Abstract
Primary biliary cholangitis (PBC) is a chronic, cholestatic, autoimmune liver disease that can progress to end-stage liver disease and its complications. A previous expert review panel collaborated on a consensus document for gastroenterologists and other healthcare professionals regarding the care of patients with PBC. Subsequently, there have been several recent important developments in the diagnosis, treatment, and monitoring of patients with PBC. These include updates to prognostic models on risk stratification, new noninvasive tools for staging of disease, updates to the appropriate use of and long-term treatment results with obeticholic acid as a second-line treatment, the emerging therapeutic role of fibrates, and the advancement of investigational agents for managing PBC. In this updated expert consensus document, we provide updates on staging, the use of noninvasive prognostic tools, and a treatment algorithm to provide evidence-based and practical tools for clinicians who manage PBC, with the ultimate goal to improve the long-term outcomes for patients with this chronic liver disease.
INTRODUCTION
Case scenario
A 62-year-old woman with a history of hypertension was seen for complaints of mild fatigue and pruritus for several months. The physical examination was unremarkable. Laboratory tests revealed alanine transaminase (ALT) = 44 U/L, aspartate aminotransferase (AST) = 45 U/L, alkaline phosphatase (ALP) = 650 U/L, total bilirubin = 0.8 mg/dL, serum albumin = 4 g/dL, and international normalized ratio = 1.0. Her symptoms and laboratory test results indicated primary biliary cholangitis (PBC) as a possibility.
PBC is a chronic, inflammatory, progressive autoimmune disease that, without treatment, can lead to cirrhosis and liver failure, resulting in liver-related death. A recent study sought to identify temporal trends in patients with PBC and disease characteristics over a 44-year period across a large international cohort of 4,805 patients with PBC. Patients were divided into 5 cohorts according to the year of diagnosis (1970–1979 [n = 143], 1980–1989 [n = 858], 1990–1999 [n = 1,754], 2000–2009 [n = 1,815], and 2010 and later [n = 235]). In recent decades, significantly more patients have presented with mild disease, according to both biochemical and histological definitions. In addition, decompensation rates significantly decreased, and 10-year transplant-free survival rates significantly increased over the 4 investigated decades. The authors postulated that these trends may, in part, be attributed to the increased routine use of serum liver biochemical tests (1). It would be expected that the availability of effective therapies may further accelerate these positive trends.
A previous review panel developed an expert consensus document for clinical gastroenterologists and other healthcare professionals who manage patients with PBC (2). Since the initial document was established, several important developments in PBC have warranted updated guidance. The previous publication included an exhaustive review of the PBC literature from 1985 to 2018. For the development of the current consensus document, a literature search was performed beginning in 2019 to the present. New data on prognostic models of risk stratification, noninvasive tools for staging the disease, the appropriate use of long-term treatment results with obeticholic acid (OCA) as a second-line treatment, and the advancement of investigational agents for managing PBC were identified. Furthermore, in 2021, the American Association for the Study of Liver Diseases (AASLD) published updated guidance recommendations (3). As a result of these advancements, included within this review are relevant data that have emerged since previous publication, recommendations from an expert panel on how to implement these findings into clinical practice, and an updated algorithm for the diagnosis and treatment of PBC. Recent guidance documents from the AASLD, Asian Pacific Association for the Study of the Liver, and European Association for the Study of the Liver also provide recommendations regarding the diagnosis and treatment of PBC (4–6).
OVERVIEW OF PBC: AN UPDATE
Although PBC is considered a rare disease, its prevalence is on the rise, and data indicate that it has become more common worldwide (7). In a recent analysis of data from 3,488 patients receiving routine clinical care in health systems participating in the Fibrotic Liver Disease Consortium, investigators found that from 2004 to 2014, the prevalence of PBC increased from 21.7 to 39.2 per 100,000 persons, while the incidence remained stable (8). A separate US study found that patients with PBC were more likely to be women (42.8 per 100,000), White (29.6 per 100,000), and between age 60 and 70 years (44.7 per 100,000) (9). PBC is characteristically associated with the presence of disease-specific antimitochondrial antibodies (AMA) (2,10). Recent studies on liver biopsy specimens from patients with PBC have demonstrated loss of the canals of Hering to be the earliest histologic change associated with PBC, with characteristic diagnostic findings becoming evident after this histologic finding (11,12). More data are needed on the clinical utility of this hypothesis.
These pathophysiologic changes result in clinical presentations that range from asymptomatic and slowly progressive to rapidly evolving fibrosis and cirrhosis typically after 10–20 years, although rates vary across studies. Progressive liver damage and other related symptoms and chronic conditions associated with PBC all require early surveillance and long-term management. A large proportion of recent publications on PBC have focused on predictors of disease progression, management strategies to slow disease progression, and options for managing symptoms.
DIAGNOSIS AND BASELINE STAGING
Diagnosis
We propose the following criteria for the diagnosis of PBC:
Scenario 1: Chronic elevation of ALP with a positive AMA (immunofluorescence assay titer of > 1:40 or enzyme immunoassay >25 units) in the absence of other liver and systemic diseases.
Scenario 2: Chronic elevation of ALP with negative AMA but positive PBC-specific antinuclear antibody (ANA) (sp-100, gp-210) tests or a reticular pattern of ANA.
Scenario 3: Chronic elevation of ALP with negative AMA and ANA tests, but a liver biopsy showing nonsuppurative cholangitis and destruction of the interlobular bile ducts.
It is known that patients with normal ALP may occasionally have a positive AMA. Several factors including test specificity, increased use of the AMA test in patients with elevated aminotransferases, or even in those with normal liver chemistries and method of testing may play a role, and the likelihood of a patient with positive AMA but normal ALP developing PBC in the future is unclear. Two recent studies shed some additional insights on this clinical scenario. In one study from the Shanghai region in China, liver biopsy performed in those individuals with positive AMA and normal ALP levels revealed 40% with marked cholangitis activity and most with some degree of fibrosis (75% had stage 2 fibrosis or more) (13). In another study from the Swiss PBC cohort consisting of 30 individuals with AMA positive and normal ALP levels, histologic features of PBC were observed in 3 of the 4 individuals (14). Although these studies provide possible interesting insights into pathophysiology, we do not recommend further evaluation with an invasive procedure such as liver biopsy in a patient with a positive AMA test with a persistently normal ALP level. Given the growing use and clinical utility of elastography in hepatology practice, liver biopsy may be considered in the subset of patients who have an otherwise unexplained increase in liver stiffness.
Staging
As recommended by the AASLD and the original American College of Gastroenterology/Chronic Liver Disease Foundation panel, a baseline assessment of PBC should include chronic elevations in liver enzymes and/or bilirubin, the presence of PBC-specific symptoms, and a physical examination that indicates hepatomegaly, splenomegaly, and/or extrahepatic signs of advanced liver disease (2,5). There is a general consensus on the need to incorporate fibrosis staging into individual risk stratification in patients with PBC soon after diagnosis (15), and historically, this has been performed through liver biopsy (16,17). In 2019, Murillo Perez et al. (15) analyzed baseline liver biopsies taken from 1,828 patients across the globe between 1980 and 2014. Advanced histologic fibrosis (stage 3 or 4) was found to be an independent predictor of lower transplant-free survival, regardless of the biochemical treatment response, with a 10-year transplant-free survival of 76.0%–86.6% compared with 94.5%–95.1% (15) in those without advanced fibrosis. However, the disadvantages of biopsy (including sampling bias, poor patient acceptance, and severe complications such as mortality, bleeding, pain, and cost [18]) continue to limit its use.
