Abstract
Introduction
This systematic review with meta-analysis aimed to compare the robotic complete mesocolon excision (RCME) to laparoscopic colectomy (LC) with (LCME) or without CME (LC non-CME) in postoperative outcomes, harvested lymph nodes and disease-free survival.
Methods
We performed a systematic review with meta-analysis according to PRISMA 2020 and AMSTAR 2 guidelines.
Results
The literature search yielded seven comparative studies including 677 patients: 269 patients in the RCME group and 408 in the LC group. The pooled analysis concluded to a lower conversion rate in the RCME group (OR=0.17; 95% CI [0.04, 0.74], p=0.02). There was no difference between the two groups in terms of morbidity (OR=1.03; 95% CI [0.70, 1.53], p=0.87), anastomosis leakage (OR=0.83; 95% CI [0.18, 3.72], p=0.81), bleeding (OR=1.90; 95% CI [0.64, 5.58], p=0.25), wound infection (OR=1.37; 95% CI [0.51, 3.68], p=0.53), operative time (mean difference (MD)=36.32; 95% CI [−24.30, 96.93], p=0.24), hospital stay (MD=−0.94; 95% CI [−2.03, 0.15], p=0.09) and disease-free survival (OR=1.29; 95% CI [0.71, 2.35], p=0.41). In the subgroup analysis, the operative time was significantly shorter in the LCME group than RCME group (MD=50.93; 95% CI [40.05, 61.81], p<0.01) and we noticed a greater number of harvested lymph nodes in the RCME group compared with LC non-CME group (MD=8.96; 95% CI [5.98, 11.93], p<0.01).
Conclusions
The robotic approach for CME ensures a lower conversion rate than the LC. RCME had a longer operative time than the LCME subgroup and a higher number of harvested lymph nodes than the LC non-CME group.
Keywords: Robotic, Laparoscopic, Colectomy, Conventional colectomy, Complete mesocolon excision, Colon cancer
Introduction
Currently, the important question in colonic surgery for cancer remains the best surgical approach and the utility of complete mesocolic excision (CME). The laparoscopic approach has shown superiority over the open approach in postoperative outcomes and has become the gold standard.1,2 CME is considered a more radical resection with oncological superiority than conventional resections.3 Xu et al3 in a randomised controlled trial (RELARC) assessed the short-term outcomes of CME versus D2 dissection in laparoscopic right colectomy for colon cancer and concluded that the CME procedure might increase the risk of intraoperative vascular injury. Still, it seems to be safe and feasible for experienced surgeons. CME required a meticulous separation of the mesocolon embryonic plane from the parietal plane with a true central ligation of the right branches of affected arteries and veins.4 Combining the open-book anatomical model, suggested by Strey et al5 with a structured dissection sequence, using critical views as safety checkpoints, may provide a safe and efficient platform for teaching laparoscopic right colectomy with CME. This separation should be performed without breaching the covering layer enveloping the lymph nodes and the lymphatic vessels. In addition, it requires careful manipulation in anatomically complex and vulnerable regions.6 For these reasons, some surgeons highlighted the need for better visualisation and improved ergonomics instruments. Robotic surgery offers favourable technical conditions for complex operations, improved instrument stability, a high degree of freedom, 3D visualisation, fluorescence imaging and other features. Although data were insufficient and randomised controlled trials were lacking, it is interesting to summarise the available results and provide a conclusion as concerns the benefits and harms of robotic CME (RCME) on behalf of laparoscopic colectomy (LC) with CME (LCME) or without CME (LC non-CME).
This systematic review with meta-analysis compared the RCME with LC with or without CME in terms of postoperative outcomes, harvested lymph nodes and disease-free survival.
Methods
This systematic review with meta-analysis is structured according to the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Review and Meta-analysis)7 and the AMSTAR 2 guidelines (Assessing the Methodological Quality of Systematic Reviews). The protocol was registered in PROSPERO under the ID: CRD42022296512.
Electronics searches
The last electronic search of the relevant literature was conducted on 5 December 2021, for the publications over the previous 20 years with no language restriction. Trials were sought in ‘Cochrane Library's Controlled Trials Registry and database of a systematic review’, ‘United States National Library of Medicine’, ‘National Institutes of Health PubMed/MEDLINE’, ‘Excerpta Medica Database’, ‘Embase’ and ‘Google Scholar’ databases. Keywords used were ‘robotic surgery’; ‘laparoscopic surgery’; ‘complete mesocolon excision’; ‘colon cancer’, ‘colectomy’, ‘outcome’, ‘morbidity’, ‘mortality’, and ‘oncological outcomes’. We screened titles and abstracts of the yielded studies for relevancy. We checked the reference list of relevant reviews manually for additional citations.
Study selection
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) comparing RCME with laparoscopic complete mesocolon excision for colon cancer were included. We excluded trials comparing robotic and LC without complete mesocolon excision. Articles published in a peer-reviewed journal were considered for inclusion. In contrast, data from non-comparative studies, review articles, editorial letters, abstracts only, comments and case series (fewer than ten cases) were excluded.
Participants
Adults (over 18 years) of either sex operated on for colon cancer and undergoing complete mesocolon excision using a robotic or laparoscopic approach with or without neoadjuvant treatment.
Interventions
We studied two approaches for complete mesocolon excision for colon cancer surgery: robotic and laparoscopic approaches.
Endpoints measures
The primary endpoint was postoperative outcomes in morbidity, operative time, conversion rate, anastomotic leak, bleeding, wound infection, conversion rate and hospital stay. Secondary endpoints were the number of harvested lymph nodes and disease-free survival.
Study selection
Two authors independently reviewed all abstracts. All studies, accompanied by the full text that met the inclusion criteria, were retained. Disagreements were resolved by discussion after consulting a third review team member.
