Abstract
Checkpoint inhibitor-related pneumonitis (CIP) is one of the most important immune checkpoint inhibitors side effects, and it is rare but fatal. Identifying patients at risk of refractory CIP before the start of CIP therapy is important for controlling CIP. We retrospectively analyzed the clinical data of 60 patients with lung cancer who developed CIP. Refractory CIP was defined as CIP with poor response to corticosteroid treatment, including CIP not relieved with corticosteroid administration or CIP recurrence during the corticosteroid tapering period. We analyzed clinical characteristics, peripheral blood biomarkers, treatment, and outcomes in nonrefractory and refractory CIP. Risk factors associated with refractory CIP were assessed. Among 60 patients with CIP, 16 (26.7%) had refractory CIP. The median onset time for patients with nonrefractory and those with refractory CIP was 16.57 (interquartile range [IQR], 6.82–28.14) weeks and 7.43 (IQR, 2.71–19.1) weeks, respectively. The level of lactate dehydrogenase (LDH) was significantly higher in the refractory CIP group at baseline (255 [222, 418] vs. 216 [183, 252], P=0.031) and at CIP onset (321.5 [216.75, 487.5] vs. 219 [198. 241], P=0.019). An LDH level >320 U/L at CIP onset was an independent risk factor of refractory CIP (odds ratio [OR], 8.889; 95% confidence interval [CI]: 1.294–61.058; P=0.026). The incidence of refractory CIP is high among patients with CIP. An increased LDH level at CIP onset is independently associated with refractory CIP. Monitoring LDH levels during immune checkpoint inhibitors treatment is recommended.
Key Words: checkpoint inhibitor-related pneumonitis, lung cancer, immunotherapy, refractory pneumonitis
Numerous clinical trials have confirmed the efficacy of immune checkpoint inhibitors (ICIs) against lung cancer at the early, locally advanced, and advanced stages. However, the widespread use of ICIs has increased the incidence of immune-related adverse events (irAEs). The incidence of checkpoint inhibitor-related pneumonitis (CIP) in patients with lung cancer is 1.0%–10.7% for any grade1,2 and 0.4%–3.5% for grade 3 or higher.3–5 Although CIP is rare, it is a leading cause of death resulting from irAEs, accounting for 35%–42% of all deaths related to irAEs.6
Refractory CIP, which is defined as CIP that is not responsive to corticosteroids or is recurrent during the corticosteroid tapering period, may be a main cause of death in CIP.7 The incidence, clinical characteristics, and outcomes of refractory CIP have not been well studied.
Refractory CIP therapy requires corticosteroid escalation and administration of other immunosuppressants when corticosteroids are ineffective,8–10 which may delay or interfere with the best time for cancer treatment. Utsumi et al11 reported a case of CIP that worsened after administration of corticosteroid for 7 days and did not improve until treated with pirfenidone and triple-combination therapy (high-dose corticosteroids, tacrolimus, and cyclophosphamide). Naidoo et al12 reported that 6 of 44 patients with CIP presented as refractory to a standard dose of corticosteroid; subsequently, >12 weeks of corticosteroid escalation or immunosuppressive treatment were required to control the CIP. Further, the control of refractory CIP may require restarting corticosteroids and prolonged corticosteroid treatment,13–15 which may increase the incidence of opportunistic infection. Administration of a prednisolone-equivalent dose ≥15 mg/day in the initial treatment of CIP was reported to the lower incidence of recurrent CIP.16 Therefore, the timely use of an immunosuppressive agent or a higher dosage of corticosteroid is essential to control refractory CIP and shorten the treatment period. Thus, it is important to identify patients at risk of refractory CIP at the onset of CIP to initiate early treatment and reduce the risk of mortality.
This study aimed to analyze the clinical characteristics, peripheral blood biomarkers, and radiologic characteristics of patients with CIP and to identify the risk factors, clinical course, and outcomes of refractory CIP.
METHODS
Patients
We analyzed the data of patients with lung cancer who presented with CIP and were treated at Guangdong Provincial People’s Hospital from January 2016 to December 2021. All included patients had been pathologically diagnosed with lung cancer, including non-small cell lung cancer (NSCLC) and small cell lung cancer. ICI treatment was used as neoadjuvant or adjuvant therapy for resectable NSCLC, while consolidation therapy was applied for unresectable locally advanced NSCLC followed by chemoradiotherapy or first-line and later-line treatment for advanced NSCLC or small cell lung cancer.
The ICI agents included programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitors and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors. All included patients received at least 1 ICI dose as a single agent or in combination with chemotherapy. Cases were managed by a multidisciplinary team comprising specialists in medical oncology, radiotherapy oncology, and radiology. The diagnosis of CIP was determined based on (1) a treatment history of ICI therapy; (2) symptoms of nonproductive cough, unresolving dyspnea, fever, and chest pain, and (3) classic and varied radiographic findings on chest computed tomography (CT).9,10 Culture and sensitivity of nasal, sputum, bronchoalveolar lavage, blood, and urine should be used to distinguish infectious pneumonia from CIP.9 Bronchoscopy is not mandatory but is highly recommended in patients with CIP grade 2 or higher and in patients with suspected infection or tumor progression.
