Abstract
Introduction
Group A streptococcus (Streptococcus pyogenes) is one of the most lethal bacterial pathogens of humans, with increased risk of progression to septic shock and multiorgan failure in the pregnant population. The objective of this study is to systematically review the outcomes and management strategies for pregnancy and puerperal group A streptococcus infections in an effort to provide further guidance for prevention and treatment of a rare but lethal infection worldwide.
Material and methods
A comprehensive search using puerperium and streptococcus pyogenes terms was completed across several registered databases. A total of 902 articles investigating pregnancy and puerperal group A streptococcus infection were identified, with 40 studies fulfilling inclusion criteria of original research articles in humans published from 1990 onwards reporting four or more unique cases of group A streptococcus in pregnancy or postpartum. This study was registered in PROSPERO: CRD42020198983.
Results
A total of 1160 patients with pregnancy and puerperal group A streptococcus infection were identified. Most infections occurred postpartum (91.9%), with 4.7% reported antepartum and 0.6% intrapartum. Bacteremia was present in 49.0% of patients and endometritis in 45.9%. Puerperal sepsis was described in 28.2% of cases and progressed to streptococcal toxic shock syndrome in one‐third of such cases. Overall, the case fatality ratio was 2.0%, with one‐third of the deaths from antenatal cases including 3/22 (13.6%) cases of septic abortion and 10/46 (21.7%) antenatal cases of group A streptococcus infection.
Conclusions
Group A streptococcus infection remains an important contributor to pregnancy and puerperal morbidity and mortality. Early recognition, diagnosis and aggressive management are important for favorable outcomes given the serious risk of sepsis and streptococcal toxic shock syndrome.
Keywords: group A streptococcus, pregnancy, puerperium, Streptococcus pyogenes
Group A streptococcal infection remains an important contributor to puerperal morbidity and mortality despite advances in infection control protocols. Early recognition, diagnosis and aggressive management are important for favorable outcomes given the serious risk of sepsis and toxic shock syndrome.
Abbreviations
- GAS
group A streptococcus
- IVIG
intravenous immunoglobulin
- RR
relative risk
- sTSS
streptococcal toxic shock syndrome
Key message.
Group A streptococcal infection remains an important contributor to puerperal morbidity and mortality despite advances in infection control protocols. Early recognition, diagnosis and aggressive management are important for favorable outcomes given the serious risk of sepsis and toxic shock syndrome.
1. INTRODUCTION
Infection is the third most common cause of pregnancy‐related mortality worldwide, representing approximately 10.7% of pregnancy‐related deaths in low‐ and middle‐income countries and 4.7% of deaths in high‐income countries. 1 Further, it is estimated that for every death, there are 50 pregnant people with life‐threatening morbidity from sepsis. 2 The incidence of puerperal sepsis has risen over the last decade, in some cases doubling, 3 with increasing rates of severe sepsis contributing to mortality. 3 , 4 Underlying this trend is increasing virulence of group A streptococcal infection, which contributed to 50% of direct sepsis deaths in two European studies. 2 , 5 , 6 This is suspected to be due to the predominance of emm1 and emm28 genotypes, which have higher associations with mortality, as well as increasing maternal risk factors for infection such as obesity and rates of cesarean section. 2
Group A streptococcus (Streptococcus pyogenes) (GAS) is one of the most lethal bacterial pathogens of humans. 7 It is a facultative, gram‐positive coccus which has reservoirs in human skin and mucous membranes. 7 Outside of pregnancy, the case fatality rate of invasive GAS infection is 15%–20% which increases to 40%–60% with progression to septic shock. 8 , 9 , 10 Pregnancy is a highly immunomodulated state which increases the risk of invasive GAS infection by 20‐fold in comparison to the non‐pregnant population. 11 Most people develop invasive GAS from ascending infection of the genital tract and endometrium, which can quickly progress to septic shock and multiorgan failure within 48–96 hours as evidenced by multiple case reports in the literature. 12
The objective of this study was to systematically review the pregnancy and puerperal outcomes and management strategies in cases of GAS infection in an effort to provide further guidance for prevention and treatment of a rare but lethal infection worldwide.
2. MATERIAL AND METHODS
This protocol was registered with PROSPERO (CRD42020198983). With the assistance of an information specialist, a comprehensive search of the Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Latin American and Caribbean Health Sciences Literature (LILACS) and PubMed (in‐process and non‐MEDLINE) databases was conducted. In addition, Clinicaltrials.gov, WHO International Clinical Trials Registry and the International Standard Randomized Controlled Trial Number Registry were reviewed along with the first 200 results from Google Scholar. Reference lists from relevant trials, reviews and commentaries were manually searched. Prior to manuscript submission, the search was updated on January 15, 2022. Subject headings and keywords were used to search for puerperium and streptococcus pyogenes terms. A detailed search strategy along with results is attached in Table S1.
Two authors independently reviewed non‐duplicate titles and abstracts and retrieved full texts of eligible studies for further evaluation. Disagreements were resolved by consensus and adjudication by a third author. All studies meeting inclusion criteria of original research articles in humans published from 1990 onwards reporting four or more unique cases of GAS in pregnancy or postpartum were evaluated. Articles recording carrier or screening status for GAS were excluded. Conference abstracts were excluded. There were no language restrictions. Study characteristics, patient demographics, infection characteristics, management strategies, and patient outcomes were extracted. Statistics were grouped according to outcomes and reported in terms of frequency of outcome with percentages calculated according to the summed number of people meeting inclusion criteria within each study. Studies with missing information were not included in the calculations for that particular outcome.
Two authors evaluated the research quality of included studies using the Newcastle–Ottawa Scale for cohort and case control studies which was converted to meet the Agency for Healthcare and Research and Quality standards with thresholds supplied by the National Center for Biotechnology Information, 13 and the National Institutes of Health assessment tool for case series (Table S2). 14
3. RESULTS
A total of 982 articles investigating puerperal GAS infection were identified, with 40 studies fulfilling inclusion criteria (Figure 1). Study design and patient population characteristics of the included studies are described in Table 1. Sixteen prospective studies including 498 patients across three cohort, three case–control and 10 case series were included in the review. Most of the studies were retrospective in nature, totaling 662 patients, with 20 case series, two cohort and two case–control studies meeting inclusion criteria. 2 , 8 , 10 , 11 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 Descriptive analysis was performed, as meta‐analysis was not possible given the heterogeneity of the patient populations, dominant study design, and study outcome definitions. 2 , 8 , 10 , 11 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50
FIGURE 1.
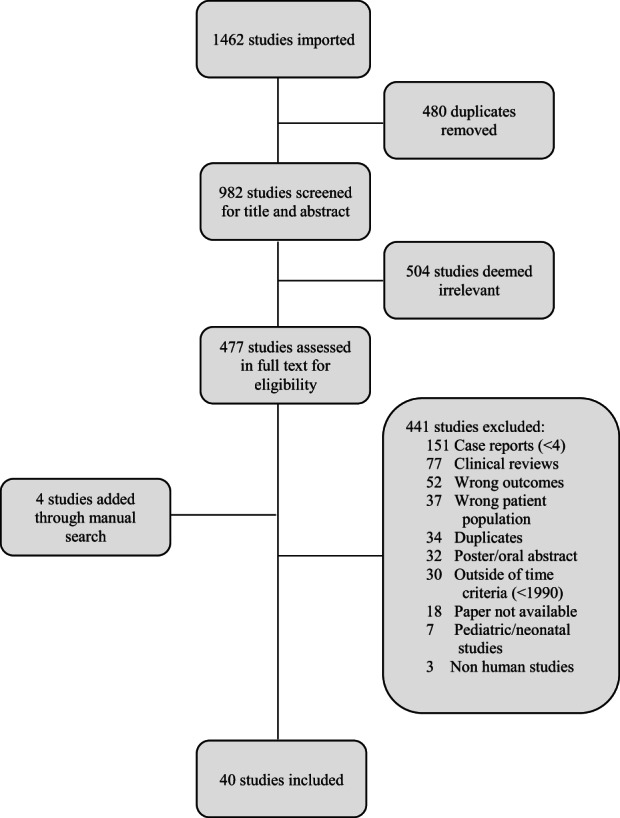
PRISMA flow chart for included studies
TABLE 1.