Assessment of study quality and risk of bias
All studies that met the selection criteria were appraised independently by two authors using the MINORS scale and Newcastle–Ottawa Scale for CCT and CONSORT for the RCTs. For bias assessment, we used the 7-piece Cochrane risk of the bias assessment tool (RoB1),8 including random sequence allocation, allocation concealing, blinding of participants and personnel, blinding of outcome assessors, incomplete outcomes data, selective outcome reporting and other potential threats to validity (including conflicts of interest) for RCT. Each piece was rated as low, high or unclear. The summarised risk of bias was considered low when all pieces were rated as low, considered high when at least one piece was rated as high and considered unclear when at least one piece was rated as unclear. Quality assessment of the CCT was assessed using the MINORS scale. Studies with a score <13/24 were excluded.
Data extraction
Two authors extracted the data, settling disparities with a senior author after discussion. For propensity score-matched studies, we used the data of the matched cohort. Studies included were fully matched for the first author name, year of publication, journal, study type, study design, country, study period, local excision technique, the number of patients included, age, gender, American Society of Anesthesiology (ASA) classification, body mass index, the site of the tumour, neoadjuvant treatment, follow-up, mortality, morbidity, anastomosis leak, conversion to open surgery, operative time, mesocolon integrity, number of harvested lymph nodes, quality of the resection, local recurrence and distant recurrence.
Certainty assessment of evidence
Two authors independently assessed the evidence. GRADE guidelines for rating the quality of evidence were used. We considered the study limitations constancy of effect, imprecision, indirectness and publication bias. We assessed the certainty of evidence as high, moderate, low or very low. If appropriate, we considered the following criteria for upgrading the certainly of evidence: large effect, dose–response gradient and plausible confounding effect. We used the methods and recommendations described in sections 8.5 and 8.7 and chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions. GRADEpro GDT software was used to prepare the summary of findings tables. We explain the reasons for downgrading or upgrading the included studies using footnotes with comments.
Assessment of heterogeneity
To assess heterogeneity, we used the Cochrane Chi2 test (Q-test), the I2 statistic and the variance Tau2 to estimate the degree of heterogeneity. Funnel plots identified studies responsible for heterogeneity after a sensitivity analysis. A subgroup analysis comparing RCME with LCME or LC non-CME was performed in case of heterogeneity among the studies when feasible.
Evaluation of effect size
We used the Review Manager 5.3.5 statistical package from the Cochrane collaboration for meta-analysis. We selected the mean difference (MD) as an effective measure for continuous data. For dichotomous variables, odds ratios (OR) with 95% confidence intervals (95% CI) were calculated. Random effects model was used. The threshold of significance was fixed to 0.05. We tested for the interaction between relevant factors and effect size estimates.
Results
Literature review
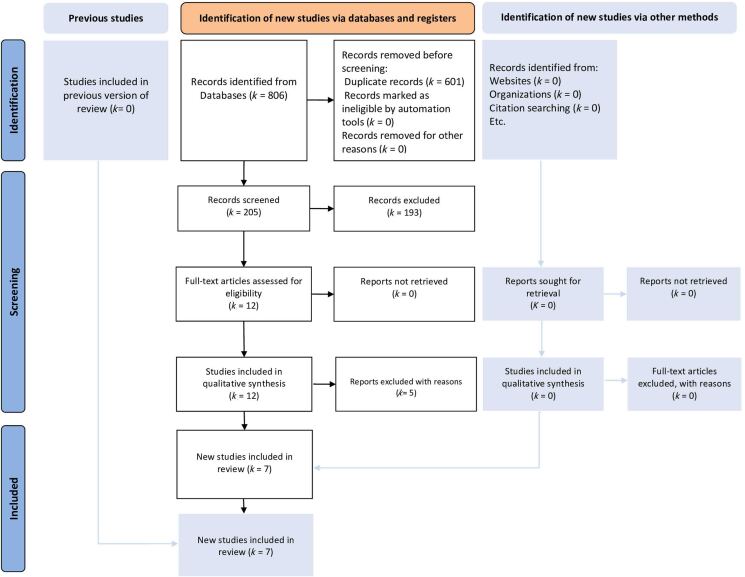
The literature search yielded 20 eligible studies. We retained seven comparative studies7,9–14 (Figure 1). Five studies were excluded for various reasons: one study presented duplicated data from the retained studies,15 one systematic review of robotic CME,8 one review article of CME in minimally invasive surgery,6 one study compared robotic and laparoscopic procedures without CME,16 and one study compared robotic CME with conventional robotic right colectomy.17 Six hundred and seventy-seven patients were retained: 269 patients in the RCME group and 408 in the LC group. The demographic data of the included patients and the quality assessment of the retained studies were reported in Tables 1 and 2, respectively. The different retained studies were published between 2017 and 2021. There were no RCTs and all the studies were CCTs. Four studies compared RCME with LCME and three studies compared RCME with LC non-CME. Five studies evaluated results of right colon cancer, one study of transverse colon and one study of left colon cancer. The mean age was 66.3 years in the RCME group and 58.8 years in the LC group with ranges between 56 and 74.6 years. The sex ratio in the study population was 1.18 with 54.1% males and 45.9% females. The majority of the included patients were not obese with a mean body mass index (BMI) between 23 and 29kg/m2. All the robotic procedures were performed using a Da Vinci Si or a Da Vinci Xi.
Figure 1 .
PRISMA 2020 flow diagram for search strategy, literature screening and study selection
Table 1 .
