Background:
Common Bile duct (CBD) measurement is a crucial aspect in the evaluation of the biliary tree. Whether the CBD undergoes any compensatory change in diameter after laparoscopic cholecystectomy or laparoscopic common bile duct exploration is still up for discussion. The aim of this study was to investigate CBD diameter changes after laparoscopic cholecystectomy (LC) and laparoscopic common bile duct exploration (LCBDE) on magnetic resonance cholangiopancreatography (MRCP).
Materials and Methods:
Our retrospective study is divided into 2 sections. The first part assessing CBD diameter changes after laparoscopic cholecystectomy due to gallstones or gallbladder polyps, involved 85 patients, who underwent MRCP procedures. These patients aged between 30 and 85 were divided into an interval LC group (group A, n=56) and a remote LC group (group B, n=29). In group A, the common CBD diameters were measured at their widest portions on MRCP obtained before and after laparoscopic cholecystectomy. Measurements of the CBD diameters were repeated on MRCP obtained twice after the surgery in group B.
Section 2 consisted of 38 patients who had choledocholithiasis and were treated with laparoscopic CBD exploration and T-tube placement. These patients aged 26 to 86 formed the interval LCBDE group (group C). The CBD widest diameters were measured on MRCP before LCBDE and after T-tube cholangiography for these individuals.
Patients in groups A and C were further divided into 5 and those in group B into 4 age-related subgroups to facilitate statistical analysis. The Pearson correlation test was performed to find any relationship between CBD diameters and age in groups A and B. Paired sample T test was used to compare the significant difference between the 2 sets of CBD diameters in each study group and their subgroups.
Results:
In the interval LC group, the post-LC mean CBD diameter was significantly wider when compared with the preoperative mean diameter (P<0.05). There was a significant difference between the first and second post-LC means CBD diameter in the remote LC group (P<0.05). In group C, the mean CBD diameter measured on T-tube cholangiography after LCBDE was significantly smaller than the preoperative dilated mean diameter (P<0.05).
Conclusions:
This study demonstrated significant dilation occurring in the common bile duct diameter after laparoscopic cholecystectomy. Furthermore, our remote LC group also supported that claim by showing significant dilation between the first and second post-cholecystectomy CBD diameter values. And lastly, our interval LCBDE sample’s initial dilation of the CBD diameters was reduced after surgery and stone extraction.
Key Words: common bile duct, common hepatic duct, diameter, laparoscopic cholecystectomy, laparoscopic common bile duct exploration, magnetic resonance cholangiopancreatography
Dilation of the common bile duct (CBD) is usually caused by obstructive changes such as CBD stones or strictures, gallbladder or pancreatic tumors, previous surgical interventions, and periampullary diverticulum.1–3 Other causes include increasing age4–7; and cholecystectomy.8–12 Post-cholecystectomy dilation was first highlighted in 1887 by Oddi.8 Later some studies supported this statement by claiming that physiological compensation occurs due to the loss of the gallbladder’s reservoir function.10,11,13
Since then, this theory in humans has been promptly debated and remains controversial in the radiologic, surgical, and sonographic literature, necessitating further examinations.10 While some studies showed a significant dilation in the CBD diameter after cholecystectomy,14–17 others denied such results.18–20 Following cholecystectomy, CBD diameter dilation has been observed in ultrasonography (US), computed tomography (CT), and magnetic resonance cholangiopancreatography (MRCP) studies.10,11,13,15
Magnetic resonance cholangiopancreatography is a noninvasive “gold standard” method that uses T2 sequence magnetic resonance imagery for assessing gallbladder and CBD diseases. It is used to examine intrahepatic and extrahepatic bile ducts.21,22 To our knowledge, MRCP has high sensitivity and specificity in detecting choledocholithiasis.23 But so far, only 1 study has utilized MRCP to assess postlaparoscopic cholecystectomy CBD dilatation.15
Our research investigated the CBD diameters retrospectively in both the postlaparoscopic cholecystectomy population (section 1) and the postlaparoscopic CBD exploration population (section 2). Section 1 of this study has 2 samples of post-LC patients. In sample 1 (interval LC), we evaluated any significant change in the CBD diameters measured on MRCP before and after laparoscopic cholecystectomy. In sample 2 (remote LC), we investigated if there was a significant change in diameter between 2 post-LC CBD diameters.
In section 2, the CBD diameters in post-LCBDE patients were assessed using MRCP. Two sets of CBD diameters measured before LCBDE and after T-tube cholangiography were recorded and compared (interval LCBDE). This study aims to measure the CBD diameters in the same patients using MRCP images to see whether there are any changes in the common bile duct diameter after (1) laparoscopic cholecystectomy and (2) laparoscopic common bile duct exploration.
