Abstract
Among sites of extrapulmonary growth of Mycobacterium tuberculosis, the liver is the least infected. Our data suggest that this is due to the complete restriction of mycobacterial growth to liver macrophages. Unlike in organs more persistently seeded by M. tuberculosis, in the liver the bacteria do not infect cell types other than professional phagocytes.
Mycobacterium tuberculosis is one of the most fatal infectious agents globally (38). More than 2 million people die from this infection annually, and the numbers are increasing. In addition, the percentage of extrapulmonary tuberculosis has risen during the last few years (7, 9, 31). Extrapulmonary or disseminated M. tuberculosis infection may enhance the risk of latent infection, thereby impairing successful chemotherapy or immune prophylaxis. It is therefore of vital importance to understand the circumstances of M. tuberculosis dissemination and persistence in more detail.
Liver tuberculosis is rarely described, and in patients with extrapulmonary tuberculosis, the liver has been shown to be the least infected internal organ (1, 9, 18). In order to investigate extrapulmonary tuberculosis, a modified protocol of the Cornell model (22, 23) was used to induce latent M. tuberculosis infection in mice.
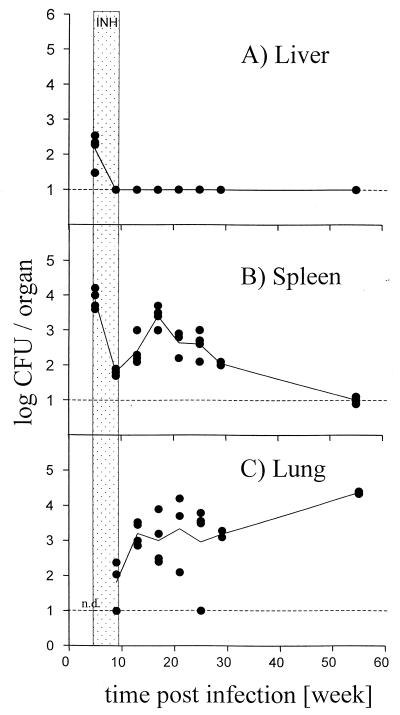
C57BL/6 mice (bred at the Bundesamt für gesundheitlichen Verbraucherschutz und Veterinärmedizin in Berlin under specific-pathogen-free conditions) were infected intravenously with 104 CFU of M. tuberculosis strain Erdman. Five weeks postinfection, mice were treated with isoniazide (0.5 g/liter) in the drinking water for 4 weeks, and bacterial titers were analyzed at the indicated time points (Fig. 1). Compared to spleen and lung, the liver carried the lowest bacterial burden, was cleared the fastest, and showed no recrudescence. These results are in line with observations in other experimental animal models for tuberculosis (6, 10, 12, 13, 19, 26, 27, 29, 35). Why the liver does not support mycobacterial persistence is even harder to understand if the size of the organ and the large number of cells is taken into account compared to lung and spleen.
FIG. 1.
M. tuberculosis titers in vivo. C57BL/6 mice were infected intravenously with 104 CFU of M. tuberculosis strain Erdman. Five weeks postinfection, mice were treated with isoniazide (INH; 0.5 g/liter) in the drinking water for 4 weeks. Log CFU were determined in liver (A), spleen (B), and lung (C) at the indicated time points. Shown are values for four individual mice per group (solid circles) and the means for four mice (line) per time point and group for all three organs. The detection limit of the assay is indicated by the broken line. Hatched area, time interval of isoniazide treatment. n.d., first time point CFU not determined for lung. Shown is one representative experiment of two similar ones.
M. tuberculosis preferentially infects and replicates inside macrophages as host cells (5, 8, 20, 21, 28, 32). However, in vitro and in vivo evidence is increasing that M. tuberculosis can infect cell types other than macrophages, such as epithelial cells/pneumocytes, endothelial cells, fibroblasts, and dendritic cells (2–4, 11, 14, 15, 24). These cells could represent a special habitat for M. tuberculosis to persist and evade immune surveillance. Infection of nonprofessional phagocytes has been especially reported for the lung, the primary organ of infection and persistence of M. tuberculosis as well as of disease manifestation (15).