Demographic data of the retained studies
| First author | Country of origin | Journal | Year of publication | Study period | Study design | Number of patients (RCME/LC) | Laparoscopic resection | Age RCME/LC | Gender (M:F) RCME/LC | BMI (mean, kg/m2) RCME/LC | ASA score RCME/LC | Type of the robot | Tumour location | Tumour size | Follow-up (mean, months) RCME/LC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceccarelli | Italy | Surgical Endoscopy | 2020 | 2014–2019 | Propensity score-matched study, single centre | 20/20* | CME | 70.6/74.6 | 14:6/13:7 | 23/24.1 | 1 2 3 4 | 2/4 11/10 7/6 0/0 | Da Vinci Si / Da Vinci Xi | Right colon | 4/4.1 | 1 |
| Khan | United Kingdom | British Journal of Surgery | 2021 | 2014–2017 | Propensity score-matched study, single centre | 40/80 | Non-CME | 69/71 | 19:21/37:43 | 26/28 | 1 2 3 | 5/3 28/53 7/24 | Da Vinci Si / Da Vinci X | Right colon | - | 36 |
| Kim | Republic of Korea | International Journal of Medical Robotics and Computer Assisted Surgery | 2017 | 2012–2017 | Retrospective study, single centre | 20/51 | CME | 58/56 | 12:8/33:18 | 25.5/24 | 1 2 3 | 8/11 12/37 0/3 | - | Left colon | 4.2/4.4 | 18/18 |
| Ngu | Singapore | Journal of Robotic Surgery | 2018 | 2015–2017 | Prospective maintained database | 16/16 | CME | 68.6/69.6 | 10:6/6:10 | 23.7/24.7 | 2 3 | 8/4 8/12 | Da Vinci Xi | Right colon | 4/4.5 | 1 |
| Ozben | Turkey | Techniques in Coloproctology | 2020 | 2011–2018 | Retrospective study, single centre | 38/80 | Non-CME | 62.3/64.1 | 27:11/47:33 | 25.3/26.7 | 1 2 3 | 10/19 19/36 9/25 | Da Vinci Xi | Transverse colon | 5.3/5.2 | 1 |
| Spinoglio | Italy | Annals of Surgical Oncology | 2018 | 2005–2015 | Prospective maintained database | 100/100 | CME | 71.2/71.2 | 44:54/54:47 | 25.1/25.8 | 1 2 3 4 | 13/10 40/36 38/43 10/12 | Da Vinci Si | Right colon | - | 60/60 |
| Yozgatli | Turkey | Journal of Laparoendoscopic and Advanced Surgical Techniques | 2019 | 2015–2017 | Retrospective study, single centre | 35/61 | Non-CME | 65/65 | 20:15/30:31 | 29/27 | - | - | Da Vinci Xi | Right colon | 5/5 | 15/16 |
* 3D laparoscopy
NA = not applicable; NR = not reported; BMI = body mass index; ASA = American Society of Anesthesiologists; RCME = robotic complete mesocolon excision; LC = laparoscopic colectomy; M = male; F = female; CME = complete mesocolon excision
Table 2 .
Risk of bias assessment of the retained clinical trials
| First author | Quality assessment: MINORS | Newcastle–Ottawa Scale | |||
|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Total | ||
| Ceccarelli | 18 | ** | * | *** | Fair quality |
| Khan | 20 | ** | * | *** | Fair quality |
| Kim | 20 | *** | * | *** | Good quality |
| Ngu | 20 | ** | * | *** | Fair quality |
| Ozben | 18 | ** | * | *** | Fair quality |
| Spinoglio | 20 | ** | * | *** | Fair quality |
| Yozgatli | 18 | ** | * | *** | Fair quality |
Morbidity
All studies reported postoperative morbidity.7,9–14 They included 269 patients in the RCME group and 408 patients in the LC group (Table 3). There was no difference between the two groups (OR=1.03; 95% CI [0.70, 1.53], p=0.87).
Table 3 .
Table of outcomes (bold denotes significance)
| Outcome | Number of studies | Participants | Odds ratio or mean difference, 95% CI | Tau2 (I2) | p-value | ||
|---|---|---|---|---|---|---|---|
| RCME | LC | ||||||
| Morbidity | All studies | 7 | 63/269 | 49/408 | 1.03 [0.70, 1.53] | 0 (0%) | 0.87 |
| CME | 4 | 45/156 | 32/187 | 1.07 [0.62, 1.85] | 0.04 (10%) | 0.82 | |
| Non-CME | 3 | 63/113 | 81/221 | 1.07 [0.56, 2.06] | 0 (0%) | 0.83 | |
| Anastomosis leak | All studies | 7 | 2/269 | 6/408 | 0.83 [0.18, 3.72] | 0 (0%) | 0.81 |
| CME | 4 | 1/156 | 1/187 | 1.00 [0.06, 16.21] | – | 1.00 | |
| Non-CME | 3 | 1/113 | 5/221 | 0.78 [0.11, 5.40] | 0.45 (15%) | 0.80 | |