MATERIALS AND METHODS
Ethics Statement
This retrospective study was performed in the Department of General Surgery of the Nanjing First Hospital, Nanjing Medical University. This manuscript has been reviewed and approved by the ethics committee of Nanjing First Hospital affiliated with Nanjing Medical University.
Data Research
In section 1 of this study, we researched MRCP images in the MRI database of the Nanjing First Hospital affiliated with Nanjing Medical University, China, between January 2011 to November 2021. Using the keyword “post-cholecystectomy”, 861 MRCPs were identified. Each post-cholecystectomy MRCP was examined using the same database by cross-matching name, gender, age, and diagnosis to find a corresponding precholecystectomy MRCP image. Furthermore, we also recorded patients having a second post-cholecystectomy MRCP.
The second part of the research was conducted by searching the term “T-tube” and 260 T-tube cholangiography were registered. We recorded T-tube cholangiography firstly as it is the most appropriate method to obtain images to measure the CBD diameters after LCBDE for our retrospective study. All the T-tube cholangiography were then assessed using the same method mentioned above to find their corresponding pre-LCBDE MRCP images, resulting in 44 cases.
Inclusion and Exclusion Criteria
Laparoscopic Cholecystectomy Sample
The LC sample was assembled by recruiting patients with the following conditions: 2 MRCP images (interval: pre-LC vs. post-LC and remote: post-LC vs. post-LC) and laparoscopic cholecystectomy due to gallstones or gallbladder polyps.
Our exclusion criteria included: Open cholecystectomy; Conversion from laparoscopic to open surgery; Extrahepatic/intrahepatic bile ducts stones; Acute cholecystitis; Cholangitis; Abnormal LFTs; Gallbladder carcinoma; Liver carcinoma; Cholangiocarcinoma; Gas accumulation or infection in the CBD; CBD stenosis; CBD injury; and Chronic or necrotizing pancreatitis. Patients were also excluded if their stones or polyps were diagnosed on CT or US without MRCP examinations.
Laparoscopic Common Bile Duct Exploration Sample
Inclusion criteria for selecting the LCBDE sample were if patients had 1 MRCP imaging before and 1 T-tube cholangiography after LCBDE, common bile duct stones, and LCBDE with stones extraction.
The exclusion criteria for this sample were open CBD exploration (OCBDE), conversion from LCBDE to OCBDE, patients with chronic pancreatitis, recurrence of choledocholithiasis, LCBDE without T-tube placement, post-LCBDE common bile duct infection, and unclear T-tube cholangiography.
Patients Grouping
Data collected were assessed using the criteria mentioned above, and the final cohort investigating any change in CBD diameter after LC included 85 patients and were divided into 2 groups (section 1). The interval LC group (group A) consisted of 56 patients aged 30 to 85 years old with 1 MRCP before and one after surgery.
The remaining 29 patients formed the remote LC group (group B), ranging from 41 to 79 years old. These patients had 2 MRCP after cholecystectomy (no MRCP was found before surgery). MRCP was performed for these patients after LC due to abdominal discomfort to check for any CBD pathology, but none was found.
The second section evaluating CBD diameter after LCBDE consisted of 44 patients with 1 MRCP before surgery and 1 T-tube cholangiography after LCBDE. Six were excluded due to CBD stones recurrence after further analysis of these patients’ medical reports. And The final cohort in the interval LCBDE group (group C) involved 38 patients aged 26 to 86 years old. A general description of the 3 study groups is shown below (Fig. 1).
FIGURE 1.
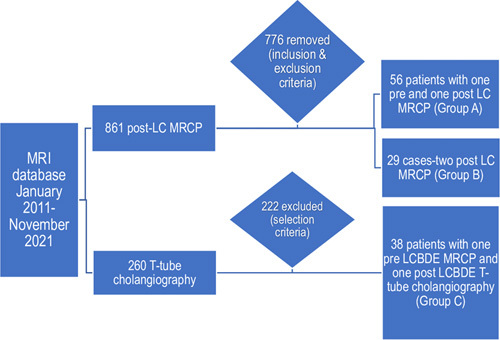
Classification of the final interval LC (group A), remote LC (group B), and interval LCBDE (group C) groups.