Characteristics of included studies
| Study, year | Collection dates | Study design | Study setting | Primary objective(s) | Secondary objective(s) |
|---|---|---|---|---|---|
| Acosta, 2014 | June 1, 2011–May 31, 2012 | Prospective Case–Control | Obstetrician‐led maternity units, UK | Estimate the incidence, describe the causative organisms and sources of infection, and identify the risk factors for severe maternal sepsis in the UK | None |
| Alberts, 2018 | 2010–2014 | Retrospective case series | Swedish insurance company reports of injury in connection with care database | Study reported obstetric care injuries related to bacterial pathogenesis | None |
| Alexander, 2018 | February 2015–March 2016 | Retrospective case series | Large academic medical center, US | Rule out potential transmission by a health care worker of GAS following a series of cases identified in postpartum women | None |
| Anteby, 1999 | June 1987–December 1994 | Retrospective case–control | University hospital, Jerusalem | Identify factors characteristic of non‐epidemic puerperal group A streptococcal infection. | None |
| Aronoff, 2008 | August 1996–August 2000 | Retrospective case series retrospective cohort | Hospitalizations, across the state of Florida | Report the detailed clinical and epidemiologic features of women hospitalized for postpartum invasive GAS disease in Florida during the 4‐year period of 1996–2000 | Secondary comparative analysis of a large Florida hospital discharge dataset obtained from the Florida Agency for Health Care Administration for women carrying the diagnosis of postpartum invasive GAS disease who were residents of Florida |
| Barnham, 2001 | 1980–1999 | Retrospective case series | Harrogate, York and Northallerton districts of North Yorkshire | To describe the features of invasive per‐partum Streptococcus pyogenes infection as it occurs in current day practice in North Yorkshire | None |
| Bauer, 2015 | 1999–2006 | Retrospective case series from cohort of maternal deaths | Various hospitals, Michigan | Identify maternal deaths due to sepsis in the state of Michigan from 1999 to 2006, review the events leading to diagnosis, and evaluate treatment to identify areas for improvement | None |
| Bengner, 2019 | 2018 | Retrospective case series | Various hospitals, Sweden | Describe two minor outbreaks of cot fever in various hospitals in Sweden, where investigation was able to detect infection from staff to patients. | None |
| Busowski, 2013 | Not reported | Retrospective case series | Single hospital, Orlando, Florida | Present recent, single institution experience with four GAS peripartum cases occurring over a 5‐year period | None |
| CDC, 1999 | July 1996–August 1997 | Retrospective case series | Single hospital, Maryland | Describe nosocomial outbreaks of GAS infection in Maryland during 1996–1997 | None |
| Chuang, 2002 | 1995–2002 | Retrospective cohort study | Various hospitals, USA | Quantify the burden of invasive postpartum GAS disease in a multistate population | None |
| Dan, 1990 | 1979–1986 | Retrospective case series | Two urban hospitals, Tel Aviv | Review our experience with the clinical spectrum produced by group A streptococcal bloodstream invasion and propose a practical classification of its various presentations | None |
| Daneman, 2005 | January 1, 1992–December 31, 2000 | Prospective, population‐based surveillance (Cohort) | All microbiology laboratories serving Ontario hospitals | Describe the epidemiology of hospital‐associated invasive GAS infections in Ontario and evaluate the risk of cross‐transmission in hospitals | None |
| Davis, 2010 | 1991–2009 | Prospective case–control | Two tertiary care centers in Salt Lake City, Utah | Investigate the association of innate immune response gene polymorphisms and puerperal group A streptococcal sepsis. | None |
| Denoude, 2005 | August 2, 2001–December 31, 2003 | Retrospective case series | Institute for Public Health Surveillance reporting across 18 establishments, France | Describe reported cases of nosocomial infections of invasive GAS | None |
| Deutscher, 2011 | 2007–2009 | Retrospective case–control | Population‐based multistate surveillance | Describe the burden and characteristics of infection in pregnant and postpartum women | To determine whether pregnant and postpartum women are at higher risk of pneumococcus, GAS and GBS infections and of complications from these infections, compared with nonpregnant women. |
| Dietz, 2003 | May–June 2002 | Retrospective case series | University Hospital, Belgium | Describe the course, diagnostics and treatment of a pseudo epidemic of puerperal GAS | Discuss measures to prevent epidemic and prevent further spread of infection |
| Eriksson, 2003 | November 1, 1996–October 31, 1997 | Prospective case–control | Laboratory‐based prospective surveillance of all public bacteriological laboratories in Sweden | Survey epidemiological and clinical characteristics of invasive GAS infections in Sweden during 1996–1997 | Study the spread of GAS clones among invasive and non‐invasive infections |
| Gordon, 1994 | Not reported | Retrospective case series | Maternity ward, UK | Report an outbreak of eight cases of GAS puerperal sepsis in which communal use of bidets during the postnatal period appears to have been responsible for the spread of infection. | None |
| Gustafson, 2017 | Retrospective case series | Denmark | To present the clinical differences in group A streptococcal infection | None | |
| Kaiser, 2018 | January 1991–September 2017 | Prospective case series | Registry from voluntary reporting by physician or microbial case log, Salt Lake City Region | Identify admission clinical and demographic characteristics associated with adverse outcomes including death, hysterectomy, ICU admission, mechanical ventilation and blood transfusion | Facilitate patient counseling, prognostication, and resource allocation |
| Knowles, 2015 | January 1, 2005–December 31, 2012 | Prospective cohort | Two tertiary referral maternity hospitals in Dublin, Ireland | Incidence, bacterial etiology, gestation/ stage at delivery, mode of delivery, antibiotic resistance, admission to augmented care, maternal, fetal and neonatal outcome | None |
| Lamagni, 2008 | January 1, 2003–December 31, 2004 | Prospective cohort | Strep‐EURO project: 11 participating European countries | Compare the epidemiological patterns of invasive S. pyogenes among the 11 participating countries in the Strep‐Euro project | None |
| LeBail, 2007 | January 1, 1997–December 31, 2005 | Prospective cohort | University hospitals, France | Incidence of postpartum infections with GAS in maternity hospitals | None |
| Leonard, 2019 | January 1, 2010–December 31, 2016 | Retrospective cross‐sectional | London and the South East of England | Describe postpartum invasive GAS infection including management of mothers and their neonates to determine whether public health guidelines have been followed | None |
| Lepoutre, 2011 | November 2006–November 2007 | Prospective cross‐sectional | Acute care hospitals (voluntary) across 22 administrative regions, France | Estimate the burden of invasive GAS infections with or without a positive blood culture, characterize the clinical presentations, assess predisposing factors and outcomes, describe the molecular characteristics and antibiotic susceptibility of GAS strains isolated from invasive infections and assess the level of implementation of the recommendations on antibiotic prophylaxis among household contacts. | None |