| Bleeding | All studies | 7 | 9/269 | 7/408 | 1.90 [0.64, 5.58] | 0 (0%) | 0.25 |
| CME | 4 | 5/156 | 5/187 | 1.49 [0.37, 5.95] | 0 (0%) | 0.57 | |
| Non-CME | 3 | 4/113 | 2/221 | 2.75 [0.47, 16.06] | 0.10 (4%) | 0.26 | |
| Operative time | All studies | 7 | 269 | 408 | 36.32 [−24.30, 96.93] | 19,889 (98%) | 0.24 |
| CME | 4 | 156 | 187 | 50.93 [40.05, 61.81] | 0 (0%) | <0.01 | |
| Non-CME | 3 | 113 | 221 | 16.37 [−144.09, 176.83] | 6,502 (98%) | 0.84 | |
| Conversion | All studies | 7 | 0/269 | 19/408 | 0.17 [0.04, 0.74] | 0 (0%) | 0.02 |
| CME | 4 | 0/156 | 6/187 | 0.16 [0.02, 1.32] | 0 (0%) | 0.09 | |
| Non-CME | 3 | 0/113 | 10/221 | 0.18 [0.02, 1.39] | 0 (0%) | 0.10 | |
| Wound infection | All studies | 7 | 15/269 | 20/408 | 1.37 [0.51, 3.68] | 0.45 (31%) | 0.53 |
| CME | 4 | 7/156 | 11/187 | 0.75 [0.25, 2.24] | 0.10 (7%) | 0.60 | |
| Non-CME | 3 | 8/113 | 9/221 | 2.41 [0.45, 12.87] | 1.00 (45%) | 0.30 | |
| Hospital stay | All studies | 7 | 269 | 408 | −0.94 [−2.03, 0.15] | 1.57 (75%) | 0.09 |
| CME | 4 | 156 | 187 | −0.22 [−0.88, 0.44] | 0 (0%) | 0.52 | |
| Non-CME | 3 | 113 | 221 | −2.06 [−4.93, 0.82] | 5.73 (90%) | 0.16 | |
| Harvested lymph nodes | CME | 4 | 156 | 187 | 2.12 [−3.39, 7.62] | 23.18 (79%) | 0.45 |
| Non-CME | 3 | 113 | 221 | 8.96 [5.98, 11.93] | 0 (0%) | <0.01 | |
| DFS | All studies | 3 | 139/160 | 195/231 | 1.29 [0.71, 2.35] | 0 (0%) | 0.41 |
RCME = robotic complete mesocolon excision; LC = laparoscopic colectomy; CME = complete mesocolon excision; DFS = disease-free survival
Anastomosis leak
All studies assessed the anastomosis leak.7,9–14 An anastomosis leak was mentioned in 2 out of 269 patients in the RCME group and 6 out of 408 patients in the LC group (Table 3). There was no difference between the RCME group and LC group in term of anastomosis leakage (OR=0.83; 95% CI [0.18, 3.72], p=0.81).
Bleeding
Postoperative bleeding was reported in seven studies.7,9–14 It was observed in 9 out of 269 patients in the RCME group and 7 out of 408 patients in the LC group (Table 3). There was no difference between the two groups with regard to the bleeding rate (OR=1.90; 95% CI [0.64, 5.58], p=0.25).
Wound infection
Seven studies reported the wound infection rate.7,9–14 It was recorded in 15 out of 269 patients in the RCME group and 20 out of 408 patients in the LC group (Table 3). There was no difference between the two surgical approaches in terms of wound infection (OR=1.37; 95% CI [0.51, 3.68], p=0.53). There was little heterogeneity among the studies (Tau2=0.45, I2=31%).
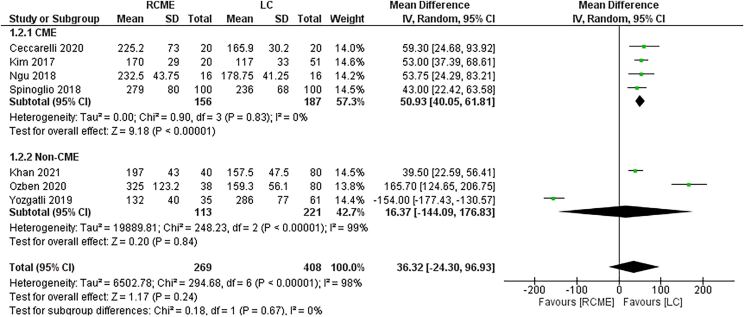
Operative time
The seven studies reported the operative time.7,9–14 The data of 269 patients in the RCME group and 408 patients in the LC group were available (Table 3). There were no differences between the two groups, in the pooled analysis, in terms of operative time (Figure 2) (MD=36.32; 95% CI [−24.30, 96.93], p=0.24). We noticed a high heterogeneity among the studies (Tau2=19,889, I2=98%). In the subgroup analysis, the operative time was significantly shorter in the LCME then RCME group (MD=50.93; 95% CI [40.05, 61.81], p<0.01) and a similar operative time between the RCME group and the LC non-CME group (MD=16.37; 95% CI [−144.09, 176.83], p=0.84). In the sensitivity analysis, the studies of Ozben et al13 and Yozgatli et al7 were the sources of the heterogeneity.
Figure 2 .
Forest plot of the operative time
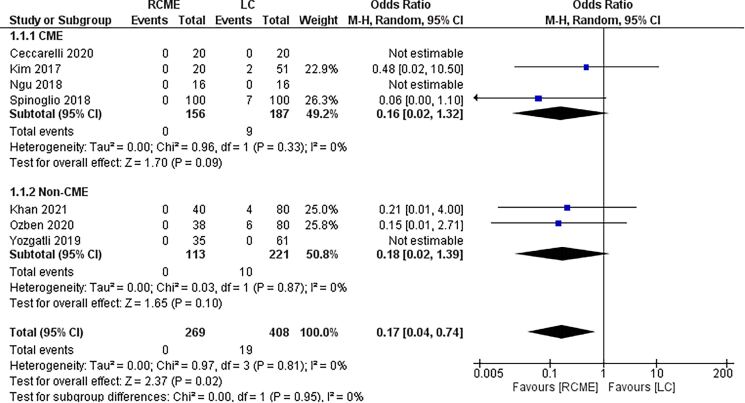
Conversion rate
The seven studies reported the conversion rate.7,9–14 No conversion was reported in the RCME group and 19 out of 408 patients in the LC group (Table 3). There was higher conversion rate in the LC group than the RCME group (OR=0.17; 95% CI [0.04, 0.74], p=0.02) (Figure 3).
Figure 3 .