In 2019, the Global PBC Study Group analyzed 1,615 patients (mean age 55.4 years) with early-stage PBC (based on normal levels of albumin and bilirubin). The proportion of patients who transitioned to moderate PBC at 1, 3, and 5 years was 12.9%, 30.2%, and 45.8%, respectively. Because approximately half of the patients with early-stage PBC progressed to a more severe stage within 5 years, the authors concluded that monitoring for progression of fibrosis is important even in early-stage PBC (19).
More recently, noninvasive tests (NIT) based on serum biomarkers, such as AST to Platelet Ratio Index, Fibrosis-4 Index, enhanced liver fibrosis (ELF), and pro-C3, have been developed and extensively studied for staging nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (20). One study found that each 1-point increase in ELF was associated with a 3-fold increase in future complications (21). Another study from Japan reported that an ELF score >10.0 was predictive of clinical outcomes and hepatic decompensation in patients with PBC. However, additional studies are needed to endorse its use as a sole NIT in PBC (22). Blood-based NIT have not gained wide acceptance by physicians or regulatory agencies regarding staging liver disease (20).
Imaging-based NIT for PBC include transient elastography (TE), shear wave elastography, and magnetic resonance elastography (MRE). Vibration-controlled TE for liver stiffness measurement has been reported to be clinically useful for identifying patients at risk of decompensation (23–28). We agree with the recommendations that both MRE and TE are useful for determining the degree of fibrosis in patients with PBC and that these NIT should be used in early-stage disease. In 2021, the European Association for the Study of the Liver published an update to the Clinical Practice Guidelines on NIT for the evaluation of liver disease severity and prognosis. For PBC, these guidelines endorse the importance of fibrosis staging for prognosis, independent of biochemical response to therapy. The guidelines state that in patients with PBC, serum biomarkers are not recommended for fibrosis staging in clinical practice. For staging purposes, liver stiffness measurement by TE, using a cutoff of 10 kPa, was proposed as a criterion for ruling in severe fibrosis/compensated advanced chronic liver disease (29). Previously published liver stiffness values that correlate to histologic stage are 7.1, 8.8, 10.7, and 16.9 kPa for F1, F2, F3, and F4, respectively (25). Both TE and MRE outperformed the biochemical markers for the prediction of advanced fibrosis, with an optimal threshold to predict hepatic decompensation of 10.2 kPa on TE and 4.30 kPa on MRE (23).
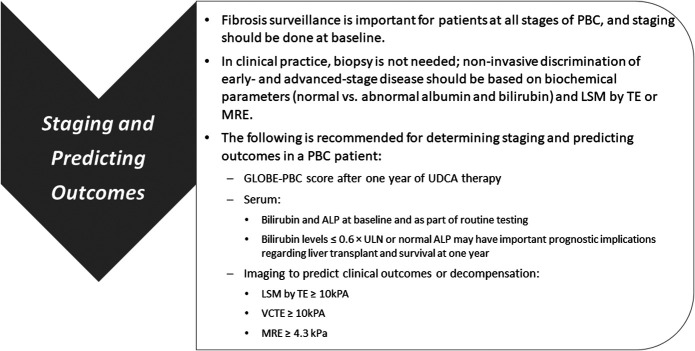
In summary, we recommend that TE or MRE be used for staging PBC at the baseline (Figure 1). We recommend that a TE of ≥10 kPa and an MRE ≥4.3 kPa would be acceptable in identifying patients with PBC with advanced fibrosis and at an increased risk of hepatic decompensation in the future.
Figure 1.
Recommendations for staging and predicting outcomes in PBC. ALP, alkaline phosphatase; kPa, kilopascal; LSM, liver stiffness measurement; MRE, magnetic resonance imaging; PBC, primary biliary cholangitis; TE, transient elastography; ULN, upper limit of normal; VCTE, vibration-controlled transient elastography.
MONITORING
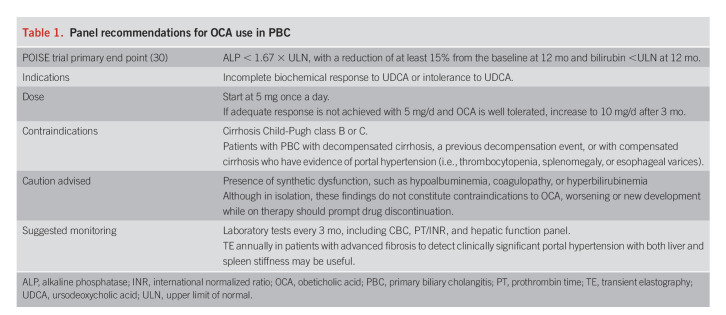
Several prognostic models that incorporate clinical and biochemical variables to predict clinical outcomes have been developed for use in PBC (2,30,31). Approval of OCA as a therapy for PBC was based on the combination of serum ALP and bilirubin levels as the primary end point in the Phase 3 Study of Obeticholic Acid in Patients With Primary Biliary Cirrhosis (POISE) trial (Table 1) (32).
Table 1.
Panel recommendations for OCA use in PBC
Earlier studies have shown that ALP >1.67 × upper limit of normal (ULN) or 2 × ULN and bilirubin >ULN were associated with adverse liver-related outcomes (32,33). A recent analysis from the Global PBC Study Group found that the 10-year survival rate of patients with bilirubin in the upper half of normal range (>0.6 × ULN) was reduced compared with patients with bilirubin in the low normal (<0.6 × ULN) range (79% vs 91%, P < 0.001). The same study also found that survival was better in patients with normal ALP compared with those with ALP above ULN but <1.67 × ULN. (93% vs 86%) (34).
As summarized in Figure 1, the GLOBE PBC score or the UK-PBC score can be calculated using online calculators (Available at https://www.globalpbc.com/globe and http://www.uk-pbc.com/resources/tools/riskcalculator/) after 1 year of ursodeoxycholic acid (UDCA) therapy (2,35). Both have similar performance characteristics and are superior to the UDCA response criteria in predicting complications of cirrhosis among patients with PBC (36). New data have augmented this recommendation, with bilirubin levels >0.6 × ULN and any elevation of ALP above ULN as important indicators of prognosis. These assessments also have important implications in monitoring PBC treatment responses, which will be discussed in a later section that follows.
Continued case scenario
Our 62-year-old female patient had further laboratory tests that showed gamma-glutamyl transferase (GGT) = 320 U/L, AMA = 1:640, and serum IgM = 500. She was diagnosed with PBC. A staging evaluation was performed, and her TE was 10.5 kPa, consistent with stage 3 fibrosis. Based on this score, she was classified as having advanced fibrosis.