Refractory CIP was indicated by poor response to corticosteroids or recurrence of CIP during the corticosteroid tapering period. Patients who responded poorly to corticosteroids were defined as patients with worse clinical symptoms or radiographic infiltrates on chest CT after the initiation of standard corticosteroid treatment according to the guidelines.9,10 Patients with recurrent CIP were defined as patients who improved after initial corticosteroid treatment but experienced aggravated CIP during the corticosteroid tapering period without rechallenge with ICI. Tumor response was evaluated at the onset of CIP and during the treatment course using the Response Criteria in Solid Tumors. Tumor progression should be carefully distinguished from refractory CIP. A chest CT scan was performed if new symptoms developed or if symptoms worsened during the CIP treatment. To distinguish CIP from infectious pneumonia or carcinomatous lymphangitis, bronchoscopy with bronchoalveolar lavage, with or without transbronchial biopsy, was performed in patients whose CT scan showed worsening pneumonitis or new lobular interstitial thickening. Thoracentesis and cytologic examination of pleural effusion were performed if a pleural effusion developed or increased. Refractory CIP was diagnosed in patients who met the criteria unless pathologically confirmed carcinomatous lymphangitis or malignant pleural effusion was diagnosed. Patients with carcinomatous lymphangitis were not included in our study.
We excluded patients with a history of interstitial lung disease before ICI treatment or patients with radiation-induced pneumonitis during ICI treatment, which is difficult to distinguish from CIP.
Data Collection
This study was approved by the ethics board of Guangdong Provincial People’s Hospital. Clinical characteristics of the patients were collected from the medical records. Baseline characteristics included performance status, age, smoking status, tumor histology, and tumor staging at the initiation of ICI treatment. Data on the history of surgery and chest radiotherapy before ICI were collected. Further, the underlying disease of composite obstructive lung disease, including chronic obstructive pulmonary disease, obstruction of spirometry, or emphysema, was documented.
The ICI treatment agents and regimens were recorded. The response to ICI treatment was evaluated using Response Evaluation Criteria in Solid Tumors 1.1 and confirmed by at least 2 physicians. The CIP grading was evaluated by a multidisciplinary team based on the Common Toxicity Criteria for Adverse Events (version 4.0). Time to CIP was defined as the time from the initial ICI administration to the onset of CIP. Data on symptoms, starting corticosteroid dose, complications, and antibiotic use were collected. Regarding blood parameters, we collected data on blood routine and lactate dehydrogenase (LDH) levels at baseline and at the onset of CIP. All patients underwent a chest CT scan at the onset of CIP, which was compared with previous examinations. Any new-onset changes in the lung were carefully distinguished and recorded by a senior radiologist (Y.H.C.). Radiologic features, including CIP involvement of the lung parenchyma lobes and area, distribution, and radiographic patterns, were recorded. The radiographic patterns of CIP were classified as cryptogenic organizing pneumonia (COP)-like pattern, hypersensitivity pneumonitis (HP)-like pattern, nonspecific interstitial pneumonia -like pattern, or acute interstitial pneumonia/acute respiratory distress syndrome-like pattern.17,18 The survival periods from the onset of CIP were recorded. The last follow-up date was April 1, 2022.
Statistical Analysis
Categorical data were compared using the χ2 or Fisher exact test. Continuous variables are described as the median [interquartile range (IQR)] and were compared using t-tests or the Mann–Whitney U test. Risk factors for refractory CIP were assessed using logistic regression through univariate and multivariable analyses. All the factors with P value<0.1 in the univariate analysis were included in the multivariable analysis. Median overall survival (OS) after the onset of CIP was estimated using the Kaplan–Meier method. Statistical analyses were conducted using SPSS version 20 (IBM Corp.). P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
From January 2016 to December 2011, 60 patients with lung cancer who presented with CIP were enrolled. Among them, 16 patients had refractory CIP. Table 1 summarizes the baseline characteristics of patients with nonrefractory and refractory CIP. Most patients with CIP were ≤65-years-old (63.3%) and male (96.7%). Further, 56.7% of the patients were diagnosed with adenocarcinoma and 86.7% were treated with PD-1 inhibitors. In addition, 80% and 70% of the patients had undergone prior surgery and chest radiotherapy, respectively. There was no significant between-group difference in sex, age, smoking status, composite obstructive lung disease history, histology, tumor staging, PD-L1 expression, Eastern Cooperative Oncology Group Performance Status, ICI agent use, ICI therapy regimen, treatment line, and proportion of prior surgery and chest radiotherapy. There was a significant between-group difference in the best response to ICI treatment (P=0.029); however, the response to ICI treatment was not evaluated in 31.2% of the patients with refractory CIP.
TABLE 1.
