INTRODUCTION:
Guidelines recommend that proton pump inhibitor-based triple regimens with clarithromycin not be used for Helicobacter pylori infection in areas where clarithromycin resistance is ≥15%, or in patients with prior macrolide use. Up-to-date information on local resistance patterns is limited, especially in the US. Here, we report resistance rates to antibiotics commonly used to treat H. pylori from a large study conducted in the US and Europe (pHalcon-HP).
METHODS:
Gastric mucosal biopsies were collected from adult participants with H. pylori infection during screening. Minimum inhibitory concentrations were determined via agar dilution for clarithromycin, amoxicillin, and metronidazole, with breakpoints ≥1 μg/mL, >0.125 μg/mL, and >8 μg/mL, respectively. Resistance rates were obtained for the US and Europe, and also for US subregions and participating European countries.
RESULTS:
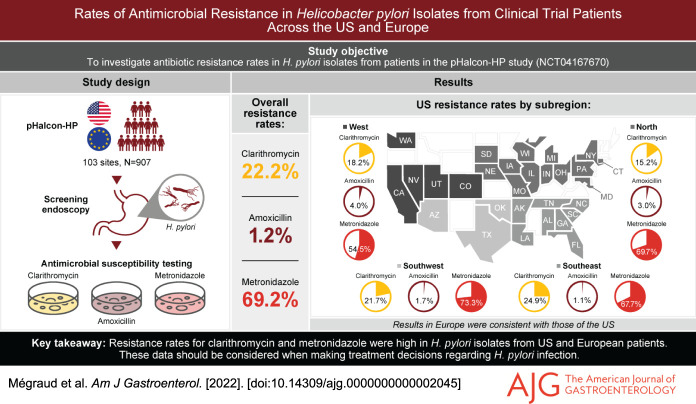
Resistance rates were established in isolates from 907 participants. Overall, 22.2% were resistant to clarithromycin, 1.2% to amoxicillin, and 69.2% to metronidazole. Resistance in the US and Europe was similar; metronidazole resistance was the most prevalent (50%–79%) and amoxicillin the least (≤5%). In all subregions, ≥15% of isolates were resistant to clarithromycin, except the UK (0/8 isolates). Among clarithromycin-resistant isolates, 75% were also metronidazole-resistant. Two US isolates were resistant to clarithromycin and amoxicillin; one of these was also metronidazole-resistant.
DISCUSSION:
The resistance rates observed in this study argue against the continued empiric use of proton pump inhibitor-based triple therapy containing clarithromycin, per treatment guidelines, and highlight the need for antibiotic resistance surveillance and novel treatment strategies for H. pylori infection in the US and Europe.
INTRODUCTION
Helicobacter pylori currently infects over half of the world's population (1). Infection causes chronic gastritis and is the major risk factor for peptic ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue lymphoma (1,2). Therapies that were developed in the 1990s include the combination of a proton pump inhibitor (PPI), clarithromycin and amoxicillin. Although these are still used globally, eradication rates have declined sharply during the last decade, due mainly to increased clarithromycin resistance (3–5).
Resistance rates to some of the antibiotics commonly used for the treatment of H. pylori infection are high; a global meta-analysis identified H. pylori primary and secondary resistance rates to clarithromycin, metronidazole and levofloxacin of more than 15% in nearly all regions (6). Furthermore, use of these antibiotics for other indications is widespread and increasing (7). In Europe, resistance of H. pylori to clarithromycin and levofloxacin has been linked to the widespread use of macrolides and fluoroquinolones, respectively (8,9). Consequently, recent guidelines have noted the effect of prior antibiotic exposure on susceptibility, and recommend that antibiotic history be taken into account in treatment decision-making (2,4).