Forest plot of the conversion rate
Hospital stay
The hospital stay was reported in seven studies.7,9–14 There were two hundred and sixty-nine patients in the RCME group and 408 in the LC group (Table 3). There were no differences between the two groups in terms of hospital stay (MD=−0.94; 95% CI [−2.03, 0.15], p=0.09). There was little heterogeneity among the studies. In the subgroup analysis, there was no difference between the RCME group and LCME group (MD=−0.22; 95% CI [−0.88, 0.44], p=0.52) or LC non-CME (MD=−2.06; 95% CI [−4.93, 0.82], p=0.16), respectively. There was moderate heterogeneity among the studies in the subgroup comparison of the RCME group with LC non-CME group. In the sensitivity analysis, the study of Yozgatli et al7 was the source of the heterogeneity.
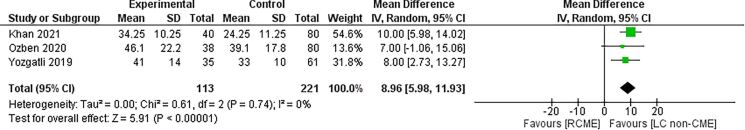
Harvested lymph nodes
The number of harvested lymph nodes was reported in the seven studies.7,9–14 The data of 269 patients in the RCME group and 408 patients in the LC group were available (Table 3). In the subgroup analysis, there was no difference between the RCME group and LCME group (MD=2.12; 95% CI [−3.39, 7.62], p=0.45) with a greater number of harvested lymph nodes in the RCME group compared with the LC non-CME group (MD=8.96; 95% CI [5.98, 11.93], p<0.01) (Figure 4). In the sensibility analysis, the study of Ngu et al12 was the source of heterogeneity.
Figure 4 .
Forest plot of the subgroup analysis of robotic complete mesocolon excision (RCME) versus laparoscopic colectomy (LC) non-CME in terms of harvested lymph nodes number
Disease-free survival
The disease-free survival was reported in three studies.10,11,14 It was mentioned in 139 patients in the RCME group and 195 in the LC group (Table 3). There was no difference between the two surgical approaches in terms of disease-free survival (OR=1.29; 95% CI [0.71, 2.35], p=0.41).
Quality assessment of the included studies and reporting the effects of robotic CME
The MINORS and the Newcastle–Ottawa Scale for the retained clinical trials were reported in Table 3. The summary of evidence findings of RCME versus LC, RCME versus LCME and RCME versus LC non-CME was reported in Tables 4, 5 and 6, respectively. First, the review shows that when the mesocolon was resected robotically, compared with LC:
-
•
It probably reduces the conversion rate with a similar anastomosis leakage.
-
•
It may provide similar morbidity, bleeding, wound infection and hospital stay.
-
•
We do not know if it led to additional operative time or disease-free survival.
Table 4 .
Summary of findings table of RCME versus LC
| RCME compared with LC for colonic cancer | |||||
|---|---|---|---|---|---|
| Patient or population: colonic cancer Setting: Intervention: RCME; Comparison: LC | |||||
| Outcomes | No. of participants (studies) |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with LC | Risk difference with RCME | ||||
| Conversion | 677 (7 observational studies) |
⨁⨁⨁◯ Moderate |
OR 0.17 (0.04 to 0.74) |
47 per 1,000 |
38 fewer per 1,000 (45 fewer to 12 fewer) |
| Operative time | 677 (7 observational studies) |
⨁◯◯◯ Very lowa,b |
– | – | MD 36.32 higher (24.3 lower to 96.93 higher) |
| Morbidity | 677 (7 observational studies) |
⨁⨁◯◯ Low |
OR 1.03 (0.70 to 1.53) |
199 per 1,000 |
5 more per 1,000 (51 fewer to 76 more) |
| Anastomosis leak | 677 (7 observational studies) |
⨁⨁⨁◯ Moderate |
OR 0.83 (0.18 to 3.72) |
15 per 1,000 |
2 fewer per 1,000 (12 fewer to 38 more) |
| Hospital stay | 677 (7 observational studies) |
⨁⨁◯◯ Lowb |
– | – | MD 0.94 lower (2.03 lower to 0.15 higher) |
| Bleeding | 677 (7 observational studies) |
⨁⨁◯◯ Low |
OR 1.90 (0.64 to 5.58) |
17 per 1,000 |
15 more per 1,000 (6 fewer to 72 more) |
| Wound infection | 581 (6 observational studies) |
⨁⨁◯◯ Lowb |
OR 0.82 (0.37 to 1.78) |
55 per 1,000 |
9 fewer
per 1,000 (34 fewer to 39 more) |
| DFS | 391 (3 observational studies) |
⨁◯◯◯ Very lowa,b |
OR 1.29 (0.71 to 2.35) |
844 per 1,000 |
31 more per 1,000 (51 fewer to 83 more) |
| DFS | All studies 3 | 139/160 | 195/231 1.29 [0.71, 2.35] | 0 (0%) | 0.41 |
RCME = robotic complete mesocolon excision; LC = laparoscopic colectomy; CI = confidence interval; MD = mean difference; OR = odds ratio; DFS = disease-free survival
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the effect estimate.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
aI2 superior to 50% with a p-value less than 0.0001 suggesting substantial heterogeneity.
bSmall sample size suggesting issues with imprecision.
Table 5 .
