Abstract
We looked at the effect of inhibiting caspases on amebic liver abscess in the mouse model of infection. A dose of the pan-caspase inhibitor benzyloxycarbonyl-V-A-D-O-methyl fluoromethyl ketone (Z-VAD-FMK; R & D Systems) given to SCID mice 2 h prior to direct hepatic inoculation with Entamoeba histolytica trophozoites, and 12 h after amebic inoculation, reduced the mean liver abscess size by 70% at 24 h compared to a control group. These data indicate that apoptosis plays a significant but not an exclusive role in amebic liver abscess formation in the mouse model.
The intestinal protozoan parasite Entamoeba histolytica kills mammalian cells in a contact-dependent manner (6, 9). How these cells die remains controversial. A careful in vitro study indicated that host cells die primarily by lytic necrosis, induced by pore-forming molecules (amoebapores) produced by E. histolytica trophozoites (1). However, E. histolytica trophozoites can also induce cells to undergo apoptosis (5, 8, 11). Apoptosis, or programmed cell death, is an ordered system of cell death, with an initiator or signaling phase, followed by an effector stage which executes cell death by degrading various cellular components. A critical component of the effector stage is the activation of caspases, cysteine proteinases with specificity for aspartate residues. Activation of caspases results in the cleavage of a number of cellular substrates leading to the apoptotic phenotype. A characteristic biochemical marker of apoptosis is endonuclease cleavage of chromatin between histone bodies, which results in a DNA ladder formation on separating gels. DNA fragmentation can also be detected by labeling of single- or double-stranded DNA breaks with terminal deoxyribonucleotidyltransferase (TUNEL assay) (10).
The major extraintestinal manifestation of amebiasis is amebic liver abscess, where E. histolytica trophozoites cause large, but circumscribed, areas of hepatocyte death with relatively few signs of diffuse liver inflammation (2). Amebic liver abscesses in mice are associated with apoptosis of hepatocytes and inflammatory cells (11). DNA ladder formation was detected in samples of abscessed liver from SCID mice by 1 h following E. histolytica inoculation (11). TUNEL staining showed areas of apoptosis within amebic liver abscesses, with staining in inflammatory cells and hepatocytes directly contacting amebic trophozoites, as well as staining in more distally located hepatocytes (11). Studies using mice lacking Fas ligand or Fas receptor and mice with targeted deletion of the tumor necrosis factor receptor 1 (TNFR1) showed that E. histolytica-induced apoptosis can occur independently of the Fas-ligand or TNFR1 pathways (11).
To further investigate the contribution of apoptotic cell death to amebic liver abscess formation, we set out to block apoptosis by inhibiting caspases in SCID mice. A group of 14 SCID mice (female; age, 8 to 10 weeks) received 100 μl of a 10 μM solution of the general caspase inhibitor benzyloxylcarbonyl-V-A-D-O-methyl fluoromethyl ketone (Z-VAD-FMK; R & D Systems) intraperitoneally 2 h before intrahepatic inoculation of 106 E. histolytica HM1:IMSS trophozoites, and a second dose of the same quantity of Z-VAD-FMK 12 h after E. histolytica inoculation (7). A second group of 14 age- and sex-matched SCID mice received 100 μl of phosphate-buffered saline (PBS) intraperitoneally, 2 h before, and 12 h after, intrahepatic inoculation of 106 E. histolytica HM1:IMSS trophozoites. A third group of five age- and sex-matched SCID mice received two doses of Z-VAD-FMK on the same schedule as groups 1 and 2, and an intrahepatic PBS inoculation instead of E. histolytica trophozoites. There was one death associated with anesthesia during the intrahepatic inoculation of E. histolytica in a Z-VAD-FMK-treated animal. Twenty-four hours after intrahepatic challenge, SCID mice in each group were sacrificed. Venous blood was obtained for measurements of serum alanine aminotransferase (ALT) from six randomly selected mice in both the Z-VAD-FMK-treated and control liver abscess groups and all mice in the Z-VAD-FMK-treated sham-injected group. Livers were removed from SCID mice and the area of liver abscess was excised and weighed to obtain the percentage of the liver occupied by abscess (4).