Time Interval Between Two MRCP
Preoperative MRCP was performed within 3 days or the same day before laparoscopic cholecystectomy and laparoscopic CBD exploration for patients in groups A and C. The Postoperative MRCP for group A’s patients ranged from 3 to 1008 days after LC. Patients in group B had the 2 MRCPs done after LC with time interval between them ranging from 164 to 3448 days. Group C’s patients had their T-tube cholangiography done between 2 to 610 days after LCBDE.
Measuring CBD Diameters on MRCP
All participants fasted for 6 to 8 hours before MRCP to minimize fluid secretions within the stomach, the duodenum, and diminished bowel peristalsis. Each patient’s first and second MRCP illustrations were displayed simultaneously to facilitate CBD diameter measurements (Fig. 2). The inner diameter of each patient’s CBD was digitally estimated from perpendicular cross-sections at a certain position on the biliary tree by positioning an electronic caliper perpendicular to the long axis at the widest visible area of the CBD on MRCP for all the patients, and the values were recorded (Fig. 3).7,24
FIGURE 2.
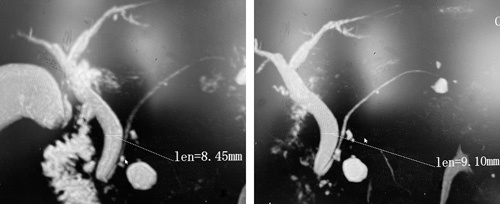
Pre (left) and post (right) laparoscopic cholecystectomy MRCPs of a 67-year-old patient while measuring CBD diameters.
FIGURE 3.
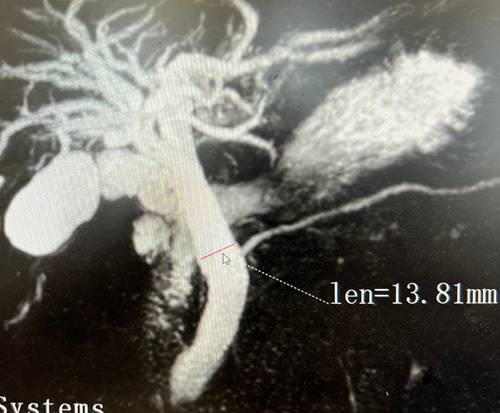
By placing an electronic caliper perpendicular to the long axis at the widest estimated portion to measure the CBD diameters on MRCP in a patient.
Recorded Information and Age Subgroups
For every patient: age, gender, 2 CBD diameters, and the time interval in days between the images were recorded for this study. All patients in the 3 study groups were stratified into age-related subgroups, regardless of gender. Group A yielded 5 subgroups: (1) 40 years or younger; (2) 41 to 50 years; (3) 51 to 60 years; (4) 61 to 70 years, and (5) 71 years or older. Due to zero patients under the age of 40 in group B and a small number of patients under 40 years old in group C, stratifications for groups B and C were as follows: (1) 50 years or younger, (2) 51 to 60 years, (3) 61 to 70 years and (4) 71 years or older; and (1) 50 years or younger, (2) 51 to 60 years, (3) 61 to 70 years, (4) 71 to 80 years and (5) 81 years or older, respectively. The collected data’s evaluation and statistical interpretation were performed independently for each study group and their corresponding age subgroups.
Statistical Analysis
Data collected were statistically analyzed using Statistical Package for the Social Sciences for Windows (SPSS Version 26.0, IBM). Percentages and frequencies are used to express categorical data. Numerical data such as ages and CBD diameters were computed as mean, SD and range. Time intervals between the 2 MRCPs were recorded in days as mean, SD, and range. The means of CBD diameter in the study groups were recorded twice. The Pearson correlation test was used to evaluate any relationship between CBD diameters and age in the interval LC (group A) and remote LC groups (group B). Significance of difference in CBD diameters before and after surgery in both groups A and C; and between the first postoperative and second postoperative CBD diameters in group B were performed using Paired samples T test. Next, the means of common bile duct diameter for each subgroup were recorded for further evaluation. And the paired sample T test was used again to assess the significance of the difference by comparing the first values of the CBD diameter to their corresponding second values for each subgroup. P<0.05 was considered statistically significant.
RESULT
Our study comprises 123 patients who had surgery and MRCP examinations in our hospital.
Section One: CBD Diameter Dilation After Laparoscopic Cholecystectomy
The laparoscopic cholecystectomy sample was divided into the interval LC (group A) (65.88%) and remote LC (group B) (34.12%) groups.
The interval LC group (group A) consisted of 56 patients (40 or 71.43% females) with a mean age of 60.57±13.176, and the average time interval between the 2 MRCPs was 424.07±257.103 days. The Pearson correlation test was significant between pre-LC CBD diameters and age (Table 1). However, after LC there was no correlation between CBD diameters and age (in Table 2).