JOURNEY OF THE PATIENT WITH PBC: THE CONTINUED NEED FOR MONITORING AND MANAGEMENT
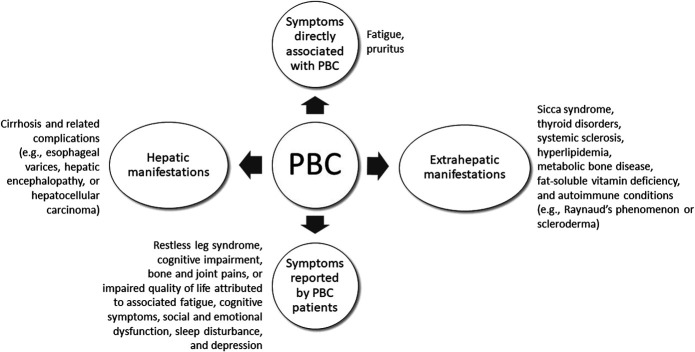
Symptoms of PBC may extend beyond those of liver disease alone, including extrahepatic complications of the disease (Figure 2) (2). Lifelong management of patients with PBC is necessary and should be personalized to the patient's disease state and symptoms. Medical therapy to prevent disease progression and complications of cirrhosis, as well as to manage the associated symptoms of the disease, is recommended (2,5,6,29).
Figure 2.
Symptoms and manifestations of PBC. Fatigue and pruritus, common complaints associated with PBC, are considered symptoms directly associated with the disease. The 3 most common extrahepatic conditions associated with PBC are sicca syndrome, thyroid disorders, and systemic sclerosis. PBC is also associated with a clustering of other autoimmune conditions (e.g., Raynaud phenomenon or scleroderma); however, a recent population-based study found that concomitant autoimmune conditions did not influence outcomes in patients with PBC (66). Other symptoms that are poorly characterized but reported by patients with PBC include restless leg syndrome, cognitive impairment, and bone and joint pain, the severity of which may not be proportionate to the severity of the underlying liver disease. Furthermore, chronic cholestasis associated with PBC may be associated with extrahepatic manifestations, such as hyperlipidemia, metabolic bone disease, and fat-soluble vitamin deficiencies. Patients with PBC can also experience impaired quality of life attributed to associated fatigue, cognitive symptoms, social and emotional dysfunction, sleep disturbances, and depression (67,68). PBC, primary biliary cholangitis.
The rate of progression of PBC from early-stage disease to cirrhosis to liver-related morbidity/mortality can vary among patients; however, progression is typically slow, with no visible symptoms. Studies of patients with PBC with cirrhosis indicate that 10 years after the initial diagnosis, progression to decompensated liver disease (ascites, bleeding, hepatic encephalopathy, jaundice, or liver failure) occurs at rates of 10%–26% (37). Treatment can slow progression and reduce the need for liver transplantation. A large international meta-analysis that included 4,845 patients with PBC demonstrated that UDCA significantly improved transplant-free survival at 5, 10, and 15 years compared with nontreated individuals (90%, 78%, and 66% vs 79%, 59%, and 32%, respectively) (33).
A recent population-based study using a claims database found that the development of portal hypertension in patients with PBC is an important predictor of liver-related adverse outcomes and survival. Titievsky et al. (38) recently presented data from 3,940 patients with PBC to evaluate the impact of cirrhosis and portal hypertension on the incidence of decompensating events, liver failure, liver transplant, and death. Of these patients, 3,303 were noncirrhotic and 547 had cirrhosis, of whom 260 had portal hypertension, while 255 did not (data were incomplete or missing in 32). The main outcomes of interest were all-cause mortality, liver failure, liver transplant, ascites, variceal bleeding, and hepatic encephalopathy. As compared to patients with PBC without cirrhosis, incidence rates per 100 patient years of all-cause mortality were higher in patients with PBC with cirrhosis: 8.3 vs 2.8. For hepatic outcomes, incidence rates (per 100 patient years) were greater among patients with PBC and cirrhosis as compared to patients with PBC without cirrhosis (liver transplant: 3.1 vs 0.3; liver failure: 34.6 vs 4.7; and decompensating events: 12.0 vs 3.7). However, the differences between patients with PBC with and without cirrhosis were primarily related to the presence or absence of portal hypertension; patients with compensated cirrhosis and no portal hypertension had risks similar to those of the noncirrhotic patients. By contrast, patients with PBC with compensated cirrhosis and portal hypertension had an incrementally higher risk of all-cause mortality, liver failure, liver transplant, ascites, variceal bleeding, and hepatic encephalopathy compared with patients with PBC without cirrhosis or with compensated cirrhosis without portal hypertension. These data suggest that it is the presence of portal hypertension, and not merely cirrhosis, that is associated with higher rates of liver-related adverse outcomes and increased mortality in patients with PBC (38).
CLINICAL IMPLICATIONS OF NEW DATA ON THE MANAGEMENT OF PBC
Since the previous document was published, new data have emerged on approved therapies and treatment strategies with approved therapies that may improve disease management. This section will review these new data, analyze their clinical implications, and conclude with a practical treatment algorithm that incorporates these advances.
Definition of an inadequate biochemical response to UDCA
Response to UDCA has historically been assessed after 12 months of treatment according to various binary response criteria and quantitative prognostic scores. Lack of biochemical response is reported in 25%–50% of treated patients (39) and has been associated with a >5-fold increase in risk of progression to cirrhosis and a 3-fold increase in age-adjusted mortality (40). At this time, clinicians are encouraged to use 2 liver biochemistries as the anchor for clinical judgment when determining candidates for second-line therapy in PBC: ALP and bilirubin levels (5,41,42). If elevated above the ULN, bilirubin is more important than ALP for prognosis and in identifying advanced stage of PBC in the absence of Gilbert syndrome or another explanation. However, most patients will have elevated ALP levels rather than elevated bilirubin levels. For example, in a patient with a normal ALP, advanced fibrosis stage, and bilirubin >ULN, adding a second-line therapy can be considered. Additional factors to consider include the patient's age and acceptance of additional medications.
When evaluated after 12 months of treatment with UDCA, serum ALP and bilirubin levels correlate closely with the risk of liver transplant or death. Current guideline documents do not recommend a specific cutoff value for serum ALP or bilirubin beyond which a second drug should be initiated, although it is important to remember that long-term survival of patients with PBC whose serum ALP is <1.5 × ULN and bilirubin is normal is similar to that of the general population (41). However, more recent data suggest that there is additional survival benefit in obtaining normalization of serum ALP and achieving a bilirubin level ≤0.6 md/dL (34).
Of note, the response to UDCA has been characteristically assessed at 12 months (43); however, there is growing evidence that the response can be reliably predicted after a shorter period of UDCA treatment. A recent study presented at the 2021 AASLD annual meeting, which included 3,516 UDCA-treated patients with PBC, was performed by the Global PBC Study Group to assess the pattern of biochemical response to treatment. POISE criteria were used to assess the response to treatment (Table 1). Of those with an inadequate response at 1 year (n = 313, 42%), 210 (67%) would already be identified at 6 months and 103 (33%) after 1 year. In their conclusion, the authors propose an ALP cutoff value of 1.9 × ULN at 6 months as a threshold for adding a second-line therapy (44). By using this cutoff at 6 months, a 90% negative predictive value is achieved, indicating only a 10% risk of over-treating patients who may not need a second-line therapy. Therefore, clinicians might consider starting second-line therapies for patients with an inadequate biochemical response after 6 months, rather than 12 months, of UDCA. (For specific recommendations, see the section that follows, Up-to-date PBC algorithm).
