Abstract
The potency of modern antiretroviral therapy (ART) allows for greater forgiveness to missed doses while still achieving, and maintaining, viral suppression. However, imperfect ART adherence, even if sufficient to maintain viral suppression, has been associated with adverse clinical outcomes. ART adherence can be objectively quantified using tenofovir diphosphate (TFV-DP) in dried blood spots (DBS), a biomarker of cumulative adherence that is predictive of future viremia—even among persons with HIV (PWH) with an undetectable HIV viral load (VL). Within a prospective cohort of PWH on tenofovir disoproxil fumarate-including ART, mismatch between drug concentration and HIV VL (i.e., low concentrations of TFV-DP in DBS in the setting of viral suppression with subsequent viremia at the following visit) was observed more frequently in PWH who were Black (36% vs. 15%; p = .04), had body mass index >30 kg/m2 (40% vs. 13%; p = .01), and reported <100% 3 months (68% vs. 50%; p = .005) and 30 days (56% vs. 31%; p = .001) adherence, compared with PWH without mismatch. Identifying PWH at risk for future viremia could help clinicians implement targeted timely interventions before episodes of breakthrough viremia.
Keywords: tenofovir diphosphate, dried blood spot, HIV, adherence, mismatch, future viremia
Historically, achieving an undetectable HIV viral load (VL) required ≥95% adherence to antiretroviral therapy (ART).1 However, the high potency and pharmacological forgiveness of new antiretroviral drugs allows for durable viral suppression with lower levels of adherence, sometimes in the range of 75%–80%.2–4 While drug forgiveness is beneficial, “suppressive”—but incomplete—adherence has been associated with higher residual inflammation, immune activation, coagulopathy, and mortality when compared with 100% adherence in persons with HIV (PWH) who are virally suppressed.5–7
Cumulative measures of adherence such as tenofovir diphosphate (TFV-DP; the phosphorylated anabolite of TFV), in dried blood spots (DBS) are predictive of future viremia in PWH-even among those who are virologically suppressed at the time of DBS collection.8–10 Thus, when a drug concentration and the HIV VL are simultaneously assessed, an HIV VL:adherence mismatch (i.e., low drug concentrations in the setting of suppression) can be identified, which could help providers intervene before the development of viremia. Given the clinical relevance of this mismatch, we aimed to identify the demographic and clinical characteristics of virologically suppressed PWH with low drug concentrations who subsequently developed viremia, a group in whom a timely intervention could potentially prevent this adverse outcome.
Within a prospective clinical cohort of PWH on tenofovir disoproxil fumarate (TDF, 300 mg/day)-including ART (any combination regimen), and after obtaining informed consent, we collected blood for DBS (4–6 mL in etheylenediaminetetraacetic acid) and HIV VL at the time of a regular clinic visit—up to three visits in a 48-week period—at the University of Colorado Hospital (UCH) Infectious Diseases Group Practice, as previously described.11 TFV-DP concentrations in DBS were quantified using validated liquid chromatography/tandem mass spectrometry (LC-MS/MS) methods, and HIV VL was assayed at the UCH clinical laboratory.11
At each study visit, self-reported three-month (3 months), 30-day (30 days), and 3-day (3 days) adherence were collected using a validated visual analog scale.11 ART type was categorized based on the anchor drug as (1) non-nucleoside reverse transcriptase inhibitors (which included efavirenz, nevirapine, and rilpivirine); (2) integrase strand transfer inhibitors (which included raltegravir, elvitegravir/cobicistat, and dolutegravir); (3) boosted protease inhibitors (which included atazanavir, lopinavir, darunavir, and fosamprenavir, all boosted with either ritonavir or cobicistat); and (4) multiclass (a combination of two or more of the previously mentioned classes).
Based on our previous research,8,12 combining pharmacological adherence measures with HIV VL can help identify PWH with high drug concentrations who remain virally suppressed and those with low concentrations who are viremic. However, this combination can also identify a population of PWH with low concentrations and viral suppression (i.e., mismatch) who are at risk of future viremia, which was the focus of this analysis.