| Lev‐Sagie 2017 | February 2008–August 2010 | Prospective cohort | Tertiary care hospitals, Hadassah University, Israel | Evaluate the incidence of long‐term vaginal carriage of GAS among women with a prior infection | None |
| Luca‐Harari, 2008 | 2003–2004 | Prospective population‐based surveillance | Microbiology departments, Denmark | Understand the epidemiology of invasive GAS disease in Denmark | None |
| O'Loughlin, 2007 | January 1, 2000–December 31, 2004 | Population‐based surveillance | Centers for Disease Control and Preventions' Active Bacterial Cord surveillance, 10 US sites | Report the current epidemiologic characteristics of invasive GAS infections and estimate potential impact of a multivalent vaccine | None |
| Rottenstreich, 2019 | 2005–2017 | Retrospective cohort | University hospitals (2), Israel | Determine incidence, associated risk factors, clinical course and outcome of pregnancy‐related GAS infections | None |
| Safar, 2011 | January 1, 2005–December 31, 2006 | Prospective cohort | Auckland public hospital (metropolitan) surveillance data | Study the effects of invasive GAS on the Auckland population | Establish the direction of further investigations and focus interventions in New Zealand |
| Schuitemaker, 1998 | 1983–1992 | Retrospective case series | Maternal Mortality Committee, Central Bureau of Statistics and Dutch Perinatal Database systems | The effect of the changed epidemiology of GAS on maternal mortality was investigated | None |
| Shinar, 2016 | January 2008–May 2015 | Retrospective case series | Tertiary care center, Israel | Describe the epidemiologic and clinical characteristics of peripartum GAS infections in an attempt to develop better preventive strategies | None |
| Strobaek, 1991 | January 1, 1987 to December 31, 1989 | Retrospective cohort | 58 general hospitals in Denmark (of 99) and one hospital in Greenland | Determine whether the epidemic of GAS was reflected within the hospitals by nosocomial cases among hospitalized patients | None |
| Strus, 2010 | October 16–23, 2007 | Retrospective case series | Hospital of the Ministry of Internal Affairs and Administration in Krakow, Poland | To analyze the characteristics of the S. pyogenes strains involved in the outbreak in Krakow Poland, including the emm gene as well as genes coding for superantigens | Pulse field gel electrophoresis (PFGE) was used to determine the genetic relatedness among the isolates. The source of infection and probable routes of transmission of the outbreak strain were investigated using fluorescent in situ hybridization (FISH) as a rapid method for detecting S. pyogenes carriage |
| Tanaka, 2019 | January 2010–December 2016 | Retrospective case series | Japanese healthcare institutions | Provide an in‐depth analysis of the maternal death cases related to sepsis reported in Japan | None |
| Thewessen, 1999 | NR | Prospective case series | University hospital, Netherlands | Describe a cluster of patients with puerperal infection and report the epidemiological and microbiological investigation | None |
| Trell, 2020 | August–December, 2018 | Retrospective case series | Single hospital in Lund, Region Skaane, Sweden | Describe the use of whole genome sequencing to investigate an outbreak of postpartum S. pyogenes emm75 infections related to an asymptomatic carrier working in a maternity ward | None |
| Viglionese, 1991 | May 1987–April 1990 | Retrospective case series | Single center, Boston, Massachusetts | Report characteristics and typing of two clusters of group A streptococcal postpartum infections | None |
| Wahl, 2007 | 1996–2002 | Retrospective cohort | National Reference Center, Germany | Identify the predominant emm types among a large collection of S. pyogenes isolates from invasive infections | Define the incidence and demographical risk‐factors for acquisition of invasive S. pyogenes infection in Germany |
| Study, year | Inclusion criteria | Exclusion criteria | Comparison group | Total eligible patients | Total patients enrolled | Total GAS infection | Total patients included | Total controls (if applicable) |
|---|---|---|---|---|---|---|---|---|
| Acosta, 2014 | All peripartum women diagnosed with severe sepsis (including septic shock) | Incomplete data | Severe sepsis without septic shock | All women giving birth in the UK (no specific number) | 365 | 32 | 32 | 757 |
| Alberts, 2018 | Suspicion of obstetric infection or inter injury between 2010–2014 | None | None | 50 | 50 | 6 | 6 | 0 |
| Alexander, 2018 | Postpartum GAS | None | None | 5 | 5 | 5 | 5 | NA |
| Anteby, 1999 | Patients from OBGYN department diagnosed with intrapartum or puerperal group A streptococcal infection | None | General population of women giving birth during that time, extracted from computerized medical records | 25 822 | 3403 | 47 | 47 | |
| Aronoff, 2008 |
‐Hospitalized with invasive GAS disease and reported to the Florida Department of Health ‐Isolation of GAS from a normally sterile site and clinically compatible presentation ‐Endometritis/postpartum sepsis noted on epidemiology surveillance form and/or pregnancy/peripartum checked in risk factor portion of the form and infection within 42 days of delivery ‐Women carrying the diagnosis of postpartum invasive GAS disease who were residents of Florida |
None | None | 257 + 2643 | 257 + 2643 | 257 + 2643 | 7 | 0 |
| Barnham, 2001 | Mother and/or baby with detected S. pyogenes bacteremia | None | None | 11 000 | 9 | 9 | 5 | 0 |
| Bauer, 2015 | Maternal sepsis | None | None | 1 047 857 live births; 558 pregnancy‐associated deaths; 151 pregnancy‐related deaths | 22 | 4 | 4 | 0 |
| Bengner, 2019 | Invasive GAS infection in puerperium | None | None | 823 | 5 | 5 | 5 | 0 |
| Busowski, 2013 | Puerperal sepsis | None | None | 4 | 4 | 4 | 4 | 0 |
| CDC, 1999 | GAS isolated from nonpharyngeal site in patient whose symptoms began more than 12 hours after admission | None | Randomly selected patients on obstetric ward during study period | 12 | 12 | 12 | 9 | 5 |
| Chuang, 2002 | Isolation of GAS from a normally sterile site (eg blood or CSF) or from a wound tissue culture when accompanied by necrotizing fasciitis or TSS in a resident of a surveillance area who was pregnant or in the postpartum period (ie </= 30 days after delivery) or who had clinician‐defined puerperal fever, chorioamnionitis, or septic abortion. | Cases in which GAS was isolated from the amniotic fluid or the placenta alone. | None | 3957 | 87 | 87 | 87 | 0 |
| Dan, 1990 | Patients with GAS bacteremia | None | None | 36 | 33 | 33 | 5 | 0 |
| Daneman, 2005 | GAS isolated from sterile‐site specimen. Cases were identified as hospital‐acquired if disease was neither present nor incubating at the time of hospital admission. Specific definitions were given for surgical site and peripartum infections. | None | None | 2351 | 291 | 291 | 86 | 0 |
| Davis, 2010 | Cases demonstrated clinical evidence of endometritis and/or had febrile illness during the postpartum period. They also had at least one positive culture for group A streptococcus from a normally sterile body site. | Unable to contact or declined participation | Racially matched healthy women with a history of term, uncomplicated deliveries and no history of puerperal infection | 48 | 28 | 28 | 28 | 54 |
| Denoude, 2005 | Invasive infection of GAS in postoperative or postpartum patients | Community infections or infections outside of specific patient population | None | 32 | 29 | 29 | 18 | 0 |
| Deutscher, 2011 | Illness in a woman aged 15–44 years with streptococcus isolated from a normally sterile body site during 2007–2009. | None | Non‐pregnant women with GAS infection | 1848 | 1848 | 439 | 90 | 349 |