In Europe, resistance rates to macrolides ranged from 4.8% to 36.9% in 2018 (8). In the US, contemporary data are limited (10), with most studies confined to small geographic regions or limited in the number of subjects evaluated (11–14). In a recent US clinical trial, antimicrobial susceptibility testing (AST) data from 345 patients showed 43.6% metronidazole resistance across the US, and 23.2% clarithromycin resistance in the Eastern US. (15). Both the Maastricht V/Florence and American College of Gastroenterology treatment guidelines recommend that the choice of regimen should be based upon knowledge of local resistance patterns (2,4). However, this information is particularly scarce in the US. (2,4). Furthermore, individual AST is currently not widely performed in the US. (4). Given the potentially serious consequences of failure to cure H. pylori infection, improved understanding of current, local resistance patterns is crucial for making treatment choices, thereby avoiding the use of unnecessary antibiotics in line with the strong recommendation of the World Health Organization, and increasing the chances of successful eradication (6,16).
A recent large clinical trial evaluated vonoprazan, a potassium-competitive acid blocker, with clarithromycin and amoxicillin as triple therapy, or with amoxicillin as dual therapy, for the treatment of H. pylori infection (pHalcon-HP; NCT04167670). In that trial, isolates were obtained from patients from multiple centers across the US and Europe for AST. Here, we report antimicrobial susceptibility data from 907 participants in 103 centers across the US and 5 European countries. This represents the largest collection of antimicrobial susceptibility data that includes participants from the US.
METHODS
Ethical practices
pHalcon-HP; NCT04167670 was conducted in compliance with the principles of the Declaration of Helsinki and in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice. Institutional review boards at each participating institution approved the trial protocol, and all participants provided written informed consent.
Participants and biopsy sampling
Participants had H. pylori infection confirmed by 13C-urea breath test and gastric biopsy specimens. Full eligibility criteria can be found in the clinical data publication (17). Gastric mucosal biopsy samples were taken at the screening endoscopy: one sample from the greater curve of the antrum and one from the lesser curve of the gastric body. The specimens were immediately introduced into Brucella broth with 20% glycerol and frozen at −20 °C or −70 °C, depending on freezer availability at the different sites, prior to transport to the central laboratory (Microbiology Specialists, Houston, TX). Upon receipt, specimens were ground, and 100 μg/mL of homogenized tissue was placed on blood agar, as well as on H. pylori medium including vancomycin, trimethoprim, cefsulodin, and amphotericin B (Anaerobe Systems, Morgan Hill, CA). Plates were placed in an Anoxomat jar (Advanced Instruments, Norwood, MA) for incubation at 35 ± 1 °C for up to 14 days. A microaerobic gas mixture (80% nitrogen, 10% carbon dioxide, 5% hydrogen and 5% oxygen) was used and an H. pylori-positive control was added to each jar. The remaining 300 μg/mL of specimen were then frozen at −70 °C.
H. pylori isolation and identification
After 72 hours, jars were opened and checked for growth. If not positive, they were re-incubated and re-observed at 7 and 14 days. If positive for small, gray and translucent colonies, they were Gram-stained, with a selection for small Gram-negative curved rods. Selected colonies were analyzed using oxidase, catalase, and urease tests. If the colonies were positive for all 3 tests, with the urease test providing a positive test within minutes, 3 plates were prepared for AST.
Antimicrobial susceptibility testing
The minimum inhibitory concentrations of amoxicillin, clarithromycin and metronidazole against each isolate were determined using the agar dilution method. Each antimicrobial was prepared at a concentration of 0.03–128 mg/L. Plates were inoculated using a Steers replicator and incubated in the Anoxomat microaerobic system for 48–72 hours. The resistance breakpoints were defined as: clarithromycin ≥1 μg/mL, amoxicillin >0.125 μg/mL and metronidazole >8 μg/mL, based on the Clinical and Laboratory Standards Institute and the European Committee on Antimicrobial Susceptibility Testing criteria (18,19).
Statistical analysis
Among participants with AST results available, resistance rates to each antibiotic were summarized overall, by region (US and Europe), and by US subregion or European country. Numbers of participants with isolates resistant to more than one antibiotic were also tabulated. Baseline characteristics were summarized for participants with isolates susceptible or resistant to each antibiotic. Statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute).