In order to assess whether or not hepatocytes have the ability to be infected by mycobacteria, the immortalized liver cell line TIB-75 (ATCC TIB-75) was infected with recombinant Mycobacterium bovis BCG expressing green fluorescent protein from Aequorea victoria (rBCG-GFP [17]). As positive control, the macrophage cell line J774A.1 (ATCC T1B-67) was infected with rBCG-GFP. Localization of intact mycobacteria in vitro as well as in vivo is best analyzed using GFP recombinant bacteria. So far, GFP recombinant M. bovis BCG but not M. tuberculosis are available. Therefore, rBCG-GFP were used for localizing mycobacteria in the following experiments.
TIB-75 and J774A.1 cells were infected at a multiplicity of infection (MOI) of 10. After 1 h and 24 h of infection, cultures were fixed with 4% paraformaldehyde (PFA), and actin was stained with phalloidin-tetramethyl rhodamine isothiocyanate (TRITC) (Molecular Probes, Eugene, Oreg.). For 24-h cultures, medium was supplemented with 25 mg of gentamicin sulfate (Biochrom, Berlin, Germany) per liter and incubated overnight. Infected cell cultures were analyzed by confocal laser microscopy for intracellular localization of rBCG-GFP. Since confocal analysis is restricted to a narrow optical section, rBCG-GFP (green fluorescing) surrounded by actin (labeled by red-fluorescing phalloidin-TRITC) indicates intracellular localization of rBCG-GFP.
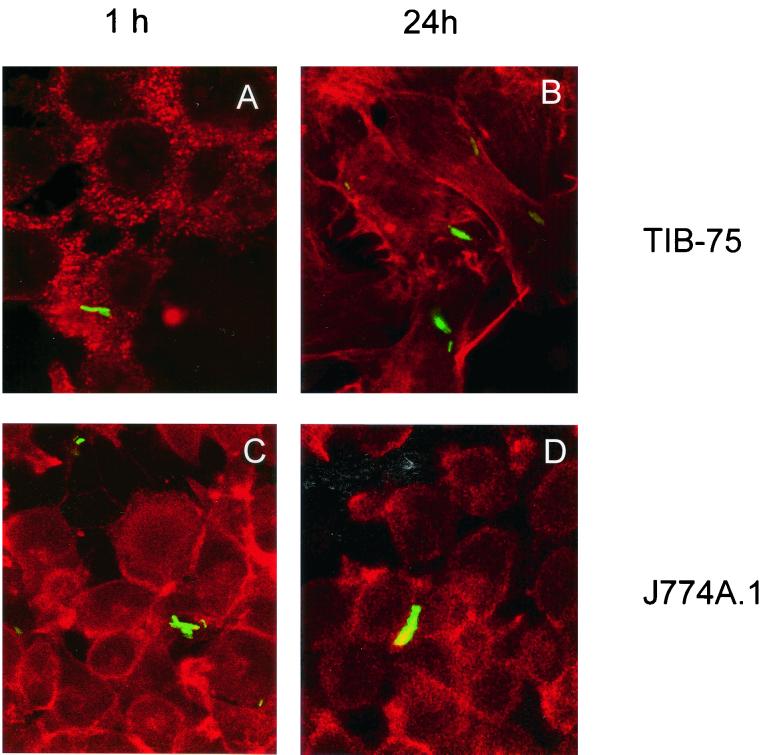
As shown in Fig. 2, TIB-75 liver cells were readily infected with rBCG-GFP after 1 h with an infection rate of 5 to 10% of the cells (Fig. 2A; projections of eight confocal scans are shown in order to increase signal intensity) and remained infected for at least 24 h (Fig. 2B). The extent of TIB-75 infection was comparable to the infection of J774A.1 macrophages (Fig. 2C and D). These experiments demonstrate that an immortalized hepatocyte cell line has the ability to be infected with mycobacteria in vitro.
FIG. 2.