Amebic liver abscesses were significantly smaller in mice receiving the caspase inhibitor Z-VAD-FMK. As summarized in Table 1, the mean liver abscess size in Z-VAD-FMK-treated SCID mice was 9.7% ± 5.4% compared to 33.5% ± 16% in SCID mice that were treated with PBS. This difference was significant at P < 0.001 (two-tailed t test). All mice in the control group (PBS) had an amebic liver abscess, while one of the mice in the Z-VAD-FMK-treated group did not have a detectable abscess. SCID mice receiving Z-VAD-FMK alone without any amebic challenge did not develop liver abscesses. Thus, pretreatment of animals with the caspase inhibitor Z-VAD-FMK significantly reduces amebic liver abscess size in the murine model of disease.
TABLE 1.
Caspase inhibition with Z-VAD-FMK reduces amebic liver abscess size in SCID micea
| Group | No. of SCID mice | E. histolytica challenge | Z-VAD-FMK treatment | % Liver abscessed [mean ± SD mean (ranged)] | ALT (U/liter)b |
|---|---|---|---|---|---|
| 1 (n = 6) | 13 | Yes | Yes | 9.7 ± 6.3 (0–18) | 329 ± 276 |
| 2 (n = 6) | 14 | Yes | No | 33.5 ± 20 (13–86) | 386 ± 172 |
| 3 (n = 5) | 5 | No | Yes | —c | 40 ± 11 |
The difference in mean abscess size between the Z-VAD-FMK-treated and control groups was significant at a P of <0.001 (two-tailed t test).
Normal range for ALT, 26 to 77.
—, no abscesses.
We also measured serum ALT levels, a marker for hepatocellular damage and liver inflammation in all three groups, to exclude a toxic effect of Z-VAD-FMK on normal livers, and as an independent marker of hepatocyte damage in SCID mice. Serum ALT levels were above normal in both groups of mice with amebic liver abscesses (Table 1). However, despite a marked difference in abscess size, there was not a significant difference in ALT levels between mice treated with the caspase inhibitor and those receiving PBS. SCID mice receiving Z-VAD-FMK and an intrahepatic inoculation with PBS did not show a significant elevation in ALT levels, excluding an independent toxic effect of VAD or hepatic inoculation on murine liver cells.
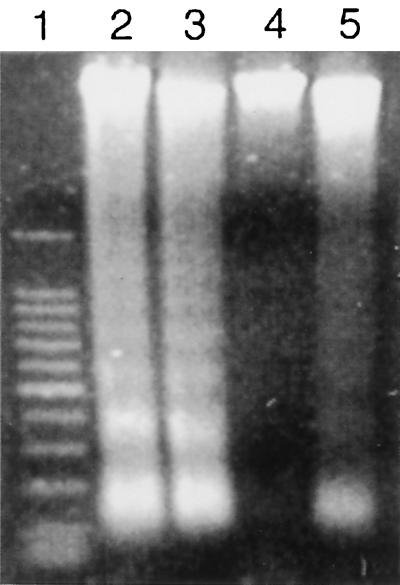
To confirm that caspase blockade had been achieved, and to determine whether the amebic liver abscesses seen in Z-VAD-FMK-treated mice had signs of apoptosis, we looked for DNA ladder formation in amebic liver abscesses from control and Z-VAD-FMK-treated mice. Genomic DNA was obtained from sections of abscessed liver as previously described (11). Five micrograms of DNA was loaded onto a 1.2% agarose gel and subjected to electrophoresis at 100 V for 75 min. Agarose gel electrophoresis of DNA obtained from amebic liver abscesses in SCID mice receiving Z-VAD-FMK showed no DNA ladder formation (Fig. 1, lane 4), or faintly visible ladders (Fig. 1, lane 5), while prominent ladder formation was present in DNA obtained at the 24-h time point in DNA from amebic liver abscess in the control group (Fig. 1, lanes 2 and 3).