RESULTS
Overview of participants with resistant and susceptible isolates
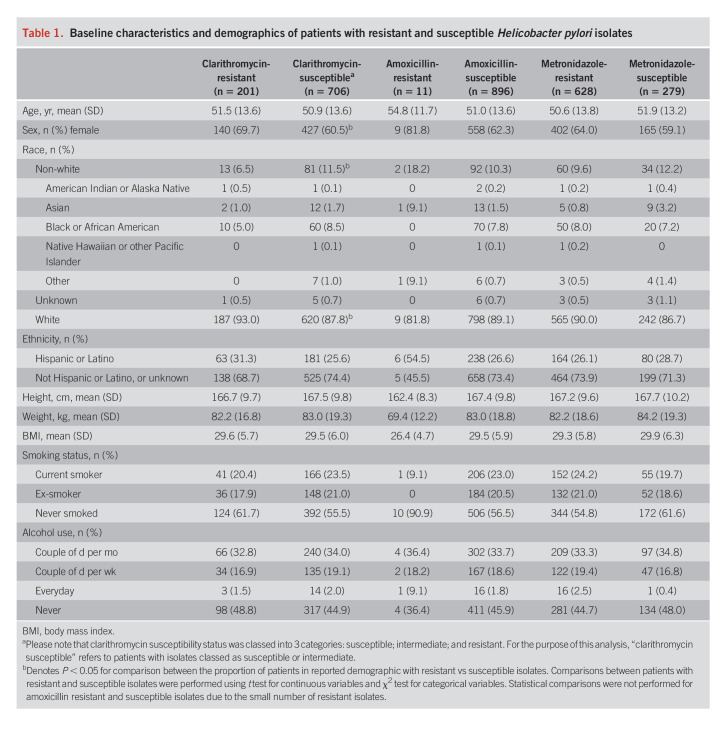
Overall, samples from 907 participants underwent AST (see Supplementary Figure 1, http://links.lww.com/AJG/C723). Clarithromycin resistance was detected in isolates from 201 participants (22.2%) (Table 1). Only 11 participants (1.2%) had isolates that were amoxicillin-resistant, but 628 (69.2%) harbored ones that were metronidazole-resistant. Baseline characteristics, including age and body mass index, were generally equally distributed between participants with isolates resistant and susceptible to the antibiotics, except for sex and white/non-white race which were unevenly distributed among those with clarithromycin-resistant and susceptible isolates (Table 1). A greater proportion of patients with clarithromycin-resistant isolates compared with susceptible isolates were female (69.7% vs 60.5%, P < 0.05) and white (93.0% vs 87.8%, P < 0.05). Due to the small number of patients with amoxicillin-resistant isolates, statistical comparisons of baseline characteristics based on amoxicillin susceptibility status were not performed. Additional demographic data stratified by antibiotic resistance or susceptibility are given in Table 1.
Table 1.
Baseline characteristics and demographics of patients with resistant and susceptible Helicobacter pylori isolates
Resistance data by geographical location
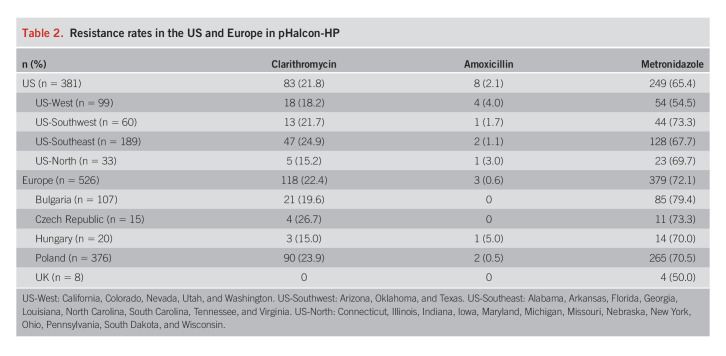
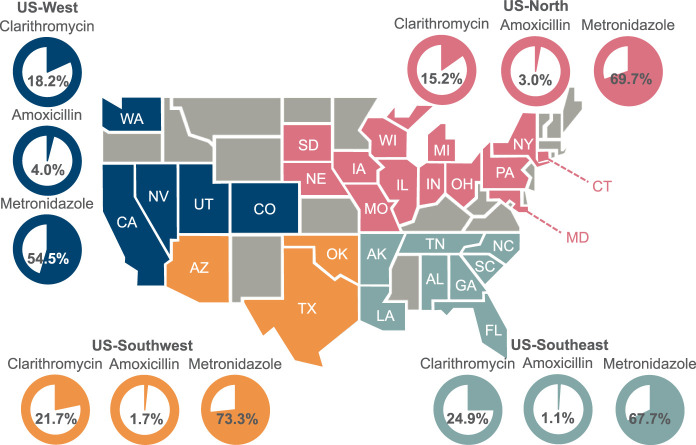
Overall, resistance rates between the US and Europe were broadly comparable (Table 2). Clarithromycin resistance was above 15% in all US subregions and in all European countries, except the UK, where none of the 8 isolates tested were clarithromycin-resistant. Among US regions, the greatest prevalence of clarithromycin resistance was in the Southeast (24.9%; 47/189) (Figure 1); among the European countries, it was in the Czech Republic (26.7%; 4/15). Two US regions (Southwest and Southeast) and 2 European countries (Czech Republic and Poland) had clarithromycin-resistant isolates in over 20% of participants.