Confocal analysis of liver cell infection in vitro. TIB-75 mouse hepatocyte and J774A.1 mouse macrophage cell lines were infected with rBCG-GFP at an MOI of 10 for 1 h, resulting in an infection rate of 5 to 10% of the cells and analyzed after 1 h (A and C) and 24 h (B and D), respectively. For 24 h of infection, cultures were supplemented after 1 h of incubation with 25 mg of gentamicin sulfate per liter. Prior to analysis, cells were fixed with 4% PFA and stained with phalloidin-TRITC in order to visualize actin. The rBCG-GFP were localized within the cells by confocal laser microscopy. Shown are projections of eight confocal laser scans. Original magnification, ×1,000. Shown is one representative experiment of two similar ones.
In order to assess whether hepatocytes have the ability to be infected with BCG in vivo, C57BL/6 mice were infected intravenously with 106 CFU of rBCG-GFP. At 15 min and 2 days postinfection, livers were fixed in 4% PFA overnight, incubated in 20% sucrose for 14 h, and snap frozen in liquid nitrogen. Antigens were unmasked by protease digestion for 2 min at 37°C (2 mg/ml in Tris-HCl buffer [Sigma, St. Louis, Mo.]) and stained using the following monoclonal antibodies (MAbs): F4/80 (ATCC HB-198; rat anti-mouse panmacrophage marker and therefore detecting all liver macrophages, Kupffer cells as well as invading monocytes), M3/84 (Pharmingen, San Diego, Calif.; rat anti-mouse Mac-3), MOMA-1 (Biomedicals, Augst, Switzerland; rat anti-mouse marginal metallophils), RB6-8C5 (rat anti-mouse Ly6G, detecting neutrophils [34]), and HL3 (Pharmingen, San Diego, Calif.; Armenian hamster anti-mouse CD11c, detecting dendritic cells).
Primary rat MAbs were detected using either an alkaline phosphatase-coupled (Fig. 3A and F) or an indocarbocyanine (Cy3.18)-coupled polyclonal (PAb) goat anti-rat antibody (Fig. 3B to E and G to J), respectively. HL3 was detected using a Cy3.18-coupled PAb goat anti-Armenian hamster antibody (all secondary antibodies from Dianova, Hamburg, Germany). Liver sections were analyzed by confocal laser microscopy for intracellular localization of rBCG-GFP. Again, due to the restriction to a narrow optical section by confocal analysis, detection of green-fluorescing rBCG-GFP surrounded by red-fluorescing F4/80-specific staining indicates intracellular localization of rBCG-GFP in liver macrophages.
FIG. 3.
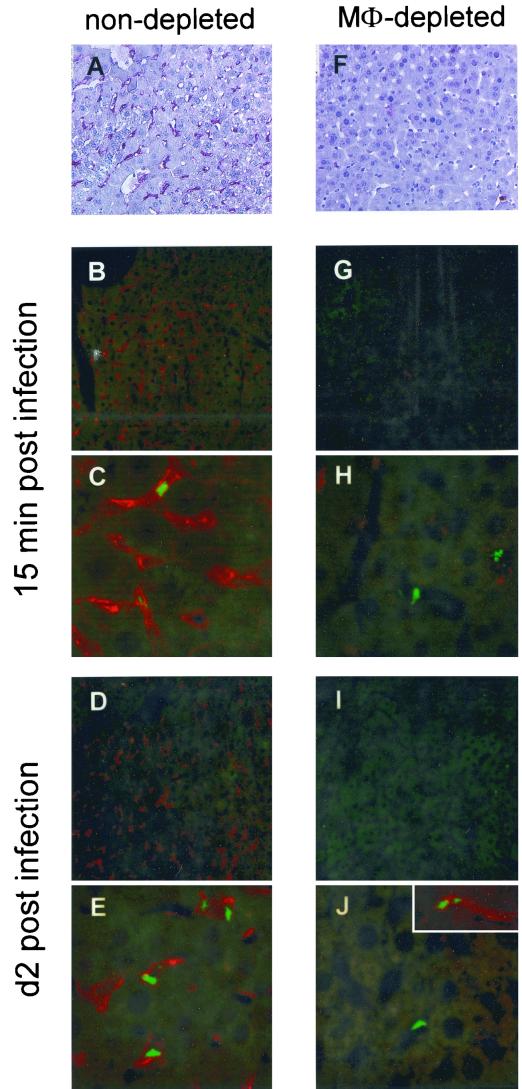
Confocal analysis of liver cell infection in vivo. C57BL/6 mice were either left untreated (A to E) or treated with 1.5 mg (F to J) of clodronate liposomes intraperitoneally at day −3. At day 0, mice were infected intravenously with 106 CFU of rBCG-GFP. At 15 min and 2 days postinfection, liver sections were stained with the rat MAb F4/80 and the secondary PAb goat anti-rat alkaline phosphatase (A and F) or PAb goat anti-rat Cy3.18 (B to E and G to J) in order to detect liver macrophages. Clusters of rBCG-GFP were detected inside the liver macrophages by confocal laser microscopy. Original magnification, ×200 (A, B, D, F, G, and I) and ×630 (C, E, H, and J). Shown is one representative experiment of two similar ones.
