Abstract
Objectives
Herpesviruses are ubiquitous and after primary infection they establish lifelong latency. The impairment of maintaining latency with short-term or long-term consequences could be triggered by other infection. Therefore, reactivation of herpesviruses in COVID-19 patients represents an emerging issue.
Design and methods
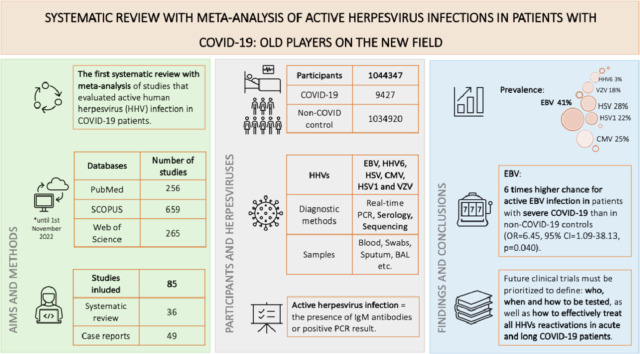
This study provided the first systematic review with meta-analysis of studies that evaluated active human herpesvirus (HHV) infection (defined as the presence of IgM antibodies or HHV-DNA) in COVID-19 patients and included 36 publications collected by searching through PubMed, SCOPUS, and Web of science until November 2022.
Results
The prevalence of active EBV, HHV6, HSV, CMV, HSV1, and VZV infection in COVID-19 population was 41% (95% CI =27%-57%), 3% (95% CI=17%-54%), 28% (95% CI=1%-85%), 25% (95% CI=1%-63%), 22% (95% CI=10%-35%), and 18% (95% CI=4%-34%), respectively. There was a 6 times higher chance for active EBV infection in patients with severe COVID-19 than in non-COVID-19 controls (OR=6.45, 95% CI=1.09-38.13, p=0.040), although there was no difference in the prevalence of all evaluated active herpesvirus infections between COVID-19 patients and non-COVID-19 controls.
Conclusions
Future research of herpesvirus and SARS-CoV-2 coinfections must be prioritized to define: who, when and how to be tested, as well as how to effectively treat HHVs reactivations in acute and long COVID-19 patients.
Keywords: CMV, COVID-19, EBV, HSV, SARS-CoV-2 infection, VZV
Graphical Abstract
Introduction
Herpesviruses are ubiquitous, double-stranded DNA, enveloped viruses that establish lifelong latency after primary infection of the host. There are nine human herpesviruses (HHVs) that can infect humans: herpes simplex virus type 1 (HSV1), herpes simplex virus type 2 (HSV2), varicella zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6A (HHV6A), human herpesvirus 6B (HHV6B), Epstein–Barr virus (EBV), human herpesvirus 7 (HHV7), and Kaposi's sarcoma herpesvirus (KSHV) or human herpesvirus 8 (HHV8). They exhibit broad cell tropism [1] and during the primary infection, as well as reactivation, they behave like lytic pathogens, destroying most of the cells that they invade [2]. At the same time, latently infected cells successfully escape immune surveillance due to suppression of viral gene expression and replication [3]. Viral conversion from a latent to lytic phase is seen in a variety of stimuli, including immunosuppression, co-infections or psychological stress [4]. This switch is rare, usually self-limiting, and without a clinical significance. However, the impairment of maintaining latency due to T cell dysfunction in immunocompromised individuals or in those whose immune imbalance has been triggered by processes such as COVID-19, could lead to long-term consequences [5,6].
Coronavirus disease 2019 (COVID-19) which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is related with one of the largest pandemics in modern history with over 668 million infected individuals, leading to more than 6.7 million deaths [7]. Since the first case was reported in December 2019 [8], an immense number of studies has been performed to investigate its causative agent and pathogenetic mechanisms. While, new SARS-CoV-2 variants effectively out-compete and replace the older ones, a variety of clinical manifestations and risk factors for adverse events are also expanding [9].
It is a common knowledge that the immune response during the acute viral illness contributes to host defense. However, the immune system might be involved in the pathogenesis of severe infections diseases like COVID-19, or even lead to critical immune dysregulation [6]. A number of studies have suggested the role of autoimmune factors and prolonged persistence of SARS-CoV-2 fragments in the development of deteriorated or extended COVID-19, but failure to return to baseline health may be a consequence of multiple pathogen activity [4,[10], [11], [12]. Of particular interest might be reactivation of already acquired microorganisms such as HHVs. Their reactivation might drive different viral genes expression, infection of additional tissues/organs and developing of new acute or chronic symptoms [6]. Finally, the possibility of developing far-reaching consequences of such uncontrolled viral replication should not be overlooked, especially in herpesviruses with oncogenic potential such as EBV and KSHV. Thus, the initial narrative that put COVID-19 in the same category with the rest of respiratory viral infections, neglected the identification of associated processes that occur simultaneously with SARS-CoV-2 infection [13].
The prevalence, the incidence and clinical relevance of active HHVs infections represent the missing link in the COVID-19 surveillance and is still in the early stage of research. Although there are some data about coinfections in COVID-19 patients, current diagnostic and treatment options are still insufficient [14,15]. To fill this gap, we conducted the systematic review and meta-analysis in order to assess all relevant published literature related to active herpesvirus infections in COVID-19 patients. Furthermore, we aimed to evaluate the prevalence of active HHVs infections in COVID-19 patients, but also the difference in that prevalence between SARS-CoV-2 infected and non-infected individuals.
Methods
PRISMA protocol [16], MOOSE guideline [17] for observational, and Cochrane handbook [18] for intervention studies were used to perform this systematic review registered at PROSPERO with registration number CRD42022348878.
Study selection and database search
Studies that evaluated active herpesvirus infections, defined as the presence of IgM antibodies or positive PCR result, in COVID-19 human populations were included in this systematic review. Studies were excluded if they: a) did not assess herpesviruses, b) did not evaluate active herpesvirus infections, c) evaluate population with active HHV infections who were SARS-CoV-2 infected afterword, d) examined other populations (animals, cell lines, etc.), and e) were abstracts, narrative or systematic reviews, or meta-analysis. Publications were screened for inclusion in the systematic review in two phases, and all disagreements were resolved by discussion at each stage with inclusion of a third reviewer.
Two biostatisticians with expertise in conducting systematic reviews and meta-analyses (AC, DM) developed the search strategy. A systematic review of peer-reviewed publications was performed through searches of three electronic databases: PubMed, Web of Science (WoS) and SCOPUS until 1st November 2022. Search queries for all three databases are available in Supplement material: Table 1 . Publications of all study types in English were considered. In addition, reference lists of articles identified through electronic retrieval were manually searched, as well as relevant reviews and editorials. Authors of relevant articles were contacted to obtain missing data or unavailable publications.
Table 1.
Systematic review.