Table 2.
Resistance rates in the US and Europe in pHalcon-HP
Figure 1.
Clarithromycin, amoxicillin and metronidazole resistance rates in the US. Gray color-coding indicates states that did not contain centers that participated in the pHalcon-HP study. Participating states are color-coded according to their assigned US-subregion.
In the US, resistance to metronidazole ranged from 54.5% (54/99) in the West to 73.3% (44/60) in the Southwest (Figure 1) and, in Europe, from 50.0% (4/8) in the UK to 79.4% (85/107) in Bulgaria.
Of the 11 participants with amoxicillin-resistant isolates, 8 (72.7%) were from the US, and half of those were in the West.
Clinical conditions
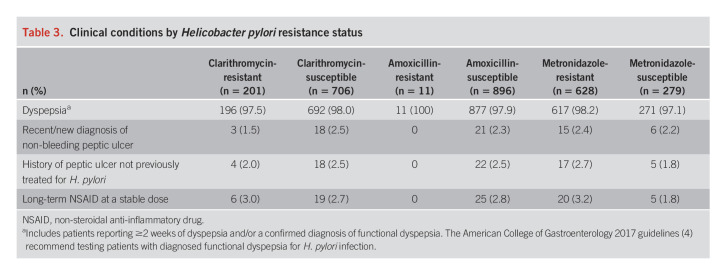
Participants with resistant and susceptible isolates had similar proportions of underlying conditions and indications for treatment of H. pylori infection at study entry (Table 3). Nearly all participants had dyspepsia, regardless of the presence or absence of resistance.
Table 3.
Clinical conditions by Helicobacter pylori resistance status
Prevalence of multi-resistance
A total of 7 of the 11 (63.6%) participants with amoxicillin-resistant isolates also had isolates resistant to at least one other antibiotic. This included one participant in the US with an isolate resistant to all 3 antibiotics, and one other US participant with resistance to both clarithromycin and amoxicillin. Five additional participants had isolates resistant to both metronidazole and amoxicillin: 3 from the US, one from Hungary and one from Poland. In most participants with clarithromycin-resistant isolates, there was also resistance to metronidazole (75.1%; 151/201). Of these 151 participants, 90 were from Europe (59.6%) and 61 were from the US (40.4%).
DISCUSSION
These data, from the largest registered clinical trial in participants with H. pylori infection to take place in the US and Europe, provide important and current insight into the prevalence of resistant H. pylori isolates. The results confirm that resistance to clarithromycin and metronidazole is high in these regions. All US subregions and European countries had clarithromycin resistance rates >15%, except the UK where the sample size was too small to provide a reliable estimate. Furthermore, most of these clarithromycin-resistant isolates were also metronidazole-resistant. Given the impact of clarithromycin resistance on treatment effectiveness, Maastricht V and American College of Gastroenterology clinical practice guidelines both recommend that triple regimens with a PPI and clarithromycin should not be used empirically where local resistance rates are greater than 15%, is unknown, or if the patient has had prior exposure to a macrolide (2,4). Triple regimens containing a PPI and clarithromycin are still commonly prescribed in the US and Europe (3,20), although, currently, AST is usually not performed (3,4). These data indicate that the current empiric management of patients with H. pylori infection is inadequate and results in a large proportion in whom treatment fails, leaving persistently infected patients at risk of potentially serious complications.