| Dietz, 2003 | Invasive infection of GAS in the puerperium | None | None | 5 | 5 | 5 | 5 | 0 |
| Eriksson, 2003 | Women with GAS isolated from a normally sterile site or from a superficial site in a patient who developed a necrotizing infection or STSS. Also included were women with clinical signs of endometritis postpartum and for whom GAS was isolated from the cervix. | None | The first five GAS isolates from throat and superficial skin infections from 3 laboratories during the first 7 months of surveillance | 255 | 255 | 255 | 20 | 144 |
| Gordon, 1994 | Mothers and infants with positive swabs for GAS whom were admitted or recently admitted to the maternity ward following the first identified case | None | None | 11 | 11 | 11 | 8 | 0 |
| Gustafson, 2017 | Postpartum GAS infection | None | None | 4 | 4 | 4 | 4 | 0 |
| Kaiser, 2018 | All patients with at least one positive culture for group A streptococci from a normally sterile body site (ie blood, urine, or endometrium) or non‐sterile body site (ie genital tract, wound) in addition to clinical suspicion for endometritis, defined as postpartum fever with no other alternative source identified | None | Women within the cohort were characterized into adverse and no adverse outcome groups* | 71 | 71 | 71 | 71 | 0 |
| Knowles, 2015 | All pregnant and postpartum women with sepsis | None | Patients without a diagnosis of sepsis | 136 897 | 276 | 12 | 12 | 136 625 |
| Lamagni, 2008 | Patients with S. pyogenes isolated from a normally sterile site or a nonsterile site in combination with clinical signs of STSS | None | None | 5522 | 3894 | 3894 | 107 | 0 |
| LeBail, 2007 | Women presenting with signs of clinical infection and GAS positive culture in the puerperium | None | None | 31 | 17 | 17 | 17 | 0 |
| Leonard, 2019 | All mothers or newborns with confirmed invasive GAS with onset within 28 days of birth, defined as either (1) isolated from a sterile site or (2) isolated from a normally nonsterile site but accompanied by a severe clinical presentation | None | None | 3 216 238 (1 598 069 live maternities) | 155 | 155 | 134 | 0 |
| Lepoutre, 2011 | Isolation of bacterium from a usually sterile site or from samples obtained from deep‐body‐site aspirates, intraoperative specimens, or a nonsterile site in association with one of the following clinical conditions: necrotizing fasciitis, clinically ascertained pneumonia, endometritis, salpingitis. or TSS not attributable to any other cause and defined according to the US Working Group on Severe Streptococcal Infections definitions. | None | None | 664 | 664 | 664 | 32 | 0 |
| Lev‐Sagie 2017 | Woman with prior vaginal GAS infection | None | Women admitted for labor without previous GAS infection, participating in a cross‐sectional study for the detection of vaginal group B streptococcus and GAS | 45 | 25 | 25 | 11 | 436 |
| Luca‐Harari, 2008 | All GAS isolation from blood or another normally sterile site of from non‐sterile sites with evidence of sepsis or sTSS recovered from hospitalized patients | None | None | 278 | 253 | 253 | 12 | 0 |
| O'Loughlin, 2007 | Isolation of GAS from a normally sterile site or from a wound specimen obtained from a surveillance area resident with necrotizing fasciitis or sTSS | None | Non | 5400 | 5400 | 5400 | 57 | 0 |
| Rottenstreich, 2019 | All symptomatic cases of culture‐proven pregnancy‐ related GAS infection | Asymptomatic patients or those with GAS positive cultures from the throat only | All other deliveries | 140 429 | 124 | 124 | 124 | 140 314 |
| Safar, 2011 | Patients with invasive GAS (isolate cultured from a previously sterile body cavity) residing in metropolitan Auckland | Women from whom GAS was isolated from amniotic fluid or placenta alone were excluded. Isolation of GAS from nonsterile site. Residence outside of metropolitan Auckland at the time of diagnosis. | None | 343 | 225 | 225 | 7 | 0 |
| Schuitemaker, 1998 | All cases of maternal death between 1983 and 1992 attributed to genital tract sepsis | None | None | 10 | 10 | 7 | 7 | 0 |
| Shinar, 2016 | All women with peripartum fever or marked abdominal tenderness and positive GAS cultures | None | None | 37 | 37 | 37 | 37 | 0 |
| Strobaek, 1991 | Patients with GAS bacteremia | No information from hospitals or when samples could not be traced back to patients. | None | 240 | 240 | 240 | 5 | 0 |
| Strus, 2010 | Patients with symptoms of suspected invasive GAS infection | None | Surgical procedures performed in same operating room period | 6 | 6 | 6 | 4 | 48 (10 CS, 38 other procedures) |
| Tanaka, 2019 | All cases of maternal death related to sepsis reported to the Japan Association of Obstetricians and Gynecologists | None | Maternal deaths related to infection from organisms other than GAS | 317 | 24 | 13 | 13 | 11 |
| Thewessen, 1999 | Women presenting with signs of clinical infection and GAS positive culture in the puerperium | None | None | 6 | 6 | 6 | 6 | 0 |
| Trell, 2020 | Women with signs and symptoms of postpartum infection in same maternity ward in southern Sweden | None | None | 6 | 6 | 6 | 6 | 0 |
| Viglionese, 1991 | Postpartum fever with positive GAS culture | None | Vaginal deliveries by physician at hospital | 9792 | 14 | 14 | 14 | 776 |
| Wahl, 2007 | Invasive GAS: isolation of S. pyogenes from either a normally sterile sample or from samples obtained from deep body site aspirates or intraoperatively. | Clinical data not available | None | 475 | 165 | 165 | 9 | 0 |
Figure 2 summarizes the reported patient outcomes by study (which are further detailed in Table S3). A total of 1160 patients with puerperal GAS infection were identified. Most infections occurred in the postpartum period (893/972; 91.9%), with 4.7% reported antepartum (46/972) and only 0.6% noted intrapartum (6/972) (Figure 3). Septic abortion (pregnancy loss secondary to infection <20 weeks gestational age) and complications from elective therapeutic abortion were both uncommon (22/972 [2.3%] and 5/972 [0.5%], respectively).
FIGURE 2.
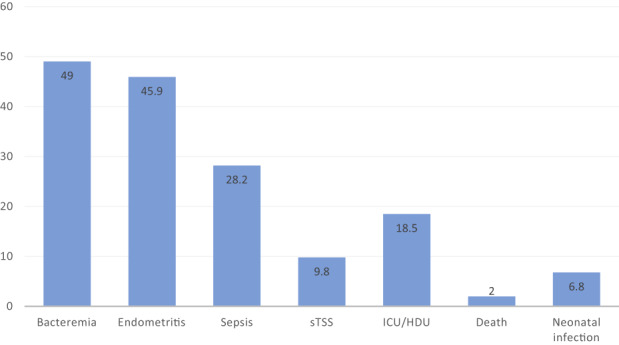
Carrier outcomes in puerperal group A streptococcal infections (%). sTSS: streptococcal toxic shock syndrome; ICU, intensive care unit; HDU high‐dependency care unit.
FIGURE 3.
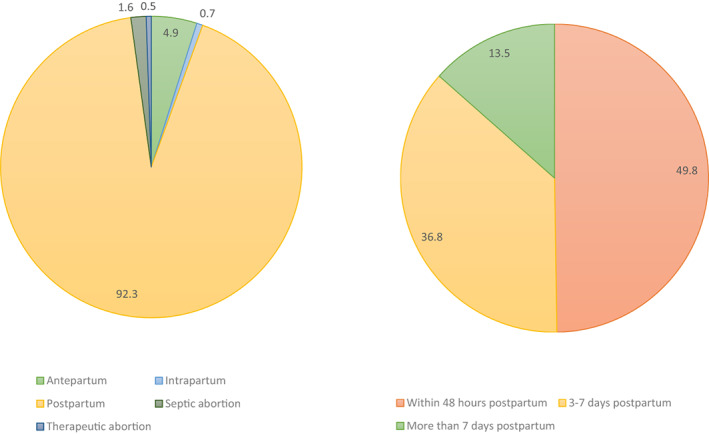
Timing of presentation in cases of group A streptococcal infection in pregnancy and postpartum (%).
When reported, the timing of antepartum infections was distributed across trimesters, with one infection in the first trimester, six in the second and six in the third. However, timing was not reported for more than two‐thirds of the antepartum infections (33/46; 71.2%). When details were provided, the majority of patients were described as healthy without pregnancy complications prior to infection. Streptococcal toxic shock syndrome (sTSS) was specifically described in three patients; however, a study by Tanaka et al. 41 reported an additional seven patients who died within 24 hours of presentation, suggesting advanced infection. Intrauterine fetal demise was diagnosed in 39.5% of pregnancies (17/44; 38.6%) and when combined with septic abortions, 57.4% of all cases resulted in not achieving a live birth (39/68). Stillbirth was most often diagnosed at time of admission to hospital.
Twenty‐one septic abortions and one septic ectopic pregnancy were described across six studies. All septic abortions underwent dilation and curettage as part of management of the infection. Three cases of sTSS were reported (3/22; 13.6%) with two progressing to death (2/22; 9%) despite surgical management of septic abortion.