| Author, year Country |
Study design | Herpesvirus | Specimen (detection method) for herpesvirus | COVID-19 Cases |
Non-COVID-19 Controls |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n Herpesvirus +Casesc | Age Gendera |
Characteristics | SARS-CoV-2 infection (Outcomeb) |
Clinical manifestation of herpesvirus infection | Durationc | Anti-herpesvirus therapy | n | n Herpesvirus + Controls |
Age Gendera |
Characteristics | ||||
| Lehner, 2020 Austria |
Case-control | EBV CMV |
Blood (RT-PCR) |
18 | 14/18 3/18 |
60.5 (52.0-64.5) med (25th-75th percentiles) NR |
COVID-19 patients treated in ICU due to respiratory failure and required invasive ventilation |
Active (NR) |
Biochemical abnormalities that resemble hepatitis and pancreatitis typically caused by herpesviruses like EBV or CMV. A subgroup of COVID-19 patients exhibit a hyperinflammatory pattern similar to sHLH |
NR | NR | 18 | 8/18 1/18 |
58.8 (47.8-72.3) med (25th-75th percentiles) NR |
Consecutive invasively ventilated ICU patients without COVID-19 |
| Majtanova, 2021 Slovakia |
Case-control | HSV1 | NR (RT-PCR) |
18 | 18/18 | 18-29 years - 3 (17%) 30-39 years - 4 (22%) 40-49 years - 4 (22%) ≥ 50 years - 7 (39%) 11 (61%) vs. 7 (39%) |
Herpes simplex keratitis COVID-19 patients | Active (NR) |
Keratitis | NR | NR | 26 | 26/26 | 18-29 years - 4 (15.4%) 30-39 years - 5 (19.2%) 40-49 years - 7 (26.9%) ≥ 50 years - 10 (38.5%) 16 (61.5%) vs. 10 (38.5%) |
Herpes simplex keratitis patients without COVID-19 |
| Naendrup, 2021 Germany |
Case-control | EBV CMV |
Whole blood (RT-PCR) *reactivation defined as DNA levels higher than 1000IU/mL |
117 (reactivation was performed in 55 cases) |
19/55 10/55 |
60 (16-80) EBV reactivation 51 (16-80) CMV reactivation median (min-max) 17 (89.5%) vs. 2 (10.5%) EBV reactivation 9 (90%) vs. 1 (10%) CMV reactivation |
Severe COVID-19 patients treated in ICU without clinical improvement, having persisting fever >2 days and/or persisting laboratory signs of hyperinflammation in the absence of alternative explanations such as pathogens in bronchoalveolar lavages, blood, or urine cultures. |
Active (37% with vs. 50% without EBV reactivation survived ICU, 29% with vs. 42% without rituximab treatment with EBV reactivation survived ICU; 55% with vs. 46% without CMV reactivation survived ICU, 86% with vs. 0% without Ganciclovir treatment with CMV reactivation survived ICU) |
NR | NR | Rituximab for EBV reactivation and Ganciclovir for CMV reactivation | 126 | 18/126 16/126 |
NR NR |
Respondents without COVID-19 |
| Abadias-Granado, 2021 Spain |
Case-control | HHV6 | Blood (Serology) |
16 | 6/16 | NR NR |
COVID-19 patients | Active (NR) |
Skin rash | 4 weeks | NR | 8 | 0/8 | NR NR |
Randomly selected hospitalized patients, of a similar median age, with COVID-19 but without cutaneous manifestations |
| Vigon, 2021 Spain |
Case-control | EBV CMV |
Blood (RT-PCR) |
61 Total 21 mild 17 severe 23 critical COVID-19 |
Total EBV: 10/61 CMV: 12/61 Severe COVID-19 EBV: 2/17 CMV: 4/17 Critical COVID-19 EBV: 8/23 CMV: 8/23 |
42.2 (26-64) mild COVID-19 74.2 (50-90) severe COVID-19 64.1 (42-88) critical COVID-19 med (IQR) 10 (47.6%(vs. 11 (52.4%) mild COVID-19 12 (70.6%) vs. 5 (29.4%) severe COVID-19 14 (60.9%) vs. 9 (39.1%) critical COVID-19 |
SARS-CoV-2 positive patients (performed with RT-qPCR assay in nasopharyngeal smear) older than 18 year with different clinical presentations of COVID-19: mild, severe or critical |
Active (Specific herpesvirus IgG Ab increased progressively in accordance to COVID-19 severity; Relative risk ratio between reactivation of EBV and/or CMV and death during hospitalization due to COVID-19 was 0.8556, although it was not statistically significant) |
NR | NR | NR | 21 | 0/21 0/21 |
NR NR |
Healthy donors (over 18 years and who have never been in contact with SARS-CoV-2 at the time of sampling) with similar age and gender distribution to mild COVID-19 patients. |
| Singh, 2021 USA | Cross-sectional | EBV CMV HSV |
nasopharyngeal, oropharyngeal, and sputum swabs (RT-PCR) |
4259 | 91/4259 3/4259 5/4259 |
45.21±20.43 mean±sd (20-49) min-max 1891(44.4%) vs. 2364 (55.5%) |
COVID-19 patients | Active (NR) |
NR | NR | NR | 46160 | 2627/46160 42/46160 37/46160 |
NR NR |
Respondents without COVID-19 |
| Brinkmann, 2022 Morocco |
Cross-sectional | EBV CMV HSV1 |
NR (RT-PCR, rapid multiplex approach (Sequencing) |
84 | 3/84 0/84 2/84 |
NR NR |
Critically ill COVID-19 patients | Active (NR) |
NR | NR | NR | 66 | 6/66 4/66 2/66 |
NR NR |
Respondents without COVID-19 |
| Kahwagi, 2022 Senegal |
Case-control | EBV VZV HSV HHV7 |
CSF, blood (RT-PCR) |
8 | 8/8 7/8 8/8 8/8 |
NR NR |
COVID-19 patients presenting clinical signs of encephalitis assessed by clinicians and requiring hospitalization. Patients presenting with an identified cause (e.g., Malaria) were excluded from the study. |
Active (NR) |
Encephalitis | NR | NR | 114 | 0/114 0/114 0/114 0/114 |
NR NR |
Patients presenting clinical signs of encephalitis assessed by clinicians and requiring hospitalization without COVID-19 |
| Aragon-Nogales, 2022 UK |
Prospective cohort study | EBV | Blood (PCR) |
181 (27 moderate, 16 severe, 17 critically ill) |
1/17 | For 17 critically ill 4.1 (6 months -15 years) med (min-max) 12 (70.6) vs. 5 (29.4) |
COVID-19 pediatric population with mild, moderate, severe, or critical (critical, if acute respiratory distress syndrome (ARDS), multiorgan failure (MOF), septic shock, or coma occurs) severity of the disease |
Active (NR) |
NR | NR | NR | 516 | NR | 1 day-5years – 207 (40%), 6-12 years – 170 (33%) >12 years - 76 (27%) NR |
COVID-19 negative pediatric population with other viral respiratory diseases |
| Katz, 2022 USA |
Cross-sectional | HSV1 VZV |
Blood (Serology) |
889 | 25/889 16/889 |
0-9 years - 17 (1.9%) 10-17 years - 22 (2.5%) 18-34 years - 355 (39.9) 35-44 years - 99 (11.1%) 45-54 years - 11 (1.2%) 55-64 years - 100 (11.2%) 65-74 years - 109 (12.3%) 75-85 years - 57 (6.5%) >85 years - 57 (6.5%) 380 (42.7%) vs. 509 (57.3%) |
COVID19 out- and in-patients | Active (NR) |
NR | NR | NR | 987849 | 7625/987849 4228/987849 |
0-9 years - 90881 (9.2%) 10-17 years – 66186 (6.7%) 18-34 years - 212388 (21.5%) 35-44 years - 94834 (9.6%) 45-54 years – 106688 (10.8%) 55-64 years - 154104 (15.62%) 65-74 years - 144226 (14.6%) 75-85 years - 83967 (8.5%) >85 years – 34575 (3.5%) 455458 (46.11%) vs. 532391 (53.89%) |
Hospital non-COVID-19 population from the Integrated Data repository (IDR) |
| Romani, 2022 Italy |
Case-control | HHV6 | NR (RT-PCR) |
68 | 1/68 | 6.5 years (0.9-11.5) Med (IQR) 38 (56%) vs. 30 (44%) |
COVID19 pediatric population with mild, moderate or severe disease | Active (NR) |
NR | NR | NR | 16 | 3/16 | 4.4 years (1.7-6.3) 10 (53%) vs. 6 (47%) |
Age matched non-COVID-19 pediatric patients |
| Zhu, 2020 China |
Cross-sectional | HSV CMV EBV |
Nasopharyngeal/ throat swab (RT-PCR) |
257 | 249/257 254/257 205/257 |
51 (2-99) med (min-max) 138 (53.7) vs. 119 (46.3) |
COVID-19 patients | Active (NR) |
NR | From -4 to 0 days from COVID-19 onset | NR | / | / | / | / |
| Wenzel, 2020 Germany |
Case series | EBV HHV6 |
Endomyocardial biopsies (RT-PCR) |
2 | 2/2 1/2 |
Case 1: 39 Case 2:36 2 males |
Patients with myocarditis who recently suffered from COVID-19 | Past (NR) |
NR | 4 weeks | NR | / | / | / | / |
| Zhao, 2020 China |
Cross-sectional | HSV CMV EBV |
Blood (Serology) |
417 | 18/417 0/417 12/417 |
45.2±17.6 mean±sd 198 (47.5) vs. 219 (52.5) |
COVID-19 patients with severe (including severe type implied one of the following conditions: (a) respiratory distress, respiratory rate ≥30 per min; (b) oxygen saturation on quiescent condition ≤ 93%; (c) partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2)≤300 mmHg (1 mmHg=0.133 kPa); (iv) and critical type implied one of the following conditions: (a) respiratory failure occurred and mechanical ventilation was required; (b) shock occurred; (c) patients had other organ dysfunction needing intensive care unit monitoring and treatment) and non-severe (including mild type implied the mild clinical symptoms with no abnormal radiological findings, and common type implied fever and respiratory symptoms and pneumonia detected on chest computed tomography) disease | Active (NR) |