FIG. 1.
Apoptotic changes are not detected in genomic DNA obtained from regions of amebic liver abscess in Z-VAD-FMK-treated SCID mice at 24 h after infection. Genomic DNA from amebic liver abscesses in control SCID mice (lanes 2 and 3) shows the characteristic 180-bp DNA ladder formation, while genomic DNA obtained from amebic liver abscesses in SCID mice treated with Z-VAD-FMK does not ladder (lane 4) or shows faint ladder formation (lane 5). Lane 1 contains the 100-bp DNA markers (Promega, Madison, Wis.).
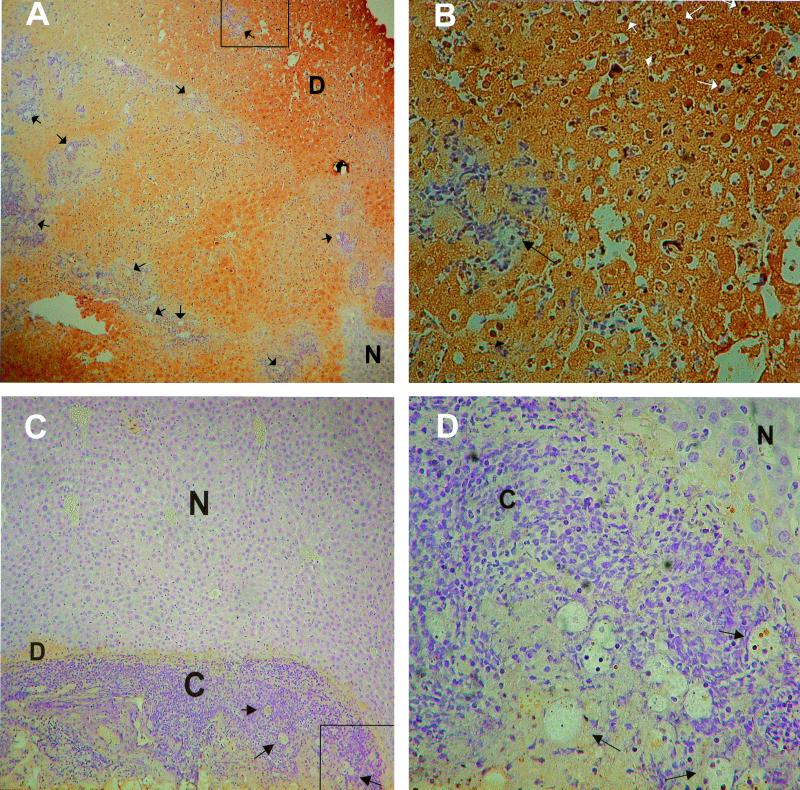
We compared the histologic findings in amebic liver abscesses from Z-VAD-FMK-treated SCID mice and control SCID mice. Sections of abscessed liver were fixed in formalin and embedded in paraffin for TUNEL staining using the in situ cell death detection kit POD (Roche, Indianapolis, Ind.) according to the manufacturer's instructions. A 10% solution of 3, 3′-diaminobenzidine in PBS (Sigma, St. Louis, Mo.) was used for detection, and all slides were counterstained with hematoxylin for 30 s. Amebic liver abscesses from control SCID mice had large areas of dead hepatocytes, which showed diffuse TUNEL staining (Fig. 2A). Small regions of normal-appearing hepatocytes could often be seen within areas of abscess. E. histolytica trophozoites were visible in sections, almost always surrounded by small numbers of inflammatory cells. At higher magnification, dead hepatocytes with condensed TUNEL-positive nuclei were seen (Fig. 2B), indicating that these hepatocytes had undergone apoptosis. Amebic liver abscesses from Z-VAD-FMK-treated SCID mice showed smaller regions of dead hepatocytes, with some cytoplasmic uptake of the TUNEL stain. The dead hepatocytes bordered areas of cellular infiltration, which was often more prominent in amebic liver abscesses from Z-VAD-FMK-treated mice (Fig. 2C). E. histolytica trophozoites were visible within the cellular infiltrate, and at a higher magnification, neutrophils were seen to be a prominent component of the inflammatory infiltrate (Fig. 2D). Little to no TUNEL staining was seen in nuclei from hepatocytes adjacent to E. histolytica trophozoites, or in the nuclei of the surrounding inflammatory cells, consistent with blockade of apoptosis by Z-VAD-FMK treatment.
