vessels (Fig. 3H). Some thrombosis was observed, but it was mostly replaced or obscured by the coagulative necrosis.
The results of these virulence experiments clearly support the hypothesis that alpha-toxin and perfringolysin O have a synergistic effect in the pathology of gas gangrene, particularly when the rate at which pathology became apparent in the footpad and the rate at which the mice were killed due to ethical intervention are taken into consideration. Indeed, no mice injected with the doubly reconstituted strain survived beyond 10 h in these experiments, so rapid and severe were the combined effects of both toxins.
It was hoped that the mechanism of this synergy could be elucidated from these experiments, but a complicating factor in the toxin reconstitution process became apparent during the histopathology studies. Specifically, the prominence of coagulative necrosis in the pathology of infection by the perfringolysin O reconstituted C. perfringens strain was markedly different from that expected from previous experiments with single toxin knockout mutants (3, 10, 17). This difference appears to be a result of the absence of alpha-toxin, since coagulative necrosis seems to be confined to the lumen of blood vessels with the double reconstituted strain. Despite this complication, the results clearly show that perfringolysin O and alpha-toxin have markedly different roles in an infected lesion. Having many of the distinctive features of gas gangrene, infection with the alpha-toxin reconstituted double mutant provides yet more evidence for the primary role that alpha-toxin plays in the progression of disease. However, vascular leukostasis and a paucity of leukocyte infiltration, both of which are very typical in gas gangrene lesions, were absent when perfringolysin O was not present. Unfortunately, it was very difficult to show that these effects were reversed in the presence of just perfringolysin O, due to the massive coagulative necrosis and vascular disruption that occurs when alpha-toxin is not present to isolate these effects to the lumen of the vasculature.
Previous studies provided evidence that the ability to produce extracellular collagenase was not a major virulence factor in clostridial myonecrosis (2). Our current results show that a double mutant unable to produce either alpha-toxin or perfringolysin O had a disease pathology almost identical to that seen in mice injected with washed, heat-killed C. perfringens type A cells. That is, a pathology consistent with a normal inflammatory response that successfully clears a bacterial infection. These studies provide further evidence that alpha-toxin and perfringolysin O are the major C. perfringens extracellular toxins involved in the pathology of gas gangrene.
Acknowledgments
J.I.R. gratefully acknowledges research grant support from the Australian National Health and Medical Research Council.
REFERENCES
- 1.Abraham L J, Rood J I. Molecular analysis of transferable tetracycline resistance plasmids from Clostridium perfringens. J Bacteriol. 1985;161:636–640. doi: 10.1128/jb.161.2.636-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awad M, Ellemor D, Bryant A, Matsushita O, Boyd R, Stevens D, Emmins J, Rood J. Construction and virulence testing of a collagenase mutant of Clostridium perfringens. Microb Pathog. 2000;28:107–117. doi: 10.1006/mpat.1999.0328. [DOI] [PubMed] [Google Scholar]
- 3.Awad M M, Bryant A E, Stevens D L, Rood J I. Virulence studies on chromosomal α-toxin and θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15:191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 4.Bannam T L, Rood J I. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid. 1993;29:223–235. doi: 10.1006/plas.1993.1025. [DOI] [PubMed] [Google Scholar]
- 5.Bryant A E, Bergstrom R, Zimmerman G A, Salyer J L, Hill H R, Tweten R K, Sato H, Stevens D L. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol Med Microbiol. 1993;7:321–336. doi: 10.1111/j.1574-695X.1993.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 6.Bryant A E, Chen R Y, Nagata Y, Wang Y, Lee C H, Finegold S, Guth P H, Stevens D L. Clostridial gas gangrene. I. Cellular and molecular mechanisms of microvascular dysfunction induced by exotoxins of Clostridium perfringens. J Infect Dis. 2000;182:799–807. doi: 10.1086/315756. [DOI] [PubMed] [Google Scholar]
- 7.Bryant A E, Chen R Y, Nagata Y, Wang Y, Lee C H, Finegold S, Guth P H, Stevens D L. Clostridial gas gangrene. II. Phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. J Infect Dis. 2000;182:808–815. doi: 10.1086/315757. [DOI] [PubMed] [Google Scholar]
- 8.Bryant A E, Stevens D L. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect Immun. 1996;64:358–362. doi: 10.1128/iai.64.1.358-362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunting M, Lorant D E, Bryant A E, Zimmerman G A, McIntyre T M, Stevens D L, Prescott S M. Alpha toxin from Clostridium perfringens induces proinflammatory changes in endothelial cells. J Clin Investig. 1997;100:565–574. doi: 10.1172/JCI119566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellemor D, Baird R, Awad M, Boyd R, Rood J, Emmins J. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect Immun. 1999;67:4902–4907. doi: 10.1128/iai.67.9.4902-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyristis M, Bryant A E, Sloan J, Awad M M, Nisbet I T, Stevens D L, Rood J I. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol Microbiol. 1994;12:761–777. doi: 10.1111/j.1365-2958.1994.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 12.Rood J I. Virulence genes of Clostridium perfringens. Annu Rev Microbiol. 1998;52:333–360. doi: 10.1146/annurev.micro.52.1.333. [DOI] [PubMed] [Google Scholar]
- 13.Saint-Joanis B, Garnier T, Cole S T. Gene cloning shows the alpha toxin of Clostridium perfringens to contain both sphingomyelinase and lecithinase activities. Mol Gen Genet. 1989;219:453–460. doi: 10.1007/BF00259619. [DOI] [PubMed] [Google Scholar]
- 14.Scott P T, Rood J I. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene. 1989;82:327–333. doi: 10.1016/0378-1119(89)90059-0. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu T, Okabe A, Minami J, Hayashi H. An upstream regulatory sequence stimulates expression of the perfringolysin O gene of Clostridium perfringens. Infect Immun. 1991;59:137–142. doi: 10.1128/iai.59.1.137-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens D L, Troyer B E, Merrick D T, Mitten J E, Olson R D. Lethal effects and cardiovascular effects of purified α- and θ-toxins from Clostridium perfringens. J Infect Dis. 1988;157:272–279. doi: 10.1093/infdis/157.2.272. [DOI] [PubMed] [Google Scholar]
- 17.Stevens D L, Tweten R, Awad M M, Rood J I, Bryant A E. Clostridial gas gangrene: evidence that α and θ toxins differentially modulate the immune response and induce acute tissue necrosis. J Infect Dis. 1997;176:189–195. doi: 10.1086/514022. [DOI] [PubMed] [Google Scholar]
- 18.Titball R, Rood J. Bacterial phospholipases. In: Aktories K, Just I, editors. Bacterial protein toxins. Berlin, Germany: Springer-Verlag; 2000. pp. 529–556. [Google Scholar]
- 19.Tweten R K. The thiol-activated clostridial toxins. In: Rood J I, McClane B A, Songer G S, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, England: Academic Press; 1997. pp. 211–221. [Google Scholar]
- 20.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]