NR | NR | NR | / | / | / | / |
| Wu, 2020 China |
Case series | CMV EBV |
nasopharyngeal/ throat swab (RT-PCR) |
74 | 3/74 3/74 |
6.0 (0.10-15.08) med (min-max) 44 (59.5) vs. 30 (40.5) |
Children without comorbidities | Active (NR) |
NR | NR | NR | / | / | / | / |
| Busani, 2020 Italy |
Case series | HSV1 CMV EBV |
Cell free plasma (RT-PCR) |
107 | 2/107 0/107 0/107 |
Case 1 - 66 Case 2 – 49 2 males |
Case 1 - history of hypertension, hypercholesterolemia and depression Case 2 - body-mass index of 32 and no significant medical history |
Active (Complete recovery) |
Ocular manifestations | NR | NR | / | / | / | / |
| Meyer, 2021 France |
Propsective cohort | HSV | blood and lower respiratory tract samples (RT-PCR) |
153 | 40/153 36/146 in blood (19/61 respiratory sample, HSV reactivation in 15/153 in blood and respiratory samples HSV-1 reactivation first in blood in 7/15, in respiratory tract in 6/15 and simultaneously in 2/15 patients) |
60.8 (50-70) med (IQR) 115 (75.2%) vs. 38 (24.8%) |
COVID-19 patients in the ICU with HSV reactivation defined as a HSV-positive PCR in blood or respiratory samples |
Active (After adjustment for mortality risk factors (age, oxygenation and ventilation characteristics, extra-respiratory components of SOFA score and corticosteroid therapy), multivariable Cox regression model showed an increased risk of mortality for HSV1 reactivation) |
NR | The median time from ICU admission to the first HSVpositive sample was 14 days (IQR 9.5–18). |
NR | / | / | / | / |
| Halawi, 2021 Saudi Arabia |
Cross-sectional | CMV HSV1 |
NR (Serology) |
417 | 2/417 123/417 |
< 30 years - 153 (37%) 30-50 years - 234 (56%) > 50 years - 30 (7%) 150 (36%) vs. 267 (64%) |
COVID-19 patients with diarrhea | Active (NR) |
NR | NR | NR | / | / | / | / |
| Paolucci, 2021 Italy |
Cross-sectional | CMV EBV HHV6 |
Serum (Serology) |
104 Total 42 ICU 62 SICU |
0/104 91/104 (40/42 ICU and 51/62 SICU) 0/104 |
ICU patients - 61.5 (55-71.25) SICU patients - 73.5 (57.8-80) med (IQR) 36 (85.7%) vs. 6 (14.3) in ICU 41 (66.1%) vs. 21 (33.9%) in SICU |
COVID-19 patients treated in ICU and SICU | Active (NR) |
NR | NR | NR | / | / | / | / |
| Saade, 2021 France |
Cross-sectional | HSV EBV CMV |
HSV, EBV, CMV=blood; HSV=blood, BAL, skin swabs (RT-PCR) |
100 | 12/100 58/100 19/100 |
59 (53–67) median (min-max) 73 (73%) vs. 23 (23%) |
Severe COVID-19 patients with malignancies and organ recipients | Active (NR) |
Cutaneous-mucous manifestations | NR | Valganciclovir for CMV reactivation, No treatment for EBV reactivation, Acyclovir and Valacyclovir for HSV reactivation | / | / | / | / |
| Xie, 2021 China |
Cross-sectional | EBV | NR (RT-PCR) |
128 | 17/128 | 62 (52-68) med (IQR) 66 (51.6%) vs. 62 (48.4%) |
COVID-19 patients treated in ICU | Active (EBV group had significantly higher 28-day and 14-day mortality than Non-EBV group (p=0.005)) |
NR | NR | NR | / | / | / | / |
| Bašić-Jukić⁎⁎, 2021 Croatia |
Prospective cohort study | VZV EBV CMV |
Serum (RT-PCR) |
104 | 1/104 9/104 27/104 |
56 (45-65) med (IQR) 56 (24-80) med (min-max) 69 (66.3%) vs. 35 (33.7) |
Renal transplant recipients after COVID-19 | Past (NR) |
Herpes Zoster and CMV colitis in one patient each | At the initial evaluation and 6 months after COVID-19 | Foscarnet, Letermovir, Hyperimmune anti-CMV globulins | / | / | / | / |
| Seeßle, 2021 USA |
Case-series | HSV1 | Serum, tracheal secretion, and bronchial lavage (RT-PCR) |
18 available specimens out of 103 COVID-19 patients | 15/18 9/18 in tracheal secretion 6/18 in bronchial lavage |
data for 15 HSV1 positive COVID-19 patients 71 (16) med (IQR) 12 (80%) vs. 3 (20%) |
Patients with laboratory confirmed SARS-CoV-2 infection with an age of 18 years or over invasively ventilated because of severe or critical pneumonia |
Active (Complete recovery) |
NR | 19.5 days mean 18 (12-28) days med (min-max) |
Acyclovir | / | / | / | / |
| Simonnet, 2021 France |
Cross-sectional | EBV CMV HHV6 |
Blood (RT-PCR) |
34 | 28/34 5/34 7/32 |
58 (26–81) med (min-max) 25 (73%) vs. 9 (27%) |
Critically ill COVID-19 patients treated in ICU | Active (No association between ICU mortality and EBV, CMV and HHV-6 reactivation) |
NR | Median delay between ICU admission and initial virus DNA detection: EBV - 4 days (range 0–20) CMV 12 days (range 1–16) HHV6 12 days (range 8–19) |
Ganciclovir or Valganciclovir for CMV reactivation cases | / | / | / | / |
| Chen, 2021 China |
Cross-sectional | EBV CMV |
Blood (Serology) |
67 | 37/67 0/67 |
37 years (IQR 30–52; range 23–81 years) 32 (47.8%) vs. 35 (52.2%) |
COVID-19 hospitalized patients The inclusion criteria in our study were as follows: (1) At least one positive result by real-time quantitative reverse-transcriptase-polymerase-chain reaction (RT-PCR) assay for SARS-CoV-2 when in hospital; (2) Measuring the antibodies against EBV VCA (IgM, IgG), EBV early antigen (EA, IgM) and EBV nuclear antigen (EBNA, IgG); (3) Time of the onset of symptoms to hospital admission less than 2 weeks. Exclusion criteria: (1) In hospital time later than February 29, 2020; (2) Most clinical information were missing. | Active (NR) |
NR | NR | NR | / | / | / | / |
| Gold⁎⁎, 2021 USA |
Cross-sectional | EBV | Blood (Serology, and for seronegative cases RT-PCR also) |
68d 39 LC 29 non-LC |
29/68 Total 26/39 LC patients 3/29 non-LC patients |
21-74 NR |
COVID-19 patients between 21 and 74 years who weren`t pregnant and not vaccinated and/or without symptoms similar to long COVID prior to testing positive for COVID-19. | Past (Long COVID) |
Long-COVID symptoms | At least 4 weeks | NR | / | / | / | / |
| Blumenthal, 2022 South Africa |
Cross-sectional | EBV HHV8 |
Blood (RT-PCR) |
104 | 81/104 21/104 |
53.0 (21.2-85.7) med (min-max) 63 (60.6%) vs. 41 (39.4%) |
Hospitalized COVID-19 patients | Active (NR) |
NR | NR | NR | / | / | / | / |
| Brooks, 2022 USA |
Prospective cohort study | EBV HHV6 |
Plasma (qPCR) |
67 | 15/67 3/67 |
60 (48-66) med (IQR) 39 (58%) vs. 28 (42%) |
COVID-19 adult patients aged ≥18 years | Active | NR | NR | NR | / | / | / | / |
| Huang, 2022 Taiwan |
Cross-sectional | CMV EBV |
Blood (PCR) |
75 | 5/75 12/75 |
NR NR |
COVID-19 patients | Active | NR | NR | NR | / | / | / | / |
| Lino, 2022 Brazil |
Cross-sectional | HHV6 | Nasopharyngeal/ throat swab (RT-PCR) |
60 | 13/60 | 60.1±18.7 mean±sd 37 (61.6%) vs. 23 (38.4%) |
Hospitalized COVID-19 patients with moderate to severe disease | Active (NR) |
NR | NR | NR | / | / | / | / |
| Su, 2022 USA |
Cohort study | EBV CMV |
Blood (RT-PCR) |
209 | 29/209 0/209 |
56±18 mean±sd 18-89 min-max 104 (48.8%) vs. 105 (50.2%) |
Patients with spectrum of acute COVID-19 severities | Active (NR) |
NR | At the clinical diagnosis of COVID-19 | NR | / | / | / | / |
| Carneiro, 2022 Brazil |
Cross-sectional | HSV1 HSV2 VZV CMV HHV6 HHV7 EBV HHV8 |
nasopharyngeal swab (RT-PCR) |
53 | 25/53 23/53 21/53 9/53 9/53 1/53 15/53 4/53 |
63.51±15.68 mean±sd 17-95 min-max 27 (50.9%) vs. 26 (49.1%) |
Patients with COVID-19 diagnosis confirmed by RT-PCR treated in ICUs and with available consent forms signed by the patient or guardian/legal representative. Pregnant women were excluded from the study. |
Active (A statistically significant association was observed between neurological changes and HHV-6 detection (p = 0.034)) |
Central nervous system symptoms | NR | NR | / | / | / | / |
| Gatto⁎⁎, 2022 Italy |
Cross sectional | CMV | whole blood bronchoalveolar lavage (RT-PCR) |
431 | 88/431 in blood 30/88 in bronchoalveolar lavage |
65 (56-72) med (IQR) 323 (74.9%) vs. 108 (25.1%) |
Patients admitted to ICUs with laboratory-confirmed SARS-CoV-2 infection and moderate to severe acute distress respiratory syndrome (ARDS), while Patients with age < 18 years, ICU length of stay (LOS) < 24 h, limitation of care or do not resuscitate order were excluded from the study |
Active (Hospital mortality was higher in patients with (67.0%) than in patients without (24.5%) CMV reactivation but the adjusted analysis did not confirm this association (HR 1141, 95% CI 0.757–1721, p = 0.528)) |
CMV-related pneumonia described as new worsening of pulmonary gas exchange, modification of chest X-ray or computed tomography compatible with new interstitial pneumonia, CMV blood reactivation and no other causes of pneumonia/worsening of pulmonary gas exchange. |