Baseline Characteristics in Patients with CIP
| Characteristic | Overall N=60 | Nonrefractory CIP N=44 | Refractory CIP N=16 | P |
|---|---|---|---|---|
| Gender, n (%) | — | — | — | 1.000 |
| Female | 2 (3.3) | 2 (4.5) | 0 (0) | — |
| Male | 58 (96.7) | 42 (95.5) | 16 (100) | — |
| Age, years, n (%) | — | — | — | 0.701 |
| >65 | 22 (36.7) | 15 (34.1) | 7 (43.8) | — |
| ≤65 | 38 (63.3) | 29 (65.9) | 9 (56.2) | — |
| Smoking status, n (%) | — | — | — | 0.240 |
| Current | 30 (50) | 20 (45.5) | 10 (62.5) | — |
| Former | 22 (36.7) | 19 (43.2) | 3 (18.8) | — |
| Never | 8 (13.3) | 5 (11.4) | 3 (18.8) | — |
| Composite obstructive lung disease history, n (%) | — | — | — | 0.681 |
| No | 27 (45.0) | 21 (47.7) | 6 (37.5) | — |
| Yes | 33 (55.0) | 23 (52.3) | 10 (62.5) | — |
| Histology, n (%) | — | — | — | 0.445 |
| Adenocarcinomas | 34 (56.7) | 25 (56.8) | 9 (56.2) | — |
| Squamous | 18 (30.0) | 14 (31.8) | 4 (25) | — |
| Small cell carcinoma | 3 (5.0) | 1 (2.3) | 2 (12.5) | — |
| Others | 5 (8.3) | 4 (9.1) | 1 (6.2) | — |
| Tumor staging, n (%) | — | — | — | 0.719 |
| II | 2 (3.3) | 2 (4.5) | 0 (0) | — |
| III | 11 (18.3) | 7 (15.9) | 4 (25) | — |
| IV | 47 (78.3) | 35 (79.5) | 12 (75) | — |
| PD-L1 expression, n (%) | — | — | — | 0.426 |
| <1% | 11 (18.3) | 7 (15.9) | 4 (25) | — |
| 1-50% | 28 (46.7) | 23 (52.3) | 5 (31.2) | — |
| >50% | 8 (13.3) | 6 (13.6) | 2 (12.5) | — |
| Unknown | 13 (21.7) | 8 (18.2) | 5 (31.2) | — |
| ECOG PS, n (%) | — | — | — | 1.000 |
| ≤1 | 42 (70.0) | 31 (70.5) | 11 (68.8) | — |
| ≥2 | 18 (30.0) | 13 (29.5) | 5 (31.2) | — |
| ICI agents, n (%) | — | — | — | 0.643 |
| PD-1 inhibitor | 52 (86.7) | 39 (88.6) | 13 (81.2) | — |
| PD-1+ CTLA-4 inhibitor | 1 (1.7) | 1 (2.3) | 0 (0) | — |
| PD-L1 inhibitor | 6 (10.0) | 3 (6.8) | 3 (18.8) | — |
| PD-L1+ CTLA-4 inhibitor | 1 (1.7) | 1 (2.3) | 0 (0) | — |
| Regimen of immune therapy, n (%) | — | — | — | 0.199 |
| ICI | 35 (58.3) | 23 (52.3) | 12 (75) | — |
| ICI+ chemotherapy | 25 (41.7) | 21 (47.7) | 4 (25) | — |
| Treatment line, n (%) | — | — | — | 0.356 |
| 1st line | 34 (56.7) | 27 (61.4) | 7 (43.8) | — |
| 2nd or later line | 26 (43.3) | 17 (38.6) | 9 (56.2) | — |
| Prior surgery, n (%) | — | — | — | 1.000 |
| No | 48 (80.0) | 35 (79.5) | 13 (81.2) | — |
| Yes | 12 (20.0) | 9 (20.5) | 3 (18.8) | — |
| Prior chest radiotherapy, n (%) | — | — | — | 1.000 |
| No | 42 (70.0) | 31 (70.5) | 11 (68.8) | — |
| Yes | 18 (30.0) | 13 (29.5) | 5 (31.2) | — |
| Best response of ICIs, n (%) | — | — | — | 0.029 |
| PR | 26 (43.3) | 21 (47.7) | 5 (31.2) | — |
| SD | 22 (36.7) | 18 (40.9) | 4 (25) | — |
| PD | 5 (8.3) | 3 (6.8) | 2 (12.5) | — |
| Unknown | 7 (11.7) | 2 (4.5) | 5 (31.2) | — |
CIP indicates checkpoint inhibitor-related pneumonitis; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor; PD, progression of disease.PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; PR, partial response; SD, stable disease.
Clinical Features and Treatment of CIP
Table 2 summarizes the clinical features and treatment of CIP. Among the 60 patients, 44 (73.3%) developed CIP >6 weeks after initiation of ICI treatment. Most patients (60%) presented with grade 1 or 2 CIP. The most common symptoms were shortness of breath (38.3%) and cough (35%). Almost half of the patients (46.7%) simultaneously presented with CIP and pneumonia. Further, almost all patients (85%) received >1 mg/kg equivalent methylprednisolone as initial treatment. Antibiotic use was common (60%). There were no significant between-group differences in CIP characteristics, except for the onset time of CIP. Figure 1 shows the distribution of the onset times of CIP in each group and in the overall sample. The median onset time of CIP for the overall sample was 14.86 [5.86–27.68] weeks. Further, the onset times of CIP for patients with nonrefractory and refractory CIP were 16.57 [6.82–28.14] and 7.43 [2.71–19.1] weeks, respectively (P=0.079). The patients were divided into those whose onset times of CIP were ≤6 weeks and those whose onset times were>6 weeks. The refractory CIP group included more patients who developed CIP within 6 weeks than the nonrefractory CIP group (50.0% vs. 18.2%, P=0.021). Shortness of breath (68.8% vs. 27.3%) and cough (43.2% vs. 12.5%) were more and less common symptoms in the refractory and nonrefractory CIP groups, respectively.
TABLE 2.