The high prevalence of resistance, including dual resistance, emphasizes the need to optimize the management of H. pylori infection by improving antimicrobial stewardship and treatment options. A study of 135 male veterans with H. pylori infection in Houston, Texas, in 2015 showed resistance rates of 16.4% to clarithromycin and 20.3% to metronidazole (14). In the current study, the US subregion that included Texas had a prevalence of clarithromycin resistance of 21.7% and metronidazole resistance of 73.3%, indicating a rise within the last 5 years. Resistance to metronidazole, which is commonly used for parasitic and other infections, has been associated with its prior use. Furthermore, the use of nitroimidazoles including metronidazole for gynecological and dental infections may further promote resistance (5,21,22). Although in vitro metronidazole resistance rates can vary widely between studies due to different microbiology techniques and difficulties with assay reproducibility, they may also indicate a trend (22). While metronidazole resistance may negatively influence eradication rates, it is not associated with treatment failure to the same extent as clarithromycin resistance (2,4). Thus, the consequences of the high rates of metronidazole resistance remain to be determined. Optimizing the management of H. pylori infection could help to address further rises in resistance rates, including multi-drug resistance, by avoiding the use of unnecessary antibiotics and thereby reducing the risk of resistance acquisition (8,16). Changes to current management could include improving antibiotic stewardship, and optimization of current therapeutic regimens, to improve eradication rates without necessitating the use of additional antibiotics. In part, this could be achieved through the more frequent use of AST to optimize treatment regimens, given the availability of real-time polymerase chain reaction kits to detect H. pylori and its resistance to clarithromycin at low cost, and the familiarity with this process in laboratories following the COVID-19 pandemic. Tailoring therapies based on known resistance has been shown to improve eradication rates and increase cost efficiency over empiric therapy when used in areas with high local resistance rates, such as the rates reported in this current study (23–25). However, AST is currently infrequently utilized in much of the US and Europe. In our opinion, this is likely due to a combination of: a lack of physician awareness, potentially due to the fact that gastroenterologists do not traditionally treat infectious diseases; dissatisfaction with historical AST services; and difficulties with reimbursement (4).
Identification of patient or clinical risk factors for resistance could be beneficial in minimizing the prescription of unnecessary antibiotics. In this study, the proportion of patients that were female was significantly higher in those with clarithromycin-resistant isolates than susceptible isolates (69.7% vs 60.5%, P < 0.05). Similarly, a study in Alaska of isolates taken between 2000 and 2016 reported that metronidazole- and clarithromycin-resistant strains (42.8% and 29.8% of isolates, respectively) were more common among women (12). In the current study, the proportion of patients that were white and non-white differed between clarithromycin-resistant and susceptible isolates (P < 0.05). There were no significant differences in the distribution of smokers and alcohol-users between resistant and non-resistant isolates in this study; smoking has previously been associated with the acquisition of metronidazole resistance in patients with H. pylori infection (26). Overall, available findings from previous studies have not indicated consistent risk factors for H. pylori antibiotic resistance. A study from Turkey investigating the effect of patient factors on resistance, found that diabetes mellitus and prior use of metronidazole and clarithromycin were associated with increased resistance to these antibiotics. It found no association between age, sex, body mass index, smoking or alcohol use and resistance (27). A Chinese study also found that age and sex had no impact on metronidazole or amoxicillin resistance. However, it found lower clarithromycin resistance in adults younger than 40 than in those older than 40 (28).
A limitation of this study is the way in which data were collected and analyzed in an exploratory manner via descriptive comparisons, as the participants were participating in a large clinical trial not primarily designed to assess resistance. Some additional data that could have been beneficial to our understanding of the characteristics associated with resistance were not collected, such as symptoms and previous antibiotic consumption. We did not test for resistance to some other antibiotics that may be used as part of treatment regimens for H. pylori infection, including levofloxacin, tetracycline and rifabutin. However, recent data suggest that, in both the US and Europe, resistance to tetracycline and rifabutin is low to non-existent (15). An additional limitation of this study was that some subgroups were small, limiting interpretation; e.g., there were only 8 participants from the UK. Furthermore, overall, only 11 participants had isolates that were resistant to amoxicillin. Similarly, the relatively small number of samples from individual states and countries are probably not generalizable to a specific provider's region of practice. Rather, this information provides estimates of the point prevalence of resistance that should reinforce general concepts around preferred treatment regimens, antibiotic stewardship, and the need for AST on a broader scale.