Thus, based on the concomitant HIV VL and TFV-DP in DBS in PWH at a study visit, we focused on a subset of our cohort categorized into three main groups using drug concentration thresholds that are predictive of future viremia8: (1) high adherence:low VL (TFV-DP ≥1,650 fmol/punch and HIV VL <20 copies/mL at every study visit); (2) low adherence:low VL with subsequent viremia (i.e., mismatch visit with TFV-DP <1,650 fmol/punch and HIV VL <20 copies/mL and documented viremia at the next study visit during our study follow-up period); and (3) low adherence:high VL (TFV-DP <1,650 fmol/punch and HIV VL ≥20 copies/mL at every study visit), as shown in Table 1.
Table 1.
Characteristics of Persons with HIV According to Tenofovir Diphosphate in Dried Blood Spot and HIV Viral Load
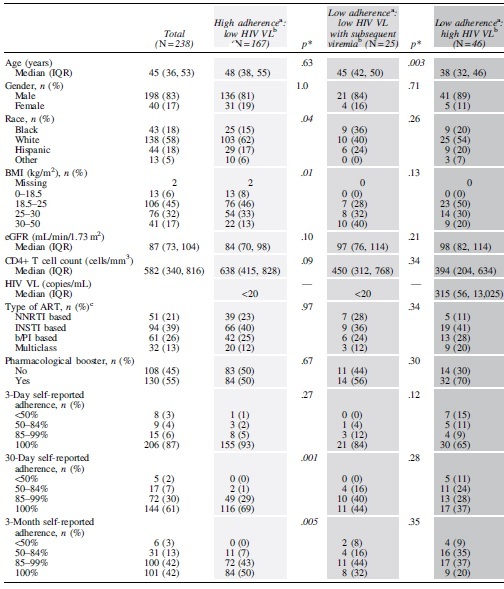
|
The light gray and dark gray columns indicate PWH without mismatch (i.e., the observed adherence matches the observed HIV VL at all visits). The medium gray column indicates PWH with mismatch who had documented viremia at their next visit (i.e., changed HIV VL status during the study follow-up).
Italics represent significant p values.
p Values represent the comparison between the groups adjacent to them (i.e., between the light gray and medium gray groups or between the medium gray and dark gray groups).
High and low adherence were defined as TFV-DP in DBS ≥1,650 or <1,650 fmol/punch, respectively.
Low and high HIV VL were defined as HIV VL <20 or ≥20 copies/mL, respectively.
NNRTI included efavirenz, nevirapine, and rilpivirine; INSTI included raltegravir, elvitegravir/cobicistat, and dolutegravir; b/PI included atazanavir, lopinavir, darunavir, and fosamprenavir; Multiclass included a combination of an NNRTI with an INSTI, and NNRTI with a b/PI or an INSTI with a b/PI.
ART, antiretroviral therapy; b/PI, boosted protease inhibitors; BMI, body mass index; DBS, dried blood spot; eGFR, estimated glomerular filtration rate; INSTI, integrase strand transfer inhibitors; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitors; PWH, persons with HIV; TFV-DP, tenofovir diphosphate; VL, viral load.
In addition, we also compared the characteristics of participants with low adherence:low VL who developed subsequent viremia versus those with low adherence:low VL who did not develop viremia. We utilized baseline characteristics to identify differences across categories and limited our analysis to PWH who had been prescribed TDF for at least 6 months. We utilized Fisher's exact test for categorical comparisons and compared median values for continuous variables using the Wilcoxon rank sum test. As this was a secondary analysis within this cohort, we did not adjust for multiple comparisons. A p value <.05 was statistically significant. Statistical analyses utilized R software (https://www.R-project.org/).
In this analysis, 238 PWH with paired TFV-DP in DBS and HIV VL at the same visit, who fit the predetermined categories, were included. The median age of the study population was 45 [interquartile range 36–53] years, and 83% were male (Table 1). More than half the cohort self-identified as White (n = 138, 58%), with similar proportions of Black (n = 43, 18%) and Hispanic (n = 44, 18%) PWH (Table 1). The largest proportion of PWH (n = 106, 45%) had a body mass index (BMI) between 18.5 and 25 kg/m2 (Table 1). The proportion of PWH who reported 100% adherence, declined from 3 days (n = 206, 87%) to 30 days (n = 144, 61%) to 3 months (n = 101, 42%). Overall, 84% of PWH in the study reported ≥85% adherence in the past 3 months (Table 1). Additional clinical characteristics of this cohort, including estimated glomerular filtration rate (estimated using modification of diet in renal disease [MDRD] equation), CD4+ T cell count, HIV VL, ART class, and the use of a pharmacological booster, are shown in Table 1.