Clinical features and treatment of CIP
| Overall N=60 | Nonrefractory CIP N=44 | Refractory CIP N=16 | P | |
|---|---|---|---|---|
| Time to CIP (wk), n (%) | — | — | — | 0.021 |
| <6 | 16 (26.7) | 8 (18.2) | 8 (50.0) | — |
| ≥6 | 44 (73.3) | 36 (81.8) | 8 (50.0) | — |
| CTCAE grade, n (%) | — | — | — | 0.211 |
| ≤2 | 36 (60.0) | 29 (65.9) | 7 (43.8) | — |
| >2 | 24 (40.0 | 15 (34.1) | 9 (56.2) | — |
| Symptoms, n (%) | — | — | — | 0.025 |
| Shortness of breath | 23 (38.3) | 12 (27.3) | 11 (68.8) | — |
| Cough | 21 (35.0) | 19 (43.2) | 2 (12.5) | — |
| Fever | 9 (15.0) | 6 (13.6) | 3 (18.8) | — |
| Others | 2 (3.3) | 2 (4.5) | 0 (0) | — |
| No symptoms | 5 (8.3) | 5 (11.4) | 0 (0) | — |
| Pneumonia, n (%) | — | — | — | 0.407 |
| Yes | 28 (46.7) | 22 (50.0) | 6 (37.5) | — |
| Suspicious | 6 (10.0) | 3 (6.8) | 3 (18.8) | — |
| No | 26 (43.3) | 19 (43.2) | 7 (43.8) | — |
| Starting dose of equivalent MP (mg/kg), n (%) | — | — | — | 0.121 |
| <1 | 9 (15.0) | 7 (15.9) | 2 (12.5) | — |
| 1-2 | 19 (31.7) | 17 (38.6) | 2 (12.5) | — |
| 2-3 | 17 (28.3) | 12 (27.3) | 5 (31.2) | — |
| ≥3 | 15 (25.0) | 8 (18.2) | 7 (43.8) | — |
| Antibiotic use, n (%) | — | — | — | 1.000 |
| Yes | 36 (60.0) | 26 (59.1) | 10 (62.5) | — |
| No | 24 (40.0) | 18 (40.9) | 6 (37.5) | — |
Statistically significant P values are in bold.
CIP indicates checkpoint inhibitor-related pneumonitis; CTCAE, Common Toxicity Criteria for Adverse Events; MP, methylprednisolone.
FIGURE 1.
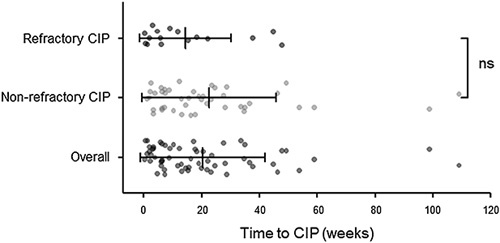
Time from initiation of ICI treatment to onset of CIP in refractory CIP, nonrefractory CIP and overall patients. ICI indicates immune checkpoint inhibitor; CIP, immune checkpoint inhibitor-related pneumonitis; ns, no significance.
Peripheral Blood Biomarkers in Patients with CIP
Table 3 presents the blood parameters at different time points in both groups. We calculated changes in blood routine and LDH levels from baseline to the onset of CIP. There were no significant between-group differences in white blood cell count, absolute neutrophil count, absolute lymphocyte count, absolute eosinophil count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio at different time points. Further, there were no significant between-group differences in the changes in blood routine from baseline to CIP onset. However, compared with the nonrefractory CIP group, the refractory CIP group showed significantly higher LDH levels at baseline (255 [222, 418] vs. 216 [183, 252], P=0.031) and at CIP onset (321.5 [216.75, 487.5] vs. 219 [198. 241], P=0.019). In addition, changes in LDH levels from baseline to CIP onset were significantly greater in the refractory CIP group than in the nonrefractory CIP group (66 [0, 165] vs. 7 [−24.5, 44], P=0.042). There were no significant differences in the blood routine and LDH levels between the baseline and CIP onset in either group (Fig. 2).
TABLE 3.
Peripheral Blood Biomarkers in Patients with CIP
| Nonrefractory CIP N=44 | Refractory CIP N=16 | P | |
|---|---|---|---|
| At baseline | |||
| WBC, median (IQR) | 7.36 (6.02, 10.35) | 8.18 (6.25, 11.1) | 0.772 |
| ANC, median (IQR) | 5.09 (3.85, 7.34) | 5.68 (3.66, 8.35) | 0.676 |
| ALC, median (IQR) | 1.46 (0.97, 1.9) | 1.29 (1.07, 1.73) | 0.831 |
| AEC, median (IQR) | 0.17 (0.11, 0.32) | 0.1 (0.06, 0.21) | 0.154 |
| NLR, median (IQR) | 3.93 (2.41, 5.91) | 3.47 (2.71, 7.84) | 1.000 |
| PLR, median (IQR) | 190.3 (111.08, 305.81) | 207.29 (149.63, 441.2) | 0.504 |
| LDH, median (IQR) | 216 (183, 252) | 255 (222, 418) | 0.031 |
| At CIP | |||
| WBC, median (IQR) | 8.21 (5.85, 11.41) | 8.38 (6.24, 9.09) | 0.635 |
| ANC, median (IQR) | 5.76 (3.37, 8.63) | 6.12 (3.86, 7.65) | 0.765 |
| ALC, median (IQR) | 1.16 (0.81, 1.66) | 0.98 (0.62, 1.63) | 0.580 |
| AEC, median (IQR) | 0.11 (0.04, 0.27) | 0.12 (0.04, 0.2) | 0.835 |
| NLR, median (IQR) | 4.71 (2.55, 10.81) | 4.5 (3.12, 9.3) | 0.993 |
| PLR, median (IQR) | 218.57 (155.5, 397.83) | 262.07 (192.34, 376.04) | 0.456 |
| LDH, median (IQR) | 219 (198, 241) | 321.5 (216.75, 487.5) | 0.019 |
| Changes from baseline to CIP development | |||
| WBC, median (IQR) | 0.45±3.38 | −0.55±3.51 | 0.335 |
| ANC, median (IQR) | 0.57±3.53 | -0.16±2.97 | 0.480 |
| ALC, median (IQR) | −0.23 (-0.54, 0.08) | −0.22 (−0.4, 0) | 0.890 |
| AEC, median (IQR) | −0.04 (−0.14, 0.11) | 0.03 (−0.08, 0.14) | 0.493 |
| LDH, median (IQR) | 7 (−24.5, 44) | 66 (0, 165) | 0.042 |
Statistically significant P and corresponding biomarker values are in bold.