Of the studies reporting patient characteristics in postpartum GAS infections, 74.5% (213/286) were multiparous, with the majority delivering vaginally (402/499; 80.6%) at term (238/273; 87.1%). Premorbid or delivery risk factors reported in studies were variable without a specific trend. However, the majority (81%) of patients in these studies had no reported risk factors for GAS infection. Approximately half (53%) of the studies reporting postpartum infections detailed time from delivery to presenting signs, with 49.9% within the first 48 hours (212/425), 36.5% within 3–7 days (155/425) and only 13.6% more than 7 days post‐delivery (58/425) (Figure 3).
Fever was the most common symptom associated with puerperal infection (297/333; 89.1%), with abdominal pain (106/333; 31.8%) and vaginal discharge (27/333; 8.1%) less frequently reported. Few details were given regarding presenting signs, but in a study by Kaiser et al., 30 comparing 71 patients with puerperal infection in terms of outcomes, they found that people presenting with tachycardia (relative risk [RR] 1.08, 95% CI 1.01–1.15, p = 0.02), hypotension (RR 0.93, 95% CI 0.89–0.97, p = 0.002), and tachypnea (RR 1.03, 95% CI 1.01–1.05, p ≤ 0.001) were more likely to experience adverse outcomes. In the two studies that detailed laboratory findings, leukocytosis and elevated C‐reactive protein (per laboratory criteria) were common, with leukocytosis reported in 81%–94% of patients 36 and elevated C‐reactive protein in 96%–100%. 38
Culture site was available for 682 patients (682/1160; 58.6%), with positive blood cultures in approximately half (338/682, 49.6%), genital tract (vaginal or cervical swab) in 43.7% (298/682) and positive cultures in more than one site in 15.4% (105/682). Positive urine (71/682; 10.4%) wound (21/682; 3.1%), intrabdominal (12/682; 1.8%), throat (10/682; 1.5%), endometrial (8/682; 1.2%) and anal cultures (2/682; 0.3%) were rare.
Of the studies reporting puerperal outcomes (Figure 2, Table S3), bacteremia was present in 49% of patients (382/780) and endometritis in 45.9% (283/617). Puerperal sepsis was described in 28.2% of cases (276/979) and progressed to streptococcal toxic shock syndrome (sTSS) in one‐third of such cases (92/942; 9.8%). Multiorgan complications included necrotizing fasciitis (n = 13), acute respiratory distress syndrome (ARDS) (9), acute liver failure (10), acute kidney injury (8), disseminated intravascular coagulopathy (10), pneumonia (3), septic embolization (1) and endocarditis (1).
Almost all studies (37/40) reported carrier death rate, with a case fatality rate of 2% (22 deaths among 1081 cases). Three case series only reported maternal deaths and therefore were not included in the case fatality ratio, given an unknown denominator of GAS infections. 19 , 41 , 49 However, they detailed another 24 deaths (including two septic abortions, nine antepartum, six postpartum infections, and seven timing unknown), which highlights the sinister nature of invasive GAS. 19 , 41 , 49 Details surrounding these additional cases are presented in Table S4. A third of the deaths were reported in cases presenting in the antenatal period, including 3/46 (6.5%) cases of septic abortion and 10/46 (21.7%) antenatal cases of GAS infection (Figure 4). Rapid deterioration occurred in 72.7% (8/11) of the antenatal cases, with death occurring within 36 hours of presentation. There were 18 deaths of the 866 postpartum cases reported (2.1%). Timing was not reported in 15 (15/46; 32.6%) cases of maternal death (Figure 4). There were strong themes of sepsis, sTSS and death within 48 hours when cases were analyzed qualitatively (Table S4). Very few studies described neonatal infections, with only 29 cases among 10 studies (29/427; 6.8%) and there were three reported neonatal deaths (3/427; 0.7%).
FIGURE 4.
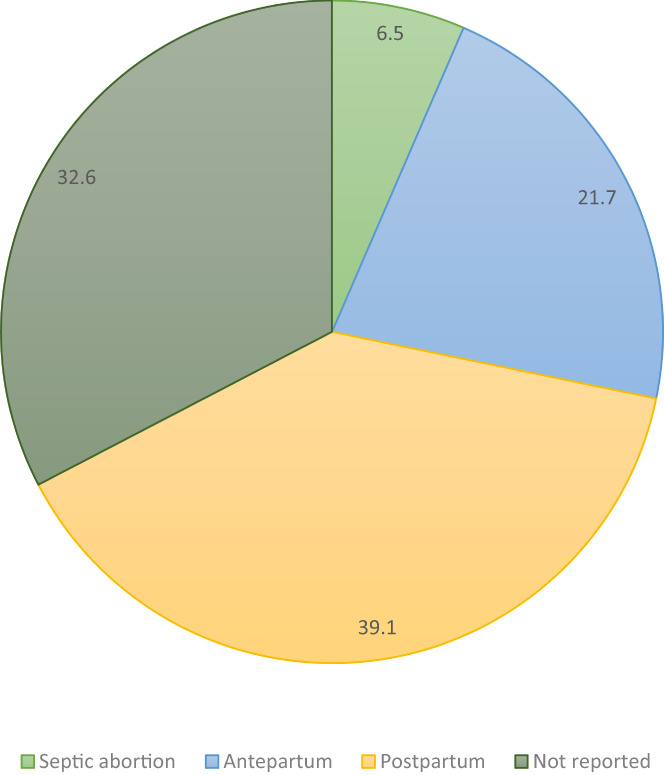
Maternal deaths by gestation at presentation (%).
In terms of management, 11 studies reported admission to an Intensive Care or High Dependency Unit in 18.5% of invasive puerperal GAS (91/492). Antibiotics were given in almost all cases (296/301; 98.3%) when management was described; however, there was a paucity of data on the time when antibiotics were administered, class of antibiotics used, time to clinical response, and duration of antibiotic course. Intravenous immunoglobulin (IVIG) was only employed in seven cases in two studies, 38 with Shinar et al. 38 describing use for the management of cases with severe complications including acute liver failure, acute renal failure, acute respiratory distress syndrome and respiratory failure. The outcomes of these cases were not specifically reported. Transfusion, mechanical ventilation and extracorporeal membrane oxygenation treatment were required in several cases. Surgical exploration occurred in 60 cases with 33 cases of hysterectomy (33/246; 13.4%), often with evidence of tissue necrosis on inspection (Table 2).
TABLE 2.
Studies reporting hysterectomy as management in puerperal group A streptococcal infections
| Study, Year | Hysterectomy n (%) | No hysterectomy n (%) | Total patients included in study | Details surrounding procedure |
|---|---|---|---|---|
| Alexander, 2018 | 0 | 5 (100) | 5 | ‐ No information on specific cases |
| Anteby, 1999 | 0 | 47 (100) | 47 | ‐ No information on specific cases |
| Aronoff, 2008 | 0 | 7 (100) | 7 | ‐ No information on specific cases |
| Bauer, 2015 | 0 | 4 (100) | 4 | ‐ No information on specific cases |
| Bengner, 2019 | 0 | 5 (100) | 5 | ‐ No information on specific cases |
| Busowski, 2013 | 2 (50) | 2 (50) | 4 | ‐ 24yo 2 days postpartum from uncomplicated SVD presenting with sTSS and NF requiring ICU admission. Blood and endometrial swab positive for GAS. Hysterectomy completed. Survived with multiple disabilities and limb amputations ‐27yo 7 days postpartum from uncomplicated SVD presenting with sTSS, NF, multi‐organ failure, thrombocytopenia and acute liver failure, requiring ICU admission. Hysterectomy on admission day 2. NF to abdominal wall following procedure requiring additional debridement. Survived and discharged after lengthy admission. |
| Davis, 2010 | 5 (17.9) | 23 (82.1) | 28 | ‐ No information on specific cases |
| Dietz, 2003 | 0 | 5 (100) | 5 | ‐ No information on specific cases |
| Gustafason, 2017 | 2 (50) | 2 (50) | 4 |
‐ 34yo 36 hours postpartum from SVD. Pregnancy complicated by cardiomyopathy. Presents with sTSS and NF requiring ICU admission. Blood and vaginal cultures positive for GAS. Multiple cardiac arrests. Hysterectomy. Survived with brain damage secondary to cardiac arrests ‐ 35yo 5 days postpartum from uncomplicated SVD. Presents with sTSS, NF and multi‐organ failure. Hysterectomy and resection of external genitalia due to NF. Blood cultures positive for GAS. Cardiac arrest requiring ECMO. Maternal death. |
| Kaiser, 2018 | 21 (29.6) | 50 (70.4) | 71 | ‐ Patients undergoing hysterectomy had significantly increased risk of capillary leakage (RR 21.77, 95% CI 3.09–153.54, p = 0.002) |
| Knowles, 2015 | 0 | 12 (100) | 12 | ‐ No information on specific cases |
| Lev‐Sagie 2017 | 0 | 11 (100) | 11 | ‐ No information on specific cases |
| Shinar, 2016 | 3 (8.1) | 34 (91.9) | 37 | ‐ Postpartum following uncomplicated SVD, no evidence of NF |
| Thewessen, 1999 | 0 | 6 (100) | 6 | ‐ No information on specific cases |
| Total | 33 (13.4) | 213 (86.6) | 246 |
Abbreviations: NF, necrotizing fasciitis; sTSS, streptococcal toxic shock syndrome.