Summary of findings table of RCME versus LCME
| RCME compared with LCME for colonic cancer | |||||
|---|---|---|---|---|---|
| Patient or population: colonic cancer Setting: Intervention: RCME and Comparison: LCME | |||||
| Outcomes | No. of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with LCME | Risk difference with RCME | ||||
| Conversion – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
OR 0.16 (0.02 to 1.32) |
48 per 1,000 |
40 fewer per 1,000 (47 fewer to 14 more) |
| Operative time – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
– | – | MD 50.93 higher (40.05 higher to 61.81 higher) |
| Morbidity – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
OR 1.07 (0.62 to 1.85) |
262 per 1,000 |
13 more per 1,000 (82 fewer to 134 more) |
| Anastomosis leak – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
OR 1.00 (0.06 to 16.21) |
5 per 1,000 |
0 fewer per 1,000 (5 fewer to 75 more) |
| Hospital stay – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
– | – | MD 0.22 lower (0.88 lower to 0.44 higher) |
| Bleeding – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
OR 1.49 (0.37 to 5.95) |
27 per 1,000 |
13 more per 1,000 (17 fewer to 114 more) |
| Wound infection – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa |
OR 0.75 (0.25 to 2.24) |
59 per 1,000 |
14 fewer per 1,000 (43 fewer to 64 more) |
| Harvested lymph nodes – CME | 343 (4 observational studies) |
⨁◯◯◯ Very lowa,b |
– | – | MD 2.12 higher (3.39 lower to 7.62 higher) |
RCME = robotic complete mesocolon excision; LCME = laparoscopic colectomy with CME; LC = laparoscopic colectomy; CI = confidence interval; MD = mean difference; OR = odds ratio
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the effect estimate.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
aSmall sample size suggesting issues with imprecision.
bI2 superior to 50% with a p-value less than 0.0001 suggesting substantial heterogeneity.
Table 6 .
Summary of findings table of RCME versus LC non-CME
| RCME compared with LC non-CME for colonic cancer | |||||
|---|---|---|---|---|---|
| Patient or population: colonic cancer Setting: Intervention: RCME and Comparison: LC non-CME | |||||
| Outcomes | No. of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with LC non-CME | Risk difference with RCME | ||||
| Conversion – Non-CME | 334 (3 observational studies) |
⨁◯◯◯ Very lowa |
OR 0.18 (0.02 to 1.39) |
45 per 1,000 |
37 fewer per 1,000 (44 fewer to 17 more) |
| Operative time – Non-CME | 334 (3 observational studies) |
⨁◯◯◯ Very lowa,b |
– | – | MD 16.37 higher (144.09 lower to 176.83 higher) |
| Morbidity – Non-CME | 334 (3 observational studies) |
⨁◯◯◯ Very lowa |
OR 1.07 (0.56 to 2.06) |
145 per 1,000 |
9 more per 1,000 (58 fewer to 114 more) |
| Anastomotic leak – Non-CME | 334 (3 observational studies) |
⨁◯◯◯ Very lowa |
OR 0.78 (0.11 to 5.40) |
23 per 1,000 |
5 fewer per 1,000 (20 fewer to 88 more) |
| Hospital stay – Non-CME | 334 (3 observational studies) |
⨁◯◯◯ Very lowa,b |
– | – | MD 2.06 lower (4.93 lower to 0.82 higher) |
| Bleeding – Non-CME | 334 (3 observational studies) |
⨁⨁◯◯ Lowa |
OR 2.75 (0.47 to 16.06) |
9 per 1,000 |
15 more per 1,000 (5 fewer to 119 more) |
| Wound infection – Non-CME | 238 (2 observational studies) |
⨁⨁◯◯ Lowa |
OR 1.28 (0.22 to 7.31) |
50 per 1,000 |
13 more per 1,000 (39 fewer to 228 more) |
| Harvested lymph nodes – Non-CME | 334 (3 observational studies) |
⨁⨁◯◯ Lowa |
– | – | MD 8.96 higher (5.98 higher to 11.93 higher) |
RCME = robotic complete mesocolon excision; LC = laparoscopic colectomy; CI = confidence interval; MD = mean difference; OR = odds ratio.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the effect estimate.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
aSmall sample size suggesting issues with imprecision.
bI2 superior to 50% with a p-value less than 0.0001 suggesting substantial heterogeneity.
Second, the review shows that when the CME was performed with a robot, compared with LCME, we do not know if it led to additional conversion rate, operative time, morbidity, anastomosis leakage, hospital stay, bleeding, wound infection or harvested lymph nodes.
Third, the review shows that RCME compared LC without CME:
-
•
It may increase the number of harvested lymph nodes with similar bleeding and wound infection rates.
-
•
We do not know if it leads to additional conversion, operative time, morbidity, anastomosis leak or hospital say.
Discussion
This systematic review with meta-analysis concluded that the robotic approach for CME ensured a lower conversion rate than the LC. There were no differences between the two groups, RCME and LC, in terms of morbidity, anastomosis leak, bleeding, wound infection, hospital stay and disease-free survival. In the subgroup analysis, RCME required a longer operative time than the LCME subgroup and ensured a higher number of harvested lymph nodes than the LC non-CME group.
In addition to the efficacy of laparoscopy in difficult procedures,18 the advantages of mini-invasive surgery in postoperative recovery after colon cancer surgery were accepted worldwide.1,19 Since its first description by Hohenberger et al,4 many studies highlight greater long-term oncological outcomes than conventional colectomy.20 This procedure remains more difficult and technically challenging. Therefore, many studies have assessed the optimal surgical approach for this procedure.9 It is well proven that the laparoscopic CME did not increase the morbidity or mortality over standard colectomy.1 To the best of the authors' knowledge, few reports are analysing the safety, feasibility and oncological outcomes comparing the advantages of the robotic approach with LCME.