TABLE 1.
The Pearson Correlation Test Shows the Relationship Between CBD Diameters and Age Before Laparoscopic Cholecystectomy in the Interval LC Group
| Correlations | ||
|---|---|---|
| Age | Pre-LC CBD diameters | |
| Age | ||
| Pearson correlation | 1 | 0.285* |
| Sig. (2-tailed) | 0.033 | |
| N | 56 | 56 |
| Pre-LC CBD diameters | ||
| Pearson correlation | 0.285* | 1 |
| Sig. (2-tailed) | 0.033 | |
| N | 56 | 56 |
Correlation is significant at the 0.05 level (2-tailed).
TABLE 2.
Pearson Correlation Test Demonstrates No Relationship Between Post-LC CBD Diameters and Age After Laparoscopic Cholecystectomy in the Interval LC Group
| Correlations | ||
|---|---|---|
| Age | Post-LC CBD diameters | |
| Age | ||
| Pearson Correlation | 1 | 0.259 |
| Sig. (2-tailed) | 0.054 | |
| N | 56 | 56 |
| Post-LC CBD diameters | ||
| Pearson correlation | 0.259 | 1 |
| Sig. (2-tailed) | 0.054 | |
| N | 56 | 56 |
Pre-LC mean CBD diameter was 6.90±2.14866 mm and post-LC mean CBD diameter was 8.20±2.45692 mm. A statistically significant increase was present between the preoperative mean CBD diameter and the postoperative mean CBD diameter (t=2.004, P<0.05). Means of preoperative and postoperative CBD diameters of the subgroups were also compared with investigate any statistical differences in the interval LC sample. There was significant dilation in CBD diameters after LC, as shown in Table 3.
TABLE 3.
Comparing Pre-LC and Post-LC CBD Diameters (Mean±SD) in the Interval LC Group (Group A)
| Age Groups in years | No. Patients | Pre-LC Mean CBD diameter in mm | Post-LC Mean CBD diameter in mm | P |
|---|---|---|---|---|
| ≤40 | 5 | 6.24±0.79878 | 8.97±1.29084 | 0.033 |
| 41-50 | 10 | 6.36±0.48747 | 7.38±0.54663 | 0.006 |
| 51-60 | 10 | 6.51±0.62331 | 6.99±0.51237 | 0.014 |
| 61-70 | 19 | 6.78±0.56448 | 7.97±0.62939 | <0.0001 |
| ≥71 | 12 | 8.14±0.61815 | 9.95±0.62015 | <0.0001 |
| TOTAL | 56 | 6.90±2.14866 | 8.20±2.45692 | <0.0001 |
The remote LC group (group B) included 29 patients (22 or 75.86% females) with a mean age of 59.59±11.262. The mean time interval between the 2 MRCPs was 2107.59±837.582 days. The Pearson correlation showed no relationship between CBD diameters and age after LC (r=0.240 and r=0.278 for the first and second post-LC CBD diameters, respectively). The total means of the 1st post-cholecystectomy and 2nd post-cholecystectomy CBD diameter were 8.58±3.73408 mm and 9.89±4.19521 mm, respectively. When comparing CBD diameters, a statistically significant dilation is found between the 2 postoperative CBD diameters (t=2.048, P<0.05). Results for each age subgroup are detailed in Table 4.
TABLE 4.
Comparison of CBD Diameters (mean±SD) Between the 2 Post-LC Values in the Remote LC Group (Group B)
| Age Groups (years) | No. Patients | First Post-LC mean CBD diameter in mm | Second Post-LC mean CBD diameter in mm | P |
|---|---|---|---|---|
| ≤50 | 7 | 6.50±1.43743 | 7.16±1.43320 | 0.030 |
| 51-60 | 8 | 9.74±1.27398 | 11.46±1.57350 | 0.017 |
| 61-70 | 8 | 8.19±1.13595 | 9.25±1.04831 | 0.035 |
| ≥71 | 6 | 9.96±1.70154 | 11.83±1.90357 | 0.007 |
| TOTAL | 29 | 8.58±3.73408 | 9.89±4.19521 | <0.0001 |
Section Two: The Dilated CBD Diameter Reduced After LCBDE
The change in the dilated CBD diameter after laparoscopic common bile duct exploration with the interval LCBDE group (group C), involved 38 patients (20 or 54.05% males) with a mean age of 64.24±16.643. The average time interval between the 2 imaging modalities was 94.76±153.542 days. The pre-LCBDE mean CBD diameter on MRCP was 10.54±3.51492 mm, and the post-LCBDE mean CBD diameter on T-tube cholangiography was 8.70±2.92332 mm.
