Heterogeneity is a familiar concept to paediatric intensive care physicians, because patients, pathologies, and treatments vary both within the paediatric intensive care unit (PICU) and within a given disease process. Paediatric acute respiratory distress syndrome (ARDS) typifies this problem. Children with ARDS vary in terms of age (and therefore lung development), underlying cause (pneumonia, sepsis, or trauma), pre-existing comorbid conditions, and baseline immunological state. There is not one pathway by which ARDS occurs or manifests, as indicated by the identification of hyperinflammatory and hypoinflammatory subtypes in adults and children.1, 2 Furthermore, treatments probably differ in their effectiveness, with interventions that are beneficial in some patients but harmful in others, leading to a null population average treatment effect.3 Thus, important questions need to be addressed to improve the management and outcomes of paediatric ARDS. In a Series paper in The Lancet Respiratory Medicine,4 Martin Kneyber and colleagues—a multinational group of paediatric ARDS experts—provide a welcome summary of where we are now as a field, and what tools are available to address challenges for the next generation of paediatric ARDS trials. Two key questions need to be considered to improve the management and outcomes of paediatric ARDS. First, can we improve outcomes by identifying mechanisms or pathways to target therapeutically within specific subgroups? And second, can this be done through a nuanced approach rather than through the use of blunt, pleiotropic instruments?
Biomarkers have been proposed as an aid to diagnosis, prognosis, and measurement of therapeutic response in paediatric ARDS. However, the practical use of biomarkers is limited by imprecision in the definition of ARDS and insufficient clinical availability of these biomarker tests. Just as no one test can identify the clinical syndrome of paediatric ARDS, no one biomarker can predict a patient's clinical course or indicate the best treatment path. Thus, biomarker-based strategies might simply be replacing one type of imprecision (clinical) with another (molecular).
Biomarkers have also been proposed for prognostic enrichment in clinical studies, to identify patients at highest risk of poor outcomes, and for predictive enrichment, to identify those most likely to benefit from a specific intervention. However, these applications might be limited by prevalence and power, because paediatric ARDS is relatively rare (occurring in 3·2% of critically ill children), hyperinflammatory subtypes account for only a proportion of those with ARDS, and mortality is increasingly rare. Previous studies of paediatric ARDS, including biomarker studies, have focused on the outcomes of short-term mortality and ventilator-free days. Given declining mortality, there is a growing appreciation of new morbidity and disability after hospital discharge among ARDS survivors, as well as a practical need to identify alternative, patient-centred outcomes. 25% of children with paediatric ARDS were reported to have a new morbidity at hospital discharge,5 and children with acute respiratory failure (some with ARDS) had lower intelligence quotient scores 3–8 years after illness than their siblings.6 In 2018, post-intensive care syndrome in paediatrics (PICS-p) was defined as a group of physical, cognitive, emotional, and social health impairments that occur after critical illness, raising awareness of the multifaceted impairments of PICU survivors.7 Studies have increasingly measured longitudinal outcomes among critically ill children and a PICU-specific core outcome measures set was recently established to guide evaluation of the long-term impact of critical illness.8, 9 However, there have been few PICS-p studies in paediatric ARDS.
Alternative strategies for patient inclusion and outcomes in clinical studies must be considered. Biomarkers are often regarded as having poor sensitivity and specificity for ARDS.10 However, we posit that the clinical definitions of critical care syndromes, including ARDS, are inherently imprecise, and that it remains to be seen whether biomarkers are more accurate at identifying a syndrome of acute inflammatory hypoxaemic lung injury with poor short-term and long-term outcomes than is the traditional, clinical definition of ARDS. This approach would require shifting inclusion criteria away from strictly clinical syndromic definitions in favour of specific molecular subtypes. The standard against which biomarker-based strategies should be measured, therefore, is not one of many syndromic definitions—ie, the Berlin definition or the Pediatric Acute Lung Injury Consensus Conference (PALICC) definition—but rather one of meaningful short-term and long-term outcomes. These include physical outcomes (eg, pulmonary function and neuromuscular weakness), new medical diagnoses, neurocognitive outcomes, health-related quality of life, health-care use, and social outcomes (eg, school or work absenteeism). And the approach must be extended to children in low-income and middle-income countries with higher mortality and poorly delineated non-mortality outcomes after ARDS, who have largely been excluded from studies thus far. By incorporating biomarkers into our syndromic definitions and purposefully recruiting in resource-limited settings, there is an opportunity to directly address enrichment, power, sample size, and generalisability for future studies.