The distribution of PWH according to the TFV-DP in DBS categories was as follows: (1) high adherence:low VL (n = 167); (2) low adherence:low VL with subsequent viremia (n = 25); and (3) low adherence:high VL (n = 46), as shown in Table 1. Compared with PWH with high adherence:low VL (n = 167), we observed low adherence:low VL with subsequent viremia (n = 25) more often in PWH who were Black (36% vs. 15%; p = .04’ Table 1) and had a higher BMI (proportion with BMI ≥30 kg/m2 was 40% vs. 13%; p = .01; Table 1).
Regarding self-reported adherence, 100% adherence in the preceding 3 months and 30 days was less frequently reported in PWH with low adherence:low VL with subsequent viremia compared with those with high adherence:low VL (32% vs. 50%; p = .005 and 44% vs. 69%; p = .001, respectively; Table 1). Similarly, ≥85% 30 days and 3 months adherence were more frequently reported in PWH with high adherence:low VL versus those with low adherence:low VL with subsequent viremia (98% vs. 84%; p = .001, and 93% vs. 76%; p = .005, respectively; Table 1).
Finally, compared with PWH with low adherence:high VL (n = 46), PWH with low adherence:low VL with subsequent viremia were older (45 vs. 38 years, p = .003; Table 1). There were no additional significant differences in self-reported adherence between these two groups. When assessing only PWH who had low adherence:low VL, CD4+ T cell count was higher among participants who did not develop subsequent viremia when compared with those who did (702 [510, 910] vs. 450 [312, 768] cells/mm3; p = .02). Comparatively, the use of a pharmacological booster was more common among participants who developed subsequent viremia versus those who did not (n = 14 [56%] vs. n = 15 [26%]; p = .01; Supplementary Table S1).
In this study, we described the clinical and demographic characteristics of PWH with mismatch between adherence and HIV VL (i.e., low drug concentrations in the setting of suppression) who developed subsequent viremia. This mismatch was more frequently observed in PWH who were Black, had a high BMI, and reported lower 3 months and 30 days adherence, compared with PWH who had high adherence and were virologically suppressed. In addition, our study showed similar self-reported adherence between PWH with low adherence:low VL and subsequent viremia and PWH with low adherence:high VL, supporting the idea that subjective adherence measures do not adequately inform virological outcomes. Collectively, these findings complement our previous research on the predictive value of TFV-DP in DBS and offer new insights on a specific patient population in whom pharmacological adherence monitoring could be of particular importance.
Another implication of our findings is that PWH who are Black and have high BMI may exhibit less pharmacological forgiveness. This observation could help clinicians identify a specific group of patients who are more susceptible to developing future viremia in whom extra care may be required to prevent an adverse clinical outcome. In addition, our findings within the group of virally suppressed PWH with low drug concentrations suggest that, among patients with low adherence:low VL, a low CD4+ T cell count in a patient who also takes a pharmacological booster could help further distinguish PWH who may be at a higher risk of future viremia if their concentration of TFV-DP in DBS were known. Thus, based on our results, a clinician could consider extending additional adherence counseling to a Black patient whose VL is <20 copies/mL, but has a high BMI and/or reports <100% adherence. This could also be considered if low TFV-DP in DBS were to become available at the point of care13 and documented in a virally suppressed PWH who takes a pharmacological booster and has low CD4+ T cell count. Similarly, our findings could support a clinic-based approach where adherence outreach is proactively focused on PWH with high BMI and/or report low adherence despite being virologically suppressed. This could better guide resource allocation and facilitate targeted outreach to patients who would have otherwise not been counseled about ART adherence by virtue of being undetectable. Finally, our results allow for the possibility of future research to evaluate dose adjustments and/or intensive drug monitoring in this population.
Several strengths are offered by our study, including a diverse large “real-world” clinical cohort of PWH on ART and the use of novel pharmacological measures to measure adherence. Limitations include the focus on TDF and the lack of other patient-level factors (social determinants of health, risk factors, etc.) that could also be associated with mismatch. Similarly, the observed low TFV-DP concentrations in Black PWH could potentially be driven by unknown genetic factors and/or drug–drug interactions—such as the use of pharmacological boosters14,15 that could influence TFV disposition and are independent of ART adherence. Finally, we do not have any follow-up data beyond the study period to assess the frequency of virological failure as defined by the Department of Health and Human Services in our cohort. Future studies that assess additional sociodemographic factors, and whether these findings extend to PWH on tenofovir alafenamide, are needed.