17 (5-26) days in blood med (IQR) |
Ganciclovir for 10 days | / | / | / | / |
| Im, 2022 South Korea |
Cross-sectional and Retrospective cohort study |
EBV | Blood (RT-PCR) |
269f Gr 1 - 212 Gr 2 - 44 Gr 3 - 10 Gr 4 - 2 Gr 5 - 1 Gr 6 - 0 |
45/269 15-29 years - 2/18 30-39 years - 0/36 40-49 years - 2/28 50-59 years - 7/36 60-69 years - 5/45 70-79 years - 15/46 ≥ 80 years - 14/60 Gradus 1 - 30/212 Gradus 2 - 8/44 Gradus 3 - 4/10 Gradus 4 - 2/2 Gradus 5 - 1/1 Gradus 6 - 0/0 |
61.6 mean 15-29 years - 18 (6.7%) 30-39 years - 36 (13.4%) 40-49 years - 28 (10.4%) 50-59 years - 36 (13.4%) 60-69 years - 45 (16.7%) 70-79 years - 46 (17.1%) ≥ 80 years - 60 (22.3%) 109 (40.5%) vs. 160 (59.5%) |
Adult COVID-19 patients (>15 years) classified according to the 6- grade systemf. Patients admitted directly to the intensive care unit (n=29) were not tested and were excluded from the analysis. Also, patients not designated for EBV PCR testing (n=53), as well as, patients who were not tested within 5 days after hospitalization (n=8) were excluded. |
Active (EBV viremia was not associated with COVID-19 progression) |
NR | 2.3 days median |
NR | / | / | / | / |
| Meng, 2022 China |
Cross-sectional | EBV CMV |
Blood (Serology) |
217 | 55/217 Not reported |
NR NR |
COVID‐19 patients defined as the presence of symptoms and positive reverse transcriptase (RT)‐PCR assays were in accordance with Chinese guidelines (sixth version) | Active (Patients with EBV reactivation have statistically nonsignificant higher mortality rate (12 [22%] vs. 18 [11%], p=0.080); As compared to patients with COVID‐19 who did not receive ganciclovir therapy, ganciclovir‐treated patients had improved survival rate (0.98, 95% CI [0.95, 1.00] vs. 0.88, 95% CI [0.81, 0.95], p = .010) |
NR | NR | NR | / | / | / | / |
| Zubchenko⁎⁎, 2022 Ukraine |
Case-control | EBV HHV6 CMV |
saliva, blood, and mucous membrane of the posterior pharyngeal wall (Serology RT-PCR) |
88 | 68/88 (51/68 reactivation) 68/88 (39/68 reactivation) 0/88 |
NR NR |
COVID-19 patients (88) were divided into: 1) post-COVID syndrome and reactivation of herpesviruses (main group) (68) and 2) post-COVID syndrome without detectable DNA of herpesviruses (control group) (20) | Past (NR) |
NR | 1-4 months | NR | / | / | / | / |
Gender is reported as n (%) males vs. n (%) females, bOutcomes regarding active herpesvirus infection that refer to death, recovery, complications, etc, as reported in the original article, cDuration between SARS-CoV-2 positive result and herpesvirus clinical manifestation cNumber of Herpesvirus positive cases is reported as number of positive cases out of the total number of cases dThe “long-term long COVID group” was defined as tested positive for COVID-19 at least 90 days prior to being enrolled, and all reported one or more of the long COVID symptoms utilized for this study. The “long-term control group” was defined as tested positive for COVID-19 at least 90 days prior to enrollment, and none reported any of the long COVID symptoms we were assessing. The “short-term long COVID group” was defined as tested positive for COVID-19 21–90 days prior to enrollment, and all reported one or more of the long COVID symptoms utilized for this study. The “short-term control group” was defined as tested positive for COVID-19 21–90 days prior to enrollment, and none reported any of the long COVID symptoms we were assessing. eLong COVID subjects were those that reported one or more of the following un-abating symptoms after recovering from initial SARS-CoV-2 infection: fatigue, insomnia, headaches, myalgia, confusion/brain fog, weakness, rash, pharyngitis, abdominal pain, tinnitus, fever over 101° F, neck lymphadenopathy, or mild-to-moderate hearing loss, fGr 1, Gr 2, Gr 3, Gr 4, and Gr 5 are Gradus 1, 2, 3, 4, 5, and 6 defines as Grade 1, symptomatic but no oxygen therapy required; Grade 2, low-flow nasal cannula oxygenation; Grade 3, high-flow nasal cannula/non-invasive ventilation; Grade 4, mechanical ventilation; Grade 5, extracorporeal membrane oxygenation; Grade 6, death.
Abbreviations: ICU – intensive care unit, SICU – sub-intensive care unit, EBV – Epstein-Barr virus, CMV – cytomegalovirus, sHLH - secondary hemophagocytic lymphohistiocytosis, NR – not reported, CSF – cerebral-spinal fluid, LC patients- Long COVID-19 patients, non-LC – patients without Long COVID-19, USA – United States of America, Ab – antibody, SOFA - Sequential Organ Failure Assessment score.
Long-COVID or post-COVID symptoms reported
Article Screening and Selection
Two reviewers independently evaluated the eligibility of all publications during title and abstract reading (AC, DM). Studies were included in the full text screening if either reviewer identified the study as being potentially eligible, or if the abstract and title did not include sufficient information for clear exclusion. The same reviewers independently performed full text reading to select articles for inclusion according to the criteria listed under Inclusion and Exclusion Criteria available in Supplement material: Table 2. Disagreements were resolved by consensus (AC, DM) or arbitration (AB).
Data Abstraction and Quality Assessment
Two reviewers independently abstracted the following data: first author, year of publication, country of research, study design, number, characteristics, age, and gender of cases/controls, evaluated HHVs, number of HHV positive cases/controls, number of HHV negative cases/controls, clinical manifestation of active HHV infection, duration between SARS-CoV-2 detection and HHV clinical manifestation or laboratory HHV confirmation, antiviral therapy, and specimen and method for herpesvirus detection. Standardized protocol for data extraction was used by both reviewers (Supplement material: Table 3). Each reviewer independently evaluated the quality and risk of bias of selected manuscripts using an adapted version of the Newcastle-Ottawa tool for observational [19] and Jadad scale for randomizes controlled trials [20].
Statistical analysis
The primary outcome of meta-analysis was to assess the prevalence of active HHV infections in COVID-19 human populations. The meta-analysis of the prevalence was performed using inverse variance method. Data that were entered for each of the studies were the original prevalence from the study and the standard error of the prevalence according to the equation, where p was the prevalence from the particular study and n was the total number of respondents from that study.
The secondary outcome was to evaluate the difference in the prevalence of active HHV infections in COVID-19 in comparison with non-COVID-19 human populations. It was achieved by Mantel-Haenszel method, a proper method for dichotomous outcomes and situations with different sample size between studies. Heterogeneity was assessed using the Chi-square Q test and I2 statistic. The categorization of heterogeneity was based on the Cochrane Handbook [18] and states that I2<30%, 30% to 60% or >60%, corresponds to low, moderate and high heterogeneity, respectively. Random effect model was used if heterogeneity was high, otherwise fixed effect model was in use. Sensitivity analysis was performed in order to assess the robustness of the primary analysis. Forest plots were constructed for each analysis showing the OR (box), 95% confidence interval (lines), and weight (size of box) for each trial. The overall effect size was represented by a diamond. Publication bias was assessed by funnel plots for every defined outcome (Supplement material: Figure 4).
A p value ≤0.05 was considered to be statistically significant. Analyses were performed using Review Manager Version 5.4 [21].
Results
Systematic review
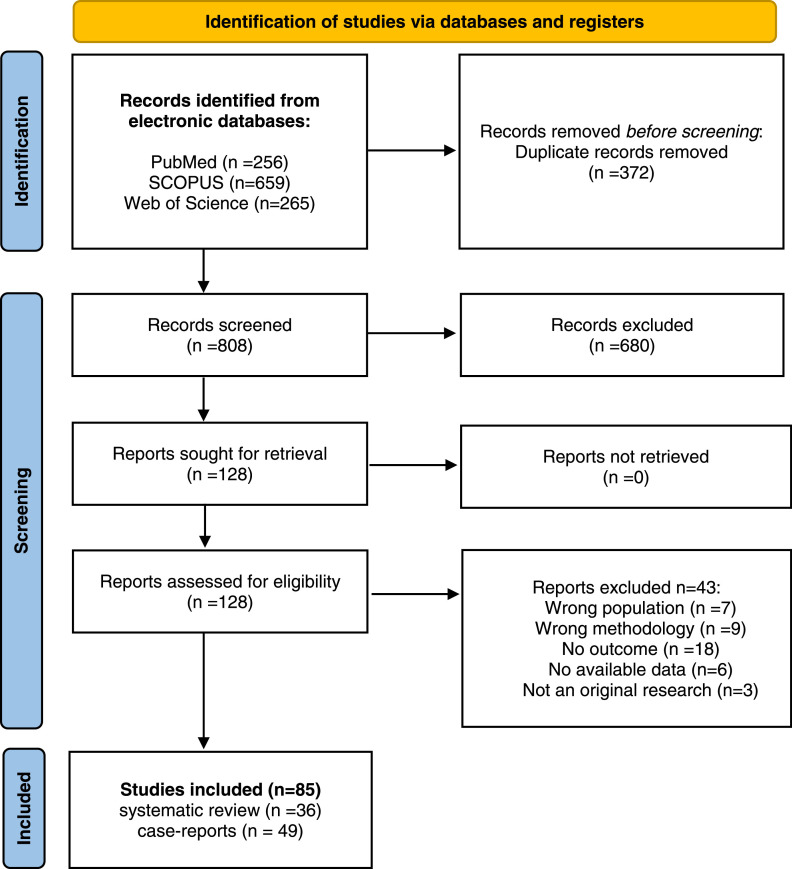
A total of 1180 potentially eligible articles were found. After duplicates (n=372) were removed, title and abstracts were evaluated for 808 articles. According to previously defined exclusion criteria 680 articles were excluded, and 128 left for full text reading. At last, 36 articles were selected for inclusion in the systematic review, and 49 case-reports were evaluated separately. A flow diagram illustrating the selection process is presented in Figure 1 .