There was a statistically significant reduction in post-LCBDE CBD diameters when compared with pre-LCBDE CBD diameters (t=2.2026, P<0.05). Hence, all patients below the age of 81 had a significant difference between their post-LCBDE CBD diameters and their preoperative CBD diameters. However, patients aged 81 and above showed no statistical difference between the 2 CBD diameters (P=0.071). Table 5 shows details for each age subgroup in the interval LCBDE sample.
TABLE 5.
Statistical Differences in CBD Diameters (mean±SD) of the Different age Subgroups in the Interval LCBDE Group (Group C)
| Age groups (years) | No. Patients | Pre-LCBDE mean CBD diameter in mm | T-tube cholangiography mean CBD diameter in mm | P |
|---|---|---|---|---|
| ≤50 | 8 | 8.19±0.74385 | 6.10±0.55819 | 0.024 |
| 51-60 | 7 | 9.99±0.93295 | 8.32±0.77738 | 0.019 |
| 61-70 | 7 | 13.03±1.91610 | 9.85±1.30846 | 0.028 |
| 71-80 | 9 | 11.52±0.97771 | 10.23±0.75832 | 0.041 |
| ≥81 | 7 | 10.05±1.25739 | 8.95±1.32741 | 0.071 |
| Total | 38 | 10.54±3.51492 | 8.70±2.92332 | <0.0001 |
DISCUSSION
The CBD diameter values vary according to the measuring techniques used. For instance, US measurements are slightly lower than those obtained on CT. The CBD diameter is measured from the inner-wall to the inner-wall on ultrasonography, in contrast to CT, which is done from the outer-wall to the outer-wall.13,25 An increase in pressure caused by the injection of contrast medium in the CBD when performing ERCP may result in a larger CBD diameter value.26,27 Two previous studies by McArthur et al and Valkovic et al reported that the CHD and the CBD could not be separately evaluated because they are not easily differentiated on sonography.10,13 Also, because the cystic duct is too small to be visible on either US or CT, special attention must be paid to not include the cystic duct in the CBD diameter measurements.28
In our study, we used MRCP, which has a high sensitivity (93%) and high specificity (94%), to detect choledocholithiasis when evaluating the biliary tree.29 We excluded patients who had open surgery or conversion from laparoscopic to open surgery due to the fact that in our own experiences, we found that patients who have had previous “open abdominal surgeries” usually present with adhesions from the peritoneum to abdominal organs because of incisions made. These adhesions usually create tension in the CBD and could affect the normal size of the CBD diameters. Patients using medications that contribute to CBD dilation in both sections were also excluded, and those with CBD stones found on MRCP and recurrence of choledocholithiasis on T-tube cholangiography after LC and LCBDE, respectively. And measurement of the extrahepatic bile ducts' inner diameter on MRCP after the section where the cystic duct joins the CHD to form the CBD contributes to our study’s data accuracy.
Two previous studies conducted on CBD diameter changes after LC by Pavlovic et al and Valkovic et al had a choledocholithiasis rate of 1% and 2%, respectively10,15; however, in our study, the rate of CBD stones when evaluating the CBD diameter was zero as patients with postoperative stones development or recurrence were excluded.
The CBD diameter significantly increased for the whole sample in the interval LC group after cholecystectomy (P<0.05). Several studies have reported similar results where the CBD diameter increased after removing the gallbladder.10–13,15,17,30 The latter physiologically acts as a tension bulb to maintain steady bile pressure in the biliary tree when the sphincter of Oddi is closed.31 And, the absence of this reservoir-acting sac causes a compensatory increase in CBD diameter to withstand the biliary pressure.
On the other hand, Valkovic et al10 stated that there is no immediate CBD dilation occurring in the early postcholecystectomy period and no need to measure the CBD diameter. However, our study found that the CBD dilated immediately after LC. Where in 2 patients, we found an increase of 1.25 and 1.27 mm in their initial pre-LC CBD diameter values, which were recorded at 3 and 5 days after surgery, respectively.