Attempts were made to track infection source in less than half of the studies reported (18/40). A community source was reported in 43.4% (190/438), nosocomial source was reported in 30.6% (134/438), and unknown source in 26% (114/438).
4. DISCUSSION
This systematic review aligns with previous literature in that the vast majority of GAS infections occur in the postpartum population following uncomplicated vaginal birth in multiparous carriers at term. Half of the reported cases presented within 48 hours of delivery, with fever and uterine tenderness as common symptoms. Bacteremia (49%) and endometritis (45.9%) were common outcomes, with blood cultures and genital swabs positive in 49.6% and 43.7% of reported cases, respectively. Sepsis developed in 28.2% of patients and a third progressed to sTSS, totaling almost 10% of cases. The case fatality rate in this review (2%) is slightly lower compared with previous population studies from the early 2000s with rates of 3.5% 23 and 4.3%. 8 However, three of the case series detailing a substantial number of fatalities were not included due to unpublished data regarding total number of infections. Although improved recognition and management have likely contributed to better survival, the burden of GAS infection continues to be significant. Historical challenges in surveillance and reporting of GAS puerperal infection at a population level are likely to contribute to an underestimation rather than a fall in case fatality rates.
A third of the deaths were antenatal cases and there was also a disproportionate number of stillbirths in the antenatal infections compared with both postpartum and non‐GAS infections in the Tanaka et al. study. 41 These findings are consistent with a review by Hamilton et al. 51 of 10 antepartum case reports from 1974 to 2009 which suggests that infections during the antenatal period result in more devastating consequences than infections occurring postpartum. Rates of septic abortion (pregnancy loss secondary to infection <20 weeks gestational age) and complications from elective therapeutic abortion were low, which as above, could be an underestimation secondary to historical challenges in reporting and labelling.
From a diagnostic perspective, recto‐vaginal carriage rates of GAS in pregnant women are low at 0.03%. 52 , 53 , 54 This is compared with skin and throat swabs which reveal colonization of GAS in 5%–30% of the population. 55 At this time, screening for asymptomatic women in pregnancy is not recommended; however, given the excessive risk during this period, further studies on necessity, modality and cost analysis of screening are needed. 56 People presenting with postpartum fever and/or abdominal pain deserve a high level of suspicion for GAS. and investigation with blood and vaginal cultures should be strongly considered and is recommended per the most recent International Society for Infectious Disease in Obstetrics and Gynecology (ISIDOG). 56 Given the potential for severe disease and rapid deterioration, the development of rapid assays for detection of GAS from vaginal specimens could allow for timely diagnosis and appropriate antibiotic therapy. Further studies on the necessity and modality of screening and treatment are needed. Positive swab results should be interpreted seriously and managed swiftly given the potential for severe disease and complications.
The optimal management strategy for puerperal GAS infection remains unclear. Most patients in this review received antibiotics; however, the timing, type and duration were not often reported and cannot be linked to outcomes. The typical regimen for invasive GAS is intravenous penicillin with clindamycin to inhibit protein production in GAS bacteria in order to avoid superantigens and M proteins. 56 Penicillin and first‐generation cephalosporins are used routinely in pregnancy for prophylaxis in preterm premature rupture of membranes, group B streptococcus carriage, as well as surgical and instrumental births. However, a prophylactic course of antibiotics for group B streptococcus is insufficient for eradication of GAS (a 10‐day course of amoxicillin plus clavulanic acid or a first‐generation oral cephalosporin is recommended) and spontaneous clearance occurs in only half of patients. 56 Therefore, this practice is unlikely to change GAS prevalence rates, but it should be considered in future population surveillance studies. At present, resistance to penicillin in GAS organisms is non‐existent; however, resistance to macrolides is upwards of 25% in some populations with increased use of these antibiotics. 57 Given that macrolides are used as an adjuvant to penicillin in managing GAS, stewardship should be employed with antibiotic use in this population to avoid developing further resistance.
The benefit of IVIG in cases of sTSS remains unknown as the few studies exploring this intervention have conflicting results. 58 , 59 , 60 , 61 In this review, IVIG was employed in seven complicated cases across two studies; however, different outcomes were reported and therefore results could not be summarized. 36 , 38 Hysterectomy was not required in the majority of GAS cases (213/246; 86.6%), even in the context of puerperal sepsis and sTSS. In the described cases where hysterectomy was performed, there was often evidence of necrotizing fasciitis in addition to sepsis, sTSS and multiorgan failure. Most experts agree that a confirmed GAS infection in the presence of organ dysfunction should be managed surgically; 7 , 51 , 62 however, severe clinical presentations have not necessarily been shown to be predictive of severe adverse outcome in the literature. 30 In a case report by Dehaene et al., a sharp rise in creatinine kinase was suggested to be a marker of tissue destruction and indicator for hysterectomy. 63 Moving forward, active registries should include initiation and duration of antibiotic administration, adherence to the Surviving Sepsis guidelines, 64 employment of IVIG therapy, and clinical and/or laboratory predictors for risk stratification for escalation to surgical management as well as outcomes following hysterectomy including duration of antibiotics, length of hospital stay, and carrier morbidity and mortality.
Historically, GAS infection has been considered a hospital‐acquired infection; however, in our study only 30.5% were confirmed nosocomial infections. Therefore, acquisition of GAS in the community is increasingly important and could be considered a target for prevention, intervention and screening. 65
The strengths of this review include the extensive search across international databases, inclusion of articles in all languages and combining more than 1000 cases. Further, international definitions for infection and sepsis were employed consistently across all studies (Table S5). Recent studies were often explicit in defining cases; however, older studies may have used common terms such as “puerperal sepsis” more loosely, which would overestimate cases of sepsis. This review was limited by the inability to perform a meta‐analysis due to dominant study design and heterogeneity. Further, the specific outcomes of interest were not universally reported across all studies and therefore the denominator for assessment is variable in studies reporting that outcome only. This review is also limited by the quality of the older studies, which were mostly large case series. Lastly, all of the studies included were performed in high‐income countries, which limits our ability to generalize the findings and recommendations to low‐ and middle‐income countries. Further study with broader geographical representation is important to guide national and international recommendations for management and public health intervention.
5. CONCLUSION
GAS infection remains an important contributor to pregnancy and puerperal morbidity and mortality despite advances in infection control protocols. There continues to be a paucity of data to guide management strategies; however, early recognition and diagnosis, aggressive management with fluid resuscitation and antibiotic therapy, and consideration of source control are expected to be important for favorable outcomes given the serious risk of sepsis and progression to sTSS.
CONFLICT OF INTEREST
None.