Figure 1.
Flow diagram.
Systematic review table presents characteristics of all 36 included publications [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57] (Table 1). They were published from the beginning of COVID-19 pandemic until 1st of November 2022, with a total of 1044347 participants (9427 COVID-19 and 1034920 non-COVID-19). Cross-sectional studies were common (18/37), then case-control studies (8/37), cohort studies (5/37), and case series (4/37). One study included retrospective cohort and cross-sectional study designs. Included studies were performed in 18 different countries, of which studies from China (6), United States of America (6), and Italy (4) were the most frequent. EBV and CMV were the most frequently analyzed herpesviruses, in 27/36 and in 21/36, respectively. HSVs were evaluated in 6/36 studies, HSV1 alone was detected in 7/37 studies also, while HSV2 was explored in only one study. HHV6 was examined in 9/37, VZV in 4, HHV8 in 2, and HHV7 in 2 studies. Blood was commonly used as a specimen for herpesvirus analysis (20/37). Nasopharyngeal and oropharyngeal swabs and sputum were rarely used (6/37), as well as tracheal secretion and bronchial lavage (3/34). Other specimens (skin swab, cerebrospinal fluid, endomyocardial biopsies, saliva) were used in one study each. Most studies used Real-Time PCR method for a detection of herpesviruses DNA (29/36), detection of antiviral antibodies was done in 9/36, and sequencing of viral DNA was applied in only one study. COVID-19 cases were predominantly hospitalized patients treated in intensive care unit. Their age varied from 2 to 99 years with almost equal distribution of genders (48% males and 52% females). Only four studies evaluated past COVID-19 (as SARS-CoV-2 negative but with history of confirmed SARS-CoV-2 infection) [24,32,52,57] and three observed and reported long-COVID (LC) symptoms [31,32,57]. Ten studies reported clinical manifestations of active herpesvirus infections. The time between determination of SARS-CoV-2 positive result and clinical manifestations and/or detection of herpesvirus infections varied from a few days up to ≥6 months. There were 49 case reports of COVID-19 patients with active HHV infections aged between 4 and 83 years (one case included neonates) and 44% males. (Supplementary material: Table 4).
Meta-analysis of the prevalence of active herpesvirus infection in COVID-19 population
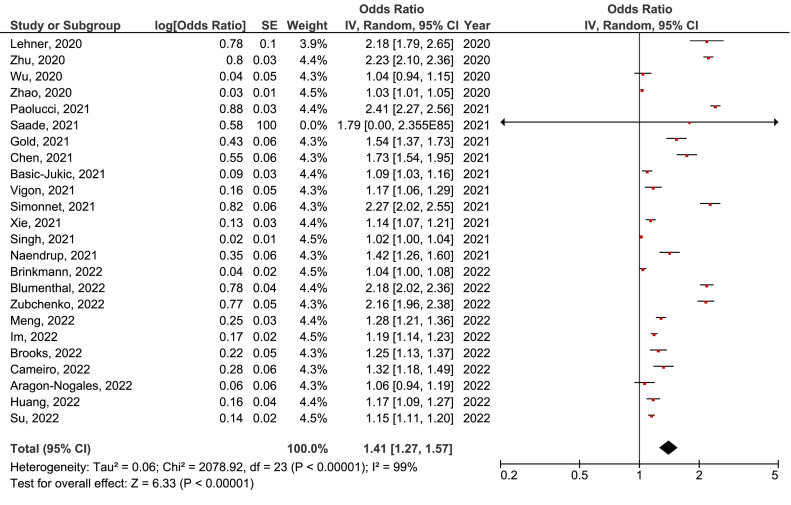
Meta-analysis of the prevalence of active EBV, CMV, HSV, HSV1, VZV, and HHV6 infection in COVID-19 population was performed. The prevalence of active EBV infection in COVID-19 population of 41% (95% CI27%-57%) (Figure 2 ) was the highest. A bit lower was the prevalence of active HHV6 infection of 34% (95% CI=18%-52%) (Figure 3 ). On the third place was the prevalence of active HSV infection 28% (95% CI=1%-85%) (Figure 4 ), followed by CMV 25% (95% CI=1%-63%) (Figure 5 ), VZV 22% (95% CI=10%-35%) (Figure 6 ), and HSV1 18% (95% CI=4%-34%) (Figure 7 ).
Figure 2.
Meta-analysis of the prevalence of active Epstein-Barr virus infection in COVID-19 population. CI, confidence interval; IV, inverse variance; SE, standard error.
Figure 3.
Meta-analysis of the prevalence of active human herpesvirus 6 infection in COVID-19 population. CI, confidence interval; IV, inverse variance; SE, standard error.
Figure 4.
Meta-analysis of the prevalence of active herpes simplex virus infection in COVID-19 population. CI, confidence interval; IV, inverse variance; SE, standard error.
Figure 5.
Meta-analysis of the prevalence of active cytomegalovirus infection in COVID-19 population. CI, confidence interval; IV, inverse variance; SE, standard error.
Figure 6.
Meta-analysis of the prevalence of active varicella zoster virus infection in COVID-19 population. CI, confidence interval; IV, inverse variance; SE, standard error.
Figure 7.
Meta-analysis of the prevalence of active herpes simplex virus type 1 infection in COVID-19 population. CI, confidence interval; IV, inverse variance; SE, standard error.
Meta-analysis of the difference in the prevalence of active herpesvirus infection in COVID-19 and non-COVID-19 human population
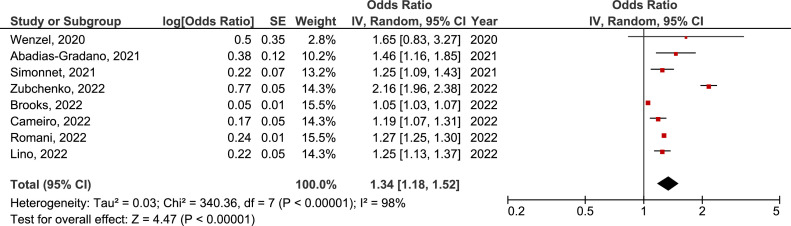
Seven studies with available data about herpesvirus activity were included in the meta-analysis of the difference in the prevalence of herpesvirus active infection in COVID-19 and non-COVID-19 groups.
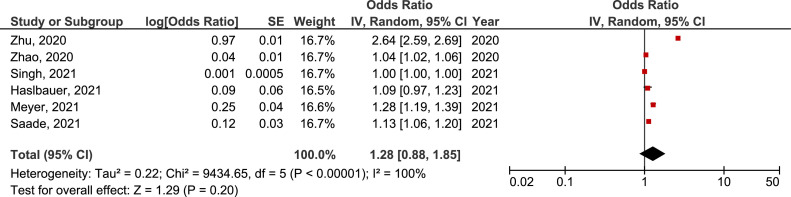
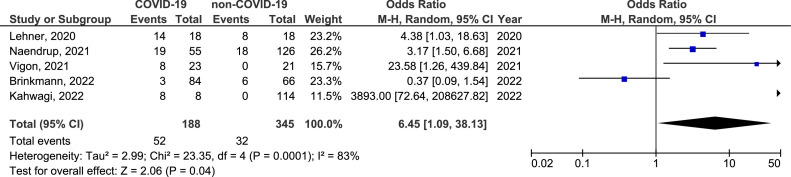
There was no significant difference in the prevalence of active EBV, CMV, and HSV infection between COVID-19 and non-COVID-19 groups (OR = 3.02, 95% CI = 0.65-14.05, p=0.160, OR = 1.27, 95% CI = 0.73-2.21, p=0.400, and OR = 24.71, 95% CI = 0.34-1802.72, p=0.140, respectively) (Supplement material: Figure 1, 2 and 3). But, in sensitivity analysis including studies that evaluated severe COVID-19 (patients hospitalized in intensive care units, those who required mechanical ventilation and/or lethal disease) only, there was a significant difference in the prevalence of active EBV infection between severe COVID-19 and non-COVID-19 groups (OR = 6.45, 95% CI = 1.09-38.13, p=0.040) (Figure 8 ). There was a 6 times higher chance for active EBV infection in COVID-19 patients with a severe form of the disease than in non-COVID-19 controls.
Figure 8.