As summarized in Fig. 3, clusters of rBCG-GFP were detected only in liver macrophages (Fig. 3 B to E; staining for F4/80 in red). Similar results were obtained with the MAb MOMA-1 and M3/84 (data not shown). The rBCG-GFP had infected the liver macrophages already after 15 min (Fig. 3B and C) and had increased in numbers by day 2 (Fig. 3D and E) postinfection in liver macrophages only. No rBCG-GFP were detectable in dendritic cells or neutrophils (data not shown). In contrast to the in vitro results, no rBCG-GFP were localized in hepatocytes visible by their weak green autofluorescence (Fig. 3B to E).
Possibly, liver parenchymal cells do not have the opportunity to phagocytose rBCG-GFP in vivo due to (i) the competition with liver macrophages, which pick up bacteria immediately and control infection, or due to (ii) the special microanatomy of the liver. The liver sinusoid is lined by endothelial cells, allowing the exchange of small molecules between the sinusoid and the liver parenchymal cells. The sinusoidal fenestrae formed by the endothelial cells are about 100 nm in diameter (37), whereas mycobacteria are 300 nm wide and 3,000 nm long. Therefore, access of mycobacteria to liver parenchymal cells may be physically prevented.
In order to distinguish between (i) competition between parenchymal cells and macrophages for bacterial uptake and (ii) physical prevention of bacterial access to parenchymal cells, C57BL/6 mice were depleted of liver macrophages prior to infection using clodronate liposomes (30). Mice received 1.5 mg of clodronate liposomes intraperitoneally at day −3 and were infected with 106 CFU of rBCG-GFP intravenously at day 0. Degree of depletion was confirmed at day 0 by immune histological staining for F4/80+ liver macrophages (Fig. 3F compared to A). Liver sections were analyzed for localization of rBCG-GFP 15 min and 2 days postinfection, the time point when macrophages started to repopulate the liver.
Compared to nondepleted control liver, in livers void of macrophages a factor of 5- to 10-fold fewer rBCG-GFP organisms were detected in one field of view (Fig. 3G to J). This was confirmed by CFU determination (data not shown). Only a few extracellularly located rBCG-GFP were visible in the sinusoids. Importantly, again no rBCG-GFP were detected inside liver parenchymal cells. As soon as a few macrophages had repopulated the liver by day 2, rBCG-GFP organisms were found in these rare F4/80+ liver macrophages (Fig. 3J, inset). These results indicate that, even in the absence of macrophages, a situation without competition for BCG uptake, liver parenchymal cells do not have the opportunity to pick up BCG in vivo. Most probably, mycobacterial access was prevented by the liver sinusoid. In comparison, mycobacterial access to the spleen must be readily available to any cell due to the open circulation of the spleen, where the marginal sinus does not restrict access of bacteria (16, 33, 36). Also, access to nonprofessional phagocytes in the lung after aerosol infection with mycobacteria is not restricted by a structure comparable to the liver sinusoid. Hence, we considered the liver sinusoid a major factor for limited mycobacterial infection of the liver.