Case Scenario Continued
The patient was started on cholestyramine with relief of her itching. Because of her fibrosis score, she was considered high risk for disease progression. Initially, she was started on UDCA at a dose of 13 mg/kg and after 1 month was tolerating it well.
Updates on the use of OCA in PBC
The only currently approved second-line therapy for PBC is OCA. OCA, a farnesoid X receptor agonist, received accelerated approval in 2016 in combination with UDCA for adults with PBC and an inadequate biochemical response to UDCA alone or as monotherapy for those with intolerance to UDCA (45). The double-blind, placebo-controlled phase 3 POISE trial demonstrated 12-month efficacy and safety of OCA in patients with PBC who had an inadequate response or were intolerant to UDCA (32), leading to the approval of OCA for PBC (46). After this study's completion, patients were offered the opportunity to enter a 5-year open-label extension study. After the 1-year double-blind phase, the patients on placebo started OCA and were then pooled with OCA-treated patients to evaluate the efficacy and safety for up to 6 years. Bowlus et al. (47) collected liver biopsies from 17 patients at the time of enrollment in POISE and after 3 years of OCA treatment. In this substudy, it was observed that long-term OCA treatment in patients with PBC was associated with the improvement or stabilization of disease features, including ductular injury, fibrosis, and collagen morphometry features.
After 5 years, the percentage of patients who met the primary end point definition of POISE (Table 1) was 46% at month 12 and 50% at months 48, 60, and 72 (32). Significant and durable reductions were observed for ALP, ALT, AST, and GGT throughout the study. The mean bilirubin level remained stable throughout 72 months of OCA treatment. The OCA treatment resulted in sustained improvement in liver biochemistry for up to 6 years of follow-up (48). The most recent analysis used propensity scores to compare patients with PBC treated with OCA in the open-label extension (n = 209) for safety to external controls from 2 large real-world databases (Global PBC, n = 1,391 and UK-PBC, n = 2,138). In univariate, multivariable, and weighted Cox regression analyses, the OCA arm had a reduced risk of liver transplantation and death compared with either external control groups. In the weighted analysis, the hazard ratio for events while on OCA was 0.20 (0.06–0.64, P = 0.001) compared with Global PBC and 0.28 (0.09–0.90, P = 0.033) compared with UK-PBC. When compared to these real-world data sets, treatment with OCA in a trial setting is associated with better transplant-free survival in patients with PBC (49). Further results of this analysis are pending publication.
Despite this growing evidence of effectiveness and safety over the 5 years postapproval, several patients treated with OCA—most with cirrhosis and advanced liver disease—experienced severe liver-related adverse outcomes, including death (50). This led to a label change in 2021 for OCA to limit use in patients with cirrhosis to only those with compensated cirrhosis and no portal hypertension. A study conducted by Tivietsky et al. emphasized the importance of portal hypertension as a negative prognostic indicator in PBC (38). OCA is now contraindicated in patients with PBC with decompensated cirrhosis, a previous decompensation event, or with compensated cirrhosis who have evidence of portal hypertension, such as gastroesophageal varices and persistent thrombocytopenia, based on the recent Food and Drug Administration (FDA) guidance. Furthermore, OCA should be permanently discontinued in patients who develop laboratory or clinical evidence of hepatic decompensation, have compensated cirrhosis and develop evidence of portal hypertension, or experience clinically significant hepatic adverse reactions while on treatment (45). The contraindications listed in the OCA label in the United States are currently not part of the OCA label in Europe (51).
The AASLD has also issued a revised guidance statement advising against the use of OCA in patients with advanced cirrhosis, defined as cirrhosis with current or previous evidence of liver decompensation (e.g., encephalopathy or coagulopathy) or portal hypertension (e.g., ascites, gastroesophageal varices, or persistent thrombocytopenia) (3). We agree with the advice to not use OCA in patients with advanced cirrhosis and that physicians should continue to consider second-line therapies in patients with cirrhosis if their liver function is normal and there are no signs of portal hypertension. Given that patients with PBC and cirrhosis seem to derive benefits from OCA—achieving similar reductions in serum ALP from the baseline when compared to their noncirrhotic counterparts (52,53)—efforts should be directed at identifying patients with cirrhosis and PBC who are not at high risk for decompensation. We believe that patients with PBC with an inadequate response to or intolerance of UDCA should be considered for second-line therapy with OCA. OCA therapy should not be used in patients with thrombocytopenia (i.e., in those with a platelet count < 120 × 109/L, ascites, esophageal varices, or hepatic encephalopathy), complications of cirrhosis (i.e., spontaneous bacterial peritonitis, hepatic encephalopathy, or variceal bleeding), or evidence of hepatic synthetic dysfunction or reduced liver function (prolonged prothrombin time, elevated serum bilirubin, or reduced serum albumin) and should be stopped if any of these develop while on treatment. Therefore, patients with cirrhosis receiving OCA should be monitored closely for any evidence of clinical or laboratory decompensation, which would indicate need to discontinue therapy. Given the evidence that OCA may increase the lithogenicity of bile, monitoring for symptomatic gallstones would also be appropriate (54). UDCA therapy may be continued in such patients if tolerated well. Table 1 clarifies dosing and monitoring recommendations, as well as current contraindications, for the use of OCA in patients with PBC.
It is becoming increasingly clear that our goal in treating PBC is evolving to target greater reductions in ALP and bilirubin than what was recommended in the past. It is our opinion that the goal after adding a second-line therapy for PBC is to achieve the lowest ALP level possible. We recommend that ALP response to UDCA be assessed 6–12 months after UDCA initiation and that the second-line therapy be considered (see the following section, Up-to-date PBC algorithm). It is also important to apply proper medical judgment and consider other factors when adding a second-line therapy. For instance, exposing an older patient with stage I disease and an ALP of 150 U/L to a second or third drug knowing that it will not alter their clinical course but will lower their ALP would not be the best approach. Given the recent restriction established on the use of OCA in patients with advanced cirrhosis, it is also important to restage the liver disease and carefully evaluate for signs of portal hypertension before starting OCA. Finally, it seems likely that our treatment goals in PBC will evolve over time to achieve complete biochemical remission (normal or near-normal ALP and bilirubin levels).
OCA postmarketing experience
A postmarketing, retrospective study of 319 patients found that the proportions of patients with a biochemical response to OCA treatment according to Toronto criteria (ALP >1.67 × ULN after 2 years on UDCA) were 48% after year 1, 58% after year 2, and 55% at the end of the follow-up period. According to Paris I criteria (ALP ≥ 3 × ULN or AST ≥ 2 × ULN or bilirubin >1.0 mg/dL), the proportions were 68%, 76%, and 69%, respectively. The authors concluded that the OCA treatment response in PBC is sustained for up to 3 years (55). In a separate study, 191 patients in the Italian PBC Registry were prospectively analyzed for at least 12 months using the POISE criteria (Table 1) (32). At 12 months, significant median reductions of ALP (−32.3%), ALT (−31.4%), and bilirubin (−11.2%) were observed, and response rates were 42.9%. Furthermore, premature discontinuation of OCA due to adverse events occurred in 17% of patients, with treatment-induced pruritus as the leading cause of OCA discontinuation (67%). The authors concluded that the efficacy and safety of OCA postmarketing mirror those in the POISE trial (56). Finally, a postmarketing international study analyzed the effects of OCA in 290 OCA-naive patients within the Global PBC Study Group. In this study, OCA demonstrated biochemical effectiveness comparable to the trial data (specifically significant reductions from baseline to month 20 in ALP, GGT, and ALT), safety, and acceptable tolerability, with pruritus being the most relevant complaint leading to discontinuation (57).