Forest plot of the difference in the prevalence of active Epstein-Barr virus infection in severe COVID-19 population in comparison with non-COVID-19 control group. CI, confidence interval; IV, inverse variance; SE, standard error.
Discussion
After almost three years of COVID-19 pandemic it is evident that beside SARS-CoV-2, the course of the acute disease and its’ consequences, is driven by some other persistent pathogens, too [58,59]. Important here is the understanding of so called „multiple hit model“ where the activity of one pathogen can induce the virulence of the next. Reactivation capacity of persistent pathogens in conditions of SARS-CoV-2-induced immune dysregulation requires a more careful and comprehensive analysis of [59]. This study provided the first systematic review with meta-analysis of active human herpesvirus infections in COVID-19 patients. The highest prevalence of active HHV infection in COVID-19 population was documented in the group of those with active EBV infection (41%). Although there was no significant difference in the prevalence of active EBV, CMV or HSV infection between COVID-19 and non-COVID-19 groups, this prevalence was nevertheless significantly higher when comparing severe form of COVID-19 and non-COVID-19 individuals. In fact, we showed that there was 6 times higher chance for EBV reactivation in severe COVID-19 than in those without COVID-19.
Clinical features of COVID-19 range from asymptomatic to critical differentiating in symptoms and signs [60]. SARS-CoV-2 can infect a variety of human cell types managing to recruit numerous mechanisms in order to disable and evade the host immune response [6]. This disturbance of the innate immune functions includes unrecognizable viral replication, dysregulation of interferon activity and/or signaling pathways initiation which leads to hyperinflammation and even cytokine storm [58,[61], [62], [63]. In addition, microvascular dysfunction and microthrombi deposition could be the result of clotting cascades stimulation and multi-organ injury [6]. When it comes to the synergistic or cumulative impact of SARS-CoV-2 and already acquired herpesviruses on clinical course and outcome of COVID-19, some similarities could be found in autoimmune diseases, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), immunosuppressive conditions, etc. The basic mechanism by which HHVs are maintained in latency is interferon-directed [64]. Thus, by disabling the host interferon response, SARS-CoV-2 may facilitate any herpesvirus to take advantage of acute COVID-19 [65]. Moreover, COVID-19-affected lymphocytes, particularly CD4+ T, CD8+ T and natural killer (NK) cells, also undergone exhaustion and reduction [66]. This encourages reactivation of latent viral infections driving additional symptoms with modification of human gene expression, immunity and metabolism [6]. Ten studies from this review reported clinical manifestations of active herpesvirus infection in active or post COVID-19 population with evaluation of outcomes that referred to death, recovery, or complications regarding HHVs involvement. In lack of large cohorts and systematic studies, the results from mentioned ten studies are still inconsistent. A few studies showed higher mortality rate in COVID-19 patients with active HHV infection such as HSV, EBV or CMV [31,42,54]. Other studies showed no association between active HHV infection and COVID-19 disease progression [35] not even hospital mortality [41,51,67].
Epstein-Barr virus infection
Based on our search of all data published so far, active EBV infection has been shown as the most prevalent HHV infection in COVID-19 population (41%). Even though EBV has a high frequency in the general population, involvement of this virus in the development of a variety of lymphoproliferative disorders, carcinomas, autoimmune diseases and in individuals with immunodeficiency, calls for caution. Therefore, after considering only the group of critically ill COVID-19 patients, the meta-analysis showed statistically significant difference in active EBV infection between this group and non-COVID-19 controls. An explanatory mechanism of more complicated disease course and even mortality increasing in patients with simultaneous EBV and SARS-CoV-2 infection is based on the lymphocytopenia-induced inability of the cellular immune system to eliminate the pathogen. This could lead to the development of dysplasia, and potential EBV-associated malignancy [68]. Correlation between reduced CD8+ T cells and NK counts, EBV DNA viremia and COVID-19 severity has already been reported [44]. Although there was no statistically significant difference in the prevalence of active EBV infection between total COVID-19 patients and non-COVID-19 groups, this prevalence was still higher in COVID-19 patients.
The role of EBV in the development of post infection sequelae such as ME/CFS and LC could be explained by the synergistic subversion and disruption of cellular and mitochondrial pathways during active EBV and SARS-CoV-2 infection [4]. Both viruses are involved in p53 degradation, altered metabolic profile and mitochondrial biogenesis with sustained inflammatory response, which facilitate EBV-induced fatigue in an already compromised individual [69,70].
Finally, among 26 case reports that described active EBV and SARS-CoV-2 infection there was a variety of EBV-associated manifestations such as lymphoproliferative disease, lymphadenopathy, splenomegaly, skin rash, polyneuropathy and infectious mononucleosis. COVID-19 patients also had heterogeneous underlying diseases like liver, renal, heart, or hematopoietic stem cell transplantation, myocarditis, cardiovascular diseases, fatigue, multisystem inflammatory syndrome in children (MIS-C), then healthy patients, neurological, ICU patients, or even consisted of patients with a fatal outcome.
HHV6 infection
This study showed a notable prevalence of active HHV6 infection in COVID-19 population of 34%. Reported cases were potentially associated with pytiriasis rosea, neurological symptoms, meningitis and hemophagocytic lymphohistocytosis. It has been suggested that cutaneous manifestations seen in COVID-19 patients, such as erythema, vesicular eruptions, pityriasis rosea, urticarial lesions, maculopapular eruptions and livedo or necrosis could be associated not only with SARS-CoV-2, but also with HHVs [22,71]. Kawasaki disease might also be added to the possible association [72]. As in moderate and critically ill patients skin lesions are not always properly valued and described in medical reports, it should be noted that those lesions could actually help to indicate HHV6 (or other HHV) co-infection with SARS-CoV-2 [39].
One of HHV6-associated complications in COVID-19 patients is HHV6-triggered encephalitis, sometimes hidden by severity of the clinical picture in sedated and intubated ICU patients [73]. Identification of HHV6 infection therefore might be crucial in determination of therapeutic approach since ganciclovir and foscarnet could be used in treatment of HHV6 encephalitis and improve disease prognosis [74]. In addition, scientific reports attempted to link HHV6 as the potential trigger of ME/CFS, as well [75,76]. Firstly, HHV6 is neurotropic virus causing a variety of neurological disorders such as dizziness, epilepsy, and encephalitis [29]. Further, HHV6 dUTPase protein has the ability to change the structure of synapses, neural communication, induce of cytokines leading to disruption of the hematoencephalic barrier and penetration of inflammatory mediators, dendritic cells, and T cells into the brain.
HSV infection
Among 322 individuals described so far with HSVs and 176 with HSV1 only active infection, during or after acute COVID-19, the prevalence was 28% and 18% for HSVs and HSV1, respectively. Meta-analysis did not show statistically significant difference in HSV prevalence between COVID-19 and non-COVID-19 groups. Patients belonging to acute and post-acute COVID-19 groups presented with skin lesions, stromal and endothelial HSV keratitis, vision loss, HSV1 meningitis or hepatitis. Some of the patients were solid organ recipients. Individuals already harboring HSV at the time of SARS-CoV-2 infection might have more difficulty in mounting an immune response that fully clears SARS-CoV-2 from all tissues. The protein ICP0 is capable to directly disrupt interferon signaling by both blocking the JAK-STAT pathway and downregulating expression of interferon-stimulated genes leading to development of chronic symptoms [37].
CMV infection
The prevalence of active CMV infection in COVID-19 population was 25%, described in only 495 patients. Even though no statistically significant difference was shown in CMV prevalence between COVID-19 and non-COVID-19 groups, the capacity of CMV to exacerbate the clinical course and induce cytokine storm was well described. The explanatory mechanism includes disruption of peripheral blood T-cell differentiation and upregulation of inflammatory cytokines with interleukin 6 (IL-6), especially in elderly [77]. In addition, serious lymphocytopenia in severe SARS-CoV-2 infection could drive CMV reactivation, immune system failure and death of T cells [68]. This systematic review revealed that active CMV infection could be associated to lymphadenopathy, splenomegaly, and colitis in renal transplant recipients, pneumonia, skin lesions, and neurological manifestations of COVID-19. Identification of CMV infection in COVID-19 patients could be effective in adopting appropriate treatment for suppressing the inflammation. Kidney transplant recipients particularly should be closely monitored to avoid organ failure, as SARS-CoV-2 and CMV co-infection in this patients may aggravate the clinical status, as described in case with myelodysplastic syndrome [78].
VZV infection
Early reports demonstrated that VZV reactivation in severe COVID-19 correlated with the onset of septic shock [79]. After more than two years, reactivation of VZV was documented only in 56 COVID-19 patients. Although Herpes Zoster (HZ) form of reactivation was dominant in this systematic review, HZ was more often the subject of publications dealing with COVID-19 vaccination as a trigger than SARS-CoV-2 infection itself. While VZV reactivation is most commonly due to age-related memory T cells decline, it could also occur in immunosuppressive conditions mostly due to lymphopenia and lymphocyte exhaustion, reported in more than 80% of COVID-19 cases [80]. Although the prevalence of active VZV infection in COVID-19 population in this systematic review was 22%, it is difficult to assess how realistic this percentage is.