In summary, using confocal laser microscopy, we were able to examine mycobacterial infection of liver cells in vitro and in vivo. In vitro, the immortalized hepatocyte cell line TIB-75 was infected with rBCG-GFP. In contrast, in vivo, liver parenchymal cells remained uninfected, and rBCG-GFP was exclusively detected in liver macrophages even under circumstances where liver macrophages had been depleted. Possibly, the immortalized cell line TIB-75 expresses an additional surface marker which facilitates the entry of mycobacteria compared to hepatocytes in vivo. More likely, the discrepancy between the in vitro and in vivo findings may be explained by the highly organized liver microanatomy. Sinusoidal endothelial cells shield parenchymal cells and may prevent initial contact between mycobacteria and liver parenchymal cells in vivo. Given that mycobacteria cannot cross the sinusoidal endothelial layer, only the liver macrophages, mainly the Kupffer cells, which are located inside the sinusoid, and possibly immigrating blood monocytes can be reached and infected by the mycobacteria.
In a situation of strong immunosuppression in hydrocortisone-treated SCID mice, infection of hepatocytes has been reported (25). It will be interesting to clarify how the liver sinusoid was affected in this situation.
Our data may explain why the liver is an unfavorable site for M. tuberculosis to disseminate or to establish latent infection. Because, in the liver, only professional phagocytes are infected, such restriction may facilitate antimycobacterial host defense. In contrast, in other organs, in particular in the lung, M. tuberculosis can infect host cells other than macrophages such as epithelial cells, pneumocytes, and fibroblasts (2–4, 14, 15, 24). These cells are not endowed with the capacity to kill and process mycobacteria and may facilitate mycobacterial persistence.
Acknowledgments
We thank Jana Zinke, Tania Torossi, and Beatrix Fauler for excellent technical assistance and L. Kremer and C. Locht for providing the rBCG-GFP. Many thanks go to H. Lobeck for helpful discussion.
P.S. is supported by the Schweizerische Stiftung für medizinisch-biologische Stipendien and by the Alexander von Humboldt-Stiftung. S.H.E.K. acknowledges financial support from BMBF Verbundprojekt “Mykobakterielle Infektionen.”
REFERENCES
- 1.Abdel-Dayem H M, Naddaf S, Aziz M, Mina B, Turoglu T, Akisik M F, Omar W S, DiFabrizio L, LaBombardi V, Kempf J S. Sites of tuberculous involvement in patients with AIDS. Autopsy findings and evaluation of gallium imaging. Clin Nucl Med. 1997;22:310–314. doi: 10.1097/00003072-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley L W. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. Science. 1993;261:1454–1457. doi: 10.1126/science.8367727. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar K A, Serbina N V, Flynn J L. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69:800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrel A. Tuberculose pulmonaire experimentale. Ann Inst Pasteur (Paris) 1893;7:593–627. [Google Scholar]
- 6.Bosio C M, Gardner D, Elkins K L. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000;164:6417–6425. doi: 10.4049/jimmunol.164.12.6417. [DOI] [PubMed] [Google Scholar]
- 7.Clark R A, Blakley S L, Greer D, Smith M H, Brandon W, Wisniewski T L. Hematogenous dissemination of Mycobacterium tuberculosis in patients with AIDS. Rev Infect Dis. 1991;13:1089–1092. doi: 10.1093/clinids/13.6.1089. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham R S, Sabin F R, Sugiyama S, Kindwall J A. The role of the monocyte in tuberculosis. Bull Johns Hopkins Hosp. 1925;37:231–283. [Google Scholar]
- 9.Farer L S, Lowell A M, Meador M P. Extrapulmonary tuberculosis in the United States. Am J Epidemiol. 1979;109:205–217. doi: 10.1093/oxfordjournals.aje.a112675. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 11.Filley E A, Bull H A, Dowd P M, Rook G A. The effect of Mycobacterium tuberculosis on the susceptibility of human cells to the stimulatory and toxic effects of tumour necrosis factor. Immunology. 1992;77:505–509. [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn J L, Scanga C A, Tanaka K E, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 13.Gale D, Lockhart E A, Reynolds D. Studies of unclassified mycobacteria. II. Survival and dissemination of typical and unclassified mycobacteria in the organs of mice inoculated intraperitoneally or intravenously. Health Lab Sci. 1966;3:48–64. [PubMed] [Google Scholar]