Use of fibrates in PBC
In recent years, the use of fibrates in combination with UDCA in PBC has demonstrated improvements in biochemical measures and symptom relief. Fibrates are traditionally lipid-lowering agents, and benefits in PBC are attributed to agonistic properties at the peroxisome proliferator–activated receptor. Bezafibrate is the most extensively studied fibrate in PBC. Limited data on fenofibrate have demonstrated improvements in GLOBE and UK-PBC scores (58); significant reduction—and in some cases, normalization—of serum ALP, ALT, AST, and proinflammatory cytokines (59); and a lower risk of cirrhosis development and hepatic deterioration (60) in patients with PBC. In 2015, a meta-analysis of 6 studies consisting of 102 patients with PBC analyzed fenofibrate use in PBC. All these studies included patients who had an inadequate response to UDCA, with fenofibrate added at a dose of 100–200 mg daily. The observed effect of fenofibrate was a complete response rate of 69% (95% CI: 53%–82%) and an odds ratio of 82.8 (95% CI: 21.6–317.2; P = 0.024). Fenofibrate use was associated with a significant decrease in ALP (−114 U/L; 95% CI: −152 to −76; P = 0.0001), a significant decrease in GGT (−92 U/L; 95% CI: −149 to −43; P = 0.0004), a significant decrease in total bilirubin (−0.11 mg/dL; 95% CI: −0.18 to −0.08; P = 0.0008), and a significant decrease in IgM levels (−88 mg/dL; 95% CI: −119 to −58; P < 0.0001). Investigators stressed the need for larger-scale, randomized trials to determine the effect of fenofibrate on disease progression, liver-related morbidity, and mortality (61).
More recently, a 24-month, double-blind, placebo-controlled, phase 3 trial titled “Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cholangitis” was conducted. Investigators randomly assigned 100 patients with PBC who had an inadequate response to UDCA according to Paris 2 criteria to receive 400 mg of bezafibrate and UDCA daily (n = 50) or placebo and UDCA (n = 50) daily. The primary outcome (complete biochemical response, defined as normal levels of total bilirubin, ALP, aminotransferases, albumin, and normal prothrombin index) was observed in 31% of the bezafibrate-treated patients compared with 0% of the placebo-treated patients (P < 0.001). In addition, 67% of the bezafibrate-treated patients and 2% of the placebo-treated patients achieved a normal ALP. Improvements in pruritus and fatigue were also noted (62). The most recent publication on bezafibrate use in patients with PBC is the investigator-initiated Fibrates for the Treatment of Cholestatic Itch (FITCH) trial, which assessed the effects of bezafibrate on pruritus in 70 patients with PBC, primary sclerosing cholangitis (PSC), and secondary sclerosing cholangitis. Investigators found that significantly more bezafibrate-treated patients achieved a ≥50% reduction of severe or moderate pruritus compared with the placebo group (45% vs 11%, respectively; P = 0.003). Regarding the secondary end points, bezafibrate reduced the morning (P = 0.01 vs placebo) and evening (P = 0.007) intensity of pruritus visual analogue scale (VAS), improved the validated 5D itch questionnaire (P = 0.002 vs placebo), and reduced serum ALP (−35%, P = 0.03 vs placebo), which correlated with improved pruritus (VAS, P = 0.01) (63).
A recently published systematic review sought to analyze the safety of fibrates in PBC and PSC. Investigators identified 37 studies (31 for PBC and 6 for PSC) that included 1,107 patients treated with fibrates (most of whom were administered bezafibrate or fenofibrate), with or without UDCA. The authors concluded that fibrates are relatively safe for use in PBC, with the most commonly reported adverse events being gastrointestinal (3.9%) and musculoskeletal (4.1%). Eight studies compared UDCA plus fibrates (n = 449) vs UDCA monotherapy (n = 1,074). In those studies, elevated aminotransferases and serum creatinine were reported more commonly in the patients treated with UDCA plus fibrates (2.6% each) vs those treated with UDCA monotherapy (0%–0.6%) (64).
Fibrates were previously not recommended for the treatment of PBC (2). Since the last publication, additional data on bezafibrate have been published, and the 2021 AASLD practice guidance update stated that fibrates may be considered off-label alternatives for patients with PBC and an inadequate response to UDCA but are discouraged in patients with decompensated liver disease (3). Bezafibrate is currently not available in the United States; therefore, fenofibrate would be the fibrate option for off-label use in the United States.
Up-to-date PBC algorithm
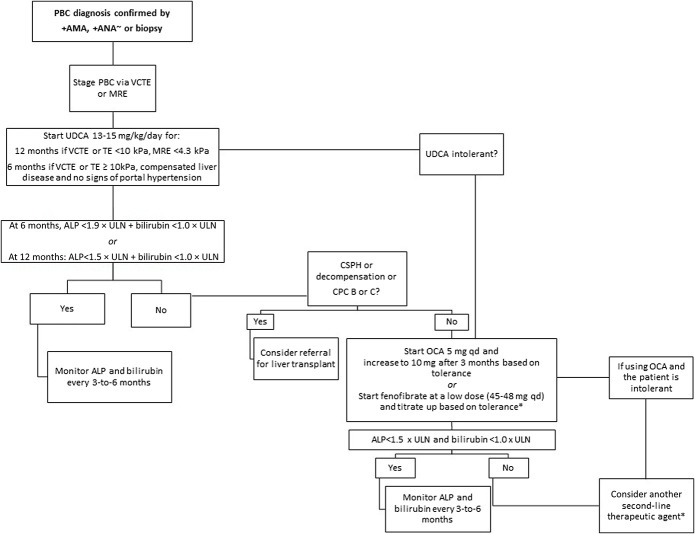
Considering the new data described above and the updated recommendations, and to further refine the appropriate use of UDCA and OCA in clinical gastroenterology practice, we propose a practical, up-to-date algorithm for first-line and second-line treatments of PBC, as depicted in Figure 3. The key (new) features of the patient algorithm include new guidance-informed suggestions for staging PBC using NIT, earlier assessment of lower thresholds to gauge UDCA response after initiation of therapy, possible earlier initiation of second-line therapy with OCA at lower levels of ALP or bilirubin, avoidance of OCA in patients with cirrhosis complicated by portal hypertension or liver decompensation, and the safety and durability of response with long-term OCA therapy and off-label use of fibrates.
Figure 3.
Updated algorithm for the treatment of PBC. ∼sp100 Gp210. *Fenofibrate is not currently approved for the treatment of PBC and use is considered off-label. ALP, alkaline phosphatase; AMA, antimicrobial antibodies; ANA, antinuclear antibodies; CPC, Child-Pugh class; CSPH, clinically significant portal hypertension; HCC, hepatocellular carcinoma; MRE, magnetic resonance elastography; OCA, obeticholic acid; PBC, primary biliary cholangitis; TE, transient elastography; UDCA, ursodeoxycholic acid; VCTE, vibration-controlled transient elastography.