AEC indicates absolute eosinophil count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CIP, checkpoint inhibitor-related pneumonitis; LDH, lactate dehydrogenase, the unit for LDH is U/L; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; WBC, white blood cell count.
FIGURE 2.
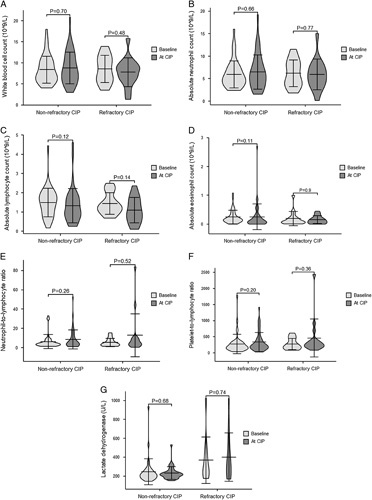
Bar plots of Peripheral blood biomarkers in patients with nonrefractory CIP and refractory CIP at baseline and the onset of CIP. A, White blood cell count; B, Absolute neutrophil count; C, Absolute lymphocyte count; D, Absolute eosinophil count; E, Neutrophil-to-lymphocyte ratio; F, Platelet-to-lymphocyte ratio; G, Lactate dehydrogenase. CIP indicates checkpoint inhibitor-related pneumonitis.
Radiologic Features in Patients with CIP
Radiologic diagnosis of CIP is challenging as there are no typical imaging findings. Some studies have investigated and described the radiographic features of CIP.17,18 We defined 4 main radiologic patterns of CIP in our study (Supplementary Fig 1, Supplemental Digital Content 1, http://links.lww.com/JIT/A705), which were also reported by Pozzessere et al.18 The first pattern is a COP-like pattern, which presented as patchy alveolar consolidations and/or ground-glass opacities (GGOs) in the peribronchovascular and/or subpleural region (Supplementary Fig 1a, Supplemental Digital Content 1, http://links.lww.com/JIT/A705). Reverse halo signs can also be recognized in some patients. The second pattern is an HP-like pattern, which presented as centrilobular micronodules, patchy hypoattenuated lobules, and GGOs distributed mostly at upper lobes (Supplementary Fig 1b, Supplemental Digital Content 1, http://links.lww.com/JIT/A705). The third pattern is an nonspecific interstitial pneumonia-like pattern, which presented as bilateral GGOs with reticulations distributed mostly in the subpleural and/or peribronchovascular regions (Supplementary Fig 1c, Supplemental Digital Content 1, http://links.lww.com/JIT/A705). And, the last pattern is acute interstitial pneumonia/acute respiratory distress syndrome, which presented as diffuse GGOs with alveolar consolidation in the parenchyma. Pleural effusion can sometimes be seen in these patients, which should be distinguished from malignant pleural effusion (Supplementary Fig 1d, Supplemental Digital Content 1, http://links.lww.com/JIT/A705)
Figure 3 shows the between-group comparisons of radiologic features. Patients in both groups tended to have involvement of more than 2 lung lobes (90.9% and 81.2%) and >25% area of the lung parenchyma (63.6% and 78.0%). Patients with refractory CIP were likely to show a diffuse pattern (68.8%), while those with nonrefractory CIP were likely to show a diffuse (45.5%) or multifocal (45.5%) pattern. Few patients with CIP showed a local distribution (9.1% and 0%). Regarding the overall CIP pattern, COP-like patterns occurred in 56.8% and 31.2% of patients with nonrefractory and refractory CIP, respectively. The HP-like pattern was more common in the refractory CIP group than in the nonrefractory CIP group (31.2% vs. 18.2%).
FIGURE 3.
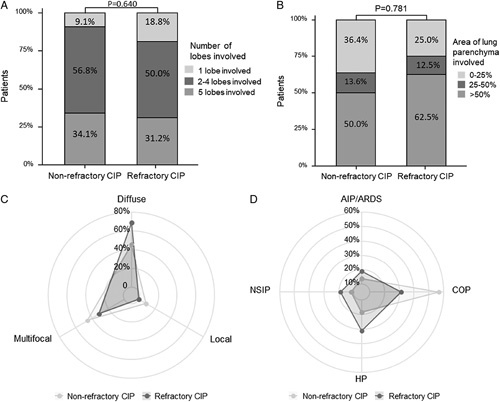
Radiological features in patients with nonrefractory CIP and refractory CIP. A and B, Number of lobes and area of lung parenchyma involved of CIP; C and D, Radar charts of distribution and overall pattern of CIP. AIP/ARDS indicates acute interstitial pneumonia/acute respiratory distress syndrome; CIP, checkpoint inhibitor-related pneumonitis; COP, cryptogenic organizing pneumonia; HP, hypersensitivity pneumonitis; NSIP, nonspecific interstitial pneumonia.
Risk Factors for Refractory CIP
Time to CIP ≤6 weeks (odds ratio (OR), 4.500; 95% confidence interval (CI): 1.297–15.611), LDH levels >320 U/L at CIP onset (OR, 11.333; 95% CI: 2.209–58.147), and changes in LDH levels >66 U/L from baseline to the onset of CIP (OR, 12.083; 95% CI: 2.053–71.114) were significantly associated with an increased risk of developing refractory CIP. The cut-off value of LDH at CIP onset (320 U/L) and changes from baseline to the onset of CIP (66 U/L) analyzed as risk factors were defined by the median LDH level of refractory CIP. Compared with cough, shortness of breath was significantly associated with refractory CIP (OR, 7.917; 95% CI: 1.473–42.538) but not fever (OR, 1.667; 95% CI: 0.330–8.423). Multivariable logistic analysis revealed that only LDH levels >320 U/L at the onset of CIP were independently associated with an increased risk of refractory CIP (OR, 8.889; 95% CI: 1.294–61.058; P=0.026; Table 4). Figure 4 shows the corresponding relationships between significant risk factors and CIP types.