- 14.Gonzalez-Juarrero M, Orme I M. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun. 2001;69:1127–1133. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, Harboe M, Rook G A, Bjune G. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–2138. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- 16.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 17.Kremer L, Baulard A, Estaquier J, Poulain-Godefroy O, Locht C. Green fluorescent protein as a new expression marker in mycobacteria. Mol Microbiol. 1995;17:913–922. doi: 10.1111/j.1365-2958.1995.mmi_17050913.x. [DOI] [PubMed] [Google Scholar]
- 18.Le Bihan G, Nouvet G, David P, Moynot Y, Le Loet C, Morere P. Tuberculosis or hepatic granulomatosis? Poumon Coeur. 1980;36:309–312. [PubMed] [Google Scholar]
- 19.Lurie M B. The fate of human and bovine tubercle bacilli in various organs of the rabbit. J Exp Med. 1928;48:155–182. doi: 10.1084/jem.48.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lurie M B. The correlation between the histological changes and the fate of living tubercle bacilli in the organs of tuberculous rabbits. J Exp Med. 1931;55:31–61. doi: 10.1084/jem.55.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maximow A. Étude comparative des cultures de tissus inoculées soit avec le bacille tuberculeux du type bovin soit avec le bacille BCG de Calmette-Guerin. Ann Inst Pasteur (Paris) 1928;42:225–245. [Google Scholar]
- 22.McCune R M, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1957;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCune R M, Tompsett R, McDermott W. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1957;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta P K, King C H, White E H, Murtagh J J, Quinn F D. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect Immun. 1996;64:2673–2679. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills J W, Ryan L, LaCourse R, North R J. Extensive Mycobacterium bovis BCG infection of liver parenchymal cells in immunocompromised mice. Infect Immun. 2001;69:3175–3180. doi: 10.1128/IAI.69.5.3175-3180.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafa T, Phyu S, Nilsen R, Jonsson R, Bjune G. A mouse model for slowly progressive primary tuberculosis. Scand J Immunol. 1999;50:127–136. doi: 10.1046/j.1365-3083.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 27.Phyu S, Mustafa T, Hofstad T, Nilsen R, Fosse R, Bjune G. A mouse model for latent tuberculosis. Scand J Infect Dis. 1998;30:59–68. doi: 10.1080/003655498750002321. [DOI] [PubMed] [Google Scholar]
- 28.Sabin F R, Doan C-A. The relation of monocytes and clasmatocytes to early infection in rabbits with bovine tubercle bacilli. J Exp Med. 1927;96:627–645. doi: 10.1084/jem.46.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scanga C A, Mohan V P, Yu K, Joseph H, Tanaka K, Chan J, Flynn J L. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seiler P, Aichele P, Odermatt B, Hengartner H, Zinkernagel R M, Schwendener R A. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–2633. doi: 10.1002/eji.1830271023. [DOI] [PubMed] [Google Scholar]
- 31.Small P M, Schecter G F, Goodman P C, Sande M A, Chaisson R E, Hopewell P C. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1991;324:289–294. doi: 10.1056/NEJM199101313240503. [DOI] [PubMed] [Google Scholar]
- 32.Suter E. The multiplication of tubercle bacilli within normal phagocytes in tissue culture. J Exp Med. 1952;96:137–155. doi: 10.1084/jem.96.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka H, Hataba Y, Saito S, Fukushima O, Miyasaka M. Phenotypic characteristics and significance of reticular meshwork surrounding splenic white pulp of mice. J Electron Microsc (Tokyo) 1996;45:407–416. doi: 10.1093/oxfordjournals.jmicro.a023459. [DOI] [PubMed] [Google Scholar]
- 34.Tepper R I, Coffman R L, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 35.van Pinxteren L A, Cassidy J P, Smedegaard B H, Agger E M, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30:3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Weiss L. Mechanisms of splenic clearance of the blood; a structural overview of the mammalian spleen. In: Bowdler A J, editor. The spleen: structure, function and clinical significance. London, England: Chapman and Hall; 1990. pp. 25–35. [Google Scholar]
- 37.Wisse E, De Zanger R B, Charels K, van der Smissen P, McCuskey R S. The liver sieve: considerations concerning the structure and function of the endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;4:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. The world health report 2000. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]