As shown in Figure 3, patients with a lower stage of fibrosis (vibration-controlled transient elastography [VCTE] or TE <10 kPa, MRE <4.3 kPa) may continue UDCA monotherapy for 12 months before determining response (ALP <1.5 × ULN and bilirubin <1 × ULN) and the need for second-line therapy. For patients with a more advanced fibrosis stage (VCTE or TE ≥10 kPa), compensated liver disease, and no signs of portal hypertension, response (ALP <1.5 × ULN and bilirubin <1 × ULN) and the need for second-line treatment should be assessed at 6 months. Based on recent data, clinicians may consider the more stringent criteria (ALP < ULN and bilirubin ≤0.6 mg/dL) to assess response in patients with more advanced disease. Second-line therapy consists of OCA, as reviewed in detail above, or off-label bezafibrate or fenofibrate in patients with an inadequate response and if there are no signs of decompensated liver disease or clinically significant portal hypertension. Because bezafibrate is not available in the United States, it is the opinion of the authors that fenofibrate could be considered as an alternative second-line therapy in the appropriate patient, at a low dose of 45–48 mg per day and titrated up as tolerated. If the patient has not responded to the first second-line option (OCA or fibrate) after 3–6 months of therapy or the patient is unable to tolerate the selected second-line treatment, then the other second-line option should be considered. Participation in clinical trials should be discussed and encouraged for appropriate patients who may need additional therapies beyond UDCA, OCA, and off-label fibrates (some examples of investigational drugs for PBC are described in Supplementary Table 1, http://links.lww.com/AJG/C752). A referral for liver transplantation evaluation may be appropriate for patients with decompensated cirrhosis, portal hypertension, or significantly affected due to severe pruritus or fatigue even if the Model for End-Stage Liver Disease score is relatively low (<15). Living donor liver transplant may be an option for some patients.
Case scenario conclusion
After 6 months of UDCA therapy, the patient's repeat ALP was 480 U/L and total bilirubin 0.9 mg/dL. Based on these laboratory values, she is considered a nonresponder to UDCA, and second-line PBC treatments can thus be considered. A repeat ALP after 12 months of UDCA therapy was 325 U/L with a total bilirubin of 0.8 mg/dL, a serum albumin of 3.8 g/dL, and a platelet count of 145 × 109/L. The patient's GLOBE score was 1.29, and her predicted 3-year survival was 87.9% compared with the mean survival of 98.2% in age-matched and sex-matched patients in the 58–66-year age group. There was a discussion with the patient about off-label treatment with fenofibrate vs on-label treatment with OCA. She was then started on OCA 5 mg/d with the plan to monitor her ALP and bilirubin in 3–6 months and consider adding fenofibrate if a complete biochemical response was not achieved.
Triple combination therapy for PBC combining UDCA, OCA, and fibrates
Emerging data have suggested that the combination of existing therapies both on-label and off-label may work in those with an inadequate treatment response. Although UDCA plus OCA is an approved regimen, off-label triple combination therapies include UDCA and OCA with either fenofibrate or bezafibrate. A recently reported multicenter, uncontrolled retrospective cohort study analyzed 58 patients with PBC to assess the potential additive effects of triple therapy using UDCA, OCA, and fibrates. All patients had previously failed to adequately respond to standard UDCA and then to a second-line option (OCA or fibrates) in combination with UDCA and had subsequently been treated with triple therapy with UDCA, OCA, and fibrates for a minimal period of 3 months. Half of the patients (n = 29) received OCA as a second-line therapy and then fibrates as a third-line therapy (Group OCA-Fibrate), whereas the other half (n = 29) experienced the inverse therapeutic sequence, namely, fibrates as a second-line therapy and then OCA as a third-line therapy (Group Fibrate-OCA). The triple therapy was associated with a ∼22% ALP reduction per year compared with the dual therapy. This additive effect seemed to be stronger in OCA followed by fibrates than in fibrates followed by OCA. Triple therapy was associated with an odds ratio of 3.4 for reaching normal ALP and was accompanied by a significant decrease in other liver biochemistries. The odds ratios of achieving the Paris 2 and the Toronto criteria of adequate biochemical response were 6.8 and 9.2, respectively. Triple therapy also improved pruritus in the OCA group, followed by the fibrates group, but not in the fibrates group, followed by the OCA group (65). It is the opinion of the authors that patients without a response to dual therapy, especially those with advanced disease but too early to be considered for liver transplantation, may be offered triple therapy with UDCA/OCA/fenofibrate after explaining the risks and benefits or the patient can be referred for a clinical trial. Further studies need to be conducted to determine whether triple combination therapy achieves similar results in larger populations at variable stages of disease and whether it improves symptoms, progression of disease stage, and clinical outcomes.
CONCLUSIONS
Several recent advances have been made in the diagnosis, staging, and treatment of PBC. VCTE has become a standard tool for noninvasive staging, and recently published guidelines have strengthened cutoff values for diagnosis of advanced disease. The indications for the introduction of second-line treatment have been refined with a trend toward earlier evaluation of UDCA response and possibly lower ALP thresholds for starting second-line therapy. The use of OCA in patients with cirrhosis is now restricted to those with compensated cirrhosis and no evidence of decompensated liver disease. Several late-stage clinical trials with novel proliferator-activated receptor agonists, such as seladelpar and elafibranor, are currently underway, and these therapies may be approved in the near future. In addition, promising clinical data are available for bezafibrate (which is currently approved in Europe and Japan). Recent guidance updates from the AASLD have included fenofibrate (available in the United States but not approved for PBC) as a possible off-label therapeutic option for PBC. These advances in therapy will hopefully help achieve the goal of complete biochemical remission in PBC, while continuing the search for additional approaches to improve symptoms and quality of life for patients with this progressive liver disease.
CONFLICTS OF INTEREST
Guarantor of the article: Kris V. Kowdley, MD.
Specific author contributions: K.V.K.: led the group of expert authors and drafted the proposed outline, identified the most recent updates in PBC data, developed the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, has approved the final draft. C.L.B.: reviewed and approved the proposed outline; performed literature searches and identified the most recent updates in PBC literature, contributed to the development of the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, approved the final draft. C.L.: reviewed and approved the proposed outline, performed literature searches and identified the most recent updates in PBC literature, contributed to the development of the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, has approved the final draft. M.J.M.: reviewed and approved the proposed outline, performed literature searches and identified the most recent updates in PBC literature, contributed to the development of the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, has approved the final draft. D.S.P.: reviewed and approved the proposed outline, performed literature searches and identified the most recent updates in PBC literature, contributed to the development of the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, has approved the final draft. R.V.: reviewed and approved the proposed outline, performed literature searches and identified the most recent updates in PBC literature, contributed to the development of the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, has approved the final draft. Z.M.Y.: reviewed and approved the proposed outline, performed literature searches and identified the most recent updates in PBC literature, contributed to the development of the updated PBC treatment algorithm and other practical recommendations in the manuscript, drafted the manuscript on updated PBC data and the use of the new algorithm, has approved the final draft.
Financial support: This manuscript was supported by an unrestricted educational grant to the Chronic Liver Disease Foundation from Intercept Pharmaceuticals. The selection of the authors and creation of this manuscript was performed independently. Intercept Pharmaceuticals did not play a role.