Overall, these data provide robust evidence to support a shift away from the default empiric prescription of triple combinations containing a PPI and clarithromycin for H. pylori infection in the US and Europe, and a shift towards more responsible antibiotic stewardship.
CONFLICTS OF INTEREST
Guarantor of the article: Francis Mégraud, MD.
Specific author contributions: F.M., D.Y.G., C.W.H., and W.D.C.: had a role in interpreting data and drafting the manuscript. E.T. and A.W.: had a role in conducting the study, collecting data and drafting the manuscript. B.H., N.S., and E.L.: had a role in planning and conducting the study, collecting and interpreting data, and drafting the manuscript. Each author approved the final draft to be submitted.
Financial support: The clinical study (NCT04167670) and this analysis were funded by Phathom Pharmaceuticals.
Potential competing interests: F.M. reported grants from Allergan, bioMerieux and Mobidiag. He is also a consultant for Phathom Pharmaceuticals. D.Y.G. is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies and has received research support for culture of H. pylori. He is also a consultant for DiaSorin regarding H. pylori diagnostics and with Otsuka Japan regarding novel breath tests. He has ongoing collaborative research projects with American Molecular regarding molecular diagnostics for H. pylori. C.W.H. discloses compensation as a consultant and advisory board/committee member for Phathom Pharmaceuticals and RedHill Biopharma; consultancy fees from Allakos, Clexio, ISOThrive, and Alfasigma; and Speaker's Bureau fees from Alnylam, RedHill Biopharma, Sanofi and Alfasigma. E.T. and A.W. are employees of Microbiology Specialists Incorporated, paid by Phathom Pharmaceuticals to undertake this work. B.H., N.S. and E.L. are employees of Phathom Pharmaceuticals; E.L. also discloses stockholder interest in Phathom Pharmaceuticals. W.D.C. reported being a Board member of the American College of Gastroenterology, GI on Demand, International Foundation of Functional GI Disorders, and the Rome Foundation; compensation as a consultant from AbbVie, Alfasigma, Allakos, Alnylam, Bayer, BioAmerica, Cosmo, Intrinsic Medicine, Ironwood Pharmaceuticals, QOL Medical, Nestle, Phathom Pharmaceuticals, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant; grant/research support from BioAmerica, Commonwealth Diagnostics International, QOL Medical, Salix, and Vibrant; stock/stock options in GI on Demand, and Modify Health; and patents relating to methods and kits for identifying food sensitivities and intolerances, digital manometry, and a rectal expulsion device.
Trial registration: Clinicaltrials.gov NCT04167670.
Study Highlights.
WHAT IS KNOWN
✓ Guidelines recommend that local resistance rates to commonly used antibiotics including clarithromycin determine treatment choice for H. pylori infection.
✓ Up-to-date information on resistance rates is limited, particularly in the US.
WHAT IS NEW HERE
✓ The prevalence of clarithromycin and metronidazole resistance in H. pylori isolates was higher than previously reported.
✓ The empiric use of proton pump inhibitor-based triple therapy containing clarithromycin should be abandoned.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients, their families, all other investigators, and all investigational site members involved in this study. Medical writing support was provided by Abigail Killen-Devine PhD and editorial support by Kyle Lambe of Synergy Medical Communications, UK, and was funded by Phathom Pharmaceuticals, Chicago, IL, USA in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The contents of this article were partially presented at the ACG Annual Meeting 2021, held on 22–27 October.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C723
Contributor Information
David Y. Graham, Email: Grdgraham@bcm.edu.
Colin W. Howden, Email: chowden@uthsc.edu.
Alice Weissfeld, Email: trevino@microbiologyspecialists.com.
Barbara Hunt, Email: bhunt@phathompharma.com.
Neila Smith, Email: nsmith@phathompharma.com.
Eckhard Leifke, Email: eleifke@phathompharma.com.