Supporting information
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Harris K, Proctor LK, Shinar S, Philippopoulos E, Yudin MH, Murphy KE. Outcomes and management of pregnancy and puerperal group A streptococcal infections: A systematic review. Acta Obstet Gynecol Scand. 2023;102:138‐157. doi: 10.1111/aogs.14500
REFERENCES
- 1. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323‐e333. [DOI] [PubMed] [Google Scholar]
- 2. Acosta CD, Kurinczuk JJ, Lucas DN, et al. Severe maternal sepsis in the UK, 2011‐2012: a national case‐control study. PLoS Med. 2014;11:e1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acosta CD, Knight M, Lee HC, Kurinczuk JJ, Gould JB, Lyndon A. The continuum of maternal sepsis severity: incidence and risk factors in a population‐based cohort study. PLoS One. 2013;8:e67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuffnell D, Rodger A, Lucas N, Lucas S, Knight M, MBRRACE‐UK Sepsis Chapter‐Writing Group . Messages for prevention and treatment of infection. In: Knight M, Bunch K, Tuffnell D, et al., eds. Saving Lives, Improving Mothers' Care ‐ lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2016‐18. National Perinatal Epidemiology Unit, University of Oxford; 2020:64‐70. [Google Scholar]
- 5. Kramer HMC, Schutte JM, Zwart JJ, Schuitemaker NWE, Steegers EAP, van Roosmalen J. Maternal mortality and severe morbidity from sepsis in The Netherlands. Acta Obstet Gynecol Scand. 2009;88:647‐653. [DOI] [PubMed] [Google Scholar]
- 6. Centre for Maternal and Child Enquiries (CMACE) . Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006‐08. The eighth report on confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118:1‐203. [DOI] [PubMed] [Google Scholar]
- 7. Rimawi BH, Soper DE, Eschenbach DA. Group A streptococcal infections in obstetrics and gynecology. Clin Obstet Gynecol. 2012;55:864‐874. [DOI] [PubMed] [Google Scholar]
- 8. O'Loughlin RE, Roberson A, Cieslak PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000‐2004. Clin Infect Dis. 2007;45:853‐862. [DOI] [PubMed] [Google Scholar]
- 9. Davies HF, McGeer A, Schwartz B, et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario group A streptococcal study group. N Engl J Med. 1996;335:547‐554. [DOI] [PubMed] [Google Scholar]
- 10. Luca‐Harari B, Ekelund K, van der Linden M, Staum‐Kaltoft M, Hammerum AM, Jasir A. Clinical and epidemiological aspects of invasive streptococcus pyogenes infections in Denmark during 2003 and 2004. J Clin Microbiol. 2008;46:79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deutscher M, Lewis M, Zell ER, Taylor TH Jr, Van Beneden C, Schrag S. Active bacterial core surveillance team. Clin Infect Dis. 2011;53:114‐123. [DOI] [PubMed] [Google Scholar]
- 12. Olp RJ, Chamales IA, Schmiedecke SS. A case study of puerperal group A streptococcal infection complicated by toxic shock syndrome. AJP Rep. 2020;10:e1‐e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newcastle‐Ottawa quality assessment form for cohort studies. https://www.ncbi.nlm.nih.gov/books/NBK115843/bin/appe‐fm3.pdf (Accessed February 1, 2021).
- 14. Newcastle‐Ottawa quality assessment form for cohort studies. https://www.ncbi.nlm.nih.gov/books/NBK294460/bin/appb‐fm4.pdf (Accessed February 1, 2021).
- 15. Alexander AJ, Myers C, Beres SB, Olsen RJ, Musser JM, Mangino JE. Postpartum group A streptococcus case series: reach out to infection prevention! Open Forum Infect Dis. 2018;5:ofy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anteby EY, Yagel S, Hanoch J, Shapiro M, Moses AE. Puerperal and intrapartum group A streptococcal infection. Infect Dis Obstet Gynecol. 1999;7:276‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aronoff DM, Mulla ZD. Postpartum invasive group A streptococcal disease in the modern era. Infect Dis Obstet Gynecol. 2008;2008:796892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnham MR, Weightman NC. Bacteraemic streptococcus pyogenes infection in the peri‐partum period: now a rare disease and prior carriage by the patient may be important. J Infect. 2001;43:173‐176. [DOI] [PubMed] [Google Scholar]
- 19. Bauer ME, Lorenz RP, Bauer ST, Rao K, Anderson FWJ. Maternal deaths due to sepsis in the state of Michigan, 1999‐2006. Obstet Gynecol. 2015;126:747‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bengnér M, Mernelius S, Claus CN, Gunnervik C, Ros A. Utbrott av barnsängsfeber på två förlossningsavdelningar. [Two nosocomial outbreaks of group A streptococcal puerperal sepsis]. Lakartidningen. 2019;116:FR9M. [PubMed] [Google Scholar]
- 21. Busowski MT, Lee M, Busowski JD, Akhter K, Wallace MR. Puerperal group A streptococcal infections: a case series and discussion. Case Rep Med. 2013;2013:751329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention . Nosocomial group A streptococcal infections associated with asymptomatic health‐care workers‐Maryland and California, 1997. MMWR Morb Mortal Wkly Rep. 1999;48:163‐166. [PubMed] [Google Scholar]
- 23. Chuang I, Van Beneden C, Beall B, Schuchat A. Population‐based surveillance for postpartum invasive group A streptococcal infections, 1995‐2000. Clin Infect Dis. 2002;35:665‐670. [DOI] [PubMed] [Google Scholar]
- 24. Dan M, Maximova S, Siegman‐Igra Y, Gutman R, Rotmensch HH. Varied presentations of sporadic group A streptococcal bacteremia: clinical experience and attempt at classification. Rev Infect Dis. 1990;12:537‐542. [DOI] [PubMed] [Google Scholar]
- 25. Daneman N, McGeer A, Low DE, Tyrrell G, Simor AE, McArthur M. Hospital‐acquired invasive group A streptococcal infections in Ontario, Canada, 1992‐2000. Clin Infect Dis. 2005;41:334‐342. [DOI] [PubMed] [Google Scholar]
- 26. Davis SM, Clark EAS, Nelson LT, Silver RM. The association of innate immune response gene polymorphisms and puerperal group A streptococcal sepsis. Am J Obstet Gynecol. 2010;202(308):e1‐e8. [DOI] [PubMed] [Google Scholar]
- 27. Eriksson BKG, Norgren M, McGregor K, Spratt BG, Normark BH. Group A streptococcal infections in Sweden: a comparative study of invasive and non‐invasive infections and analysis of dominant T28 emm28 isolates. Clin Infect Dis. 2003;37:1189‐1193. [DOI] [PubMed] [Google Scholar]
- 28. Gordon G, Dale BA, Lochhead D. An outbreak of group A haemolytic streptococcal puerperal sepsis spread by the communal use of bidets. Br J Obstet Gynaecol. 1994;101:447‐448. [DOI] [PubMed] [Google Scholar]
- 29. Gustafson LW, Blaakær J, Helmig RB. Group A streptococci infection. A systematic clinical review exemplified by cases from an obstetric department. Eur J Obstet Gynecol Reprod Biol. 2017;215:33‐40. [DOI] [PubMed] [Google Scholar]
- 30. Kaiser JE, Bakian aV, Silver RM, Clark EAS. Clinical variables associated with adverse maternal outcomes in puerperal group A streptococci infection. Obstet Gynaecol. 2018;132:179‐184. [DOI] [PubMed] [Google Scholar]
- 31. Knowles SJ, O'Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015;122:663‐671. [DOI] [PubMed] [Google Scholar]
- 32. Lamagni TL, Darenberg J, Luca‐Harari B, et al. Epidemiology of severe streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46:2359‐2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leonard A, Wright A, Saavedra‐Campos M, et al. Severe group A streptococcal infections in mothers and their newborns in London and the south east, 2010‐2016: assessment of risk and audit of public health management. BJOG. 2019;126:44‐53. [DOI] [PubMed] [Google Scholar]