TABLE 4.
Factors Associated with Refractory CIP
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Best response of ICI | 0.558 | - | - | |
| PR vs. PD | 0.357 (0.074- 2.740) | 0.322 | - | - |
| SD vs. PD | 0.333 (0.041- 2.699) | 0.303 | - | - |
| Time to CIP (<6w vs. ≥6w) | 4.500 (1.297- 15.611) | 0.018 | 3.812 (0.193- 75.405) | 0.380 |
| Symptoms | 0.213 | - | - | |
| Shortness of breath vs. cough | 7.917 (1.473- 42.538) | 0.016 | 7.506 (0.340- 165.578) | 0.202 |
| Shortness of breath vs. fever | 1.667 (0.330- 8.423) | 0.537 | - | - |
| LDH at baseline (≥255 vs. <255) | 3.733 (0.917- 15.207) | 0.066 | 0.926 (0.090- 9.512) | 0.948 |
| LDH at CIP (≥320 vs. <320) | 11.333 (2.209- 58.147) | 0.004 | 8.889 (1.294- 61.058) | 0.026 |
| Changes of LDH from baseline to CIP development (≥66 vs. <66) | 12.083 (2.053- 71.114) | 0.006 | 5.160 (0.598- 44.514) | 0.136 |
Statistically significant P and corresponding odds ratio values are in bold.
CI indicates confidence interval; CIP, checkpoint inhibitor-related pneumonitis; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; OR, odds ratio; PD, progression of disease; PR, partial response; SD, stable disease.
FIGURE 4.
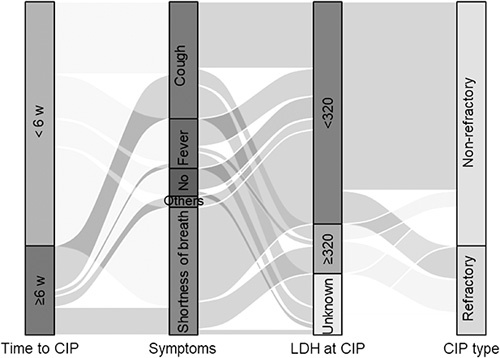
Sankey diagram of the clinical feature and peripheral blood biomarker in refractory and nonrefractory CIP patients. w indicates weeks; CIP, immune checkpoint inhibitor-related pneumonitis; LDH, lactate dehydrogenase, the unit for LDH is U/L.
Clinical Course and Outcomes of Refractory CIP
Figure 5 shows the clinical course from the onset of CIP to the clinical outcomes. All patients were treated with sufficient doses of prednisone or methylprednisolone based on guidelines9,10 or the doctor’s clinical experience. Eleven patients responded to the initial corticosteroid treatment; however, they showed recurrence of CIP or worsening symptoms during the corticosteroid tapering period. After recurrence, the corticosteroid dose was increased. Among them, 2 patients were treated with infliximab in addition to corticosteroids; further, 5 patients recovered and achieved long survival for >1 year, while the remaining 6 patients died during the corticosteroid tapering period because of CIP (n=3), cancer progression (n=2), and infection (n=1). Among the 16 patients with refractory CIP, 5 did not respond to the initial corticosteroid therapy. Among them, 1 was also treated with infliximab. All 5 patients died within 2 months, with 4 dying because of CIP and 1 dying because of infection. The median OS of the refractory group was 12.00 weeks (95% CI: 5.47–18.53 wk); further, 7 patients died of CIP, 2 of infection, 4 of cancer progression, and 3 patients were alive at the last follow-up. The rate of CIP mortality in the refractory group was 43.8% (7/16).
FIGURE 5.
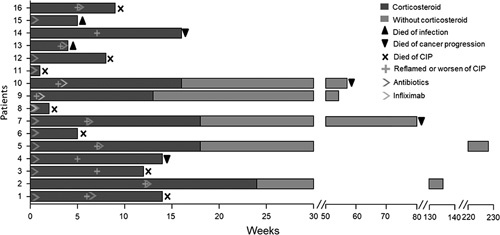
Clinical course and outcomes of patients with refractory CIP. CIP indicates checkpoint inhibitor pneumonitis.
DISCUSSION
To our knowledge, this is the first study to investigate the risk factors for refractory CIP. An LDH level greater than 320 U/L at the onset of CIP was found to be an independent risk factor of refractory CIP. Generally, corticosteroid administration is the first-line treatment for CIP. In our study, the incidence of refractory CIP at initial corticosteroid treatment and during the corticosteroid tapering period was 26.7%, which is consistent with the findings of a previous report.19
The risk factors for CIP development are well-established. Regarding baseline characteristics, tumor invasion in the central airway,20 history of chronic obstructive pulmonary disease21 or chronic pulmonary diseases,22,23 PD-L1 levels ≥50%,21 age 60 years or younger,24 Eastern Cooperative Oncology Group Performance Status ≥2,23 and use of inhaled corticosteroids at ICI onset25 are independently associated with an increased incidence of CIP. A controversial study reported an age 65 years or older as an independent risk factor for CIP.25 Adenocarcinoma19 could be a protective factor for CIP. However, none of these aforementioned characteristics were associated with refractory CIP in our study. Moreover, we found that the best response to ICI treatment may be related to refractory CIP; however, the response was not evaluated in >30% of patients with refractory CIP.