HHV7 and HHV8 infections
HHV7 or HHV8 active infections in SARS-CoV-2 positive individuals were reported sporadically. There were 30 HHV8 positive patients among COVID-19 hospitalized and patients with central nervous system (CNS) symptoms and signs, those with Kaposi sarcoma, HIV, and liver dysfunction after transplantation. Patients with HHV7 (11) were reported in studies that included COVID-19 patients with CNS signs and symptoms or encephalitis. As the number of studies that analyzed these types of infections is insufficient, the prevalence of active HHV7 and HHV8 infections may be underestimated, especially in critically ill COVID-19 patients with neurological manifestations [29]. Neuroinflammatory potential of HHV7 is recently even identified in Alzheimer's brain autopsy [81]. Oncogenic and angiogenic properties of HHV8 should be kept in mind when it comes to purplish-violaceus lesions which are characteristic of Kaposi sarcoma, but may be confused with other conditions seen in COVID-19 patients [82].
This study has several limitations that should be considered when interpreting the results. They refer to the quality of included studies that depends on their methodology (design, characteristics of study groups, collected and reported variables). Prospective studies have a higher quality in comparison with all retrospective or cross-sectional studies. COVID-19 patients with the same severity of the disease and healthy respondents for controls should be recommended for meta-analysis in this field. Confounding variables should be reported in order to control estimated effect from the meta-analysis in the meta-regression. Unreported data about specimen and method for virus detection could diminish the validity of the result and make discrepancies in the interpretation.
Conclusions
COVID-19 is a new disease with the capacity to trigger immune disbalance. In such an environment the impairment of maintaining human herpesvirus latency could lead to short-term or even long-term consequences. Due to shown high prevalence of active HHVs infection in SARS-CoV-2 positive patients, up to 41%, and 6 times higher chance for active EBV infection in critically ill COVID-19 patients than in non-COVID-19 controls, reactivation of already harbored pathogens in COVID-19 patients should represent an emerging issue. To address the knowledge gap about the exact role and consequences of herpesvirus reactivation during COVID-19 pathogenesis, as well as to establish therapeutic protocols, large cohorts and systematic studies are required. Future clinical trials focused on research of herpesvirus and SARS-CoV-2 coinfections must be prioritized to define: who, when and how to be tested, as well as how to effectively treat all HHVs reactivations in acute and long COVID-19 patients.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
All detailed information regarding the search queries, reasons for inclusion/exclusion, and protocol for data extraction are available as supplement material within the article.
Author contributions
AB and AC conceived the work. DM and AC developed the search strategy, performed study selection, database search, article screening and selection. AC performed data abstraction, quality assessment and qualitative and quantitative analysis. AB wrote the original draft. AC made the figures. DM reviewed the manuscript. All authors read the final version of the submitted manuscript.
Acknowledgements
The authors are supported by the Science Fund of the Republic of Serbia, PROMIS, grant number 6060866, ROLERS.
Ethics approval statement
Not applicable.
Patient consent statement
Not applicable.
Permission to reproduce material from other sources
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.036.
Appendix. Supplementary materials
References
- 1.Connolly SA, Jardetzky TS, Longnecker R. The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 2021;19(2):110–121. doi: 10.1038/s41579-020-00448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croen KD. Latency of the human herpesviruses. Annu. Rev. Med. 1991;42:61–67. doi: 10.1146/annurev.me.42.020191.000425. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Pablos M, Paiva B, Montero-Mateo R, Garcia N, Zabaleta A. Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.656797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafiee A, Aghajanian S, Athar MMT, Gargari OK. Epstein–Barr virus and COVID-19. J. Med. Virol. 2022;94(9):4040–4042. doi: 10.1002/jmv.27823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Khanna R. Immune regulation of human herpesviruses and its implications for human transplantation. Am. J. Transplant. 2013;13(SUPPL. 3):9–23. doi: 10.1111/ajt.12005. [DOI] [PubMed] [Google Scholar]
- 6.Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 Data Explorer - Our World in Data [Internet]. [cited 2022 Nov 11];Available from: https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&country=∼OWID_WRL&hideControls=true&Interval=New+per+day&Relative+to+Population=false&Metric=Confirmed+cases&Color+by+test+positivity=false.
- 8.Zhou P, Lou Yang X, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshee S, Vatti N, Chang C. Long-Term Effects of COVID-19. Sustain. 2022;97(3):579–599. doi: 10.1016/j.mayocp.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumbein H, Kümmel LS, Fragkou PC, Thölken C, Hünerbein BL, Reiter R, et al. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2022:e2365. doi: 10.1002/rmv.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023:1–14. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malekifar P, Pakzad R, Shahbahrami R, Zandi M, Jafarpour A, Rezayat SA, et al. Viral Coinfection among COVID-19 Patient Groups: An Update Systematic Review and Meta-Analysis. Biomed Res. Int. 2021 doi: 10.1155/2021/5313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pakzad R, Malekifar P, Shateri Z, Zandi M, Akhavan Rezayat S, Soleymani M, et al. Worldwide prevalence of microbial agents’ coinfection among COVID-19 patients: A comprehensive updated systematic review and meta-analysis. J. Clin. Lab. Anal. 2022;36(1) doi: 10.1002/jcla.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch VA. John Wiley & Sons; 2019. Cochrane handbook for systematic reviews of interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O'connell D, Peterson J W V. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014;
- 20.Jadad AR, Andrew Moore R, Carroll D, Jenkinson C, John Reynolds DM, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.RevMan | Cochrane Training.
- 22.Abadías-Granado I, Navarro-Bielsa A, Morales-Callaghan AM, Roc L, Suso-Estívalez CC, Povar-Echeverría M, et al. COVID-19-associated cutaneous manifestations: does human herpesvirus 6 play an aetiological role? Br. J. Dermatol. 2021;184(6):1187–1190. doi: 10.1111/bjd.19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aragón-Nogales R, Zurita-Cruz J, Vázquez-Rosales G, Arias-Flores R, Gómez-González C, Montaño-Luna V, et al. Clinical presentation of pediatric patients with symptomatic SARS-CoV-2 infection during the first months of the COVID-19 pandemic in a single center in Mexico City. Front. Pediatr. 2022;10 doi: 10.3389/fped.2022.912784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basic-Jukic N, Juric I, Furic-Cunko V, Katalinic L, Radic J, Bosnjak Z, et al. Follow-up of renal transplant recipients after acute COVID-19—A prospective cohort single-center study. Immunity, Inflamm. Dis. 2021;9(4):1563–1572. doi: 10.1002/iid3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal MJ, Lambarey H, Chetram A, Riou C, Wilkinson RJ, Schäfer G. Kaposi's Sarcoma-Associated Herpesvirus, but Not Epstein-Barr Virus, Co-infection Associates With Coronavirus Disease 2019 Severity and Outcome in South African Patients. Front. Microbiol. 2022;12 doi: 10.3389/fmicb.2021.795555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkmann A, Uddin S, Ulm SL, Pape K, Förster S, Enan K, et al. RespiCoV: Simultaneous identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and 46 respiratory tract viruses and bacteria by amplicon-based Oxford-Nanopore MinION sequencing. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0264855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks B, Tancredi C, Song Y, Mogus AT, Huang MLW, Zhu H, et al. Epstein–Barr Virus and Human Herpesvirus-6 Reactivation in Acute COVID-19 Patients. Viruses. 2022;14(9):1872. doi: 10.3390/v14091872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busani S, Tosi M, Mighali P, Vandelli P, D'Amico R, Marietta M, et al. Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: A structured summary of a study protocol for a randomised cont. Trials. 2020;21:724. doi: 10.1186/s13063-020-04645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carneiro VC de S, Alves-Leon SV, Sarmento DJ de S, Coelho WL da CNP, Moreira O da C, Salvio AL, et al. Herpesvirus and neurological manifestations in patients with severe coronavirus disease. Virol. J. 2022;19(1):1–9. doi: 10.1186/s12985-022-01828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen T, Song J, Liu H, Zheng H, Chen C. Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021;11:10902. doi: 10.1038/s41598-021-90351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatto I, Biagioni E, Coloretti I, Farinelli C, Avoni C, Caciagli V, et al. Cytomegalovirus blood reactivation in COVID-19 critically ill patients: risk factors and impact on mortality. Intensive Care Med. 2022;48(6):706–713. doi: 10.1007/s00134-022-06716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long covid prevalence and its relationship to epstein-barr virus reactivation. Pathogens. 2021;10:763. doi: 10.3390/pathogens10060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halawi M, Al-Hazmi A, Aljuaid A, Allahyani M, Abdulaziz O, Almalki AA, et al. Seroprevalence of toxoplasma gondii, rubella, group a streptococcus, cmv and hsv-1 in covid-19 patients with vitamin d deficiency. Pakistan J. Biol. Sci. 2021;24(11):1169–1174. doi: 10.3923/pjbs.2021.1169.1174. [DOI] [PubMed] [Google Scholar]