There was a lower conversion rate in the RCME group than the LC group in our study. This advantage is relevant, and it could be explained by the ergonomics and added advantages of the robotic platform. We did not find any differences between the RCME and the LC groups concerning 30-day postoperative morbidity, even in the subgroup analysis comparing RCME to LCME or LC non-CME. We found a similar rate of anastomosis leakage, postoperative bleeding or wound infection. In particular, the retained studies have not reported superior mesenteric vein lesions during the dissection using the robotic or laparoscopic approach. The operative time was similar between the RCME group and the LC group. There was high heterogeneity among the studies. In the subgroup analysis, the operative time was significantly shorter in the LCME than in the RCME group, with a similar operative time in the RCME group and LC non-CME. In the sensitivity analysis, the two studies of Ozben et al13 and Yozgatli et al7 were the sources of the heterogeneity. This heterogeneity could be explained by the studies’ absence of standardised measure features. In addition, there are no data regarding the learning curve of the involved surgeons, which is longer in the robotic approach. These findings concerning the lower conversion rate and longer operative time after a RCME compared to LCME were similar to several results assessing the benefits and harms of robotic complete mesorectal excision in patients with rectal cancer. Huang et al21 and Eltair et al22 reported a longer operative time in the robotic complete mesorectal excision group than the laparoscopic complete mesorectal excision group. As concerns the conversion rate, Huang et al21 in a recent updated systematic review and meta-analysis of RCTs found a lower conversion rate in the robotic complete mesorectal excision group. Furthermore, Gavriilidis et al23 in a systematic review by updated meta-analysis concluded that although the robotic complete mesorectal excision group included patients with higher BMI, more distal rectal cancer and more patients undergoing neoadjuvant therapy, this group demonstrated lower conversion rates to open surgery when compared to laparoscopic complete mesorectal excision. These findings highlight the importance of the robotic approach to reduce the conversion rate in colorectal surgery.
The hospital stay was similar between the RCME and LC groups. There was little heterogeneity among the retained studies. In the subgroup analysis, we do not find any difference between the RCME, LCME or LC non-CME groups. It was noticed that there was moderate heterogeneity among the studies in the subgroup comparison of the RCME group with LC non-CME group. In the sensitivity analysis, the study by Yozgatli et al7 was the source of the heterogeneity. This heterogeneity could be explained by the absence of data concerning the postoperative recovery applied in each department and the criteria to discharge patients after surgery.
There is evidence that CME in colonic cancer ensures improved oncological outcomes owing to more harvested lymph nodes than conventional resection that does not focus on removing an intact and complete mesocolon.24 In our review, it was not rational to compare the RCME group and the LC group in terms of harvested lymph nodes number. Furthermore, the comparison for this outcome could present residual confounding and biases. The subgroup analysis showed no difference between the RCME and LCME groups with more harvested lymph nodes in the RCME group than the LC non-CME group. This is in harmony with the available data in the literature confirming that robotic or laparoscopic CME provided a high number of harvested lymph nodes.25,26 In the sensibility analysis, Ngu et al12 was the source of the heterogeneity. This heterogeneity could be explained by including 4 out of 16 patients who underwent LC non-CME in the RCME group.
The disease-free survival was reported in only three studies.10,11,14 It was impossible to perform subgroup analysis. There was no difference between the RCME group and the LC group. Owing to the lack of evidence regarding oncological data, it is not easy to make conclusions, and further studies were mandatory with mid and long-term outcomes.
This is the first meta-analysis comparing RCME and LC to the best of the authors' knowledge. Several limitations should be considered. Owing to the absence of RCTs, we included CCTs with a risk of bias and low to moderate heterogeneity in some outcomes. We used the MINORS to assess the quality of the retained studies and the Newcastle–Ottawa Scale for risk of bias assessment to only studies with great quality. To overcome the heterogeneity among the studies, we have performed a subgroup and sensitivity analysis to provide the highest level of evidence according to the available data in the literature. Therefore, our findings should be considered with caution, and it is recommended to assess the efficacy of RCME in long-term oncological outcomes. RCTs with larger sample sizes and longer follow-up were mandatory for better placement of RCME therapeutic features of colonic cancer. Currently, the RoLaCaRT1, an international randomised phase III trial that is comparing robotic-assisted right colectomy versus laparoscopic-assisted colectomy for resection, will provide more information for this question.
In conclusion, no international surgical society had recommended colectomy with CME as the ‘gold standard’. It is consensual that the main criteria to exclude patients were: a metastatic disease, aged patients, ASA superior to 3 and comorbidities inhibiting major surgery.27 The robotic approach for CME ensures a lower conversion rate than LC. It required a longer operative time than the LCME subgroup and ensured more harvested lymph nodes than the LC non-CME group.
Authors’ contributions
All authors participated in the study on the conception, design of the research and data acquisition. All the authors validated the final version of the article.