For our remote laparoscopic cholecystectomy patients, a statistically significant dilatation was found between the first and second post-LC CBD diameters (P<0.05). This group included 9 patients who were diagnosed with gallstones on CT and US before LC. Our study did not include these patients in the interval LC group due to the lack of their pre-LC CBD diameters. Also, we ensured that the Liver function tests (LFTs) of all the 85 patients included in this section were normal. As high values of γ-aminotransferase (>90 U/L) and ALP increases the possibility of choledocholithiasis.11,32–37
The postcholecystectomy mean CBD diameter values in both the interval (8.20±2.45692 mm) and remote cholecystectomy (9.89 ± 4.19521 mm) samples are within <10 mm, which is consistent with the study done by Park et al.11 Therefore, we can confirm a threshold upper limit of CBD diameter of 10 mm after laparoscopic cholecystectomy. The remote LC group of our study shows statistically significant differences in CBD diameters among all age groups when comparing the 2 post-cholecystectomy CBD diameters (Table 4). It also indicates that the CBD continues to dilate after cholecystectomy as time goes by.
In section 1 of our research, the interval LC group showed that the CBD undergoes a compensatory mechanism postgallbladder removal. The significant difference in our 2 post-LC values (remote LC group) begs whether this phenomenon is caused by an adaptive response of the CBD in the absence of the gallbladder or whether the increase in our second post-LC CBD diameters is due to a physiological dilation. We found no studies inquiring about this mechanism, thus opening the door for future studies. However, the Pearson correlation test demonstrated that post-LC CBD diameters in both the interval and remote LC groups have no relationship with age.
After stones extraction through an incision made in the CBD during the LCBDE procedure, many surgeons opt to insert a T-tube in the cut before stitching up the CBD.38 The T-tube allows the drainage of residual stones which may have been undetected or impossible to extract during the surgery.39,40 Other reasons include decompression of the CBD, incision support, prevention of bile building up due to temporary swelling, and its leakage through the incision.39,40 T-tube cholangiography also helps us to obtain CBD images after LCBDE, whereas for primary closure after LCBDE usually needs us to do MRCP again to obtain the images, which is costly in real-world clinical settings.
In section 2, we measured the CBD diameter on MRCP obtained before LCBDE and after LCBDE with T-tube insertion to assess whether, postsurgery, the dilated CBD diameter reverted back to under the accepted upper limit set by previous studies.5,7,41 The difference between the pre-LCBDE and the post-LCBDE CBD diameter was statistically significant for the whole sample (Table 5) (P<0.05), proving a significant decrease of the dilated CBD diameter size after LCBDE.
However, in analyzing by subgroups, we found no statistically significant decrease between the 2 CBD diameter values (P=0.071) in patients above 81 years old. It is known that longitudinal smooth muscle bands and their intervening connective tissue breaks with increasing age and are accompanied by loss of the ductal wall reticuloendothelial network.42 Therefore, this inability in our eldest group of patients to experience a significant reversion in CBD diameter can be theorized due to the loss of the ductal wall’s reticuloendothelial network with increasing age. In such a situation, it can be hypothesized that the CBD diameter change in older patients post-LCBDE is age-related rather than being surgically-related.
The first section of our study suggests that CBD undergoes a potential compensatory adaptive mechanism in response to the removal of the gallbladder. Although this likeliest phenomenon is supported by our results, future studies involving methods evaluating the intraductal pressure changes pre-LC and post-LC will help strengthen the compensatory adaptive theory.
The limitations in our study include a nondefined time interval between the 2 images. However, as this study is being performed retrospectively, we recorded the longest time interval to be 3448 days which is within 10 years, as Wu et al5 mentioned that the CBD physiologically increases by 1 mm every decade. In addition, the common bile duct diameter was measured at a single point, being the widest estimated portion of the extrahepatic bile ducts. Next, we did not consider the respiratory phase during data collection. And finally, this investigation was conducted in a single-center environment, with only Chinese participants. This result may be different in people with different ethnic backgrounds.
CONCLUSION
In conclusion, this study demonstrated that the CBD diameter dilates after laparoscopic cholecystectomy. In the interval laparoscopic cholecystectomy group, we found the increase in CBD diameters after LC to be significant, and we concluded that this dilation was due to the compensatory adaptation of the CBD in the absence of the gallbladder.
The increase in our remote LC subgroups further supports the claim of an increase in CBD diameters post-LC. However, this could also be an effect of the physiological dilation of the CBD as one ages.
Footnotes
This manuscript has been reviewed and approved by the ethics committee of Nanjing First Hospital affiliated with Nanjing Medical University. The institutional review board of the Nanjing First Hospital classified this study as exempt, and informed consent was not required.
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
N.K.P. and S.S.G. are co-first authors.
The authors declare no conflicts of interest.
Contributor Information
Nayagan Kavidassen Pallaneeandee, Email: naya.pallaneeandee@hotmail.com.
Shankara Subramanyam Govindan, Email: shankara-no7@hotmail.com.