- 34. Lepoutre A, Doloy A, Bidet P, et al. Epidemiology of invasive streptococcus pyogenes infections in France in 2007. J Clin Microbiol. 2011;49:4094‐4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lev‐Sagie A, Hochner‐Celnikier D, Stroumsa D, Khalaileh A, Daum H, Moses AE. Group A streptococcus: is there a genital carrier state in women following infection? Eur J Clin Microbiol Infect Dis. 2017;36:91‐93. [DOI] [PubMed] [Google Scholar]
- 36. Rottenstreich A, Benenson S, Levin G, Kleinstern G, Moses AE, Amit S. Risk factors, clinical course and outcomes of pregnancy‐related group A streptococcal infections: retrospective 13‐year cohort study. Clin Microbiol Infect. 2019;25:251.e1‐251.e4. [DOI] [PubMed] [Google Scholar]
- 37. Safar A, Lennon D, Stewart J, et al. Invasive group A streptococcal infection and vaccine implications, Auckland, New Zealand. Emerg Infect Dis. 2011;17:983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shinar S, Fouks Y, Amit S, et al. Clinical characteristics of and preventative strategies for Peripartum group A streptococcal infections. Obstet Gynecol. 2016;127:227‐232. [DOI] [PubMed] [Google Scholar]
- 39. Strøbaek S, Zimakoff J, Kristensen KF, Borgen H, Sørensen L. Udredning of en epidemi ved hjoelp af en case‐kontrolundersøgelse. [puerperal fever. A survey of an epidemic using a case‐controlled study]. Ugeskr Laeger. 1997, 22;159:4117. [PubMed] [Google Scholar]
- 40. Strus M, Drzewiecki A, Chmielarczyk A, Tomusiak A, Romanek P, Kosowski K. Microbiological investigation of a hospital outbreak of invasive group A streptococcal disease in Krakow, Poland. Clin Microbiol Infect. 2010;16:1442‐1447. [DOI] [PubMed] [Google Scholar]
- 41. Tanaka H, Katsuragi S, Hasegawa J, et al. The most common causative bacteria in maternal sepsis‐related deaths in Japan were group A streptococcus: a nationwide survey. J Infect Chemother. 2019;25:41‐44. [DOI] [PubMed] [Google Scholar]
- 42. Trell K, Jorgensen J, Rasmussen M, Senneby E. Management of an outbreak of postpartum streptococcus pyogenes emm75 infections. J Hosp Infect. 2020;105:752‐756. [DOI] [PubMed] [Google Scholar]
- 43. Viglionese A, Nottebart VF, Bodman HA, Platt R. Recurrent group A streptococcal carriage in a health care worker associated with widely separated nosocomial outbreaks. Am J Med. 1991;91:329S‐333S. [DOI] [PubMed] [Google Scholar]
- 44. Wahl RU, Lütticken R, Stanzel S, van der Linden M, Reinert RR. Epidemiology of invasive streptococcus pyogenes infections in Germany, 1996‐2002: results from a voluntary laboratory surveillance system. Clin Microbiol Infect. 2007;13:1173‐1178. [DOI] [PubMed] [Google Scholar]
- 45. Alberts A, Blanck Olerup A, Gustafson P. Klassisk barnsängsfeber existerar fortfarande ‐ 9 fall bland anmälningar till LÖF under 2010‐2014. Lakartidningen. 2018;115:EUU9. [PubMed] [Google Scholar]
- 46. Denoeud L, Lepoutre A, Bouvet A, Coignard B. Signalements d'infections nosocomiales invasives à Streptococcus pyogenes en post‐opératoire ou post‐partum en France du 1er août 2001 au 31 décembre 2003. Behaviour. 2005;33:165‐166. [Google Scholar]
- 47. Dietz V, Derks JB, Mascini EM, Bruinse HW. A pseudo‐epidemic of puerperal sepsis. Ned Tijdschr Geneeskd. 2003;147:2505‐2508. [PubMed] [Google Scholar]
- 48. Le Bail M, Pouedras P, Quemener A. Infections du post‐partum àstreptocoque béta‐hémolytique du groupe A: résultats d'une étude épidémiologique prospective. Hygiènes. 2007;15:51‐56. [Google Scholar]
- 49. Schuitemaker N, van Roosmalen J, Dekker G, van Dongen P, van Geijn H, Gravenhorst JB. Increased maternal mortality in The Netherlands from group A streptococcal infections. Eur J Obstet Gynecol Reprod Biol. 1998;76:61‐64. [DOI] [PubMed] [Google Scholar]
- 50. Thewessen EAPM, Bontekoe‐Hoornstra J, Smelting‐Nagtzaam AE, Bilkert‐Mooiman MA, Admiraal JF. Een cluster van patiënten met kraamvrouwenkoorts in Gouda; een hernierwde les van Semmelweis. Ned Tijdschr Geneeskd. 1999;143:1700‐1705. [PubMed] [Google Scholar]
- 51. Hamilton SM, Stevens DL, Bryant AE. Pregnancy‐related group A streptococcal infections: temporal relationships between bacterial acquisition, infection onset, clinical findings, and outcome. Clin Infect Dis. 2013;57:870‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mead PB. Streptococcal screening obstetrics. Obstet Gynecol. 2001;98:721‐723. [DOI] [PubMed] [Google Scholar]
- 53. Saab J, Bell SM, Lahra MM. Vaginal carriage rate of streptococcal pyogenes in 1600 pregnant women. Pathology. 2012;44:567‐568. [DOI] [PubMed] [Google Scholar]
- 54. Hassan IA, Onon TS, Weston D, et al. A quantitative descriptive study of the prevalence of carriage (colonisation) of haemolytic streptococci groups a, B, C and G in pregnancy. J Obstet Gynaecol. 2011;31:207‐209. [DOI] [PubMed] [Google Scholar]
- 55. Tanz RR, Shulman ST. Chronic pharyngeal carriage of group A streptococci. Pediatr Infect Dis J. 2007;26:175‐176. [DOI] [PubMed] [Google Scholar]
- 56. Donders G, Greenhouse P, Donders F, Engel U, Paavonen J, Mendling W. Genital tract GAS infection ISIDOG guidelines. J Clinl Med. 2021;10:2043‐2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Desjardins M, Delgaty KL, Ramotar K, Seetaram C, Toye B. Prevalence and mechanisms of erythromycin resistance in group A and group B streptococcus: implications for reporting susceptibility results. J Clin Microbiol. 2004;42:5620‐5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Darenberg J, Ihendyane N, Sjölin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double‐blind, placebo‐controlled trial. Clin Infect Dis. 2003;37:333‐340. [DOI] [PubMed] [Google Scholar]
- 59. Madsen MB, Hjortrup PB, Hansen MB, et al. Immunoglobulin G for patients with necrotising soft tissue infection (INSTINCT): a randomised, blinded, placebo‐controlled trial. Intensive Care Med. 2017;43:1585‐1593. [DOI] [PubMed] [Google Scholar]
- 60. Parks T, Wilson C, Curtis N, Curtis N, Norrby‐Teglund A, Sriskandan S. Polyspecific intravenous immunoglobulin in clindamycin‐treated patients with streptococcal toxic shock syndrome: a systematic review and meta‐analysis. Clin Infect Dis. 2018;67:1434‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hua C, Bosc R, Sbidian E, et al. Interventions for necrotizing soft tissue infections in adults. Cochrane Database Syst Rev. 2018;5:CD011680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anderson BL. Puerperal group A streptococcal infection. Obstet Gynecol. 2014;123:874‐882. [DOI] [PubMed] [Google Scholar]
- 63. Dehaene I, Loccufier A, Temmerman M, Keersmaecker D, De Baene L. Creatinine kinase as an indicator for hysterectomy in postpartum endomyometritis due to group A streptococci: a hypothesis illustrated by a case report. Gynecol Obstet Invest. 2012;73:82‐88. [DOI] [PubMed] [Google Scholar]
- 64. Howell MD, Davis AM. Management of sepsis and septic shock. JAMA. 2017;317:847‐848. [DOI] [PubMed] [Google Scholar]
- 65. Cohen R, Cohen S, Afraimov M, et al. Screening asymptomatic households for streptococcus pyogenes pharyngeal carriage as a part of in‐hospital investigation of puerperal sepsis. Am J Infect Control. 2019;47:1493‐1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.