Dry cough, shortness of breath, and fever are common CIP symptoms. A study showed that 88.1% and 78.6% of patients with CIP had cough and dyspnea, respectively.26 Consistent with these findings, we found that cough (35.0%) and shortness of breath (38.3%) were the most common symptoms. Further, shortness of breath showed a nonindependent association with refractory CIP.
The onset of CIP was nonsignificantly earlier in the refractory group than in the nonrefractory group (16.57 vs. 7.43 wk, P=0.079). CIP with an onset ≤6 weeks and >6 weeks after ICI treatment was defined as early-onset and late-onset CIP, respectively.18,27 In addition, 92.9% of patients with early-onset CIP had grade 3 or higher CIP, with the mortality rate being as high as 50.0%.27 Early-onset CIP has a much poorer prognosis than late-onset CIP. Our study demonstrated that the incidence of early-onset CIP was higher in the refractory group than in the nonrefractory group (50.0% vs. 18.2%, P=0.021), which could explain the higher mortality rate in the refractory CIP group. Early-onset CIP may be an indicator of refractory CIP; however, the definitions of early-onset and late-onset CIP remain controversial. One study defined early and late-onset of CIP as <6 and >6 months, respectively, from the initiation of ICI therapy.19 Compared with low-grade CIP, high-grade CIP (grade 3 or higher) showed an earlier onset. We observed no between-group differences after dividing the CIP cohort as previously described. Defining the early-onset of CIP as <6 weeks may be more appropriate for identifying refractory CIP.
Peripheral blood biomarkers are associated with the incidence and prognosis of CIP. Baseline interleukin-8 (IL-8) levels21 are negatively associated with the incidence of CIP, while baseline absolute eosinophil count levels (≥0.125 × 10(9) cells/L) are positively associated with the risk of CIP and clinical outcomes.28 Increased IL-6, IL-10, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio levels, as well as decreased absolute lymphocyte count and Albumin levels, are associated with an increased risk of CIP; additionally, high IL-6 levels and low ALB levels at the onset of CIP are associated with high-grade CIP and poor prognosis.29 In our hospital, IL levels are not routinely tested; accordingly, IL-8 and IL-6 were not included in the analysis. Among the peripheral blood biomarkers, only the LDH levels were associated with refractory CIP. Increased LDH levels are associated with lung injury and autoimmune pneumonia.30,31 Lin et al29 reported a significant increase in LDH levels from baseline to the onset of CIP (223.80 U/L [IQR, 177.03–398.93] to 257.85 U/L [IQR, 189.03–311.83]; P=0.049]; however, no changes in LDH levels were observed among patients without CIP. We observed no significant differences between LDH levels at baseline and the onset of CIP in either group. However, compared with the nonrefractory CIP group, the refractory CIP group showed significantly higher LDH levels at baseline and CIP onset. In addition, the change in LDH levels from baseline to CIP onset was significantly greater in the refractory CIP group than in the nonrefractory group. Further analysis revealed that an LDH level >320 U/L at CIP onset was an independent risk factor for refractory CIP. Accordingly, closely monitoring changes in LDH levels may allow early detection of patients with refractory CIP.
Patients with refractory CIP require immune suppression agents and prolonged steroid treatment. In our study, 3 patients were treated with infliximab in addition to steroids; among them, 2 recovered and showed a long survival period (≥1 y from the CIP onset). For the other patients, the corticosteroid dose was increased. Since a high starting dose and long duration of corticosteroids (≥30 mg/day equivalent methylprednisolone; P=0.001) are associated with an opportunistic infection,26 62.5% (10/16) of the patients were treated with antibiotics at the onset of CIP. The low infection-related mortality (12.5%, 2/16) could be attributed to the high usage of antibiotics, with 75% (12/16) of the patients receiving antibiotics during CIP treatment.
The CIP-related mortality among patients with refractory CIP was high (43.8% [7/16]); contrastingly, the mortality rate for the overall sample was 11.7% (7/60), which is lower than the previously reported value of 22.7%.7 The effect of CIP occurrence on survival remains unclear. Li et al32 reported that CIP occurrence did not significantly affect OS (hazard ratio, 1.14 [0.70–1.86]). Tone et al7 reported that the median progression-free survival and OS were significantly shorter in patients with severe grade CIP than in those without. In addition, CIP development is demonstrated to be significantly associated with increased progression-free survival and OS.33,34 In the refractory CIP group, some patients showed a long survival period (>5 y), while others died within 12 weeks. This indicates that successfully curing refractory CIP may increase the survival period.
This study has several limitations. First, this was a retrospective study with some missing clinical data, including blood parameters and chest CT images. Moreover, the treatment regimens for CIP were not standardized. These aforementioned factors may result in bias during the analysis. Second, this study had a small sample size, with only 16 patients with refractory CIP being included. Further, 5 patients did not respond to the initial steroid treatment, while 11 patients showed worsening symptoms during the steroid tapering period. These 2 groups could not be analyzed separately because of the small sample sizes. Since the 2 subtypes of refractory CIP could involve different mechanisms, future studies are warranted.
CONCLUSION
Refractory CIP is a common occurrence among patients with CIP. Further, patients with refractory CIP showed very high CIP-related mortality; therefore, it is important to identify this subset of patients with CIP. We found that an LDH level >320 U/L at CIP onset was an independent risk factor for refractory CIP. Therefore, monitoring LDH levels is recommended during the ICI treatment. Large-cohort studies are required to verify our findings.
Supplementary Material
Acknowledgments
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
This study was supported by GDPH Supporting Fund for NSFC Program (8190120260), Guangzhou Science and Technology Plan Foundation (2021-02-01-04-1002-0017), National Natural Science Foundation of China (Grant No. 81972970 and No. 82172671) and the Natural Science Foundation of Guangdong Province of China (Grant No. 2020A1515010186). The funding sources have no involvement in the study.