William D. Chey, Email: wchey@med.umich.edu.
REFERENCES
- 1.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017;153:420–9. [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Megraud F, O'Morain CA, et al. , European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 3.Nyssen OP, Bordin D, Tepes B, et al. , Hp-EuReg Investigators. European registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021;70:40–54. [DOI] [PubMed] [Google Scholar]
- 4.Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- 5.Thung I, Aramin H, Vavinskaya V, et al. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016;43:514–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savoldi A, Carrara E, Graham DY, et al. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in world Health organization regions. Gastroenterology 2018;155:1372–82.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742–50. [DOI] [PubMed] [Google Scholar]
- 8.Megraud F, Bruyndonckx R, Coenen S, et al. , European Helicobacter pylori Antimicrobial Susceptibility Testing Working Group. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021;70:1815–22. [DOI] [PubMed] [Google Scholar]
- 9.Megraud F, Coenen S, Versporten A, et al. , Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34–42. [DOI] [PubMed] [Google Scholar]
- 10.Saleem N, Howden CW. Update on the management of Helicobacter pylori infection. Curr Treat Options Gastroenterol 2020;18:476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Sangitha R, Nachamkin I, et al. Resistance patterns of refractory Helicobacter pylori infection in a referral centre in the Delaware Valley. GastroHep 2020;2:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosites E, Bruden D, Morris J, et al. Antimicrobial resistance among Helicobacter pylori isolates in Alaska, 2000-2016. J Glob Antimicrob Resist 2018;15:148–53. [DOI] [PubMed] [Google Scholar]
- 13.Nayar DS. Current eradication rate of Helicobacter pylori with clarithromycin-based triple therapy in a gastroenterology practice in the New York metropolitan area. Infect Drug Resist 2018;11:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiota S, Reddy R, Alsarraj A, et al. Antibiotic resistance of Helicobacter pylori among male United States veterans. Clin Gastroenterol Hepatol 2015;13:1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulten KG, Lamberth LB, Kalfus IN, et al. National and regional United States antibiotic resistance to Helicobacter pylori. Lessons from a clinical trial. Gastroenterology 2021;161:342–4.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics (Basel) 2020;9:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chey WD, Megraud F, Laine L, et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the US and Europe: Randomized clinical trial. Gastroenterology 2022;163:608–619. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. CLSI M100 29Ed: Performance Standards for Antimicrobial Susceptibility Testing 29th Edition (https://www.clsi.org/) (2019). Accessed March, 2020. [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters v10.0(https://eucast.org/clinical_breakpoints/). (2020) Accessed March 1, 2020. [Google Scholar]
- 20.Murakami TT, Scranton RA, Brown HE, et al. Management of Helicobacter Pylori in the United States: Results from a national survey of gastroenterology physicians. Prev Med 2017;100:216–22. [DOI] [PubMed] [Google Scholar]
- 21.McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med 2003;139:463–9. [DOI] [PubMed] [Google Scholar]
- 22.Mégraud F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004;53:1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosme A, Montes M, Martos M, et al. Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clin Microbiol Infect 2013;19:379–83. [DOI] [PubMed] [Google Scholar]
- 24.Romano M, Marmo R, Cuomo A, et al. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol 2003;1:273–8. [PubMed] [Google Scholar]
- 25.Wenzhen Y, Yumin L, Quanlin G, et al. Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med 2010;49:1103–9. [DOI] [PubMed] [Google Scholar]
- 26.Witteman EM, Hopman WP, Becx MC, et al. Short report: Smoking habits and the acquisition of metronidazole resistance in patients with Helicobacter pylori-related gastritis. Aliment Pharmacol Ther 1993;7:683–7. [DOI] [PubMed] [Google Scholar]
- 27.Erkut M, Uzun DY, Kaklıkkaya N, et al. Sociodemographic characteristics and clinical risk factors of Helicobacter pylori infection and antibiotic resistance in the Eastern Black Sea region of Turkey. Turk J Gastroenterol 2020;31:221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu DS, Wang YH, Zeng ZR, et al. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: A multiregion prospective 7-year study. Clin Microbiol Infect 2018;24:780.e5–e8. [DOI] [PubMed] [Google Scholar]