Compliance with ethical standards
This research involves Human participants. It is a retrospective analysis of published cases and did not require informed consent. Ethics approval and consent to participate were not applicable in this review.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Chaouch MA, Dougaz MW, Bouasker Iet al. . Laparoscopic versus open complete mesocolon excision in right colon cancer: a systematic review and meta-analysis. World J Surg 2019; 43: 3179–3190. [DOI] [PubMed] [Google Scholar]
- 2.Anania G, Arezzo A, Davies RJet al. . A global systematic review and meta-analysis on laparoscopic vs open right hemicolectomy with complete mesocolic excision. Int J Colorectal Dis 2021; 36: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L, Su X, He Zet al. . Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): a randomised, controlled, phase 3, superiority trial. Lancet Oncol 2021; 22: 391–401. [DOI] [PubMed] [Google Scholar]
- 4.Hohenberger W, Weber K, Matzel Ket al. . Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 2009; 11: 354–364. [DOI] [PubMed] [Google Scholar]
- 5.Strey CW, Wullstein C, Adamina Met al. . Laparoscopic right hemicolectomy with CME: standardization using the ‘critical view’ concept. Surg Endosc 2018; 32: 5021–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm D, Vogel T, Neumann P-Aet al. . Complete mesocolic excision in minimally invasive surgery of colonic cancer: do we need the robot? Eur Surg 2021; 53: 166–174. [Google Scholar]
- 7.Yozgatli TK, Aytac E, Ozben Vet al. . Robotic complete mesocolic excision versus conventional laparoscopic hemicolectomy for right-sided colon cancer. Journal of Laparoendoscopic & Advanced Surgical Techniques 2019; 29: lap.2018.0348. [DOI] [PubMed] [Google Scholar]
- 8.Petz W, Borin S, Fumagalli Romario U. Updates on robotic CME for right colon cancer: A qualitative systematic review. J Pers Med 2021; 11: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceccarelli G, Costa G, Ferraro Vet al. . Robotic or three-dimensional (3D) laparoscopy for right colectomy with complete mesocolic excision (CME) and intracorporeal anastomosis? A propensity score-matching study comparison. Surg Endosc 2021; 35: 2039–2048. [DOI] [PubMed] [Google Scholar]
- 10.Khan JS, Ahmad A, Odermatt Met al. . Robotic complete mesocolic excision with central vascular ligation for right colonic tumours – a propensity score-matching study comparing with standard laparoscopy. BJS Open 2021; 5: zrab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JC, Lee JL, Yoon YSet al. . Robotic left colectomy with complete mesocolectomy for splenic flexure and descending colon cancer, compared with a laparoscopic procedure. Int J Med Robotics Comput Assist Surg 2018; 14: e1918. [DOI] [PubMed] [Google Scholar]
- 12.Ngu JC-Y, Ng YY-R. Robotics confers an advantage in right hemicolectomy with intracorporeal anastomosis when matched against conventional laparoscopy. J Robot Surg 2018; 12: 647–653. [DOI] [PubMed] [Google Scholar]
- 13.Ozben V, de Muijnck C, Sengun Bet al. . Robotic complete mesocolic excision for transverse colon cancer can be performed with a morbidity profile similar to that of conventional laparoscopic colectomy. Tech Coloproctol 2020; 24: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 14.Spinoglio G, Bianchi PP, Marano Aet al. . Robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann Surg Oncol 2018; 25: 3580–3586. [DOI] [PubMed] [Google Scholar]
- 15.Spinoglio G, Marano A, Priora Fet al. . Right colectomy with complete mesocolic excision: four-arm technique. In: Spinoglio G. Robotic Surgery [Internet]. Milano: Springer Milan; 2015. pp. 125–132 (Updates in Surgery). http://link.springer.com/10.1007/978-88-470-5714-2_13 (cited January 2023). [Google Scholar]
- 16.Scotton G, Contardo T, Zerbinati Aet al. . From laparoscopic right colectomy with extracorporeal anastomosis to robot-assisted intracorporeal anastomosis to totally robotic right colectomy for cancer: The evolution of robotic multiquadrant abdominal surgery. Journal of Laparoendoscopic & Advanced Surgical Techniques 2018; 28: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 17.Larach JT, Flynn J, Wright Tet al. . Robotic complete mesocolic excision versus conventional robotic right colectomy for right-sided colon cancer: a comparative study of perioperative outcomes. Surg Endosc 2021; 36: 2113–2120. https://link.springer.com/10.1007/s00464-021-08498-8 (cited January 2023). [DOI] [PubMed] [Google Scholar]
- 18.Chaouch MA, Jerraya H, Dougaz MWet al. . A systematic review of laparoscopic cholecystectomy in situs inversus. J Invest Surg 2021; 34: 324–333. [DOI] [PubMed] [Google Scholar]
- 19.Chaouch MA, Kellil T, Jeddi Cet al. . How to prevent anastomotic leak in colorectal surgery? A systematic review. Ann Coloproctol 2020; 36: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ow ZGW, Sim W, Nistala KRYet al. . Comparing complete mesocolic excision versus conventional colectomy for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2021; 47: 732–737. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y-J, Kang Y-N, Huang Y-Met al. . Effects of laparoscopic vs robotic-assisted mesorectal excision for rectal cancer: An update systematic review and meta-analysis of randomized controlled trials. Asian Journal of Surgery 2019; 42: 657–666. [DOI] [PubMed] [Google Scholar]
- 22.Eltair M, Hajibandeh S, Hajibandeh Set al. . Meta-analysis and trial sequential analysis of robotic versus laparoscopic total mesorectal excision in management of rectal cancer. Int J Colorectal Dis 2020; 35: 1423–1438. [DOI] [PubMed] [Google Scholar]
- 23.Gavriilidis P, Wheeler J, Spinelli Aet al. . Robotic vs laparoscopic total mesorectal excision for rectal cancers: has a paradigm change occurred? A systematic review by updated meta-analysis. Colorectal Dis 2020; 22: 1506–1517. [DOI] [PubMed] [Google Scholar]
- 24.Di Buono G, Buscemi S, Cocorullo Get al. . Feasibility and safety of laparoscopic complete mesocolic excision (CME) for right-sided colon cancer: short-term outcomes. A randomized clinical study. Ann Surg 2021; 274: 57–62. [DOI] [PubMed] [Google Scholar]
- 25.Díaz-Vico T, Fernández-Hevia M, Suárez-Sánchez Aet al. . Complete mesocolic excision and D3 lymphadenectomy versus conventional colectomy for colon cancer: A systematic review and meta-analysis. Ann Surg Oncol 2021; 28: 8823–8837. [DOI] [PubMed] [Google Scholar]
- 26.Magistro C, Bertoglio CL, Giani Aet al. . Laparoscopic complete mesocolic excision versus conventional resection for right-sided colon cancer: a propensity score matching analysis of short-term outcomes. Surg Endosc 2021; 36: 3049–3058. [DOI] [PubMed] [Google Scholar]
- 27.Jamali FR. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg 2008; 143: 762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.