FIG. 2.
Histologic findings in amebic liver abscesses from control and Z-VAD-FMK-treated SCID mice 24 h following infection. (A) TUNEL staining of a section of amebic liver abscess from a SCID mouse. Most of the liver is occupied by dead hepatocytes (D), which have taken up the TUNEL stain (brown coloration). Multiple E. histolytica trophozoites (some of which are indicated by the arrows) are visible throughout the liver, usually surrounded by pockets of inflammatory cells. A small region of normal-appearing liver (N) is indicated. The portion of the abscess examined in panel B is indicated by the box. Magnification, ×50. (B) Detail from panel A, showing a region of dead hepatocytes surrounding an E. histolytica trophozoite and a small number of inflammatory cells. Multiple apoptotic nuclei stained by TUNEL are indicated by the small arrows (white and black), while a single amebic trophozoite is indicated by the large arrow. Magnification, ×340. (C) TUNEL staining of a section of amebic liver abscess from a Z-VAD-FMK-treated SCID mouse. A small rim of dead hepatocytes (D) surrounds a region of increased cellularity (C) and E. histolytica trophozoites (some of which are indicated by arrows). Some take-up of TUNEL stain (brown coloration) is seen in the dead hepatocytes. Most of the field is occupied by normal-appearing liver (N). The portion of the abscess examined in panel D is indicated by the box. Magnification, ×85. (D) Detail from panel C, showing multiple amebic trophozoites, (some of which are indicated by arrows) surrounded by inflammatory cells (C) including many neutrophils. There is little to no TUNEL staining in nuclei of inflammatory cells, or in the adjacent region of normal-appearing hepatocytes (N). Magnification, ×340.
Taken as a whole, these findings suggest that hepatocyte apoptosis plays a significant role in the formation of amebic liver abscesses in the mouse model of disease. They also indicate that amebic activation of caspases is a key event in liver abscess formation. These data are consistent with recent studies indicating that caspase activation is seen in Jurkat cells within minutes of contact with E. histolytica trophozoites, and that blockade of caspase 3 could block Jurkat cell death (5).
It should be emphasized that our data also indicate that apoptosis is not the sole pathway for amebic liver abscess formation in the mouse model. Amebic abscesses were seen in livers from mice treated with the caspase inhibitor. We cannot absolutely exclude the possibility that some of these abscesses could be secondary to apoptosis associated with residual caspase activity, due to incomplete blockade by Z-VAD-FMK. However, the complete absence of ladder formation in DNA samples obtained from some abscesses in Z-VAD-FMK-treated mice, as well as the absence of nuclear TUNEL-staining in histologic sections, makes it unlikely that residual caspase activity was solely responsible for amebic liver abscess formation in these animals. A more likely mechanism is direct cytolysis of hepatocytes by E. histolytica trophozoites through the action of the amebapore molecule (1). We hypothesize that both E. histolytica-induced cytolysis and apoptosis may contribute to amebic liver abscess formation in the mouse model. Direct contact with amebic trophozoites may cause either hepatocyte necrosis or apoptosis, but hepatocyte death distal to areas of immediate contact between E. histolytica trophozoites and hepatocytes may be primarily due to apoptosis. This would explain the predominant histologic findings in amebic liver abscesses in control SCID mice: large areas of dead hepatocytes, many with TUNEL-positive nuclei, but relatively few in direct contact with E. histolytica trophozoites. Apoptosis in these cells could be secondary to molecules released by amebae, such as amebapore or proteinases (13, 14), molecules released by host cells that were lysed by E. histolytica trophozoites (3, 12), or ischemia and infarction caused by amebic disruption of the liver vasculature.