All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
P.T. and W.H. are co-first authors.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
Contributor Information
Peixin Tan, Email: tpxsaxin@163.com.
Wei Huang, Email: huangwei_0118@163.com.
Xinyan He, Email: yummy_HXY@163.com.
Fengquan Lv, Email: lvfengquan678@163.com.
Yanhai Cui, Email: cuiyanhai_gpph@163.com.
Shasha Du, Email: duss0202@163.com.
REFERENCES
- 1. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. [DOI] [PubMed] [Google Scholar]
- 3. Yin J, Wu Y, Yang X, et al. Checkpoint Inhibitor Pneumonitis Induced by Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer: Occurrence and Mechanism. Front Immunol. 2022;13:830631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. The. N Engl J Med. 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 5. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 6. Domagała-Kulawik J, Leszek P, Owczarek W, et al. Immunotherapy of solid tumors: safety of treatment. Polish Arch Intern Med. 2020;130:766–778. [DOI] [PubMed] [Google Scholar]
- 7. Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. 2019;10:2006–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miao K, Xu Y, Xu W, et al. Treatment of steroid-resistant checkpoint inhibitor pneumonitis with pirfenidone: A case report. Thorac Cancer. 2021;12:2214–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for. Med Oncol. 2018;29(Suppl 4):iv264–iv266. [DOI] [PubMed] [Google Scholar]
- 11. Utsumi H, Araya J, Okuda K, et al. Successful treatment of steroid-refractory immune checkpoint inhibitor-related pneumonitis with triple combination therapy: a case report. Cancer Immunol Immunother. 2020;69:2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naidoo J, Cottrell TR, Lipson EJ, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer. 2020;8:e000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jong C, Peters BJM, Schramel F. Recurrent episodes of nivolumab-induced pneumonitis after nivolumab discontinuation and the time course of carcinoembryonic antigen levels: A Case of a 58-Year-Old Woman with Non-Small Cell Lung Cancer. Chemotherapy. 2018;63:272–277. [DOI] [PubMed] [Google Scholar]
- 14. Asher N, Marom EM, Ben-Betzalel G, et al. Recurrent pneumonitis in patients with melanoma treated with immune checkpoint inhibitors. Oncologist. 2019;24:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan PX, Huang W, Liu PP, et al. Dynamic changes in the radiologic manifestation of a recurrent checkpoint inhibitor related pneumonitis in a non-small cell lung cancer patient: a case report. World J Clin Cases. 2021;9:9108–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tao H, Li F, Wu D, et al. Rate and risk factors of recurrent immune checkpoint inhibitor-related pneumonitis in patients with lung cancer. Transl Lung Cancer Res. 2022;11:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chuzi S, Tavora F, Cruz M, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res. 2017;9:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pozzessere C, Lazor R, Jumeau R, et al. Imaging features of pulmonary immune-related adverse events. J Thorac Oncol. 2021;16:1449–1460. [DOI] [PubMed] [Google Scholar]
- 19. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol. 2018;13:1930–1939. [DOI] [PubMed] [Google Scholar]
- 20. Moda M, Saito H, Kato T, et al. Tumor invasion in the central airway is a risk factor for early-onset checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Thorac Cancer. 2020;11:3576–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao Y, Zhou J, Hsu S, et al. Risk factors for immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer. Transl Lung Cancer Res. 2022;11:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest. 2021;160:731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang C, Gao F, Jin S, et al. Checkpoint inhibitor pneumonitis in Chinese lung cancer patients: clinical characteristics and risk factors. Ann Palliat Med. 2020;9:3957–3965. [DOI] [PubMed] [Google Scholar]
- 24. Asada M, Mikami T, Niimura T, et al. The risk factors associated with immune checkpoint inhibitor-related pneumonitis. Oncology. 2021;99:256–259. [DOI] [PubMed] [Google Scholar]
- 25. Li M, Spakowicz D, Zhao S, et al. Brief report: inhaled corticosteroid use and the risk of checkpoint inhibitor pneumonitis in patients with advanced cancer. Cancer Immunol, Immunother. 2020;69:2403–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Zhao Y, Zhang X, et al. Clinical characteristics and management of immune checkpoint inhibitor-related pneumonitis: a single-institution retrospective study. Cancer Med. 2021;10:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang A, Xu Y, Zang X, et al. Radiographic features and prognosis of early- and late-onset non-small cell lung cancer immune checkpoint inhibitor-related pneumonitis. BMC Cancer. 2021;21:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chu X, Zhao J, Zhou J, et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung cancer. 2020;150:76–82. [DOI] [PubMed] [Google Scholar]
- 29. Lin X, Deng H, Yang Y, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol. 2021;11:698832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tokano Y, Ogasawara H, Ando S, et al. Cyclosporin A therapy for interstitial pneumonitis associated with rheumatic disease. Mod Rheumatol. 2002;12:305–310. [DOI] [PubMed] [Google Scholar]
- 31. Drent M, Cobben NA, Henderson RF, et al. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736–1742. [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Zhang Y, Jia X, et al. Effect of immune-related adverse events and pneumonitis on prognosis in advanced non-small cell lung cancer: A comprehensive systematic review and meta-analysis. Clin Lung Cancer. 2021;22:e889–e900. [DOI] [PubMed] [Google Scholar]
- 33. Cui P, Huang D, Wu Z, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920922033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ono K, Ono H, Toi Y, et al. Association of immune-related pneumonitis with clinical benefit of anti-programmed cell death-1 monotherapy in advanced non-small cell lung cancer. Cancer Med. 2021;10:4796–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]