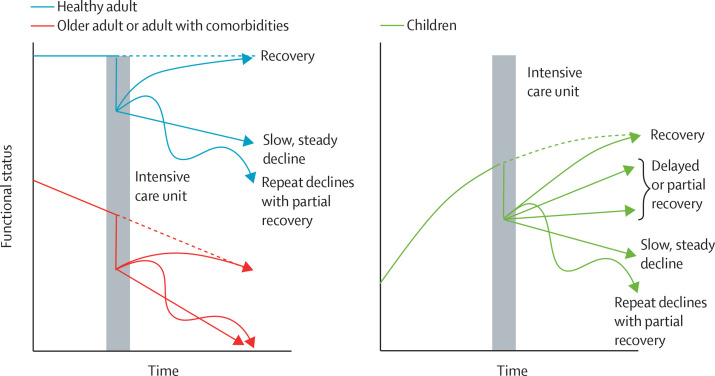
Finally, adult paradigms break down when assessing post-discharge outcomes in children and adolescents, requiring the paediatric critical care community to develop its own conceptual models. Trajectories of recovery and dysfunction postulated in adult critical illness assume static or declining baseline trajectories that are suddenly impacted by critical illness.11, 12 By contrast, the baseline trajectory of children surviving PICU is typically upward, because progression from infancy to childhood to young adulthood is characterised by improving functional status (figure ). Thus, a full understanding of how ARDS affects PICS-p, and what aspects of the development of PICS-p are modifiable, requires a better delineation of a child's anticipated trajectory, defined in part by comorbidities (or lack thereof). Longitudinal assessment of functional trajectory can be readily performed within some subgroups. Children with immunocompromising diagnoses, for example, are at high risk for ARDS and for poor outcomes, and have frequent medical encounters. Better characterisation of these children's functional trajectory pre-PICU is both warranted and feasible.
Figure.
Functional trajectories after critical illness in adults and children
The natural trajectory of functional status among adults is proposed to include a period of stability followed by a decline over time, especially among older adults and adults with comorbid conditions. By contrast, the functional status of children is expected to improve from infancy through young adulthood.
Just as there is heterogeneity in patients, pathophysiology, and treatment efficacy in ARDS, so there is probably heterogeneity in the trajectory and types of PICS-p among ARDS survivors. Biomarkers might provide an opportunity to identify mechanistically meaningful subgroups and to assess how they differ in terms of these short-term and long-term characteristics. Future studies must strive for routine follow-up of the longitudinal impact of ARDS, establishing which outcomes should be followed and for how long to identify meaningful subgroups for targeted intervention, and identifying which individuals are at highest risk for particular outcomes (appendix).

© 2023 Ian Hooton/Science Photo Library
For the Acute Respiratory Distress Syndrome 2022 Series see thelancet.com/series/ARDS-2022
EFC (K12HL138039, KL2TR002241, UL1TR002240) and NY (K23-HL136688, R01-HL148054) have received grants to their institutions from the US National Institutes of Health.
Supplementary Material
References
- 1.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahmer MK, Yang G, Zhang M, et al. Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis. Lancet Respir Med. 2022;10:289–297. doi: 10.1016/S2213-2600(21)00382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194:147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kneyber MCJ, Khemani RG, Bhalla A, et al. Understanding clinical and biological heterogeneity to advance precision medicine in paediatric acute respiratory distress syndrome. Lancet Respir Med. 2022 doi: 10.1016/S2213-2600(22)00483-0. published online Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keim G, Watson RS, Thomas NJ, Yehya N. New morbidity and discharge disposition of pediatric acute respiratory distress syndrome survivors. Crit Care Med. 2018;46:1731–1738. doi: 10.1097/CCM.0000000000003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson RS, Beers SR, Asaro LA, et al. Association of acute respiratory failure in early childhood with long-term neurocognitive outcomes. JAMA. 2022;327:836–845. doi: 10.1001/jama.2022.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing post intensive care syndrome in children—the PICS-p framework. Pediatr Crit Care Med. 2018;19:298–300. doi: 10.1097/PCC.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 8.Maddux AB, Pinto N, Fink EL, et al. Postdischarge outcome domains in pediatric critical care and the instruments used to evaluate them: a scoping review. Crit Care Med. 2020;48:e1313–e1321. doi: 10.1097/CCM.0000000000004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink EL, Maddux AB, Pinto N, et al. A core outcome set for pediatric critical care. Crit Care Med. 2020;48:1819–1828. doi: 10.1097/CCM.0000000000004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar J, Slutsky AS. GOLDEN anniversary of the acute respiratory distress syndrome: still much work to do! Curr Opin Crit Care. 2017;23:4–9. doi: 10.1097/MCC.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 11.Prescott HC, Carmichael AG, Langa KM, Gonzalez R, Iwashyna TJ. Paths into sepsis: trajectories of presepsis healthcare use. Ann Am Thorac Soc. 2019;16:116–123. doi: 10.1513/AnnalsATS.201806-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.