Liu Zi Jun, Email: 2534619071@qq.com.
REFERENCES
- 1. Campbell WL, Foster RG, Miller WJ, et al. Changes in extrahepatic bile duct caliber in liver transplant recipients without evidence of biliary obstruction. AJR Am J Roentgenol. 1992;158:997–1000. [DOI] [PubMed] [Google Scholar]
- 2. Reinus WR, Shady K, Lind M, et al. Ultrasound evaluation of the common duct in symptomatic and asymptomatic patients. Am J Gastroenterol. 1992;87:489–492. [PubMed] [Google Scholar]
- 3. Rajnakova A, Goh PM, Ngoi SS, et al. ERCP in patients with periampullary diverticulum. Hepatogastroenterology. 2003;50:625–628. [PubMed] [Google Scholar]
- 4. Bachar GN, Cohen M, Belenky A, et al. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med. 2003;22:879–882; quiz 883-5. [DOI] [PubMed] [Google Scholar]
- 5. Wu CC, Ho YH, Chen CY. Effect of aging on common bile duct diameter: a real-time ultrasonographic study. J Clin Ultrasound. 1984;12:473–478. [DOI] [PubMed] [Google Scholar]
- 6. Perret RS, Sloop GD, Borne JA. Common bile duct measurements in an elderly population. J Ultrasound Med. 2000;19:727–730; quiz 731. [DOI] [PubMed] [Google Scholar]
- 7. Govindan S, Tamrat NE, Liu ZJ. Effect of ageing on the common bile duct diameter. Dig Surg. 2021;38:368–376. [DOI] [PubMed] [Google Scholar]
- 8. Oddi R. D’une disposition a sphincter speciale de l’ouverture du canal choledoque. Arch Ital Biol. 1887;8:317–322. [Google Scholar]
- 9. Daradkeh S, Tarawneh E, Al-Hadidy A. Factors affecting common bile duct diameter. Hepatogastroenterology. 2005;52:1659–1661. [PubMed] [Google Scholar]
- 10. Valkovic P, Miletic D, Zelic M, et al. Dynamic changes in the common bile duct after laparoscopic cholecystectomy: a prospective longitudinal sonographic study. Ultraschall Med. 2011;32:479–484. [DOI] [PubMed] [Google Scholar]
- 11. Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc. 2012;83:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng B, Song Q. Does the common bile duct dilate after cholecystectomy? Sonographic evaluation in 234 patients. AJR Am J Roentgenol. 1995;165:859–861. [DOI] [PubMed] [Google Scholar]
- 13. McArthur TA, Planz V, Fineberg NS, et al. CT evaluation of common duct dilation after cholecystectomy and with advancing age. Abdom Imaging. 2015;40:1581–1586. [DOI] [PubMed] [Google Scholar]
- 14. Chawla S, Trick WE, Gilkey S, et al. Does cholecystectomy status influence the common bile duct diameter? a matched-pair analysis. Dig Dis Sci. 2010;55:1155–1160. [DOI] [PubMed] [Google Scholar]
- 15. Pavlović T, Trtica S, Troskot Perić R. Bile duct diameter changes after laparoscopic cholecystectomy: a magnetic resonance cholangiopancreatography prospective study. Croat Med J. 2020;61:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Senturk S, Miroglu TC, Bilici A, et al. Diameters of the common bile duct in adults and postcholecystectomy patients: a study with 64-slice CT. Eur J Radiol. 2012;81:39–42. [DOI] [PubMed] [Google Scholar]
- 17. Niederau C, Müller J, Sonnenberg A, et al. Extrahepatic bile ducts in healthy subjects, in patients with cholelithiasis, and in postcholecystectomy patients: a prospective ultrasonic study. J Clin Ultrasound. 1983;11:23–27. [DOI] [PubMed] [Google Scholar]
- 18. Edmunds R, Katz S, Garciano V, et al. The common duct after cholecystectomy. Interval report. Arch Surg. 1971;103:79–81. [DOI] [PubMed] [Google Scholar]
- 19. Puri SK, Gupta P, Panigrahi P, et al. Ultrasonographic evaluation of common duct diameter in pre and post cholecystectomy patients. Trop Gastroenterol. 2001;22:23–24. [PubMed] [Google Scholar]
- 20. Majeed AW, Ross B, Johnson AG, et al. Common duct diameter as an independent predictor of choledocholithiasis: is it useful? Clin Radiol. 1999;54:170–172. [DOI] [PubMed] [Google Scholar]
- 21. Shanmugam V, Beattie GC, Yule SR, et al. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br J Radiol. 2005;78:888–893. [DOI] [PubMed] [Google Scholar]