Potential competing interests: K.V.K.: advisory role: Cymabay, Genfit, HighTide, Intercept, Mirum, Gilead, Madrigal, and NGM; consultant: Calliditas; research funding: Cymabay, Genfit, Glaxo Smith Kline, HighTide, Mirum, Gilead, Pliant, Viking, Pfizer, Intercept, Hanmi, and Madrigal. C.L.B.: research funding: Gilead, BMS, Cymabay Therapeutics, Genfit, Glaxo Smith Kline, Mirum, Pliant, Novartis, BiomX, Boston Scientific, COUR Pharmaceuticals, and Target PharmaSolutions. C.L.: advisory role: Calliditas, Cara, Cymabay, Genfit, Glaxo Smith Kline, Intercept, Mirum, and Target; research funding: Calliditas, Cara, Cymabay, Genfit, Gilead Sciences, Glaxo Smith Kline, HighTide, Intercept, Mirum, Target, and Zydus. M.J.M.: advisory role: Cymabay, and Glaxo Smith Kline; research funding: Cymabay, Genfit, Glaxo Smith Kline, Mirum, Target Pharmaceuticals, and Intercept. D.S.P.: consultant: Mediar Therapeutics; research funding: Lygenesis, Cara, Gilead Sciences, Cymabay, Genfit, and HighTide. R.V.: advisory role: Echosens; consultant: Data safety monitoring boards for Labcorp, Medpace, and COUR Pharmaceuticals; research funding: Institutional funding from Zydus Therapeutics, Pliant, Eli Lilly, Galectin, Terns, and Cara Therapeutics. Z.M.Y.: consultant: Novo Nordisk, Merck, Siemens, Intercept, Gilead Sciences, Astra Zeneca, Madrigal, BMS, AbbVie, Abbott, and Novartis
Supplementary Material
ACKNOWLEDGEMENTS
Rachel E. Bejarano, PharmD, and Lisa D. Pedicone, PhD, provided medical writing assistance.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C752
Contributor Information
Christopher L. Bowlus, Email: clbowlus@ucdavis.edu.
Cynthia Levy, Email: clevy@med.miami.edu.
Marlyn J. Mayo, Email: marlyn.mayo@utsouthwestern.edu.
Daniel S. Pratt, Email: dspratt@mgh.harvard.edu.
Raj Vuppalanchi, Email: rvuppala@iu.edu.
Zobair M. Younossi, Email: zobair.younossi@inova.org.
REFERENCES
- 1.Murillo Perez CF, Goet JC, Lammers WJ, et al. Milder disease stage in patients with primary biliary cholangitis over a 44-year period: A changing natural history. Hepatology 2018;67:1920–30. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Bernstein D, Shiffman ML, et al. Diagnosis and management of primary biliary cholangitis. Am J Gastroenterol 2019;114:48–63. [DOI] [PubMed] [Google Scholar]
- 3.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2021 practice guidance update from the American association for the study of liver diseases. Hepatology 2021;75:1012–3. [DOI] [PubMed] [Google Scholar]
- 4.EASL Clinical Practice Guidelines. The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- 5.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology 2019;69:394–419. [DOI] [PubMed] [Google Scholar]
- 6.You H, Ma X, Efe C, et al. APASL clinical practice guidance: The diagnosis and management of patients with primary biliary cholangitis. Hepatol Int 2022;16:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J Hepatol 2012;56:1181–8. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Zhou Y, Haller IV, et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin Gastroenterol Hepatol 2018;16:1342–50.e1. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Li J, Haller IV, et al. Factors associated with prevalence and treatment of primary biliary cholangitis in United States health systems. Clin Gastroenterol Hepatol 2018;16:1333–41.e6. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda S, Nakamura M, Ishibashi H, et al. Molecular mimicry of mitochondrial and nuclear autoantigens in primary biliary cirrhosis. Gastroenterology 2003;124:1915–25. [DOI] [PubMed] [Google Scholar]
- 11.Theise ND, Crawford JM, Nakanuma Y, et al. Canal of hering loss is an initiating step for primary biliary cholangitis (PBC): A hypothesis. Med Hypotheses 2020;140:109680. [DOI] [PubMed] [Google Scholar]
- 12.Chazouilleres O, Wendum D, Serfaty L, et al. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features and response to therapy. Hepatology 1998;28:296–301. [DOI] [PubMed] [Google Scholar]
- 13.Sun C, Xiao X, Yan L, et al. Histologically proven AMA positive primary biliary cholangitis but normal serum alkaline phosphatase: Is alkaline phosphatase truly a surrogate marker? J Autoimmun 2019;99:33–8. [DOI] [PubMed] [Google Scholar]
- 14.Terziroli Beretta-Piccoli B, Stirnimann G, Mertens J, et al. Primary biliary cholangitis with normal alkaline phosphatase: A neglected clinical entity challenging current guidelines. J Autoimmun 2021;116:102578. [DOI] [PubMed] [Google Scholar]
- 15.Murillo Perez CF, Hirschfield GM, Corpechot C, et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther 2019;50:1127–36. [DOI] [PubMed] [Google Scholar]
- 16.Scheuer P. Primary biliary cirrhosis. Proc R Soc Med 1967;60:1257–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol 1978;379:103–12. [DOI] [PubMed] [Google Scholar]
- 18.Zhou JH, Cai JJ, She ZG, et al. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol 2019;25:1307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatselis NK, Goet JC, Zachou K, et al. Factors associated with progression and outcomes of early stage primary biliary cholangitis. Clin Gastroenterol Hepatol 2020;18:684–92.e6. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Noureddin M, Bernstein D, et al. Role of noninvasive tests in clinical gastroenterology practices to identify patients with nonalcoholic steatohepatitis at high risk of adverse outcomes: Expert panel recommendations. Am J Gastroenterol 2021;116:254–62. [DOI] [PubMed] [Google Scholar]
- 21.Mayo MJ, Parkes J, Adams-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 2008;48:1549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujinaga Y, Namisaki T, Takaya H, et al. Enhanced liver fibrosis score as a surrogate of liver-related complications and mortality in primary biliary cholangitis. Medicine (Baltimore) 2021;100:e27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osman KT, Maselli DB, Idilman IS, et al. Liver stiffness measured by either magnetic resonance or transient elastography is associated with liver fibrosis and is an independent predictor of outcomes among patients with primary biliary cholangitis. J Clin Gastroenterol 2021;55:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez-Dominguez E, Mendoza J, García-Buey L, et al. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Aliment Pharmacol Ther 2008;27:441–7. [DOI] [PubMed] [Google Scholar]
- 25.Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56:198–208. [DOI] [PubMed] [Google Scholar]
- 26.Joshita S, Yamashita Y, Sugiura A, et al. Clinical utility of FibroScan as a non-invasive diagnostic test for primary biliary cholangitis. J Gastroenterol Hepatol 2020;35:1208–14. [DOI] [PubMed] [Google Scholar]
- 27.Cristoferi L, Calvaruso V, Overi D, et al. Accuracy of transient elastography in assessing fibrosis at diagnosis in naive patients with primary biliary cholangitis: A dual cut-off approach. Hepatology 2021;74:1496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manesis EK, Schina M, Vafiadis I, et al. Liver stiffness measurements by 2-dimensional shear wave elastography compared to histological and ultrasound parameters in primary biliary cholangitis. Scand J Gastroenterol 2021;56:1187–93. [DOI] [PubMed] [Google Scholar]
- 29.EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659–89. [DOI] [PubMed] [Google Scholar]