- 34.Huang R-C, Chiu C-H, Chiang T-T, Tsai C-C, Wang Y-C, Chang F-Y, et al. Hospital-acquired infections in patients hospitalized with COVID-19: First report from Taiwan. J. Chinese Med. Assoc. 2022;85(9):922–927. doi: 10.1097/JCMA.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Im JH, Nahm CH, Je YS, Lee J-S, Baek JH, Kwon HY, et al. The effect of Epstein–Barr virus viremia on the progression to severe COVID-19. Medicine (Baltimore) 2022;101(18):e29027. doi: 10.1097/MD.0000000000029027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahwagi J, Seye AO, Mbodji AB, Diagne R, Mbengue E, Fall M, et al. Surveillance of Viral Encephalitis in the Context of COVID-19 : Viruses. 2022;14:871. [DOI] [PMC free article] [PubMed]
- 37.Katz J, Yue S, Xue W. Herpes simplex and herpes zoster viruses in COVID-19 patients. Ir. J. Med. Sci. 2022;191(3):1093–1097. doi: 10.1007/s11845-021-02714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehner GF, Klein SJ, Zoller H, Peer A, Bellmann R, Joannidis M. Correlation of interleukin-6 with Epstein–Barr virus levels in COVID-19. Crit. Care. 2020;24:657. doi: 10.1186/s13054-020-03384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lino K, Alves LS, Raposo J V, Medeiros T, Souza CF, Silva AA d, et al. Presence and clinical impact of human herpesvirus-6 infection in patients with moderate to critical coronavirus disease-19. J. Med. Virol. 2022;94(3):1212–1216. doi: 10.1002/jmv.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majtanova N, Kriskova P, Keri P, Fellner Z, Majtan J, Kolar P. Herpes simplex keratitis in patients with SARS-CoV-2 infection: A series of five cases. Med. 2021;57:412. doi: 10.3390/medicina57050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng M, Zhang S, Dong X, Sun W, Deng Y, Li W, et al. COVID-19 associated EBV reactivation and effects of ganciclovir treatment. Immunity, Inflamm. Dis. 2022;10(4):e597. doi: 10.1002/iid3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer A, Buetti N, Houhou-Fidouh N, Patrier J, Abdel-Nabey M, Jaquet P, et al. HSV-1 reactivation is associated with an increased risk of mortality and pneumonia in critically ill COVID-19 patients. Crit. Care. 2021;25:417. doi: 10.1186/s13054-021-03843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naendrup JH, Borrega Jorge G, Dennis Alexander E, Alexander SV, Matthias K, Boris B. Reactivation of EBV and CMV in Severe COVID-19—Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically ill Patients. J. Intensive Care Med. 2021;18 doi: 10.1177/08850666211053990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paolucci S, Cassaniti I, Novazzi F, Fiorina L, Piralla A, Comolli G, et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis. 2021;104:315–319. doi: 10.1016/j.ijid.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romani L, Del Chierico F, Macari G, Pane S, Ristori MV, Guarrasi V, et al. The Relationship Between Pediatric Gut Microbiota and SARS-CoV-2 Infection. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.908492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saade A, Moratelli G, Azoulay E, Darmon M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect. Dis. Now. 2021;51(8):676–679. doi: 10.1016/j.idnow.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeßle J, Hippchen T, Schnitzler P, Gsenger J, Giese T, Merle U. High rate of HSV-1 reactivation in invasively ventilated COVID-19 patients: Immunological findings. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonnet A, Engelmann I, Moreau A, Garcia B, Six S, Kalioubie A El. High incidence of Epstein–Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51(1):296–299. doi: 10.1016/j.idnow.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh V, Upadhyay P, Reddy J, Granger J. SARS-CoV-2 respiratory co-infections: Incidence of viral and bacterial co-pathogens. Int. J. Infect. Dis. 2021;105:617–620. doi: 10.1016/j.ijid.2021.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895. doi: 10.1016/j.cell.2022.01.014. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vigón L, García-Pérez J, Rodríguez-Mora S, Torres M, Mateos E, Castillo de la Osa M, et al. Impaired Antibody-Dependent Cellular Cytotoxicity in a Spanish Cohort of Patients With COVID-19 Admitted to the ICU. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.742631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenzel P, Kopp S, Gobel S, Jansen T, Geyer M, Hahn F, et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc. Res. 2020 Aug 1;116(10):1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q, Xing Y, Shi L, Li W, Gao Y, Pan S, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146(1) doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 54.Xie Y, Cao S, Dong H, Lv H, Teng X, Zhang J, et al. Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation. BMC Infect. Dis. 2021;21:955. doi: 10.1186/s12879-021-06638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, Wang F, Dong G, Sheng Q, Feng S. A disease progression prediction model and nervous system symptoms in coronavirus disease 2019 patients. Am. J. Transl. Res. 2021;12(12):8192–8207. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu J, Wu Y. COVID-19 Epidemic: Clinical Characteristics of Patients in Pediatric Isolation Ward. Clin. Pediatr. (Phila). 2020;59(12):1069–1073. doi: 10.1177/0009922820941228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zubchenko S, Kril I, Nadizhko O, Matsyura O, Chopyak V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol. Int. 2022;42(9):1523–1530. doi: 10.1007/s00296-022-05146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 59.Proal A, Marshall T. Myalgic encephalomyelitis/chronic fatigue syndrome in the era of the human microbiome: Persistent pathogens drive chronic symptoms by interfering with host metabolism, gene expression, and immunity. Front. Pediatr. 2018;6:373. doi: 10.3389/fped.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taefehshokr N, Taefehshokr S, Hemmat N, Heit B. Covid-19: Perspectives on Innate Immune Evasion. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16(7) doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Quach T. COVID-19 cytokine storm syndrome: a threshold concept. Lancet Microbe. 2021;2(2):e49–e50. doi: 10.1016/S2666-5247(20)30223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le-Trilling VTK, Trilling M. Attack, parry and riposte: Molecular fencing between the innate immune system and human herpesviruses. Tissue Antigens. 2015;86(1):1–13. doi: 10.1111/tan.12594. [DOI] [PubMed] [Google Scholar]
- 65.Acharya D, Liu GQ, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'souza S, Lau KCK, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020;26(38):5759–5783. doi: 10.3748/wjg.v26.i38.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aghbash PS, Eslami N, Shirvaliloo M, Baghi HB. Viral coinfections in COVID-19. J. Med. Virol. 2021;93(9):5310–5322. doi: 10.1002/jmv.27102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardozo CM, Hainaut P. Viral strategies for circumventing p53: The case of severe acute respiratory syndrome coronavirus. Curr. Opin. Oncol. 2021;33(2):149–158. doi: 10.1097/CCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nunn AVW, Guy GW, Botchway SW, Bell JD. SARS-CoV-2 and EBV; the cost of a second mitochondrial “whammy”? Immun. Ageing. 2021;18(1):40. doi: 10.1186/s12979-021-00252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: Pityriasis rosea and Kawasaki disease. Dermatol. Ther. 2020;33(4):2019–2021. doi: 10.1111/dth.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Wang D, Tao X. Human herpesvirus 6B encephalitis in a liver transplant recipient: A case report and review of the literature. Transpl. Infect. Dis. 2021;23(1):1–4. doi: 10.1111/tid.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costa BK da, Sato DK. Viral encephalitis: a practical review on diagnostic approach and treatment. J. Pediatr. (Rio. J). 2020;96(S1):12–19. doi: 10.1016/j.jped.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapenko S, Krumina A, Logina I, Rasa S, Chistjakovs M, Sultanova A, et al. Association of active human herpesvirus-6, -7 and parvovirus B19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv. Virol. 2012;2012 doi: 10.1155/2012/205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ariza ME. Myalgic encephalomyelitis/chronic fatigue syndrome: The human herpesviruses are back! Biomolecules. 2021;11(2):185. doi: 10.3390/biom11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kadambari S, Klenerman P, Pollard AJ. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev. Med. Virol. 2020;30(5):1–5. doi: 10.1002/rmv.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juric I, Katalinic L, Furic-Cunko V, Basic-Jukic N. Myelodysplastic syndrome in a kidney transplant recipient after SARS-CoV-2 infection: can SARS-CoV-2 induce myelodysplastic syndrome? Int. Urol. Nephrol. 2022;54(7):1775–1776. doi: 10.1007/s11255-021-03069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu R, Zhou Y, Cai L, Wang L, Han J, Yang X, et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br. J. Dermatol. 2020;183(6):1145–1147. doi: 10.1111/bjd.19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diez-Domingo J, Parikh R, Bhavsar AB, Cisneros E, McCormick N, Lecrenier N. Can COVID-19 Increase the Risk of Herpes Zoster? A Narrative Review. Dermatol. Ther. (Heidelb). 2021;11(4):1119–1126. doi: 10.1007/s13555-021-00549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Readhead B, Haure-Mirande J, Funk C, Richards M, Shannon P, Haroutunian V, et al. Multiscale analysis of three independent Alzheimer's cohorts reveals disruption of molecular, genetic, and clinical networks by Human herpesvirus. Physiol. Behav. 2017;176(3):139–148. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magri F, Giordano S, Latini A, Muscianese M. New-onset cutaneous kaposi's sarcoma following SARS-CoV-2 infection. J. Cosmet. Dermatol. 2021;20(12):3747–3750. doi: 10.1111/jocd.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All detailed information regarding the search queries, reasons for inclusion/exclusion, and protocol for data extraction are available as supplement material within the article.