- 22. Park MS, Kim TK, Kim KW, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234–240. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Mo JJ, Lin L, et al. Diagnostic value of magnetic resonance cholangiopancreatography in choledocholithiasis. World J Gastroenterol. 2015;21:3351–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiu NC, Chiou YY. Role of MRCP in the measurement of the CBD diameter. J Chin Med Assoc. 2012;75:423–424. [DOI] [PubMed] [Google Scholar]
- 25. McGillicuddy EA, Schuster KM, Brown E, et al. Acute cholecystitis in the elderly: use of computed tomography and correlation with ultrasonography. Am J Surg. 2011;202:524–527. [DOI] [PubMed] [Google Scholar]
- 26. Itoi T, Kamisawa T, Fujii H, et al. Extrahepatic bile duct measurement by using transabdominal ultrasound in Japanese adults: multi-center prospective study. J Gastroenterol. 2013;48:1045–1050. [DOI] [PubMed] [Google Scholar]
- 27. Mueller PR, Ferrucci JT Jr, Simeone JF, et al. Observations on the distensibility of the common bile duct. Radiology. 1982;142:467–472. [DOI] [PubMed] [Google Scholar]
- 28. Horrow MM. Ultrasound of the extrahepatic bile duct: issues of size. Ultrasound Q. 2010;26:67–74. [DOI] [PubMed] [Google Scholar]
- 29. Kaltenthaler E, Vergel YB, Chilcott J, et al. A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography. Health Technol Assess. 2004;8:1–89; iii. [DOI] [PubMed] [Google Scholar]
- 30. Co CS, Shea WJ, Jr, Goldberg HI. Evaluation of common bile duct diameter using high resolution computed tomography. J Comput Assist Tomogr. 1986;10:424–427. [PubMed] [Google Scholar]
- 31. JUDD ES. Condition of the common duct after cholecystectomy. JAMA. 1923;81:704–709. [Google Scholar]
- 32. Freitas ML, Bell RL, Duffy AJ. Choledocholithiasis: evolving standards for diagnosis and management. World J Gastroenterol. 2006;12:3162–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alponat A, Kum CK, Rajnakova A, et al. Predictive factors for synchronous common bile duct stones in patients with cholelithiasis. Surg Endosc. 1997;11:928–932. [DOI] [PubMed] [Google Scholar]
- 34. Chang CW, Chang WH, Lin CC, et al. Acute transient hepatocellular injury in cholelithiasis and cholecystitis without evidence of choledocholithiasis. World J Gastroenterol. 2009;15:3788–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nebiker CA, Baierlein SA, Beck S, et al. Is routine MR cholangiopancreatography (MRCP) justified prior to cholecystectomy? Langenbecks Arch Surg. 2009;394:1005–1010. [DOI] [PubMed] [Google Scholar]
- 36. Peng WK, Sheikh Z, Paterson-Brown S, et al. Role of liver function tests in predicting common bile duct stones in acute calculous cholecystitis. Br J Surg. 2005;92:1241–1247. [DOI] [PubMed] [Google Scholar]
- 37. Song SH, Kwon CI, Jin SM, et al. Clinical characteristics of acute cholecystitis with elevated liver enzymes not associated with choledocholithiasis. Eur J Gastroenterol Hepatol. 2014;26:452–457. [DOI] [PubMed] [Google Scholar]
- 38. Zhang JF, Du ZQ, Lu Q, et al. Risk factors associated with residual stones in common bile duct via T tube cholangiography after common bile duct exploration. Medicine (Baltimore). 2015;94:e1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gurusamy KS, Koti R, Davidson BR. T-tube drainage versus primary closure after laparoscopic common bile duct exploration. Cochrane Database Syst Rev. 2013;6:Cd005641. [DOI] [PubMed] [Google Scholar]
- 40. Williams JA, Treacy PJ, Sidey P, et al. Primary duct closure versus T-tube drainage following exploration of the common bile duct. Aust N Z J Surg. 1994;64:823–826. [DOI] [PubMed] [Google Scholar]
- 41. Bowie JD. What is the upper limit of normal for the common bile duct on ultrasound: how much do you want it to be. Am J Gastroenterol. 2000;95:897–900. [DOI] [PubMed] [Google Scholar]
- 42. Kialian G, Aznaurian A. The age-related characteristics of the muscular layer of the common bile duct in man. Morfologiia (Saint Petersburg, Russia). 1995;108:10–12. [PubMed] [Google Scholar]