In conclusion, we observed low adherence:low VL with subsequent viremia (i.e., mismatch visit) more frequently in PWH who were Black, had a higher BMI, and reported <100% adherence. These findings could allow clinicians to identify a population of suppressed PWH that would benefit from early adherence counseling. Additional research is needed to confirm these findings and evaluate the impact of preemptive adherence interventions in this population.
Supplementary Material
Authors' Contributions
E.G. cowrote the first version of the article and contributed to all the edits for subsequent drafts. M.M. performed data and statistical analysis and interpretation, generated figures and tables, and made substantial edits and critical revisions of the article. S.M. contributed to the study design and conceptualization, performed the sample size calculation, data management, statistical analysis and interpretation, generated figures and tables, and made substantial edits and critical revisions of the article. R.P.C. and S.S.C performed participant consent, data and sample collection, data management, data analysis and interpretation and made substantial edits and critical revisions of the article. J.H.Z., L.E., and L.R.B. led the sample processing, pharmacological analysis, and data validation for the drug concentrations, and made substantial edits and critical revisions of the article. J.J.K. participated in the study design, adherence, and pharmacological data interpretation, and performed article editing and critical revisions. P.L.A. co-led the study conception and design, assisted with obtaining the funding, supported the study monitoring and logistics, directed and supported all aspects of the pharmacological and drug concentration analysis, collaborated with data interpretation, and made substantial edits and critical revisions of the original article and all its subsequent versions. J.R.C.M. led the conception and study design, obtained the funding and regulatory approvals, led all aspects regarding study monitoring, logistics, data and sample collection, result interpretation, cowrote the first article draft, and performed all the edits for all the subsequent drafts.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
J.J.K and P.L.A. have received research support from Gilead Sciences paid to their institution. Other authors reported no conflicts of interest.
Funding Information
This study was supported by the National Institutes of Health (K23AI104315 and R01AI145453 to J.R.C.M.; R01AI122298 to P.L.A.).
Supplementary Material
References
- 1. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 2. Byrd KK, Hou JG, Hazen R, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. JAIDS J Acquir Immune Defic Syndr 2019;82(3):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viswanathan S, Justice AC, Alexander GC, et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2015;69(4):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parienti J-J, Fournier AL, Cotte L, et al. Forgiveness of dolutegravir-based triple therapy compared with older antiretroviral regimens: A prospective multicenter cohort of adherence patterns and HIV-RNA replication. Open Forum Infect Dis 2021;8(7):ofab316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo-Mancilla JR, Cavassini M, Schneider MP, et al. Association of incomplete adherence to antiretroviral therapy with cardiovascular events and mortality in virologically suppressed persons with HIV: The Swiss HIV cohort study. Open Forum Infect Dis 2021;8(2):ofab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016;63(12):1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Int AIDS Soc 2019;22(6):e25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrow M, MaWhinney S, Coyle RP, et al. Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2019;220(4):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Odayar J, Orrell C, Phillips TK, et al. Use of tenofovir diphosphate levels to predict viremia during the postpartum period in women living with HIV: A nested case-control study. Clin Infect Dis 2022;75(5):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jennings L, Robbins RN, Nguyen N, et al. Tenofovir diphosphate in dried blood spots predicts future viremia in persons with HIV taking antiretroviral therapy in South Africa. AIDS (London, England) 2022;36(7):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2018;68(8):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kristofich M, Anderson PL, Castillo-Mancilla JR. Beyond HIV viral load: application of pharmacologic measures to identify ART adherence mismatch. Ther Adv Infect Dis 2021;8:20499361211010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pu F, Pandey S, Bushman LR, et al. Direct quantitation of tenofovir diphosphate in human blood with mass spectrometry for adherence monitoring. Anal Bioanal Chem 2020;412(6):1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kiser JJ, Carten ML, Aquilante CL, et al. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther 2008;83(2):265–272. [DOI] [PubMed] [Google Scholar]
- 15. Coyle RP, Morrow M, MaWhinney S, et al. Cumulative tenofovir diphosphate exposure in persons with HIV taking single- vs. multiple-tablet regimens. Pharmacotherapy 2022;42(8):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