- 30.Roll J, Boyer JL, Barry D, et al. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med 1983;308:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Dickson ER, Grambsch PM, Fleming TR, et al. Prognosis in primary biliary cirrhosis: Model for decision making. Hepatology 1989;10:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631–43. [DOI] [PubMed] [Google Scholar]
- 33.Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014;147:1338–49.e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 34.Murillo Perez CF, Harms MH, Lindor KD, et al. Goals of treatment for improved survival in primary biliary cholangitis: Treatment target should Be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol 2020;115:1066–74. [DOI] [PubMed] [Google Scholar]
- 35.Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 2015;149:1804–12.e4. [DOI] [PubMed] [Google Scholar]
- 36.Efe C, Taşçilar K, Henriksson I, et al. Validation of risk scoring systems in ursodeoxycholic acid-treated patients with primary biliary cholangitis. Am J Gastroenterol 2019;114:1101–8. [DOI] [PubMed] [Google Scholar]
- 37.Prince M, Chetwynd A, Newman W, et al. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: Follow-up for up to 28 years. Gastroenterology 2002;123:1044–51. [DOI] [PubMed] [Google Scholar]
- 38.Titievsky L, Ness E, Law A, et al. Incidence of hepatic outcomes in patients with cirrhosis due to primary biliary cholangitis: A population-based epidemiology study. J Hepatol 2021;75:S434. [Google Scholar]
- 39.Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009;136:1281–7. [DOI] [PubMed] [Google Scholar]
- 40.Örnolfsson KT, Lund SH, Olafsson S, et al. Biochemical response to ursodeoxycholic acid among PBC patients: A nationwide population-based study. Scand J Gastroenterol 2019;54:609–16. [DOI] [PubMed] [Google Scholar]
- 41.Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014;147:1338–49.e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the L. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- 43.Zhang LN, Shi TY, Shi XH, et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: Results of a 14-year cohort study. Hepatology 2013;58:264–72. [DOI] [PubMed] [Google Scholar]
- 44.Murillo Perez CF, Ioannou S, Hassanally I, et al. Early identification of insufficient biochemical response to ursodeoxycholic acid in patients with primary biliary cholangitis. Presented at: The American Association for the Study of Liver Diseases Annual Meeting; November 12–15, 2021.
- 45.Ocalavia [package Insert]. New York, NY: Intercept Pharmaceuticals, Inc; 2021. [Google Scholar]
- 46.FDA Approves Ocaliva for Rare, Chronic Liver Disease. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-ocaliva-rare-chronic-liver-disease. Accessed January 3, 2022. [Google Scholar]
- 47.Bowlus CL, Pockros PJ, Kremer AE, et al. Three years of obeticholic acid (OCA) therapy results in histological improvements in patients with primary biliary cholangitis: Further analysis of the POISE biopsy substudy. Dig Liver Dis 2019;51:e19. [Google Scholar]
- 48.Nevens F, Shiffman ML, Drenth J, et al. Durable response in the markers of cholestasis through 5 years of open-label extension study of obeticholic acid in primary biliary cholangitis. Dig Liver Dis 2020;52:e30. [Google Scholar]
- 49.Murillo Perez C, Fisher H, Hiu S, et al. Patients with primary biliary cholangitis treated with long-term obeticholic acid in a trial setting demonstrate better transplant-free survival than external controls from the Global PBC and UK-PBC study groups. Late-breaking abstract 08 presented at: The American Association for the Study of Liver Diseases Annual Meeting; November 12-15, 2021.
- 50.Ocaliva (obeticholic acid) by Intercept Pharmaceuticals: Drug safety communication—due to risk of serious liver injury, FDA restricts use of ocaliva in primary biliary cholangitis patients with advanced cirrhosis. Available at: https://www.fda.gov/safety/medical-product-safety-information/ocaliva-obeticholic-acid-intercept-pharmaceuticals-drug-safety-communication-due-risk-serious-liver. Accessed February 7, 2021.
- 51.Eurpean Medicines Agency. Ocaliva. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/ocaliva. Accessed April 4, 2022. [Google Scholar]
- 52.D'Amato D, De Vincentis A, Malinverno F, et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep 2021;3:100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trauner M, Nevens F, Shiffman ML, et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol 2019;4:445–53. [DOI] [PubMed] [Google Scholar]
- 54.Al-Dury S, Wahlström A, Panzitt K, et al. Obeticholic acid may increase the risk of gallstone formation in susceptible patients. J Hepatol 2019;71:986–91. [DOI] [PubMed] [Google Scholar]
- 55.Gish RG, Law A, Adekunle F, et al. Real-world effectiveness of obeticholic acid in patients with primary biliary cholangitis. Hepatology 2020;72:S762A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Amato D, De Vincentis A, Malinverno F, et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep 2021;3:100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulamhusein AF, Roberts SB, Haliday N, et al. Real world effectiveness of obeticholic acid in patients with primary biliary cholangitis: The global experience. Hepatology 2020;72:S761A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Sun K, Tian A, et al. Fenofibrate improves GLOBE and UK-PBC scores and histological features in primary biliary cholangitis. Minerva Med 2021. doi: 10.23736/S0026-4806.21.07316-X. [DOI] [PubMed] [Google Scholar]
- 59.Ghonem NS, Auclair AM, Hemme CL, et al. Fenofibrate improves liver function and reduces the toxicity of the bile acid pool in patients with primary biliary cholangitis and primary sclerosing cholangitis who are partial responders to ursodiol. Clin Pharmacol Ther 2020;108:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung SW, Lee JH, Kim MA, et al. Additional fibrate treatment in UDCA-refractory PBC patients. Liver Int 2019;39:1776–85. [DOI] [PubMed] [Google Scholar]
- 61.Grigorian AY, Mardini HE, Corpechot C, et al. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol 2015;39:296–306. [DOI] [PubMed] [Google Scholar]
- 62.Corpechot C, Chazouilleres O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018;378:2171–81. [DOI] [PubMed] [Google Scholar]
- 63.de Vries E, Bolier R, Goet J, et al. Fibrates for itch (FITCH) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial. Gastroenterology 2021;160:734–43.e6. [DOI] [PubMed] [Google Scholar]
- 64.Carrion AF, Lindor KD, Levy C. Safety of fibrates in cholestatic liver diseases. Liver Int 2021;41:1335–43. [DOI] [PubMed] [Google Scholar]
- 65.Soret PA, Lam L, Carrat F, et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment Pharmacol Ther 2021;53:1138–46. [DOI] [PubMed] [Google Scholar]
- 66.Efe C, Torgutalp M, Henriksson I, et al. Extrahepatic autoimmune diseases in primary biliary cholangitis: Prevalence and significance for clinical presentation and disease outcome. J Gastroenterol Hepatol 2021;36:936–42. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005;353:1261–73. [DOI] [PubMed] [Google Scholar]
- 68.Sivakumar T, Kowdley KV. Anxiety and depression in patients with primary biliary cholangitis: Current insights and impact on quality of life. Hepat Med 2021;13:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]