Acknowledgments
This study was supported by NIH grant AI30084 to S.L.S. and grant DK52574 to the Washington University Digestive Diseases Research Center. S.L.S. is a Burroughs Wellcome Scholar in Molecular Parasitology.
REFERENCES
- 1.Berninghausen O, Leippe M. Necrosis versus apoptosis as the mechanism of target cell death induced by Entamoeba histolytica. Infect Immun. 1997;65:3615–3621. doi: 10.1128/iai.65.9.3615-3621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt H, Pérez Tamayo R. Pathology of human amebiasis. Hum Pathol. 1970;1:351–385. doi: 10.1016/s0046-8177(70)80072-7. [DOI] [PubMed] [Google Scholar]
- 3.Burchard G D, Prange G, Mirelman D. Interaction between trophozoites of Entamoeba histolytica and the human intestinal cell line HT-29 in the presence and absence of leukocytes. Parasitol Res. 1993;79:140–145. doi: 10.1007/BF00932260. [DOI] [PubMed] [Google Scholar]
- 4.Cieslak P R, Virgin IV H W, Stanley S L., Jr A severe combined immunodeficient (SCID) mouse model for infection with Entamoeba histolytica. J Exp Med. 1992;176:1605–1609. doi: 10.1084/jem.176.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huston C D, Houpt E R, Mann B J, Hahn C S, Petri W A., Jr Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2000;2:617–625. doi: 10.1046/j.1462-5822.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 6.Jarumilinta R, Kradolfer F. The toxic effect of Entamoeba histolytica on leucocytes. Ann Trop Med Parasitol. 1964;58:375–381. doi: 10.1080/00034983.1964.11686259. [DOI] [PubMed] [Google Scholar]
- 7.Kubo S, Sun M, Miyahara M, Umeyama K, Urakami K, Yamamoto T, Jakobs C, Matsuda I, Endo F. Hepatocyte injury in tyrosinemia type 1 is induced by fumarylacetoacetate and is inhibited by caspase inhibitors. Proc Natl Acad Sci USA. 1998;95:9552–9557. doi: 10.1073/pnas.95.16.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragland B D, Ashley L S, Vaux D L, Petri W A., Jr Entamoeba histolytica: target cells killed by trophozoites undergo DNA fragmentation which is not blocked by Bcl-2. Exp Parasitol. 1994;79:460–467. doi: 10.1006/expr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 9.Ravdin J I, Croft B Y, Guerrant R L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980;152:377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte-Hermann R, Bursch W, Grasl-Kraupp B. Active cell death (apoptosis) in liver biology and disease. Prog Liver Dis. 1995;13:1–35. [PubMed] [Google Scholar]
- 11.Seydel K B, Stanley S L., Jr Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect Immun. 1998;66:2980–2983. doi: 10.1128/iai.66.6.2980-2983.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley S L., Jr Pathophysiology of amoebiasis. Trends Parasitol. 2001;17:280–285. doi: 10.1016/s1471-4922(01)01903-1. [DOI] [PubMed] [Google Scholar]
- 13.Stanley S L, Jr, Reed S L. VI. Entamoeba histolytica: parasite-host interactions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1049–G1054. doi: 10.1152/ajpgi.2001.280.6.G1049. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Wang L, Seydel K B, Li E, Ankri S, Mirelman D, Stanley S L., Jr Entamoeba histolytica cysteine proteinases with interleukin-1 beta converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis. Mol Microbiol. 2000;37:542–548. doi: 10.1046/j.1365-2958.2000.02037.x. [DOI] [PubMed] [Google Scholar]